Abstract
We examined the ability of the plasma of a 52-yr-old male Tangier patient to effect the conversion of radiolabeled pro-apolipoprotein A-I (apo A-I), isolated from hepatoma cell culture media, into mature apo A-I. The conversion was assessed by amino-terminal sequence analysis, isoform patterns with two-dimensional gel electrophoresis, and a rapid assay based on the different solubilities of intact pro-apo A-I and its hexapeptide prosegment in 10% trichloroacetic acid. We found that the converting activity of Tangier plasma was comparable to that exhibited by control normolipidemic plasma and that in both cases pro-apo A-I was correctly processed at the Gln-Asp bond. After ultracentrifugal fractionation of Tangier plasma at d = 1.21 g/ml, the pro-apo A-I-to-mature apo A-I converting activity was mainly recovered in the middle fraction of d = 1.225 g/ml and was at least 10-fold more effective than the top and bottom fractions. In contrast, in normal plasma the activity was only present in the top and bottom fractions. It has been previously established that in Tangier plasma the pro-apo A-I/apo A-I ratio is significantly higher than normal (1 vs. 0.02). Our studies suggest that this abnormal ratio is not the result of a reduced converting enzyme activity and may relate to differences in turnover rates between Tangier and normal plasma apolipoproteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer H. B., Jr, Fairwell T., LaRue A., Ronan R., Houser A., Bronzert T. J. The amino acid sequence of human APOA-I, an apolipoprotein isolated from high density lipoproteins. Biochem Biophys Res Commun. 1978 Feb 14;80(3):623–630. doi: 10.1016/0006-291x(78)91614-5. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Fairwell T., Meng M., Kay L., Ronan R. Human proapoA-ITangier: isolation of proapoA-ITangier and amino acid sequence of the propeptide. Biochem Biophys Res Commun. 1983 Jun 29;113(3):934–940. doi: 10.1016/0006-291x(83)91088-4. [DOI] [PubMed] [Google Scholar]
- Edelstein C., Gordon J. I., Toscas K., Sims H. F., Strauss A. W., Scanu A. M. In vitro conversion of proapoprotein A-I to apoprotein A-I. Partial characterization of an extracellular enzyme activity. J Biol Chem. 1983 Oct 10;258(19):11430–11433. [PubMed] [Google Scholar]
- Gordon J. I., Sims H. F., Lentz S. R., Edelstein C., Scanu A. M., Strauss A. W. Proteolytic processing of human preproapolipoprotein A-I. A proposed defect in the conversion of pro A-I to A-I in Tangier's disease. J Biol Chem. 1983 Mar 25;258(6):4037–4044. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
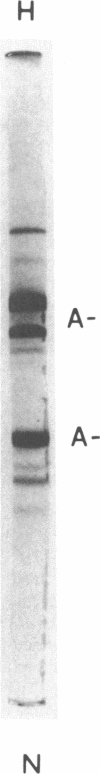
- Kay L. L., Ronan R., Schaefer E. J., Brewer H. B., Jr Tangier disease: a structural defect in apolipoprotein A-I (apoA-I Tangier). Proc Natl Acad Sci U S A. 1982 Apr;79(8):2485–2489. doi: 10.1073/pnas.79.8.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Law S. W., Gray G., Brewer H. B., Jr cDNA cloning of human apoA-I: amino acid sequence of preproapoA-I. Biochem Biophys Res Commun. 1983 Apr 15;112(1):257–264. doi: 10.1016/0006-291x(83)91824-7. [DOI] [PubMed] [Google Scholar]
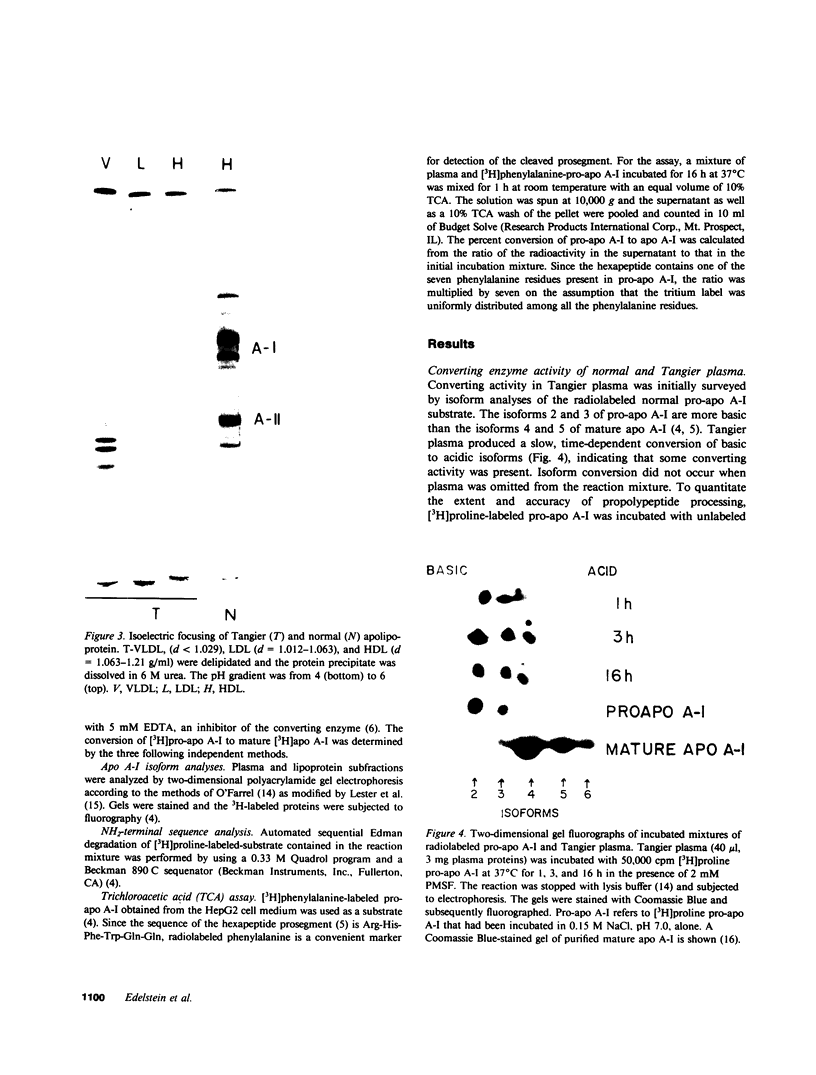
- Lester E. P., Lemkin P., Lipkin L., Cooper H. L. Computer-assisted analysis of two-dimensional electrophoreses of human lymphoid cells. Clin Chem. 1980 Sep;26(10):1392–1402. [PubMed] [Google Scholar]
- Lopes-Virella M. F., Stone P., Ellis S., Colwell J. A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977 May;23(5):882–884. [PubMed] [Google Scholar]
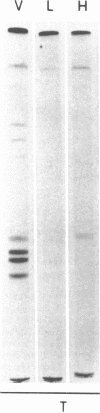
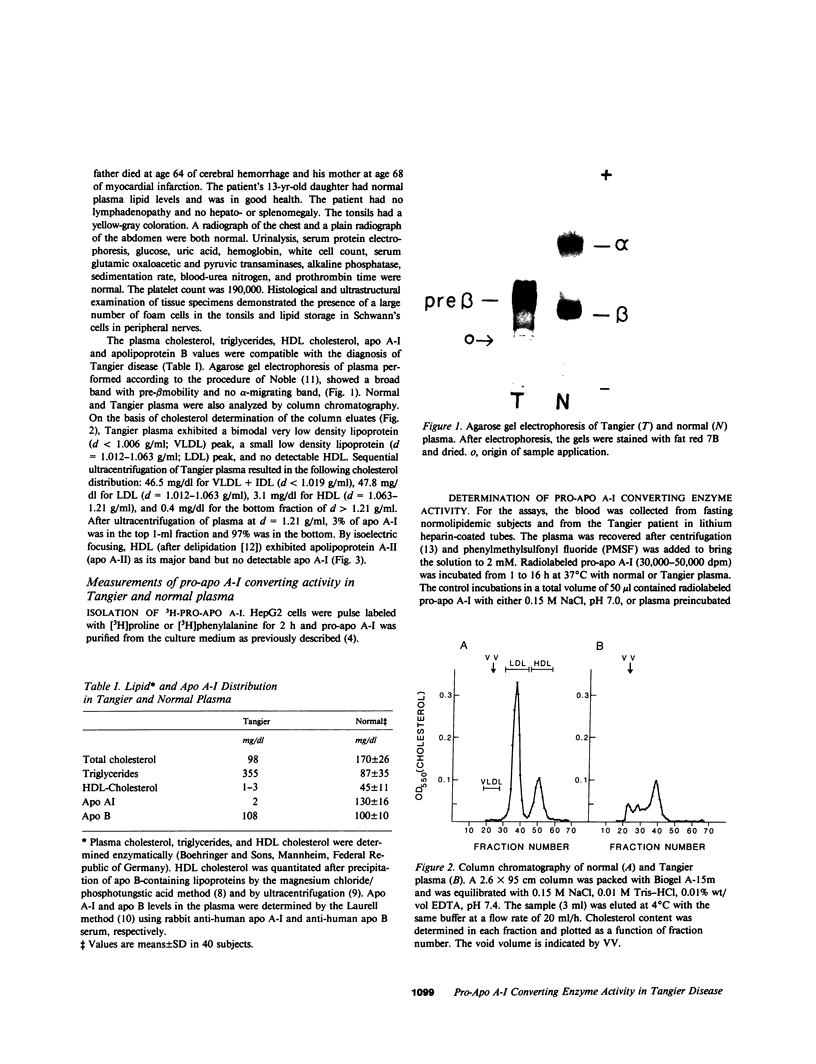
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Polacek D., Edelstein C., Scanu A. M. Rapid fractionation of human high density apolipoproteins by high performance liquid chromatography. Lipids. 1981 Dec;16(12):927–929. doi: 10.1007/BF02534999. [DOI] [PubMed] [Google Scholar]
- Scanu A. Forms of human serum high density lipoprotein protein. J Lipid Res. 1966 Mar;7(2):295–306. [PubMed] [Google Scholar]
- Schaefer E. J., Blum C. B., Levy R. I., Jenkins L. L., Alaupovic P., Foster D. M., Brewer H. B., Jr Metabolism of high-density lipoprotein apolipoproteins in Tangier disease. N Engl J Med. 1978 Oct 26;299(17):905–910. doi: 10.1056/NEJM197810262991701. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Kay L. L., Zech L. A., Brewer H. B., Jr Tangier disease. High density lipoprotein deficiency due to defective metabolism of an abnormal apolipoprotein A-i (ApoA-ITangier). J Clin Invest. 1982 Nov;70(5):934–945. doi: 10.1172/JCI110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Assmann G., Rall S. C., Jr, Mahley R. W. Tangier disease: defective recombination of a specific Tangier apolipoprotein A-I isoform (pro-apo A-i) with high density lipoproteins. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6081–6085. doi: 10.1073/pnas.80.19.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani C. G., Plancher A. C., Zuin M., Cattaneo M., Tramaloni C., Maccari S., Roma P., Catapano A. L. Bile lipid composition and haemostatic variables in a case of high density lipoprotein deficiency (Tangier disease). Eur J Clin Invest. 1984 Feb;14(1):49–54. doi: 10.1111/j.1365-2362.1984.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Karathanasis S. K., Keutmann H. T., Goldberger G., Breslow J. L. Intracellular and extracellular processing of human apolipoprotein A-I: secreted apolipoprotein A-I isoprotein 2 is a propeptide. Proc Natl Acad Sci U S A. 1983 May;80(9):2574–2578. doi: 10.1073/pnas.80.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis V. I., Lees A. M., Lees R. S., Breslow J. L. Abnormal apoprotein A-I isoprotein composition in patients with Tangier disease. J Biol Chem. 1982 May 10;257(9):4978–4986. [PubMed] [Google Scholar]