Abstract
It has been a few decades since Ca2+ was identified as one of the important factors that can accelerate gastric wound repair as well as contribute to epithelial homeostasis and regulation of gastric secretions. The mechanistic basis has remained largely unexplored in vivo because it was not possible to track in real time either intracellular Ca2+ mobilization or wound repair in living tissues. Recent advances in technology, such as combining high resolution light microscopy and genetically encoded Ca2+ reporters in mice, now allow the monitoring of Ca2+ mobilization during gastric epithelial cell restitution. Ca2+ is a ubiquitous second messenger that influences numerous cellular processes, including gastric acid/bicarbonate secretion, mucus secretion, and cell migration. We have demonstrated that cytosolic Ca2+ mobilization within the restituting gastric epithelial cells is a central signal driving small wound repair. However, extracellular Ca2+ is also mobilized in the juxtamucosal luminal space above a wound, and evidence suggests extracellular Ca2+ is a third messenger that also promotes gastric epithelial restitution. Interplay between intracellular and extracellular Ca2+ is necessary for efficient gastric epithelial restitution.
Introduction
Two processes, proliferation and restitution, are responsible for maintaining epithelial continuity and consequently an epithelial cell barrier in the stomach. Proliferation requires mitosis and is therefore a fairly protracted process, while restitution is a much more rapid process dependent on cell migration. In response to acute disruption of the gastric epithelium, cell migration is always the first responder towards restoring epithelial continuity and barrier function [1].
Epithelial restitution is a highly regulated process that is energy-dependent and engages both intracellular and extracellular biomolecules to signal and drive repair (e.g. prostaglandins (PG), cytoskeletal rearrangements, ion transporters, and other cellular processes [2-5]). The Ca2+-dependence of gastric epithelial restitution is not surprising since Ca2+ is a ubiquitous second messenger that influences numerous cellular processes [6,7]. In the field of gastric restitution, this broad set of roles has the potential to bring convergence to a disparate literature, as a common mediator for the wide range of factors that have been reported to stimulate restitution [8-11].
Despite this exciting potential, there are few reports measuring Ca2+ in the gastric epithelium. While the intracellular loading of conventional acetoxymethylester Ca2+ sensitive fluorescent probes (Fura2, Fluo4, etc) can be used for in vitro study, these fail to load effectively in vivo [12]. The reasons for this are unknown: the chemicals may be cleaved prematurely in the extracellular space, leak rapidly from gastric cells (like the SNARF probe [13]), or never get effectively cleaved to the active form in the intracellular space. Consequently, there was a 30 year interval with virtually no in vivo study exploring the role of intracellular Ca2+ in gastric epithelial cells. In 1997, genetically encoded yellow cameleon (YC) protein was developed in which cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) are linked by calmodulin and a M13 calmodulin-binding domain [14]. Upon an increase in Ca2+ concentration, Ca2+ binds calmodulin and interacts with M13, which results in a conformational change of the protein that increases the efficiency of Förster resonance energy transfer (FRET) from CFP to YFP. A YC transgenic mouse has been created that allows direct observation of intracellular Ca2+ dynamics in real time in vivo [15]. There are even fewer reports evaluating the role of extracellular Ca2+. The pioneering work of Curci's group using Ca2+ microelectrodes introduced the concept of Ca2+ being mobilized in the lumen of gastric glands as a signaling mechanism to regulate gastric secretion [16]. Our work and that of others has shown that there is also a pH microdomain adjacent to the surface epithelium, altered in the presence of epithelial damage [4,5,17,18]. Based on these advances, the conceptual and experimental foundation for evaluating luminal Ca2+ microdomains has solidified in recent years.
Restitution model using two photon confocal microscopy
The restitution process has been difficult to observe with high fidelity due to both the speed of the process and the inherent heterogeneity of damaged and healthy cells. Generally, in vitro cell culture studies have used a scratch-induced wound and start to measure the area that is covered by migrating cells at least 2 hr after wounding. This is a great tool to monitor cell migration, including cell shape changes [19-22]. Gastric restitution is classically measured morphologically in fixed tissue sections, or by transmucosal electrical resistance and potential difference between the luminal and serosal side using isolated gastric mucosa mounted in Ussing chambers. In such models, restitution required >4 hr to complete [2,22,23]. When gastric restitution was measured in ex vivo chambered stomach in anesthetized animals by detecting transmucosal potential difference, repair of damage was completed in 1-2 hr [24,25]. The different time courses may be related to different modes of inducing damage, or variable potency of tissue preparations to mount a robust damage response. Overall, these electrophysiological approaches are very reasonable models to monitor restitution since results monitor time-dependent closing of denuded/injured area of gastric epithelium. Since such outcomes also indicate that the restitution process starts within 10 min after induction of injury, the work also encourages exploration of approaches that allow higher resolution of early events in restitution.
Recent imaging advances led us to establish a new gastric restitution model that makes it possible to visualize both the repair progression and microenvironment changes using fluorescent probes. The microscopic precision of two-photon absorption allows the timed creation of microlesions (Photodamage) at any selected gastric surface epithelial region, followed by visualizing in real time of damage expansion, exfoliation of dead cells, and restitution (Figure 1A). Although the mechanism underlying two-photon-induced damage is still unknown, absorption of light energy by targeted fluorescent proteins or endogenous mitochondrial NAD(P)H results in photobleaching and potentially leads to induction of reactive oxygen species to cause cellular apoptosis or necrosis. Similar optical strategies are used in phototherapy to selectively kill tumor cells [26,27]. We have reported that two-photon absorption in response to high power laser scanning immediately bleaches NAD(P)H in a set of targeted gastric surface epithelial cells and damage expands to an area that is 5-10 times the original area of illumination [5]. Subsequently, exfoliation of dead cells and closing of the wound area were observed, and this entire process is completed within 15 min. Importantly, gastric restitution after this photodamage was inhibited in cyclooxygenase (COX) inhibitors or COX1 knockout mice [4,5,12], consistent with findings using different damage models [19,28].
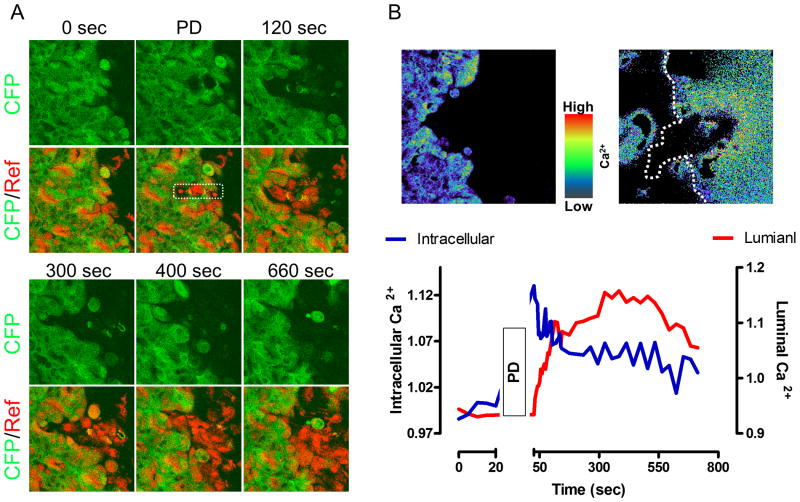
Figure 1. Change in intracellular and extracellular Ca2+ during gastric epithelial restitution induced by photodamage.
Images were collected at the indicated times in anesthetized YC mouse stomach using dyes and imaging parameters as previously described [12]. A. CFP (Green) and reflectance (Red) images (Ex: two photon 840 nm) Gastric surface cells were photodamaged (PD) in the 258 μm2 region marked by white rectangle. B. Pseudocolor FRET/CFP and Fura-Red F458/F488 images showed intracellular and luminal Ca2+ changes at 2 min after PD, respectively. In addition, the time course of intracellular (Blue line) and luminal (Red Line) Ca2+ changes was calculated from images. B: taken from [12].
The further advantage of this model is that while measuring restitution, additional parameters can be monitored using different fluorescent probes. For instance, several reports demonstrated that gastric luminal pH increased in response to damage [28,29], but these results obtained from measuring collected gastric fluid contents. In contrast, in the focal damage model after photodamage an increase of pH was observed only adjacent to the damage site [4,5]. Thus, the restitution model using two-photon microscope allows us to visualize the entire restitution process as well as microenvironmental changes in a real time setting.
Role of Intracellular Ca2+ in gastric epithelial restitution
Based on results from multiple tissue types, there is no doubt about the involvement of cytosolic Ca2+ mobilization in cell migration. In the stomach, one report using primary cultured rabbit gastric epithelial cells showed that intracellular Ca2+ was significantly higher in migrating cells at the edge of an imposed scratch wound at 2 hr after damage, and observed that verapamil, calmidazolium (Ca2+/calmodulin complex inhibitor), and calphostin-C (protein kinase C : PKC inhibitor) significantly inhibited cell migration speed observed at 24 hr after monolayer wounding [21]. Until recently, there was no extension of this 2002 work to evaluate intracellular Ca2+ mobilization in vivo. This gap in knowledge was approached using the two-photon microscopy restitution model applied to transgenic mice with gastric expression of the YC Ca2+ sensor.
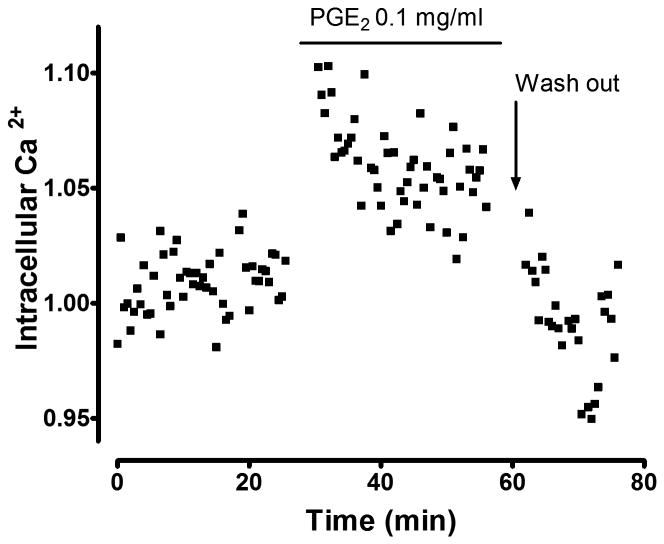
Use of high irradiance with 840 nm light caused two-photon light absorption by the CFP fluorophore, YC photobleaching, and induced cell death (Figure 1A). The intracellular Ca2+ selectively increased in the viable cells adjacent to fatally damaged cells (< 50 μm), in a verapamil or BAPTA/AM inhibitable manner, but not in the cells located far away from damage (> 100 μm) (Figure 1B) [12]. PLC inhibitor, IP3 receptor antagonist, or COX inhibitor slowed repair and also blocked the Ca2+ mobilization stimulated in restituting gastric epithelial cells [12]. These drug effects are all likely to be mediated by inhibiting activation of the Gq protein. PLC is usually linked to Gq and its activation leads to IP3 turnover, by which Ca2+ is released from the endoplasmic reticulum (ER). Many receptors, such as the EP1 receptor subtype of the PGE receptor family, are coupled to Gq [30]. In the scheme of gastric mucosal protection, bicarbonate secretion is mediated by EP1 receptor in a verapamil inhibitable manner [31]. Even in the absence of imposed damage, we observed that PGE2 stimulates intracellular Ca2+ in intact YC mouse gastric epithelial cells (Figure 2), suggesting this pathway is broadly available in an unperturbed epithelium but only selectively activated in some cells of the restituting epithelium. Furthermore, gastric damage induced by taurocholate is more severe in EP1 receptor knockout mice [28]. These data suggest that the increase of intracellular Ca2+ during restitution is likely mediated by PGE2 /EP1 receptor acting downstream of PLC/IP3 activation. It should also be noted that in vitro studies of gastric epithelial cells reported that PGE2 release is inhibited by a PLC inhibitor; suggesting that the increase of intracellular Ca2+ in response to damage will enhance PGE2 production late in the repair cycle, predicted to stimulate repair while sustaining high Ca2+ levels [32,33]. However, because we observed that PLC inhibition blocked the intracellular Ca2+ increase in response to damage [12], another factor besides intracellular Ca2+ must be invoked as a mediator of any initial release of PGE2 in response to damage.
Figure 2. PGE2stimulates intracellular Ca2+ mobilization in the intact stomach.
Intracellular Ca2+ (Ratio FRET/CFP) was monitored in anesthetized YC mouse gastric surface epithelial cells as previously described [12]. PGE2 (0.1 mg/ml) was topically applied to gastric tissue at the time indicated and then removed (washed out).
The routes and mechanisms of stimulating Ca2+ influx from the extracellular compartment remain somewhat speculative in the gastric epithelium, with many potential candidates. Evidence (from use of inhibitors) suggests that some Ca2+ influx important to cell migration occurs through voltage-gated Ca2+ channels in vivo [12,21]. However, it has also been reported that in vitro the voltage-gated K+ channel Kv1.1, is involved in rat gastric epithelial (RGM-1) cell migration [34] as well as intestinal epithelial cell migration [35], and the work showed that Kv1.1 causes Ca2+ influx independently of voltage-gated Ca2+ channels in these models [34,35]. It is possible that other Ca2+ channels such as transient receptor potential (TRP) channels regulate Ca2+ influx. TRPC seems to function as store-operated Ca2+ channel (SOC) in many cells, but TRP subtype expression profiles are still unknown in gastric surface epithelial cells [36,37]. Furthermore, the recently identified Ca2+ release-activated channel protein1 (Orai1), also activates in response to Ca2+ store depletion, which is mediated by translocation of a ER Ca2+ sensor, stromal interaction molecule 1 (STIM1) [38]. Recently, TRPC has been shown to associate with Orai1 and STIM1 in several models [39-41]. In the small intestine, it is reported that TRPC1 interacts with STIM1 in response to Ca2+ store depletion, resulting in stimulating Ca2+ entry and enhancing epithelial migration [42]. All these proteins remain viable candidates to mediate or modulate the Ca2+ influx that stimulates wound repair.
PKC inhibitors also slow gastric cell migration in vitro in response to wounding [21], and we further confirmed that blockade of PKC (by Chelerythrine 5 mg/kg, i.p.) inhibits photodamage-induced restitution in vivo (unpublished). Interestingly, PKC inhibitor treatment simultaneously inhibited mobilization of intracellular Ca2+. Several studies showed that PKC regulates the action of SOCs including TRPC1 [43], suggesting that TRPCs/Orai1/STIM1 may be involved in gastric epithelial Ca2+ influx. However, lacking studies in the gastric epithelial cells or most other regions of the gastrointestinal tract, the mechanisms of Ca2+ influx by gastric epithelial cells remains to be elucidated.
Extracellular Ca2+ in gastric epithelial restitution
A pivotal early observation was that extracellular Ca2+ chelation decreased gastric mucosal potential difference, and this drop was recovered by addition of excess extracellular Ca2+ [25], suggesting that gastric luminal Ca2+ is necessary to maintain integrity of gastric epithelial homeostasis. It has been reported more recently that Ca2+ release into the lumen of gastric glands can occur as part of the regulatory control of normal physiologic functions. Using Ca2+- sensitive microelectrodes, it has been possible to monitor changes in local Ca2+ levels in the extracellular spaces surrounding amphibian gastric glands [44]. In our complementary observations in mice, the luminal environment adjacent to the surface epithelium also sustains a pH and Ca2+ microenvironment as a consequence of the surface unstirred layer [12,45] (Figure 3). It seems that an extracellular Ca2+ gradient exists in a variety of locations within the gastric luminal compartment, and this Ca2+ source has at least a physiologic role in promoting mucus and HCO3- secretion in intact tissue as one contribution to the first line of gastric defenses.
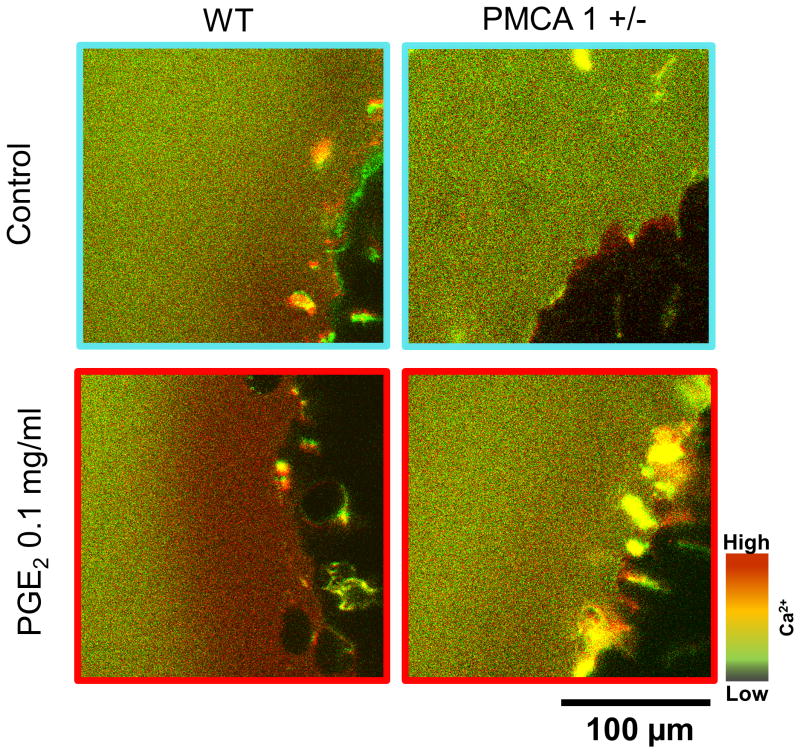
Figure 3. Luminal Ca2+ gradient in response to PGE2.
Luminal Ca2+ (Ratio F458/F488) was monitored by addition of 100 μM Fura-Red added to the gastric luminal perfusate. PGE2 (0.1 mg/ml) was topically applied to gastric tissue.
Extracellular Ca2+ also has a role in injured tissue. Critchlow et al. first demonstrated almost 30 years ago that an adequate extracellular Ca2+ level (changed conditions on the serosal side of isolated tissues in Ussing chamber) is required for complete restitution of isolated frog gastric mucosa after hyperosmolar injury, and that restitution occurs by migration of persisting viable gastric pit cells [2]. Similarly, in response to pervasive surface gastric damage caused by taurocholate, or 30% ethanol, the gastric luminal Ca2+ increased in the collected gastric effluent [24,46]. In the focal damage model after photodamage, we recently reported that luminal Ca2+ increased (Figure 1B) [12]. Importantly, the luminal Ca2+ increase was localized to areas adjacent to the focal damage. The increase of luminal Ca2+ during gastric restitution was inhibited by verapamil, COX inhibitor, or PLC inhibitor pretreatment which can also inhibit intracellular Ca2+ increase during restitution [12]. Furthermore, chelation of intracellular Ca2+ by BAPTA/AM inhibited the increase of luminal Ca2+ [12], suggesting that increase of luminal Ca2+ that benefits epithelial repair is dependent on the intracellular Ca2+ increase, likely the result of active Ca2+ efflux from the surviving epithelial cells that repair the epithelium.
In virtually all cells, there are only two classes of transporter that are candidates to extrude Ca2+ across the cell: plasma membrane Ca2+ ATPases (PMCA) and Na+/Ca2+ exchangers (NCX). The latter class is not expressed in the gastric surface epithelium [12], focusing attention on the PMCAs. It has been reported that PMCA1 serves essential housekeeping functions for cellular Ca2+ homeostasis, whereas PMCA4 performs specialized physiological functions [47]. Caroppo et al. showed that Ca2+ extrusion into the gastric gland lumen is induced by carbachol and is mediated by PMCA [16]. Interestingly, (see Figure 3), PGE2 failed to increase luminal Ca2+ release in PMCA1 (+/-) mouse stomach. We reported that PMCA1 plays an important role in gastric restitution and in regulation of extracellular Ca2+ following damage [12]. In contrast, full ablation of PMCA4 did not affect the speed of gastric restitution, although PMCA1 protein is upregulated, resulting in higher basal luminal Ca2+ levels. These results suggest that although PMCA1 is responsible for Ca2+ efflux across the basolateral membrane of the gastric epithelium, either transmembrane or paracellular Ca2+ flux is allowing access of this pool of extracellular Ca2+ to the lumen even in a healthy epithelium. The tight junction protein claudin-16 has Ca2+ permeability as shown in the kidney and small intestine [48,49], but we could not detect claudin-16 mRNA in the stomach (unpublished). After imposing focal damage, PMCA1 (+/-) mice had a diminished increase in luminal Ca2+ compared to wild-type mice [12]. Since the lateral membranes of cells are exposed to the gastric lumen after photodamage and disruption of epithelial continuity, enhanced paracellular permeability is the simplest route predicted to cause the high Ca2+ concentrations we observe in the damage site microenvironment.
Cross-talk between intracellular and extracellular Ca2+
One of the most striking findings from recent work is to reconcile long-standing scientific observations about the role for luminal Ca2+ as a necessary factor to promote gastric repair versus the lack of need to continually take Ca2+ supplements to promote health and sustain the gastric barrier. Observations in the focal damage model suggest that the resolution to this paradox is that (at least for modest injuries) the stomach is able to recruit endogenous Ca2+ to all the spaces (intracellular and extracellular) that are needed to promote efficient repair and epithelial barrier function. Exogenous luminal Ca2+ is helpful, but not necessary.
The distinct roles of extracellular versus intracellular Ca2+ on restitution remain uncertain. Lack of methods to create a pure separation of intracellular and extracellular Ca2+ mobilization requires cautious conclusions, but results do suggest that luminal Ca2+ plays a potentially independent role to stimulate the restitution. The two spaces display distinct time signatures of Ca2+ mobilization, in that extracellular Ca2+ has a slower time course (peak ∼6 min) than intracellular Ca2+ levels (peak at ∼1 min) after damage (Figure 1B) [12]. Additionally, luminal Ca2+ chelation by HEDTA partially blocked the increase of intracellular Ca2+ only at the late phase yet had a substantial effect to inhibit restitution [12]. Additionally, exogenous luminal CaCl2 administration also promotes gastric repair of damage whether a pervasive damage model [25,46] or our focal damage model [12]. This supplemental luminal Ca2+ also rescued the inhibition of microscopic wound repair caused by verapamil. Importantly, Ca2+ supplement only partially restores intracellular Ca2+ responsiveness in the late phase; again suggesting separable roles of the Ca2+ acting from these two locations. Since a voltage-activated Ca2+ channel blocker also slows repair and blunts intracellular Ca2+ mobilization in the migrating cells, results suggest that luminal Ca2+ could have an important secondary role in later stimulation of Ca2+-dependent signals in the restituting epithelial cells. We already know that PMCA1 efflux is a source of mobilized extracellular Ca2+, but this would provide a second explanation of why it appears almost impossible to separate Ca2+ mobilization in the intracellular versus luminal compartments.
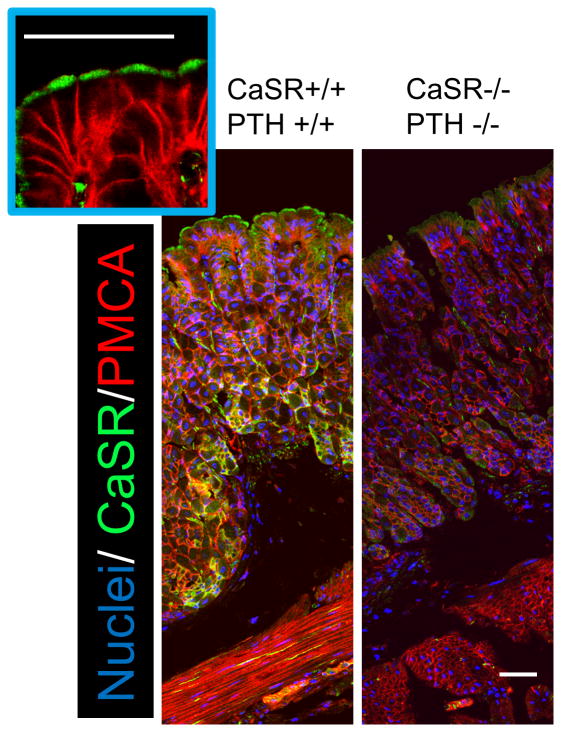
The route of extracellular Ca2+ action remains speculative, but there are some tantalizing possibilities. It has been reported that the Calcium sensing receptor (CaSR) and PMCA protein co-localize with H+/K+ ATPase in the oxynopeptic cells, and evidence has been provided for an autocrine feedback loop that involves luminal Ca2+ sensing to regulate alkali and pepsinogen secretion, and drive water transport [16,44,50]. The mouse surface epithelium is likely to act differently. In Figure 4, CaSR clearly expresses in the apical membrane of gastric surface epithelial cells, distinct from PMCA1 localization. However it has been shown in other cell types that activation of the CaSR can lead to intracellular Ca2+ mobilization, providing yet another route for cross-talk between the two compartments. Extracellular Ca2+ may be physiologically important as it has been proposed that the protective effects of milk on the stomach are due to high Ca2+, rather than buffering of pH [46]. We speculate that the gastric mucosal protective action of milk or Ca2+ administration on the stomach is mediated by CaSR. Some protein, such as casein also can stimulate CaSR leading to increase intracellular Ca2+ [51], while casein itself does not show gastric mucosal protection [46]. However, it is possible that casein potentiate Ca2+ effect on CaSR. Thus, a tempting speculation is that the CaSR may respond to the luminal Ca2+ signals, a topic to be pursued in further study.
Figure 4. Immunofluoresence staining of Calcium sensing receptor in the stomach.
Cryostat sections of stomachs from wild-type mouse are compared to sections from a dual knockout mouse lacking Calcium sensing receptor (CaSR -/-) and Parathyroid hormone (PTH -/-) [66]. were stained with primary antibodies that recognize all PMCA (5F10, Thermo Sci) and CaSR (Millipore). Both anti-CaSR or PMCA primary antibodies were used at 1:100 dilution and visualized with secondary antibodies at 1:100 dilution, Alexa Fluor 488-labelded goat anti-rabbit IgG or Alexa Fluor 555-labelded goat anti-mouse IgG2a, respectively. Nuclear staining was performed by incubation with Hoechst 33342 at 1 μg/ml. Scar bar = 50 μm.
Conclusion
In conventional signaling models, most physiological changes are triggered by intracellular second messengers, such as cAMP, cGMP and Ca2+. All these signaling pathways are involved in the regulation of gastric acid secretion [52,53], pepsinogen secretion [54-56], and mucus secretion [57-59]. The gastric mucosal bicarbonate secretion is mediated by cGMP and Ca2+ [60,61]. it has been shown that cGMP-induced bicarbonate secretion is mediated by COX/PGE2/EP1 receptor [62], so based on known signaling by this receptor, Ca2+ is likely to be the most downstream messenger to elicit receptor actions. In contrast, evidence from cultured cells suggests that neither cAMP nor cGMP plays any role in gastric epithelial cell migration [21,63]. Conversely, luminal Ca2+ likely stimulates cNOS-dependent nitric oxide/cGMP production resulting in facilitating gastric restitution due to inhibition of acid secretion or increase of bicarbonate secretion [24,64]. This set of results leaves space for Ca2+ and its signaling to take a central role in gastric epithelial cell restitution.
Overall, endogenous Ca2+ benefits gastric epithelial repair in vivo. Intact gastric epithelial cells respond to focal damage through the increase of intracellular Ca2+, followed by the release of Ca2+ into the gastric lumen via PMCA1. Subsequently, luminal Ca2+ cooperates to complete restitution. Thus, we have expanded the concept that luminal Ca2+ acts as a ‘Third messenger’ [65] to provide an even more broad range of beneficial effects in both healthy and damaged stomach.
Highlights.
Two-photon microscopy allows unique opportunities for monitoring of gastric events
Two-photon damage model reveals functional microdomains of Ca2+ mobilization
Intracellular Ca2+ increases selectively on restituting gastric epithelial cells
Gastric luminal Ca2+ accelerates gastric epithelial cell restitution
Acknowledgments
We thank Dr. Edward M. Brown (Brigham and Women's Hospital, Boston) for the gift of CaSR/PTH wild-type and its double knockout mouse gastric tissue. The work was supported by the National Institutions of Health Grant RO1-DK-54940 (M.H.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ito S, Lacy ER, Rutten MJ, Critchlow J, Silen W. Rapid repair of injured gastric mucosa. Scand J Gastroenterol Suppl. 1984;101:87–95. [PubMed] [Google Scholar]
- 2•.Critchlow J, Magee D, Ito S, Takeuchi K, Silen W. Requirements for restitution of the surface epithelium of frog stomach after mucosal injury. Gastroenterology. 1985;88:237–249. doi: 10.1016/s0016-5085(85)80177-3. This is the first paper that demostrates the requirement of Ca2+ for gastric restitution in vitro. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AM, Morrison SW, Yang DX, Hagen SJ. Energy dependence of restitution in the gastric mucosa. Am J Physiol Cell Physiol. 2001;281:C430–438. doi: 10.1152/ajpcell.2001.281.2.C430. [DOI] [PubMed] [Google Scholar]
- 4.Xue L, Aihara E, Podolsky DK, Wang TC, Montrose MH. In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase. Gut. 2010;59:1184–1191. doi: 10.1136/gut.2009.205625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Starodub OT, Demitrack ES, Baumgartner HK, Montrose MH. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–232. doi: 10.1152/ajpcell.00395.2006. This study introduces two-photon-induced gastric restitution model in live animals and this model can monitor restitution process as well as microenvironments changes in real time. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol. 2005;205:129–137. doi: 10.1007/s00232-005-0778-z. [DOI] [PubMed] [Google Scholar]
- 7.Belkacemi L, Bedard I, Simoneau L, Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium. 2005;37:1–8. doi: 10.1016/j.ceca.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–2938. doi: 10.1007/s00018-005-5481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paimela H, Goddard PJ, Silen W. Present views on restitution of gastrointestinal epithelium. Digestive diseases and sciences. 1995;40:2495–2496. doi: 10.1007/BF02063263. [DOI] [PubMed] [Google Scholar]
- 10.Tarnawski A, Ahluwalia A, Jones MK. Gastric cytoprotection beyond prostaglandins: cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr Pharm Des. 2013;19:126–132. doi: 10.2174/13816128130117. [DOI] [PubMed] [Google Scholar]
- 11.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 12•.Aihara E, Hentz CL, Korman AM, Perry NP, Prasad V, Shull GE, Montrose MH. In vivo epithelial wound repair requires mobilization of endogenous intracellular and extracellular calcium. J Biol Chem. 2013;288:33585–33597. doi: 10.1074/jbc.M113.488098. This is the key publication that demonstrates the importance of both intracellular and extracellular Ca2+ in the gastric restitution in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demitrack ES, Soleimani M, Montrose MH. Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl-/HCO3- Am J Physiol Gastrointest Liver Physiol. 2010;299:G255–264. doi: 10.1152/ajpgi.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 15.Tsien RY. Breeding molecules to spy on cells. Harvey Lect. 2003;99:77–93. [PubMed] [Google Scholar]
- 16.Caroppo R, Gerbino A, Debellis L, Kifor O, Soybel DI, Brown EM, Hofer AM, Curci S. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia. Embo J. 2001;20:6316–6326. doi: 10.1093/emboj/20.22.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksnas J, Phillipson M, Petersson J, Engstrand L, Holm L. An in vivo model for gastric physiological and pathophysiological studies in the mouse. Acta Physiol Scand. 2005;184:151–159. doi: 10.1111/j.1365-201X.2005.01434.x. [DOI] [PubMed] [Google Scholar]
- 18.Phillipson M, Atuma C, Henriksnas J, Holm L. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am J Physiol Gastrointest Liver Physiol. 2002;282:G211–219. doi: 10.1152/ajpgi.00223.2001. [DOI] [PubMed] [Google Scholar]
- 19.Luo JC, Cho CH, Ng KM, Hsiang KW, Lu CL, Chen TS, Chang FY, Lin HC, Perng CL, Lee SD. Dexamethasone inhibits tumor necrosis factor-alpha-stimulated gastric epithelial cell migration. J Chin Med Assoc. 2009;72:509–514. doi: 10.1016/S1726-4901(09)70419-8. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi S, Nakamura E, Kubo Y, Takahashi N, Yamaguchi A, Matsui H, Hagen SJ, Takeuchi K. Impairment by allyl isothiocyanate of gastric epithelial wound repair through inhibition of ion transporters. J Physiol Pharmacol. 2008;59:691–706. [PubMed] [Google Scholar]
- 21•.Ranta-Knuuttila T, Kiviluoto T, Mustonen H, Puolakkainen P, Watanabe S, Sato N, Kivilaakso E. Migration of primary cultured rabbit gastric epithelial cells requires intact protein kinase C and Ca2+/calmodulin activity. Dig Dis Sci. 2002;47:1008–1014. doi: 10.1023/a:1015025704589. This study shows intracellular Ca2+ increase and its signaling pathway is involved in gastric cell migration in vitro. This is the only paper investigating intracellular Ca2+ mobilization in gastric cell migration in past 30 years. [DOI] [PubMed] [Google Scholar]
- 22.Ragasa R, Nakamura E, Marrone L, Yanaka S, Hayashi S, Takeuchi K, Hagen SJ. Isothiocyanate inhibits restitution and wound repair after injury in the stomach: ex vivo and in vitro studies. J Pharmacol Exp Ther. 2007;323:1–9. doi: 10.1124/jpet.107.121640. [DOI] [PubMed] [Google Scholar]
- 23.Hagen SJ, Morrison SW, Law CS, Yang DX. Restitution of the bullfrog gastric mucosa is dependent on a DIDS-inhibitable pathway not related to HCO3- ion transport. Am J Physiol Gastrointest Liver Physiol. 2004;286:G596–605. doi: 10.1152/ajpgi.00390.2002. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi K, Kato S, Konaka A, Sugawa Y. Luminal calcium in regulation of nitric oxide release and acid secretion in rat stomachs after damage. Dig Dis Sci. 1999;44:515–522. doi: 10.1023/a:1026645004918. [DOI] [PubMed] [Google Scholar]
- 25•.Takeuchi K, Nobuhara Y, Okabe S. Role of luminal Ca2+ on normal and damaged gastric mucosa in the rat. Dig Dis Sci. 1985;30:1072–1078. doi: 10.1007/BF01315605. This study suggests the exsistance of Ca2+ in the gastric lumen and it plays critical role in the gastric restitution. [DOI] [PubMed] [Google Scholar]
- 26.Mir Y, Houde D, van Lier JE. Two-photon absorption of copper tetrasulfophthalocyanine induces phototoxicity towards Jurkat cells in vitro. Photochem Photobiol Sci. 2006;5:1024–1030. doi: 10.1039/b607113a. [DOI] [PubMed] [Google Scholar]
- 27.Starkey JR, Rebane AK, Drobizhev MA, Meng F, Gong A, Elliott A, McInnerney K, Spangler CW. New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse. Clin Cancer Res. 2008;14:6564–6573. doi: 10.1158/1078-0432.CCR-07-4162. [DOI] [PubMed] [Google Scholar]
- 28.Komoike Y, Nakashima M, Nakagiri A, Takeuchi K. Prostaglandin E receptor EP1 subtype but not prostacyclin IP receptor involved in mucosal blood flow response of mouse stomachs following barrier disruption. Digestion. 2003;67:186–194. doi: 10.1159/000072057. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi K, Okabe S. Role of luminal alkalinization in repair process of ethanol-induced mucosal damage in rat stomach. Digestive diseases and sciences. 1983;28:993–1000. doi: 10.1007/BF01311728. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi K, Yagi K, Kato S, Ukawa H. Roles of prostaglandin E-receptor subtypes in gastric and duodenal bicarbonate secretion in rats. Gastroenterology. 1997;113:1553–1559. doi: 10.1053/gast.1997.v113.pm9352857. [DOI] [PubMed] [Google Scholar]
- 32.Sekiguchi F, Saito S, Takaoka K, Hayashi H, Nagataki M, Nagasawa K, Nishikawa H, Matsui H, Kawabata A. Mechanisms for prostaglandin E2 formation caused by proteinase-activated receptor-1 activation in rat gastric mucosal epithelial cells. Biochem Pharmacol. 2007;73:103–114. doi: 10.1016/j.bcp.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Ota S, Hata Y, Terano A, Yoshiura K, Hiraishi H, Kawabe T, Mutoh H, Shiina S, Sugimoto T. Roles of Ca2+ and protein kinase C in regulation of prostaglandin E2 release by cultured rabbit gastric epithelial cells. Dig Dis Sci. 1993;38:1426–1434. doi: 10.1007/BF01308599. [DOI] [PubMed] [Google Scholar]
- 34.Wu WK, Li GR, Wong TM, Wang JY, Yu L, Cho CH. Involvement of voltage-gated K+ and Na+ channels in gastric epithelial cell migration. Mol Cell Biochem. 2008;308:219–226. doi: 10.1007/s11010-007-9631-2. [DOI] [PubMed] [Google Scholar]
- 35.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JX, Wang JY. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. American journal of physiology Cell physiology. 2002;282:C885–898. doi: 10.1152/ajpcell.00361.2001. [DOI] [PubMed] [Google Scholar]
- 36.Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, Turner DJ, Yuan JX, Wang JY. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G782–792. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- 37.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zbidi H, Lopez JJ, Amor NB, Bartegi A, Salido GM, Rosado JA. Enhanced expression of STIM1/Orai1 and TRPC3 in platelets from patients with type 2 diabetes mellitus. Blood Cells Mol Dis. 2009;43:211–213. doi: 10.1016/j.bcmd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao JN, Rathor N, Zhuang R, Zou T, Liu L, Xiao L, Turner DJ, Wang JY. Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. American journal of physiology Cell physiology. 2012;303:C308–317. doi: 10.1152/ajpcell.00120.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583:25–36. doi: 10.1113/jphysiol.2007.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Caroppo R, Gerbino A, Fistetto G, Colella M, Debellis L, Hofer AM, Curci S. Extracellular calcium acts as a “third messenger” to regulate enzyme and alkaline secretion. J Cell Biol. 2004;166:111–119. doi: 10.1083/jcb.200310145. This study directly measures luminal Ca2+ in the intact stomach using microelectrodes, and shows that luminal Ca2+ regulates normal gastric functions. They suggest the concept that extracellular Ca2+ acts as a ‘third messenger’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumgartner HK, Kirbiyik U, Coskun T, Chu S, Montrose MH. Endogenous cyclooxygenase activity regulates mouse gastric surface pH. J Physiol. 2002;544:871–882. doi: 10.1113/jphysiol.2002.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Koo MW. The effects of milk and calcium on ethanol-induced gastric mucosal damage. Pharmacol Res. 1994;29:217–224. doi: 10.1016/1043-6618(94)80045-6. This study demonstrates gastric luminal Ca2+ increases in response to damage, and supplemental Ca2+ promotes gastric restitution. [DOI] [PubMed] [Google Scholar]
- 47.Prasad V, Okunade G, Liu L, Paul RJ, Shull GE. Distinct phenotypes among plasma membrane Ca2+-ATPase knockout mice. Ann N Y Acad Sci. 2007;1099:276–286. doi: 10.1196/annals.1387.029. [DOI] [PubMed] [Google Scholar]
- 48.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerbino A, Fistetto G, Colella M, Hofer AM, Debellis L, Caroppo R, Curci S. Real time measurements of water flow in amphibian gastric glands: modulation via the extracellular Ca2+-sensing receptor. J Biol Chem. 2007;282:13477–13486. doi: 10.1074/jbc.M610585200. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima S, Hira T, Hara H. Calcium-sensing receptor mediates dietary peptide-induced CCK secretion in enteroendocrine STC-1 cells. Mol Nutr Food Res. 2012;56:753–760. doi: 10.1002/mnfr.201100666. [DOI] [PubMed] [Google Scholar]
- 52.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Berg A, Redeen S, Grenegard M, Ericson AC, Sjostrand SE. Nitric oxide inhibits gastric acid secretion by increasing intraparietal cell levels of cGMP in isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1061–1066. doi: 10.1152/ajpgi.00230.2005. [DOI] [PubMed] [Google Scholar]
- 54.Inoue M, Fong J, Shah G, Hirschowitz BI. Mediation of muscarinic stimulation of pepsinogen secretion in the frog. Am J Physiol. 1985;248:G79–86. doi: 10.1152/ajpgi.1985.248.1.G79. [DOI] [PubMed] [Google Scholar]
- 55.Fiorucci S, Distrutti E, Chiorean M, Santucci L, Belia S, Fano G, De Giorgio R, Stanghellini V, Corinaldesi R, Morelli A. Nitric oxide modulates pepsinogen secretion induced by calcium-mediated agonist in guinea pig gastric chief cells. Gastroenterology. 1995;109:1214–1223. doi: 10.1016/0016-5085(95)90581-2. [DOI] [PubMed] [Google Scholar]
- 56.Gritti I, Banfi G, Roi GS. Pepsinogens: physiology, pharmacology pathophysiology and exercise. Pharmacological research : the official journal of the Italian Pharmacological Society. 2000;41:265–281. doi: 10.1006/phrs.1999.0586. [DOI] [PubMed] [Google Scholar]
- 57.Heim HK, Sewing KF. Pharmacological regulation of gastric mucous glycoprotein secretion. Eur J Gastroenterol Hepatol. 1995;7:1105–1122. doi: 10.1097/00042737-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi S, Takeuchi K, Okabe S. EP4 receptor mediation of prostaglandin E2-stimulated mucus secretion by rabbit gastric epithelial cells. Biochemical pharmacology. 1999;58:1997–2002. doi: 10.1016/s0006-2952(99)00286-5. [DOI] [PubMed] [Google Scholar]
- 59.Brown JF, Keates AC, Hanson PJ, Whittle BJ. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol. 1993;265:G418–422. doi: 10.1152/ajpgi.1993.265.3.G418. [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi K, Takehara K, Kato S, Yagi K. PACAPs stimulate duodenal bicarbonate secretion at PACAP receptors in the rat. Am J Physiol. 1997;272:G646–653. doi: 10.1152/ajpgi.1997.272.3.G646. [DOI] [PubMed] [Google Scholar]
- 61.Flemstrom G, Garner A. Gastroduodenal HCO3- transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982;242:G183–193. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- 62.Kita K, Takahashi K, Ohashi Y, Takasuka H, Aihara E, Takeuchi K. Phosphodiesterase isozymes involved in regulation of formula secretion in isolated mouse stomach in vitro. The Journal of pharmacology and experimental therapeutics. 2008;326:889–896. doi: 10.1124/jpet.108.138941. [DOI] [PubMed] [Google Scholar]
- 63.Kiviluoto T, Watanabe S, Hirose M, Sato N, Mustonen H, Puolakkainen P, Ronty M, Ranta-Knuuttila T, Kivilaakso E. Nitric oxide donors retard wound healing in cultured rabbit gastric epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1151–1157. doi: 10.1152/ajpgi.2001.281.5.G1151. [DOI] [PubMed] [Google Scholar]
- 64.Yanaka A, Muto H, Fukutomi H, Ito S, Silen W. Role of nitric oxide in restitution of injured guinea pig gastric mucosa in vitro. Am J Physiol. 1995;268:G933–942. doi: 10.1152/ajpgi.1995.268.6.G933. [DOI] [PubMed] [Google Scholar]
- 65.Hofer AM, Lefkimmiatis K. Extracellular calcium and cAMP: second messengers as “third messengers”? Physiology (Bethesda) 2007;22:320–327. doi: 10.1152/physiol.00019.2007. [DOI] [PubMed] [Google Scholar]
- 66.Quinn SJ, Thomsen AR, Egbuna O, Pang J, Baxi K, Goltzman D, Pollak M, Brown EM. CaSR-mediated interactions between calcium and magnesium homeostasis in mice. Am J Physiol Endocrinol Metab. 2013;304:E724–733. doi: 10.1152/ajpendo.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]