Abstract
Most patients relapse to opioids within one month of opioid agonist detoxification, making the antecedents and parallel processes of first use critical for investigation. Craving and withdrawal are often studied in relationship to opioid outcomes, and a novel analytic strategy applied to these two phenomena may indicate targeted intervention strategies. Specifically, this secondary data analysis of the Prescription Opioid Addiction Treatment Study used a discrete-time mixture analysis with time-to-first opioid use (survival) simultaneously predicted by craving and withdrawal growth trajectories. This analysis characterized heterogeneity among prescription opioid-dependent individuals (N=653) into latent classes (i.e., latent class analysis [LCA]) during and after buprenorphine/naloxone stabilization and taper. A 4-latent class solution was selected for overall model fit and clinical parsimony. In order of shortest to longest time-to-first use, the 4 classes were characterized as 1) high craving and withdrawal 2) intermediate craving and withdrawal 3) high initial craving with low craving and withdrawal trajectories and 4) a low initial craving with low craving and withdrawal trajectories. Odds ratio calculations showed statistically significant differences in time-to-first use across classes. Generally, participants with lower baseline levels and greater decreases in craving and withdrawal during stabilization combined with slower craving and withdrawal rebound during buprenorphine taper remained opioid-free longer. This exploratory work expanded on the importance of monitoring craving and withdrawal during buprenorphine induction, stabilization, and taper. Future research may allow individually tailored and timely interventions to be developed to extend time-to-first opioid use.
Keywords: opioid, relapse, buprenorphine, latent class, craving, withdrawa
1. Introduction
A majority of patients (>70%) receiving treatment for opioid dependence relapse to opioids within 6 months following detoxification from agonist therapy (Chutuape, Jasinski, Fingerhood, & Stitzer, 2001; Tuten, DeFulio, Jones, & Stitzer, 2011), and most relapses occur within 1 month (Gossop, Stewart, Browne, & Marsden, 2002). Despite clinical guidelines for opioid-dependent patients to remain on long-term pharmacotherapy (Amato, Davoli, Ferri, Gowing, & Perucci, 2004; Stotts, Dodrill, & Kosten, 2009), many patients seek, and community treatment providers often insist upon, agonist detoxification (Mannelli et al., 2009; Peterson et al., 2010). Data support that sustained opioid abstinence is an attainable goal with appropriate intervention (Amato, Minozzi, Davoli, & Vecchi, 2011; Donovan et al., 2012; Smyth et al., 2005; Stotts et al., 2012); however, relapse rates are still quite high (Amato, et al., 2011).
Opioid agonist treatment and detoxification encompasses numerous stages, including pharmacotherapy induction, stabilization, taper, and ultimately pharmacological cessation. The first use of an illicit opioid following initiation of agonist detoxification is often an indicator that a relapse to pre-treatment opioid use is imminent (Gossop, Marsden, Stewart, & Treacy, 2001; Gossop, et al., 2002; Gruber, Delucchi, Kielstein, & Batki, 2008; Kertesz, Horton, Friedmann, Saitz, & Samet, 2003). While not discounting individuals who engage in isolated one-time use (i.e., a “slip”) or several slips and then re-establish abstinence (Gossop, et al., 2002), the overwhelming majority of opioid users who engage in a first “slip” will go on to relapse. Thus, a better understanding of the antecedents and parallel processes that accompany first use may assist in meaningfully advancing opioid-dependence treatment.
Craving and withdrawal have long been studied as potential moderators and mediators of opioid-use outcomes (Gowing, Ali, & White, 2009; Hyman, Fox, Hong, Doebrick, & Sinha, 2007; Kosten, Schottenfeld, Ziedonis, & Falcioni, 1993; Krupitsky et al., 2011; McMillan & Gilmore-Thomas, 1996; Rounsaville, Kosten, & Kleber, 1985; Scherbaum, Heppekausen, & Rist, 2004; Sinha, 2007; Soyka, Zingg, Koller, & Kuefner, 2008; Strobbe, Brower, & Galen, 2003; Wasan et al., 2009; Whitley et al., 2010; Ziedonis et al., 2009). Craving is hypothesized to play a central role in relapse to opioids (Hyman, et al., 2007; McMillan & Gilmore-Thomas, 1996; Ren, Shi, Epstein, Wang, & Lu, 2009; Sinha, 2007; Wasan, et al., 2009). For example, individuals on non-therapeutic doses of medications used to treat opioid dependence (e.g., 1-mg of buprenorphine), placebo, or medications currently considered less efficacious (e.g., clonidine) often report greater levels of craving and experience earlier dropout or relapse (Fudala et al., 2003; Krupitsky, et al., 2011; Ling et al., 2005; Ling et al., 1998) compared to individuals on therapeutic doses of opioid-agonist medications (Dole, 1994).
Similar to craving, withdrawal symptoms tend to be lower in groups receiving efficacious opioid-agonist treatment compared to those on inadequate or no pharmacotherapy (e.g., Oreskovich et al., 2005). Many studies have reported that withdrawal severity may significantly relate to opioid-use outcomes (Gowing, et al., 2009; Kosten, et al., 1993; Soyka, et al., 2008; Whitley, et al., 2010; Ziedonis, et al., 2009), such that individuals with withdrawal symptoms that quickly abate or decline after receiving treatment often fare better, especially when receiving buprenorphine (Gowing, et al., 2009; Whitley, et al., 2010). Also, Ziedonis et al. (2009) showed that individuals with higher baseline withdrawal levels who received buprenorphine-naloxone fared better than those with fewer symptoms.
Opioid users demonstrate significant variability across craving (e.g., McMillan & Gilmore-Thomas, 1996; Ren, et al., 2009) and withdrawal experiences (e.g., Nielsen, Hillhouse, Mooney, Fahey, & Ling, 2012). Furthermore, craving and withdrawal, appear to be distinct, yet related, phenomena (e.g., Swift & Stout, 1992). For example, Schuster et al. (1995) found that craving increased following naloxone-precipitated withdrawal in a sample of methadonemaintained patients. Taken as a whole, investigations to date suggest associations between craving, withdrawal, and opioid use outcomes; however, more sophisticated statistical tools are needed to fully explore these relationships and potentially adapt the information for interventions. Longitudinal models may help characterize the timing and magnitude of changes in withdrawal and craving related to first opioid use during and after a buprenorphine stabilization and taper.
Latent class analyses (i.e., mixture modeling) have shown promise for explaining heterogeneous substance dependence treatment outcomes and offer novel targets for clinical intervention. Stated simply, latent class analyses (LCA) create subgroups of individuals (classes) from a larger diverse population based on constructs of interest, such as substance use patterns (e.g., frequency, polysubstance use), routes of administration, and patterns of health-risk behaviors (Trenz et al., 2013). Several latent class analyses with opioid-dependent populations have been conducted. Monga et al. (2007) examined latent classes of opioid users on drug use, depression, pain, HIV/Hepatitis infection, and homelessness; Banta-Green et al. (2009) examined health and pain; and, Shand et al. (2011) explored abuse/dependence symptom subtypes. However, none has modeled craving, withdrawal, and opioid use concurrently.
In a broader context, this exploratory research is relevant to the national agenda to find approaches to “individualize medicine.” Organizations such as the Patient-Centered Outcomes Research Institute (PCORI) support evidence-based approaches to improve health care delivery and patient outcomes. Identifying trends during and after opioid-agonist stabilization and taper may help generate hypotheses about how different subgroups (and ultimately individuals) respond to treatment. Empirically based latent class analyses are an important first step.
This secondary data analysis of the Prescription Opioid Addiction Treatment Study (POATS; Weiss et al., 2011; Weiss et al., 2010) explored latent classes of prescription opioid users. Specifically, we used discrete-survival, growth mixture modeling with time-to-first opioid use (survival) predicted by growth trajectories of craving and withdrawal (Muthen & Muthen, 2012) during and after stabilization and taper from buprenorphine/naloxone. Exploring heterogeneity across these constructs may better characterize the variability in opioid use outcomes and provide direction for intervention (Amato, et al., 2011).
2. Methods
POATS was a multisite, randomized clinical trial (NCT00316277) funded through the NIDA Clinical Trials Network. The institutional review boards at each of the 10 study sites approved the study, and all participants gave written informed consent. Full details of the trial design and primary outcome are published elsewhere (Weiss, et al., 2011; Weiss, et al., 2010).
2.1 Participants & Procedures
Sociodemographic and clinical characteristics of the population separated by intervention condition have previously been reported (see Weiss, et al., 2011). Briefly, 653 participants were randomized to standard medical management alone (SMM) or SMM plus individual opioid dependence counseling (SMM+ODC). A majority of the sample was male (60.0%), White (91.3%), and employed full-time (62.9%). The average age was 33.2 years (SD=10.2) with a mean education of 13.0 years (SD=2.2). The average days of opioid analgesics use in the past 30 days at baseline was 28.1 (SD=4.0), while heroin was 0.1 (SD=0.6).
The main trial consisted of 2 phases, and only phase 1 data were used in the current study. Phase 2 had differing aims and methods and a smaller sample size, precluding its use in current investigation. In phase 1, following screening and completion of baseline assessments, participants underwent a 1-day buprenorphine/naloxone induction (participants received between 4-12 mg [in 4-mg doses] on the day of induction, depending on initial response). Participants continued to increase their dosage based on opioid use, withdrawal symptoms, side effects, and craving (consistent with standard dosing guidelines) up to a maximum dose of 32 mg/day (SAMHSA, 2004). A 2-week stabilization period (weeks 1 and 2) was followed by a 2-week taper (weeks 3 and 4). Participants were followed for up to an additional 8 weeks (weeks 5-12). Participants randomized to SMM attended a 1-hour initial visit, followed by weekly 15-20 minute visits during weeks 1-4, and weekly 15-20 minute visits during follow-up at weeks 6 and 8. Participants randomized to SMM+ODC followed the same visit schedule as those in SMM with the addition of 45-minute individual drug counseling sessions twice a week during weeks 1-4, and 45-minute individual drug counseling sessions once a week during follow-up at weeks 6 and 8. Participants completed weekly assessments during weeks 1-4 and then attended study visits and completed assessments every other week during the 8-week follow-up period (weeks 6, 8, 10, and 12). Study visits were conducted by research associates and intervention visits were completed by trained staff. Participants received compensation for completing assessment visits.
2.2 Measures
The 3-item Opioid Craving Scale (OCS) was adapted from the 3-item Cocaine Craving Scale (Weiss et al., 2003) for use with opioids. The original 5-item version was found to be valid and unidimensional among cocaine-dependent individuals (Weiss, Griffin, & Hufford, 1995; Weiss et al., 1997). Individual OCS items’ responses ranged from 0-10, a 1-point increase from Weiss et al. (see Table 1), and total possible scores ranged from 0 to 30 with greater scores indicating higher opioid craving.
Table 1. Opiate Craving Scale (OCS) and Clinical Opiate Withdrawal Scale (COWS) Items and Responses.
| Question Stem | Response Options | ||
|---|---|---|---|
| OCS (Possible Range: 0 – 30) | |||
| How much do you currently crave opiates? | Range: 0-10 0 = Not at all 10 = Extremely |
||
| In the past week, please rate how strong your desire to use opiates has been when something in the environment has reminded you of opiates (example: seeing a medication bottle, using the Internet, visiting a doctor's office, going to a place where you used to buy drugs)? | Range 0-10 0 = Not at all 10 = Extremely |
||
| Please imagine yourself in the environment in which you previously used opiates (examples: a party, a hangout, a particular room where you live). If you were in this environment today and if it were the time of day that you typically used opiates, what is the likelihood that you would use opiates today? | Range: 0 – 10 0 = Not at all 10 = Extremely |
||
| COWS (Possible Range: 0- 46)* | |||
| Resting pulse rate: measured after participant is sitting or lying for one minute | 0 = Pulse rate 80 or below 1 = Pulse rate 81-100 2 = Pulse rate 101-120 4 = Pulse rate higher than 120 |
||
| GI upset: over the last 1/2 hour | 0 = No GI symptoms 1 = Stomach cramps 2 = Nausea or loose stool 3 = Vomiting or diarrhea 5 = Multiple episodes of diarrhea or vomiting |
||
| Sweating: over the past 1/2 hour not accounted for by room temperature or participant activity | 0 = No report of chills or flushing 1 = Subjective report of chills or flushing 2 = Flushed or observable moisture on face 3 = Beads of sweat on brow or face 4 = Sweat streaming off face |
||
| Tremor: observation of outstretched hands | 0 = No tremor 1 = Tremor can be felt, but not observed 2 = Slight tremor observable 4 = Gross tremor or muscle twitching |
||
| Restlessness: observation during assessment | 0 = Able to sit still 1 = Reports difficulty sitting still, but is able to do so 2 = Frequently shifting or extraneous movements of legs/arms 3 = Unable to sit still for more than a few seconds |
||
| Yawning: observation during assessment | 0 = No yawning 1 = Yawning once or twice during assessment 2 = Yawning three or more times during assessment 4 = Yawning several times/minute |
||
| Pupil size | 0 = Pupils pinned or normal size for room light 1 = Pupils possibly larger than normal for room light 2 = Pupils moderately dilated 5 = Pupils so dilated that only the rim of the iris is visible |
||
| Anxiety or irritability | 0 = None 1 = Participant reports increasing irritability or anxiousness 2 = Participant obviously irritable or anxious 4 = Participant is so irritable or anxious that participation in the assessment is difficult |
||
| Bone or joint aches: If participant was having pain previously, only the additional component attributed to opiate withdrawal is scored | 0 = Not present 1 = Mild diffuse discomfort 2 = Participant reports severe diffuse aching of joints/muscles 4 = Participant is rubbing joints or muscles and is unable to sit still because of discomfort |
||
| Gooseflesh skin | 0 = Skin is smooth 3 = Pilocerection of skin can be felt or hairs standing up on arms 5 = Prominent pilocerection |
||
| Runny nose or tearing: not accounted for by cold symptoms or allergies | 0 = Not present 1 = Nasal stuffiness or unusually moist eyes 2 = Nose running or tearing 4 = Nose constantly running or tears streaming down cheeks |
||
Note. The COWS is clinician-administered and based on participants’ current signs and symptoms of opiate withdrawal.
Due to a slight change in scoring from the original measure, the total possible score on this measure in the POATS trial was 46 instead of 48, per Tompkins et al (2009).
The 11-item Clinical Opiate Withdrawal Scale (COWS) was used to assess withdrawal symptoms (Tompkins et al., 2009; Wesson & Ling, 2003) with greater scores indicating higher levels of withdrawal. Possible scores ranged from 0 to 46 (see Table 1). The COWS has been shown to be a valid measure for opioid withdrawal (Tompkins, et al., 2009).
A urine drug screen (UDS) at each visit and timeline follow-back (TLFB) self-report (Sobell, Maisto, Sobell, & Cooper, 1979) were used to capture drug use. Time-to-first opioid use has been used previously as a post-detoxification outcome of opioid resumption (Kertesz, et al., 2003). First opioid use was defined as an opioid-positive UDS, self-reported opioid use, or a missing UDS or TLFB, as found in other studies (Krupitsky, et al., 2011; Peirce et al., 2006). Missing data were treated as positive; the overwhelming majority of participants (94.3%) treated as positive due to missing data were positive (on TLFB or UDS) or missing additional substance use data at the following visit, suggesting relapse. From an analytic standpoint this definition resulted in no missing data for the survival variable. Use of opioids prior to week 2 (i.e., the induction and first week of stabilization); however, was not considered a “first use”, giving participants time to stabilize on the medication; a high proportion of the sample had an opioidpositive UDS in the week following baseline.
3. Data Preparation and Statistical Analyses
Data were analyzed with MPlus, version 7 (Muthen & Muthen, 2012). No effects of treatment were found in the primary outcome analyses (Weiss, et al., 2011); therefore, participants were collapsed across the 2 arms of the study. Discrete-time survival growth mixture modeling analyzed change in craving and withdrawal trajectories, which predicted timeto-first opioid use. Mixture modeling was applied to these longitudinal trajectories and survival data to characterize heterogeneity in these processes (i.e., growth and survival factors) in terms of latent classes (see Figure 1) (Muthen & Muthen, 2012). For simplicity we refer to this as “latent class analysis” (LCA) from here forward. This approach accrues benefits by simultaneously modeling longitudinal processes and time-to-first use, which yields unbiased estimates for all three processes (Ibrahim, Chu, & Chen, 2010). Prior to simultaneous modeling of the constructs, model fitting of craving and withdrawal were conducted separately. Specifically, we made determinations about whether intercept, linear, quadratic, and/or cubic parameters were needed to evaluate the functional form of change of each construct over time.
Figure 1.
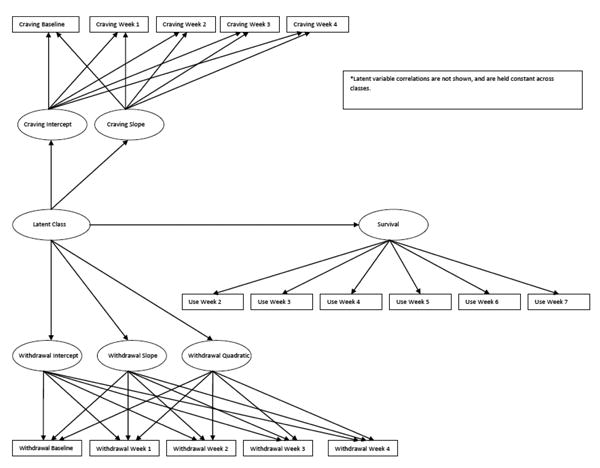
Latent class model of craving, withdrawal, and survival (time-to-first use).
For each class solution, probabilistic assignment of individuals to discrete classes permitted evaluation of distinct participant subtypes according to particular response patterns. Robust sandwich estimators (White, 1980) addressed correlation induced via clustering by study site. This precluded the use of the bootstrap likelihood ratio test (unavailable in MPlus version 7) requiring the use of other indices including the BIC, entropy, inspection of transition matrices and overall interpretability of the solution. Recommendations were followed to select the number of classes (Lubke & Muthén, 2005; Nagin, 2005; Nylund, Asparouhov, & Muthen, 2007) and our approach was similar to other LCA with opioids and other substances (Banta-Green, et al., 2009; Green, Black, Grimes Serrano, Budman, & Butler, 2011; Sherman et al., 2009).
Using LCA the initial model space consisted of solutions with 1 through 10 classes. Similar to other LCA research (e.g., Banta-Green, et al., 2009), the widely used Bayesian Information Criterion (BIC; Kass & Raftery, 1995) provided some guidance in identifying best number of classes for characterizing the data. Entropy summarized the average estimated conditional probability of each participant being categorized into classes and ranges from 0 to 1, with values closer to 1 indicating clear classification across classes (Ramaswamy, Desarbo, Reibstein, & Robinson, 1993); values above 0.80 indicate good classification. Transition matrices crosstabulate class membership (based on highest membership probability) in k classes with membership in k + 1 classes (Lubke & Muthén, 2005). Specifically this allowed observance of how participants from a model with fewer classes (k) sorted into a model with more classes (k+1). Graphical and quantitative inspection of the longitudinal processes of craving, withdrawal, and opioid-free survival further permitted evaluation of solutions for clinical relevance and parsimony. Interpretability of a given solution based on clinical knowledge of opioid use, craving, and withdrawal, along with the stated aims, were used to identify the best model as recommended and used by other LCA analyses with opioids (e.g., Banta-Green, et al., 2009; Green, et al., 2011) and other substances (de Dios et al., 2010; Sherman, et al., 2009).
Intermittent missing data (<1%) for craving and withdrawal measures were handled with Full Information Maximum Likelihood, robust to data missing at random. Missing data due to participant dropout were handled by modeling this process using the survival curve. Due to the decline in participant data points over time, craving and withdrawal data beyond week 4 were not modeled.
4. Results
Craving, withdrawal, and time-to-first use were modeled simultaneously to form each class (i.e., each contributed to class identification). However, to improve understanding these phenomena are at times discussed separately.
4.1 Model Selection
Model fitting for 1 through 10 latent classes (Table 2) demonstrated that the 8-class solution had the best BIC value. However, many of the classes had similar growth trajectories or survival curves that suggested variation along a quantitative dimension but did not demonstrate qualitative differences in shape. This, coupled with several small class sizes (i.e., 5 classes had 35 or fewer participants and 2 classes had fewer than 20) strained the interpretability and clinical relevance of this solution. Beginning with the k = 8 class model and sequentially working backward, comparisons of graphs for craving, withdrawal, and time-to-first use permitted evaluation of each k model relative to the k-1 model. Patterns in the graphs helped determine whether latent class trajectory variations were differences in “type” of trajectory versus differences in “dimensionality” (magnitude differences). This process resulted in the selection of the 4-class model as representing the most clinically parsimonious model which also had good entropy and acceptable fit indices.
Table 2. Fit Indices for 1- through 10-Class Models.
| Model | AIC | BIC | aBIC | ΔBIC | Entropy | Parameters |
|---|---|---|---|---|---|---|
| 1 Class | 34741.2 | 34902.5 | 34788.2 | N/A | 0.80 | 36 |
| 2 Class | 34477.1 | 34638.4 | 34524.1 | 264.1 | 0.63 | 36 |
| 3 Class | 34599.3 | 34792.0 | 34655.5 | -153.6 | 0.84 | 43 |
| 4 Class** | 34516.1 | 34740.1 | 34581.4 | 51.9 | 0.82 | 50 |
| 5 Class | 34126.6 | 34395.5 | 34205.0 | 344.7 | 0.78 | 60 |
| 6 Class | 34047.1 | 34351.9 | 34136.0 | 43.6 | 0.80 | 68 |
| 7 Class | 33982.9 | 34323.4 | 34082.1 | 28.4 | 0.81 | 76 |
| 8 Class* | 33935.3 | 34311.8 | 34045.1 | 11.7 | 0.79 | 84 |
| 9 Class | 33910.9 | 34323.2 | 34031.1 | -11.4 | 0.77 | 92 |
| 10 Class | 33870.7 | 34318.9 | 34001.4 | 4.3 | 0.76 | 100 |
Note. AIC = Akaike's Information Criterion; BIC = Bayesian Information Criterion; aBIC = Adjust BIC; ΔBIC = Change in BIC scores from one class to the next (positive values = fit improvements; negative values = fit decrements).
Best fitting model using BIC criterion
Most parsimonious model
Subsequently we examined transition matrices for the 1- through 8-class solutions. Transitioning from 1-to-2 classes, 2-to-3 classes, and 3-to-4 classes resulted in distinct class formation for each subsequent model. Specifically, each new class had unique patterns of craving and withdrawal processes and time-to-first use, and the number of individuals who transitioned from the 3-class model into the 4th class in the 4-class model suggested it was a clinically distinct addition. Models with classes greater than 4 did not result in additional distinct groups; therefore, the 4-class model was retained.
4.2 Four-Class Solution
Examination of the parameter estimates (Table 3) in combination with the graphical representation of craving and withdrawal (Figure 2) and opioid-free survival (Figure 3) suggested distinct values on the intercept, slope, and quadratic properties for both craving and withdrawal, as a function of class membership. That is, characterization of heterogeneity is possible as a function of where people start as well as how they change on craving and withdrawal.
Table 3. Parameter Estimates for a 4-Latent Class Model.
| Parameter Estimate, SE (95% CI Lower Limit, 95% CI Upper Limit) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Class | n | COWS Intercept | COWS Slope | COWS Quadratic | OCS Intercept | OCS Slope | Factor Means |
| Class 1 | 40 | 17.3, SE=2.6 (95% CI: 12.1,22.5) |
-16.9, SE=3.7 (95% CI: -24.2,-9.5) |
4.6, SE=1.1 (95% CI: 2.3,6.8) |
26.2, SE=0.9 (95% CI: 24.5,28.0) |
-2, SE=0.4 (95% CI: -2.8,-1.1) |
0.3, SE=0.4 (95% CI: -0.5,1.1) |
| Class 2 | 441 | 11.5, SE=0.3 (95% CI: 10.8,12.2) |
-9.3, SE=0.3 (95% CI: -10.0,-8.6) |
2.0, SE=0.1 (95% CI: 1.8,2.2) |
25.7, SE=0.3 (95% CI: 25.1,26.4) |
-5.3, SE=0.2 (95% CI: -5.7,-4.8) |
-0.8, SE=0.1 (95% CI: -1.1,-0.6) |
| Class 3 | 100 | 12.0, SE=0.6 (95% CI: 10.8,13.2) |
-9.4, SE=0.5 (95% CI: -10.3,-8.5) |
1.9, SE=0.1 (95% CI: 1.8,2.1) |
13.7, SE=1.0 (95% CI: 11.7,15.7) |
-2.5, SE=0.3 (95% CI: -3.2,-1.8) |
-0.9, SE=0.2 (95% CI: -1.4,-0.4) |
| Class 4 | 72 | 12.2, SE=0.4 (95% CI: 11.5,13.0) |
-5.0, SE=0.4 (95% CI: -5.9,-4.2) |
1.0, SE=0.2 (95% CI: 0.7,1.4) |
23.9, SE=1.1 (95% CI: 21.6,26.1) |
-3.2, SE=0.4 (95% CI: -3.9,-2.4) |
0.0, SE=0.0 (95% CI: 0.0,0.0) |
Note. The Factor Mean for class 4 was set to 0 for model identification. COWS = Clinical Opiate Withdrawal Scale; OCS = Opioid Craving Scale.
Figure 2.
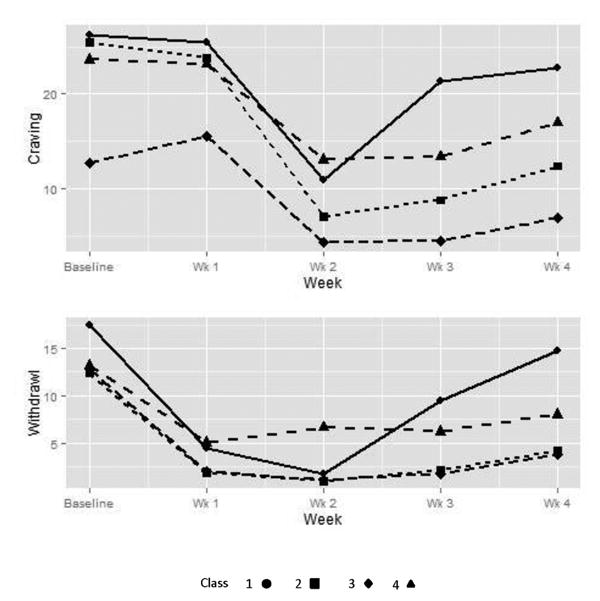
Craving and withdrawal trajectories for the 4 classes of opioid users. Number of participants decreased over time due to opioid use as follows: baseline and week 1 (N=653), week 2 (n=388), week 3 (n=258), and week 4 (n=122).
Figure 3.
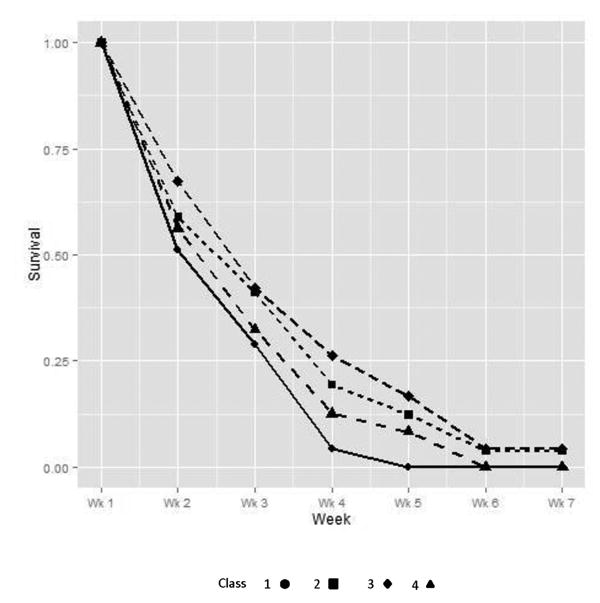
Survival (time-to-first use) trajectories for the 4 classes of opioid users. Class sample sizes were as follows: Class 1 (n = 40); class 2 (n = 441); class 3 (n = 100); and Class 4 (n = 72).
Class 1 (n = 40) had the highest initial levels of craving and withdrawal, with decreases during stabilization, and a quick rebound in withdrawal and craving at the beginning of taper, with all class members lapsing by week 5. Class 4 (n = 72) is distinguishable from class 1 by a lower baseline withdrawal level along with more gradual rebound symptoms on both craving and withdrawal and a slightly longer time-to-first-use curve with all class members lapsing by week 6. Class 2 (n = 441) and class 3 (n = 100) are distinguished by a lower baseline craving score and a lower level of ongoing craving for class 3. Also, not all participants lapsed in these two classes: classes 2 and 3 had 3.85% and 4.00% opioid-free survival beyond week 6, respectively. A majority of all participants (>65%) were placed in class 2 by the LCA, suggesting this as the most typical experience of craving, withdrawal, and survival for this sample. Noteworthy, all classes had a sharp decrease in withdrawal from baseline to week 1; however, craving lagged behind withdrawal and sharp decreases were not apparent until the interval between weeks 1 and 2.
The odds ratios for class comparison on time-to-first use (reference group=class 1 [shortest opioid-free survival period]; see Table 4), ranged in magnitude from 0.35 (for class 3 [longest opioid-free survival period]) to 0.74 (for class 4). Specifically, values below 1.0 indicate lower odds of opioid use at each time point.
Table 4. Odds Ratio of Survival (i.e., No Opioid Use).
| Class Comparison | OR (95% CI) | z | p |
|---|---|---|---|
| Class 1 to Class 2 | 0.37 (95% CI: 0.26-0.52) | -5.74 | <0.001 |
| Class 1 to Class 3 | 0.35 (95% CI: 0.27-0.45) | -8.15 | <0.001 |
| Class 1 to Class 4 | 0.74 (95% CI: 0.55-1.00) | -1.98 | <0.05 |
Note. Class 1 is the reference group for all comparisons. Class 1 was used as the reference group because members of this class overall used opioids earliest. These odds ratios were generated by discrete survival analysis at each time point and they give the odds of opioid use occurring.
5. Discussion
This study used LCA to characterize the heterogeneity in craving and withdrawal and opioid use over consecutive phases of opioid-agonist stabilization, taper, and post-taper. Individuals were successfully parsed into clusters experiencing similar levels of craving, withdrawal, and time-to-first use. The four latent classes each had unique patterns across the three domains, which suggested the possibility of opioid-dependent subgroups following different trajectories. The previous point is underscored by the magnitude of the odds ratio differences on time-to-first use.
The clinical utility of these findings is uncertain at this time. Approximately two-thirds of all opioid-dependent individuals in this sample were grouped into a single class, potentially highlighting homogeneity across a majority of opioid-dependent individuals. On the other hand, one-third of the participants (n=212) followed different trajectories. At this stage, this work is hypothesis-generating and needs replication before tailored treatment approaches based on latent class characteristics can be tested to improve buprenorphine-detoxification outcomes.
The approach to simultaneously modeling the change processes of craving and withdrawal along with opioid use is a novel characteristic of this study. Each of the 4 classes exhibited relatively distinct relationships with regard to the three constructs studied. The group with the highest baseline craving and withdrawal levels and the most significant early rebound experience of these symptoms had the earliest use, and the group with the lowest baseline craving and withdrawal and minimal early rebound of symptoms had the best opioid-use outcomes. Similar associations have been proposed for alcohol dependence. Specifically, non-cue dependent alcohol craving has been suggested as an explanatory subtype of alcohol dependence that may account for significant variation in alcohol use outcomes (Oslin, Cary, Slaymaker, Colleran, & Blow, 2009). Our data tend to support that some opioid-dependent individuals experience relatively greater levels of craving and withdrawal before beginning opioid-agonist treatment and experience a significant and pronounced rebound of these symptoms during taper compared to other opioid-dependent subtypes. More intensive interventions (e.g., Stotts, et al., 2012) will likely be needed for those who have more distress and faster rebound.
Current outpatient detoxification strategies do not appear sufficient for a majority of opioiddependent individuals, particularly those individuals with quick and pronounced rebound of craving and withdrawal who tend to use within 1-3 weeks of taper initiation as seen in this study. It is possible that regardless of dose-reduction schedule (7-day, 14-day, 28-day), or opioid type (heroin, prescription opioid), or perhaps treatment (buprenorphine/naloxone, methadone, clonidine), there are common subtypes worthy of further investigation. For example, Whitley et al. (2010) found few differences (i.e., pre- and post-induction withdrawal scores) between prescription opioid- and heroin-dependent individuals. Proposing common opioid-dependence subtypes (across treatment, schedule, and opioid type) is speculative, however, and confirmatory research is needed.
It is worth noting that withdrawal scores dropped sharply from induction/baseline to week 1 and that decreases in craving were not observed until one week later, highlighting the distinct nature of these phenomena. All 4 classes tended to experience large decreases in withdrawal from baseline to week 1; however, only the 2 classes who fared the best on opioid use outcomes had relatively low levels of craving by week 2. The two classes with the worst opioid use outcomes had more modest decreases in craving from baseline to week 2, with nearly 50% of the participants in these classes using opioids by this week. From week 2 onward, craving and withdrawal trajectories tended to mirror one another. Future investigations may further establish the separation between withdrawal and craving (and better characterize how each drives lapses) by modeling additional time points between induction and the end of the taper and dynamically modeling these 2 constructs.
Sample heterogeneity has not been sufficiently examined in previous explorations with craving and withdrawal. This study adds to the LCA literature within opioid dependence which has reported other investigations of constructs in relation to opioid dependence, such as prescription opioid user subtypes (Green, et al., 2011), illicit opioid user subtypes (Monga, et al., 2007), and mental health and pain (Banta-Green, et al., 2009). Limitations are noted, however. As this was a largely unsuccessful detoxification trial, few individuals survived past week 4, which minimized the data available to model latent classes beyond this point. We could have chosen to model full relapse, although choosing a uniformly agreed upon definition is challenging. A full relapse definition would have extended survival; although, approximately 95% of participants continued using or missing visits in the week following the first missed visit. The overall patterns in each of the classes would likely have been unchanged. Also, we chose to model craving and withdrawal in tandem with opioid use; however, there were other important variables not included (e.g., psychosocial stress; Hyman, et al., 2007). Also, current modeling limitations remain a challenge for all latent class analyses. After accounting for clustering, some statistical approaches were unavailable and there are no uniformly accepted rules for making model determinations. However, the additional rigor of examining transition matrices and understanding the creation of classes across the separate models was a strength.
Presently a majority of outpatient-detoxified opioid users will return to opioid use following a buprenorphine taper, but the precipitants and the timing of return may help differentiate groups. Craving and withdrawal symptoms across phases of buprenorphine treatment and detoxification (pre-induction, induction, stabilization, taper, and post-taper) can follow numerous trajectories. Future research will help determine whether or not tailored interventions based on individuals’ craving and withdrawal experiences increase detoxification success rates during and after buprenorphine treatment. Given the dire state of current efforts to detoxify individuals from opioid-agonist treatments, these findings contribute to the understanding of detoxification, particularly in the early phases. In a broader context, this preliminary research is relevant to the national agenda to find approaches to “individualize medicine.” Empirically-based latent class analyses are an important first step in this process.
Highlights.
Antecedents of first post-detoxification opioid use were simultaneously modeled
Opioid users were parsed in to classes during and after agonist pharmacotherapy
Four classes were characterized on craving, withdrawal, and opioid-free survival
Odds ratios showed significant differences in time-to-first use across classes
Future research may allow timely interventions to extend time-to-first opioid use
Acknowledgments
Role of Funding Sources: The Prescription Opioid Addiction Treatment Study (POATS) was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers U10 DA15831 and K24 DA022288 (PI: Weiss). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors: Dr. Northrup conceptualized the manuscript, in collaboration with Drs. Stotts, Potter, Walker, and Trivedi. Dr. Green served as the primary biostatistician, with Dr. Northrup assisting, and helped write significant portions of the Data Analysis and Results. Dr. Northrup wrote the initial draft of the full manuscript, with significant editing assistance from Drs. Stotts and Green. Drs. Potter and Weiss, along with Elise Marino, helped draft parts of the Methods relevant to the POATS trial. Dr. Weiss, PI of the POATS trial, and Drs. Walker and Trivedi provided edits on several revisions of the paper. All authors have approved the final version of this manuscript.
Conflict of Interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato L, Davoli M, Ferri M, Gowing L, Perucci CA. Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug and Alcohol Dependence. 2004;73(3):219–226. doi: 10.1016/j.drugalcdep.2003.11.002.. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database of Systematic Reviews. 2011;9:1–53. doi: 10.1002/14651858.CD005031.pub4.. [DOI] [PubMed] [Google Scholar]
- Banta-Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Opioid use behaviors, mental health and pain--development of a typology of chronic pain patients. Drug and Alcohol Dependence. 2009;104(1-2):34–42. doi: 10.1016/j.drugalcdep.2009.03.021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. American Journal of Drug and Alcohol Abuse. 2001;27(1):19–44. doi: 10.1081/ADA-100103117.. [DOI] [PubMed] [Google Scholar]
- de Dios MA, Anderson BJ, Herman DS, Hagerty CE, Caviness CM, Budney AJ, Stein M. Marijuana use subtypes in a community sample of young adult women. Women′s Health Issues. 2010;20(3):201–210. doi: 10.1016/j.whi.2010.02.001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP. What have we learned from three decades of methadone maintenance treatment? Drug and Alcohol Review. 1994;13(1):3–4. doi: 10.1080/09595239400185661.. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Wells EA. Primary outcome indices in illicit drug dependence treatment research: Systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107(4):694–708. doi: 10.1111/j.1360-0443.2011.03473.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Tusel D. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New England Journal of Medicine. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164.. [DOI] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, Treacy S. Outcomes after methadone maintenance and methadone reduction treatments: Two-year follow-up results from the National Treatment Outcome Research Study. Drug and Alcohol Dependence. 2001;62(3):255–264. doi: 10.1016/S0376-8716(00)00211-8.. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x.. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews. 2009;(3):CD002025. doi: 10.1002/14651858.CD002025.pub4.. [DOI] [PubMed] [Google Scholar]
- Green TC, Black R, Grimes Serrano JM, Budman SH, Butler SF. Typologies of prescription opioid use in a large sample of adults assessed for substance abuse treatment. PLoS ONE. 2011;6(11):e27244. doi: 10.1371/journal.pone.0027244.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug and Alcohol Dependence. 2008;94(1–3):199–206. doi: 10.1016/j.drugalcdep.2007.11.021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KA, Doebrick C, Sinha R. Stress and drug-cueinduced craving in opioid-dependent individuals in naltrexone treatment. Experimental and Clinical Psychopharmacology. 2007;15(2):134–143. doi: 10.1037/1064-1297.15.2.134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. Journal of Clinical Oncology. 2010;28(16):2796–27801. doi: 10.1200/JCO.2009.25.0654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RE, Raftery AE. Bayes factors. Journal of the American Statistical Association. 1995;90(430):773–795. doi: 10.2307/2291091.. [DOI] [Google Scholar]
- Kertesz SG, Horton NJ, Friedmann PD, Saitz R, Samet JH. Slowing the revolving door: Stabilization programs reduce homeless persons' substance use after detoxification. Journal of Substance Abuse Treatment. 2003;24(3):197–207. doi: 10.1016/S0740-5472(03)00026-6.. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. Journal of Nervous and Mental Disease. 1993;181(6):358–364. doi: 10.1097/00005053-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9.. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, Ziedonis D. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: Findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100(8):1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, Segal D. Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomized clinical trial. Addiction. 1998;93(4):475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Lubke GH, Muthén B. Investigating population heterogeneity with factor mixture models. Psychological Methods. 2005;10:21–39. doi: 10.1037/1082-989X.10.1.21.. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Peindl K, Gorelick DA, Wu LT, Gottheil E. Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addiction Biology. 2009;14(2):204–213. doi: 10.1111/j.1369-1600.2008.00119.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE, Gilmore-Thomas K. Stability of opioid craving over time as measured by visual analog scales. Drug and Alcohol Dependence. 1996;40:235–239. doi: 10.1016/0376-8716(96)01218-5.. [DOI] [PubMed] [Google Scholar]
- Monga N, Rehm J, Fischer B, Brissette S, Bruneau J, El-Guebaly N, Bahl S. Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug and Alcohol Dependence. 2007;88(1):1–8. doi: 10.1016/j.drugalcdep.2006.08.029.. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User′s Guide. Sixth. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Nagin DS. Group-based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nielsen S, Hillhouse M, Mooney L, Fahey J, Ling W. Comparing buprenorphine induction experience with heroin and prescription opioid users. Journal of Substance Abuse Treatment. 2012;43(3):285–290. doi: 10.1016/j.jsat.2011.12.009.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14(4):535–569. doi: 10.1080/10705510701575396.. [DOI] [Google Scholar]
- Oreskovich MR, Saxon AJ, Ellis ML, Malte CA, Reoux JP, Knox PC. A double-blind, double-dummy, randomized, prospective pilot study of the partial mu opiate agonist, buprenorphine, for acute detoxification from heroin. Drug and Alcohol Dependence. 2005;77(1):71–79. doi: 10.1016/j.drugalcdep.2004.07.008.. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Cary M, Slaymaker V, Colleran C, Blow FC. Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug and Alcohol Dependence. 2009;103:131–136. doi: 10.1016/j.drugalcdep.2009.03.009.. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201.. [DOI] [PubMed] [Google Scholar]
- Peterson JA, Schwartz RP, Mitchell SG, Reisinger HS, Kelly SM, O'Grady KE, Agar MH. Why don't out-of-treatment individuals enter methadone treatment programmes? International Journal of Drug Policy. 2010;21(1):36–42. doi: 10.1016/j.drugpo.2008.07.004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy V, Desarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Science. 1993;12(1):103–124. doi: 10.1287/mksc.12.1.103.. [DOI] [Google Scholar]
- Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in painsensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204(3):423–429. doi: 10.1007/s00213-009-1472-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten T, Kleber H. Success and failure at outpatient opioid detoxification. Evaluating the process of clonidine- and methadone-assisted withdrawal. Journal of Nervous and Mental Disease. 1985;173(2):103–110. doi: 10.1097/00005053-198502000-00007. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Center for Substance Abuse Treatment Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
- Scherbaum N, Heppekausen K, Rist F. Is premature termination of opiate detoxification due to intensive withdrawal or craving? Fortschritte der Neurologie-Psychiatrie. 2004;72(1):14–20. doi: 10.1055/s-2003-812451.. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Greenwald MK, Johanson CE, Heishman SJ. Measurement of drug craving during Naloxone-precipitated withdrawal in methadone-maintained volunteers. Experimental and Clinical Psychopharmacology. 1995;3(4):424–431. doi: 10.1037//1064-1297.3.4.424.. [DOI] [Google Scholar]
- Shand FL, Slade T, Degenhardt L, Baillie A, Nelson EC. Opioid dependence latent structure: Two classes with differing severity? Addiction. 2011;106(3):590–598. doi: 10.1111/j.1360-0443.2010.03217.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, Sutcliffe CG, German D, Sirirojn B, Aramrattana A, Celentano DD. Patterns of risky behaviors associated with methamphetamine use among young Thai adults: A latent class analysis. Journal of Adolescent Health. 2009;44(2):169–175. doi: 10.1016/j.jadohealth.2008.06.021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current Psychiatry Reports. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Smyth BP, Barry J, Lane A, Cotter M, O'Neill M, Quinn C, Keenan E. Inpatient treatment of opiate dependence: Medium-term follow-up outcomes. British Journal of Psychiatry. 2005;187:360–365. doi: 10.1192/bjp.187.4.360.. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. International Journal of Neuropsychopharmacology. 2008;11(5):641–653. doi: 10.1017/s146114570700836x.. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: Options in pharmacotherapy. Expert Opinion on Pharmacotherapy. 2009;10(11):1727–1740. doi: 10.1517/14656560903037168.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Green C, Masuda A, Grabowski J, Wilson K, Northrup TF, Schmitz JM. A Stage I pilot study of acceptance and commitment therapy for methadone detoxification. Drug and Alcohol Dependence. 2012;125:215–222. doi: 10.1016/j.drugalcdep.2012.02.015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobbe S, Brower KJ, Galen LW. Predicting completion of outpatient opioid detoxification with clonidine. American Journal on Addictions. 2003;12(3):260–269. [PubMed] [Google Scholar]
- Swift RM, Stout RL. The relationship between craving, anxiety, and other symptoms in opioid withdrawal. Journal of Substance Abuse. 1992;4:19–26. doi: 10.1016/0899-3289(92)90024-R.. [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug and Alcohol Dependence. 2009;105(1-2):154–159. doi: 10.1016/j.drugalcdep.2009.07.001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenz RC, Scherer M, Duncan A, Harrell PT, Moleko AG, Latimer WW. Latent class analysis of polysubstance use, sexual risk behaviors, and infectious disease among South African drug users. Drug and Alcohol Dependence. 2013;132(3):441–448. doi: 10.1016/j.drugalcdep.2013.03.004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuten M, DeFulio A, Jones H, Stitzer M. Abstinence-contingent recovery housing and reinforcement-based treatment following opioid detoxification. Addiction. 2011;107:973–982. doi: 10.1111/j.1360-0443.2011.03750.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss R, Greenfield S, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clinical Journal of Pain. 2009;25(3):193–198. doi: 10.1097/AJP.0b013e318193a6c4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C. Craving in hospitalized cocaine abusers as a predictor of outcome. American Journal of Drug and Alcohol Abuse. 1995;21(3):289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C, Muenz LR, Najavits LM, Jansson SB, Thompson HJ. Early prediction of initiation of abstinence from cocaine. Use of a craving questionnaire. American Journal on Addictions. 1997;6(3):224–231. [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Mazurick C, Berkman B, Gastfriend DR, Frank A, Moras K. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. American Journal of Psychiatry. 2003;160:1320–1325. doi: 10.1176/appi.ajp.160.7.1320.. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Ling W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): Rationale, design, and methodology. Contemporary Clinical Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35(2):253–259. doi: 10.1080/02791072.2003.10400007.. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. doi: 10.2307/1912934.. [DOI] [Google Scholar]
- Whitley SD, Sohler NL, Kunins HV, Giovanniello A, Li X, Sacajiu G, Cunningham CO. Factors associated with complicated buprenorphine inductions. Journal of Substance Abuse Treatment. 2010;39(1):51–57. doi: 10.1016/j.jsat.2010.04.001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis DM, Amass L, Steinberg M, Woody G, Krejci J, Annon JJ, Ling W. Predictors of outcome for short-term medically supervised opioid withdrawal during a randomized, multicenter trial of buprenorphine-naloxone and clonidine in the NIDA clinical trials network drug and alcohol dependence. Drug and Alcohol Dependence. 2009;99(1-3):28–36. doi: 10.1016/j.drugalcdep.2008.06.016.. [DOI] [PMC free article] [PubMed] [Google Scholar]


