Summary
When wounded, eukaryotic cells reseal in a few seconds. Ca2+ influx induces exocytosis of lysosomes, a process previously thought to promote repair by “patching” wounds. New evidence suggests that resealing involves direct wound removal. Exocytosis of lysosomal acid sphingomyelinase triggers endocytosis of lesions, followed by intracellular degradation. Characterization of injury-induced endosomes revealed a role for caveolae, sphingolipid-enriched plasma membrane invaginations that internalize toxin pores and are abundant in mechanically stressed cells. These findings provide a novel mechanistic explanation for the muscle pathology associated with mutations in caveolar proteins. Membrane remodeling by the ESCRT complex was also recently shown to participate in small wound repair, emphasizing that cell resealing involves previously unrecognized mechanisms for lesion removal, which are distinct from the “patch” model.
Keywords: injury, resealing, endocytosis, caveolae, muscular dystrophy
Injury-mediated Ca2+ entry, exocytosis and plasma membrane repair
It has been known for several decades that wounded eukaryotic cells repair plasma membrane wounds in a few seconds, by a mechanism strictly dependent on extracellular Ca2+ 1, 2. Subsequent imaging experiments demonstrated that Ca2+ entering cells through lesions triggers localized bursts of exocytosis, a process that is required for plasma membrane repair 3, 4. Ultrastructural analysis revealed the presence of large intracellular vesicles close to wound sites 3, leading to the suggestion that Ca2+ influx induces homotypic fusion of intracellular vesicles, forming a large exocytic membrane “patch” that promotes resealing when fused with the wound 5. However, most of these early investigations did not utilize specific markers to identify the injury-induced intracellular vesicles, and to confirm their exocytic origin. Recent studies revisited this issue, and generated surprising observations, as discussed below. These studies confirmed the essential role of exocytosis in the resealing of wounded cells but revealed additional mechanisms that appear to be involved in direct removal of lesions, and not simply in “patching” the damaged membrane.
Plasma membrane repair is mediated by lysosomal exocytosis
Ca2+-triggered exocytosis near plasma membrane wounds was initially detected by imaging, in cells labeled for several hours with lipophilic dyes. Such dyes label many intracellular organelles, so these early experiments did not allow identification of the vesicles involved 3. Subsequent studies revealed that specific markers of lysosomes, such as luminal epitopes of the glycoprotein Lamp1, appear on the cell surface after injury 6. This observation, while initially surprising, was confirmed in several cell types after different forms of injury, including those inflicted by bacterial and protozoan pathogens 7, 8. Lysosomal hydrolases are released into the supernatant of cells wounded in the presence, but not in the absence of Ca2+ 9, consistent with the finding that conventional lysosomes behave as Ca2+-dependent secretory vesicles 10. Prior to these studies, Ca2+-dependent exocytosis was thought to involve only lysosome-related organelles in specialized cells, such as cytotoxic lymphocytes, melanocytes and lung alveolar cells 11. It is now clear that the ability to fuse with the plasma membrane upon elevation in intracellular Ca2+ is a property of lysosomes found in many different cell types, including cells not specialized in Ca2+-regulated secretion 12-14. As discussed below, Ca2+-dependent lysosomal exocytosis also occurs in injured muscle fibers 15, 16.
Ca2+-triggered exocytosis of lysosomes was directly visualized by total internal reflection fluorescence microscopy (TIRF-M), and these assays showed that most lysosomes that fuse with the plasma membrane belong to a pre-existing membrane-proximal population 17. Ca2+-regulated lysosomal exocytosis occurs in two forms, complete fusion or kiss-and-run 18, and can be regulated by two lysosomal membrane proteins, the Ca2+ sensor Syt VII and the cation transporter mucolipin 1. Syt VII proved a useful tool to demonstrate that Ca2+-regulated lysosomal exocytosis is required for plasma membrane repair 6. It has been widely assumed that lysosomes promote plasma membrane resealing by giving rise to an intracellular membrane patch, which would be applied directly to the wounded membrane, repairing the injury 5. However, in experiments involving localized wounding with a microinjection needle, the lysosomal marker Lamp1 was detected in a punctate pattern surrounding the wound site - an observation that is not consistent with the fusion of a large lysosome-derived “patch” directly onto the wound 6. In addition, as discussed below, the patch model of plasma membrane repair does not satisfactorily explain how lesions formed by the insertion of stable, protein-lined transmembrane pores can be repaired.
Wounded membrane is actively removed
Ca2+-triggered exocytosis reduces membrane tension, a process proposed to facilitate spontaneous bilayer resealing in wounded cells 19. This scenario provided a plausible alternative to the patch hypothesis of plasma membrane repair, because it did not postulate the hard-to-envision process of direct merging of intracellular vesicle membranes with the irregular margins of a wound. However, lesions formed by insertion of pore-forming proteins are also repaired in a Ca2+-dependent manner, with rapid kinetics similar to that observed during mechanical wound repair 20, 21. The realization that stable, protein-lined transmembrane pores cannot be eliminated by simply reducing plasma membrane tension, prompted a closer examination of the resealing mechanism in cells permeabilized by the bacterial toxin streptolysin O (SLO). These studies revealed a surprising response: In addition to lysosomal exocytosis, SLO-permeabilized cells undergo massive endocytosis, with a rapid intracellular accumulation of endocytic vesicles. Injury-induced endocytic vesicles were detected as soon as 12-20 seconds after cell permeabilization, and these vesicles appeared as large (300-500 nm), uncoated intracellular compartments 4-5 minutes after cell wounding 22. Similar observations were made in cells permeabilized by perforin, the pore-forming protein that mediates target cell killing by cytotoxic lymphocytes (CTL). Exposure to perforin triggered intracellular Ca2+-transients, lysosomal exocytosis, and the formation of numerous endosomes that carried the pores intracellularly, and later appeared as large vesicles 2 minutes after cell injury 13. Interestingly, endocytosis of perforin-induced lesions caused a shift from necrotic to apoptotic death in CTL targets, suggesting that restoration of plasma membrane integrity is required for the subsequent signaling steps that lead to apoptosis 13. The involvement of endocytosis in plasma membrane repair is not exclusive to mammalian cells, having been also observed in wounded crayfish neurons 23 and in the nematode C. elegans, where resealing of intestinal cells permeabilized with the Bacillus thuringiensis pore-forming toxin Cry21A was shown to require RAB-5 and RAB11-dependent endocytosis 24.
The patch hypothesis postulates that exocytosis of intracellular membrane is sufficient to repair plasma membrane wounds. However, it has been difficult to envision how fusion of a large intracellular vesicle, through a single radially-expanding fusion pore, could restore continuity of a lipid bilayer containing an irregular wound. To address this difficulty, it was suggested that patch-mediated plasma membrane repair might involve a vertex fusion process. In this model, multiple fusion pores would form around the periphery of the wounded region, between the intracellular “patch” vesicle and the plasma membrane. Expansion and merging of these fusion pores would cause extracellular release of a membrane fragment containing the wound, and a residual portion of the patch vesicle 25 (see Figure 1D). This mechanism, in principle, could be involved in the removal of stable, proteinaceous pores from the plasma membrane. However, several lines of evidence argue against a vertex ring exocytosis and membrane shedding mechanism as the major event responsible for the removal of pores formed by bacterial toxins. In mammalian cells, electron microscopy and biochemistry experiments showed that after cell permeabilization and resealing streptolysin O (SLO) traffics intracellularly in endosomes that gradually increase in size, undergoes ubiquitination, and is sorted into luminal vesicles of multivesicular bodies/lysosomes for degradation 26 (Figure 2). Furthermore, imaging experiments showed that plasma membrane blebs induced by SLO fully retract back into the cell body during cell resealing, instead of being shed 22. A similar expansion of micron-sized blebs quickly followed by retraction was observed after plasma membrane damage with a UV laser 27. These observations agree with the earlier demonstration that transient rearrangements of the cortical actin cytoskeleton, a process induced by cytosolic Ca2+ transients, control the expansion and subsequent shrinkage of plasma membrane blebs 28. When plasma membrane repair blocks further Ca2+ entry, the cortical cytoskeleton is restored, promoting retraction of toxin pore-induced membrane blebs. Further strengthening this view, disruption of the cortical actin cytoskeleton abolished blebbing but did not prevent resealing of SLO-permeabilized cells 22.
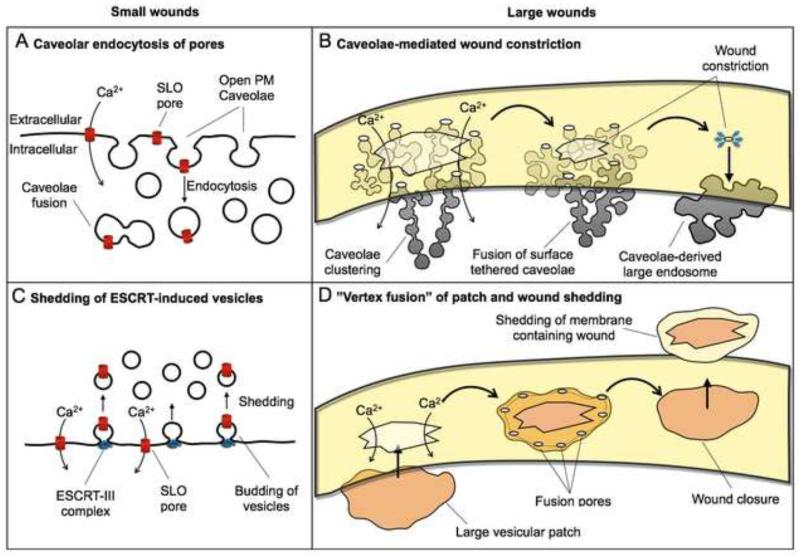
Figure 1. Proposed mechanisms for plasma membrane repair.
Small wounds (<100 nm) such as those generated by pore-forming toxins were proposed to be repaired by two mechanisms: (A) endocytosis of caveolar vesicles 16, and (C) ESCRT-mediated vesicle budding 27. Large wounds (>100 nm) were proposed to be repaired by: (B) a mechanism involving endocytosis, clustering and fusion of caveolae that leads to wound constriction 16, and (D) patching by a large intracellular exocytic vesicle, proposed to fuse at several points at the periphery of the wound, resulting in shedding of the damaged membrane 25. Internalization of SLO pores in caveolar vesicles has been observed 16, but direct evidence for extracellular shedding of wounded plasma membrane is still not available.
Figure 2. Transmembrane pores formed by SLO are internalized in caveolar vesicles which gradually traffic into lysosomes for degradation.
During the first 30 seconds after cell permeabilization with SLO in the presence of Ca2+, individual caveolae accumulate at the cell periphery. In subsequent minutes, caveolar vesicles carrying SLO pores merge, forming larger compartments. SLO pores are ultimately sorted into luminal vesicles of multivesicular bodies, and degraded in mature lysosomes 26. The images show transmission EM of endocytic vesicles observed in NRK cells at increasing time points after permeabilization with SLO; the arrows point to BSA-gold sued as an endocytic tracer.
It remains possible, however, that membrane fragments containing lesions are shed from cells during plasma membrane repair. This has been proposed to occur in the form of annexin-positive microvesicles 29 or more recently by membrane budding mediated by the ESCRT complex 27 (see Figure 1C). Extracellular shedding of intestinal microvilli was also observed in C. elegans after exposure to pore-forming proteins, but the process did not require Ca2+, a major requisite for plasma membrane repair 24. One study proposed that extracellular budding and shedding of large vesicles (in the micrometer diameter range) might represent the primary mechanism by which cells defend themselves from pore forming toxins 30. However, that study described SLO-induced plasma membrane bleb formation in chemically fixed cells 30, raising important questions regarding the physiological significance of the observations. Several studies demonstrated that plasma membrane repair is strictly ATP dependent 22, 31. Given the frequent observation of large transient blebs in live wounded cells that expand and then retract, shedding-mediated plasma membrane repair may largely involve the release of smaller membrane buds, such as those predicted to be generated by the ESCRT membrane-deforming pathway 27, 32. Indeed, it was found that ESCRT III components are required for the repair of small (<100 nm), but not large plasma membrane lesions 27. Several issues, however, remain to be clarified before lesion removal can be unequivocally attributed to ESCRT-mediated plasma membrane budding and shedding. For example, plasma membrane protrusions of 200-500 nm or more in diameter were recently reported 27. However, according to the available data, vesicles generated by the ESCRT pathway are expected to be significantly smaller, around 50 nm in diameter 33, 34. Furthermore, the reported kinetics of ESCRT recruitment to the plasma membrane after laser wounding is slower than the few seconds required for the repair of several types of lesions 5,27,16,25, raising the possibility that ESCRT-mediated budding may represent a plasma membrane remodeling process that occurs after resealing. Direct evidence for the shedding of plasma membrane wounds is still not available, because it has been challenging to distinguish plasma membrane fragments containing lesions from membrane that is constitutively shed under normal conditions, or passively released from the cytosol of wounded cells. Interestingly, the ESCRT I protein Tsg101 and the AAA ATPase Vps4 were found to be involved in microvesicle shedding from the surface of cells, independently of viral infections or plasma membrane wounding 35. Whether ESCRT-dependent plasma membrane budding and shedding represents a constitutive process that is co-opted, and perhaps intensified during plasma membrane repair, is an intriguing topic that deserves further investigation.24
Collectively, evidence generated by independent laboratories strongly indicates that plasma membrane repair may not be mediated simply by the addition of an exocytic patch. Rather, endocytosis followed by intracellular degradation of the lesion and/or ESCRT-mediated vesicle shedding appear to play an active role in wound repair. Given the very similar kinetics and common requirement for factors such as Ca2+ and cholesterol in the repair of toxin pores and of mechanical tears 9, 22, it is conceivable that remodeling and active removal of injured portions of the membrane represents a general strategy used by eukaryotic cells to maintain their plasma membrane integrity.
Sphingomyelinase emerges as a major regulator of lesion removal by endocytosis
The uncoated, irregularly shaped endosomes seen intracellularly a few minutes after cell injury 22 are morphologically similar to vesicles formed at the periphery of cells exposed to bacterial sphingomyelinase 36. This observation suggested, for the first time, a mechanism by which Ca2+-triggered exocytosis of lysosomes might promote lesion removal by endocytosis: By remodeling the plasma membrane through secretion of acid sphingomyelinase (ASM). Sphingomyelin is an abundant plasma membrane lipid found in association with cholesterol, in sphingolipid-enriched membrane domains known as lipid rafts 37. Enzymatic cleavage of the phosphorylcholine head group of sphingomyelin by sphingomyelinase generates ceramide, a lipid that tends to coalesce on membranes forming inward budding microdomains (Box 1). Ceramide-driven membrane invagination was directly demonstrated in artificial giant liposomes 38 and also shown to be involved inthe formation of intraluminal vesicles in multi-vesicular bodies, a process mediated by cytosolic neutral sphingomyelinase 39. There is substantial evidence from several laboratories suggesting that the lysosomal enzyme ASM may trigger plasma membrane invagination and endocytosis in wounded cells, in response to Ca2+ influx. ASM is translocated from a lysosomal/endosomal location to the cell surface when cells are exposed to membrane-damaging stress 40, and can act on the outer leaflet of the plasma membrane generating ceramide-enriched microdomains 41-44. ASM inhibitors block formation of the plasma membrane-associated ceramide platforms triggered by Ca2+ influx in SLO-permeabilized cells 45, and inhibit plasma membrane repair 9. In coronary arterial endothelial cells, translocation of lysosomal V1 H+-ATPase to the plasma membrane was associated with the creation of an extracellular acidic microenvironment, proposed to facilitate extracellular ASM activity and formation of ceramide-enriched, lipid raft-associated redox signalosomes 46.
Box 1. Ceramide generation and plasma membrane invagination.
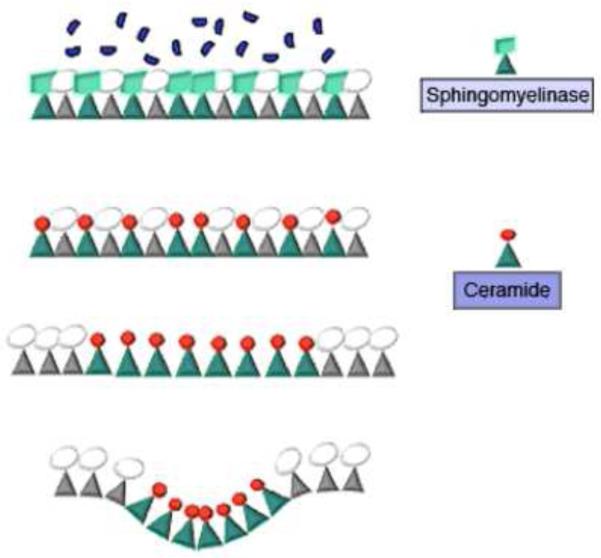
Sphingomyelin is a major sphingolipid of animal cells, present at high concentration in the outer leaflet of the plasma membrane 69. Sphingomyelinase, a sphingomyelin-specific type C phospholipase, cleaves the phosphorylcholine headgroup of sphingomyelin generating ceramide, a small lipid with a less hydrated head group and two long, saturated hydrophobic chains. Ceramide increases the order of acyl chains in lipid bilayers, leading to a tighter packing and self-aggregation process that results in the formation of ceramide-enriched microdomains. The smaller area occupied by ceramide when compared to other membrane lipids can induce outer membrane leaflets to condense and form an inverted nonlamelar phase, a process that can cause membrane invagination (see Figure I). The driving force for invagination may be provided by the area difference between the adjacent monolayers, and by the negative spontaneous curvature of the ceramide-enriched domain. Through this mechanism, growth of the ceramide-enriched microdomain can lead to formation of an enclosed cavity, as demonstrated experimentally in giant liposomes 38. In agreement with this proposed mechanism, the exogenous addition of C6-ceramide or sphingomyelinase to fibroblasts and macrophages results in endocytic vesicle formation 36, 70. Although ceramide has been suggested to have second-messenger roles in cells, it is important to note that it does not transfer spontaneously between lipid bilayers, and has a slow flip-flop rate (estimated as about 22 min – 71). This suggests that ceramide is initially restricted to the bilayer side where it is generated, indicating that it may act primarily through membrane remodeling events such as microdomain formation, membrane vesiculation and vesicular trafficking 43.
Experiments using ASM-deficient cells directly demonstrated the importance of lysosomal ASM for the restoration of plasma membrane integrity. ASM-depleted cells generated by siRNA or derived from Niemann-Pick Type A human patients (Box 2) were deficient in injury-induced endocytosis and in plasma membrane repair. Remarkably, exposure to purified ASM or bacterial sphingomyelinase was sufficient to restore membrane integrity in ASM-depleted cells 9. Exogenous administration of sphingomyelinase promoted plasma membrane repair even in the absence of Ca2+, demonstrating that sphingomyelinase activity can bypass the need for Ca2+-triggered lysosomal exocytosis 47. These findings suggest that the essential contribution of lysosomes to plasma membrane repair is to release ASM, which remodels the cell surface thereby promoting lesion removal.
Box 2. Acid sphingomyelinase and Niemann-Pick A and B disease.
In 1914, Albert Niemann described the first patient with the genetic disorder now known as Niemann-Pick Disease (NPD). Both forms of NPD, Type A and Type B, are caused by recessive mutations in SMPD1, the gene encoding lysosomal enzyme sphingomyelinase (ASM). The variations in severity between the two NPD forms are likely to result from differences in residual ASM activity. NPD Type A is characterized by a progressive neurodegeneration that leads to death of affected children by age 2-3. Type B NPD is a milder, later onset form in which patients have reduced neurological symptoms but develop visceral abnormalities such as hepatosplenomegaly, pulmonary insufficiency and cardiovascular disease. The clinical findings in NPD are largely attributed to the accumulation of sphingomyelin, cholesterol and other lipids within lysosomes, consistent with its classification as a lysosomal storage disease. However, it is becoming increasingly clear that ASM secretion can result in sphingomyelin hydrolysis in the outer leaflet of the plasma membrane generating ceramide, and that defects in this process may influence the pathophysiology of NPD 72. To explain the extracellular release of ASM, it was suggested that mammalian cells contain two enzyme forms, one lysosomal and another secreted 73. However, ASM is encoded by a single gene, SMPD1, and no alternative spliced transcripts have been identified 72. A plausible explanation is that secreted ASM originates from Ca2+-triggered exocytosis of lysosomes. Several studies have shown that when cells are exposed to stress, ASM relocates from the lysosomes to the cell surface 40. Wounding cells with pore-forming toxins triggers exocytosis of ASM, a process dependent on Ca2+ influx from the extracellular medium. Importantly, cells derived from NPD patients or depleted in ASM by RNAi are impaired in Ca2+-dependent endocytosis and plasma membrane repair, and these defects can be reversed by extracellular addition of ASM or B. cereus sphingomyelinase 9. Despite the low pH optimum of ASM, there is evidence that this lysosomal enzyme can act extracellularly, possibly due to the simultaneous translocation of lysosomal V1 H+-ATPase to the plasma membrane 46. The recently emerged role of ASM in plasma membrane repair suggests that defective resealing of wounded cells may account for some aspects of NPD pathology, such as the reduced muscle tone observed in NPD Type A patients 74.
Interestingly, recent electrophysiological studies also reported massive endocytosis in cells subjected to large intracellular Ca2+ transients, or exposed to bacterial sphingomyelinase 48, 49. This rapid endocytic process, designated as MEND (massive endocytosis), did not involve the actin cytoskeleton or the endocytosis-regulatory proteins clathrin and dynamin 48. These findings are similar to that observed for the sphingomyelinase-dependent endocytic process that mediates plasma membrane repair, which is also dynamin-independent 9, 16, 22. A dynamin-independent form of Ca2+-regulated endocytosis was also reported in cortical astrocytes 50, adding to the evidence that this process may play a central role in the resealing of many different cell types after injury. It remains unclear how wounding-induced, sphingomyelinase-dependent endosomes pinch off from the plasma membrane, but experiments in artificial liposomes show that sphingomyelinase is sufficient to induce membrane invagination and the budding of luminal vesicles 38. Thus, it is conceivable that forces generated during invagination of ceramide-enriched membrane domains may be sufficient for membrane scission, bypassing the need for dynamin. Alternatively, other molecules not yet identified may promote the pinching-off of vesicles involved in plasma membrane repair.
Experiments with inhibitors suggested that the massive Ca2+-dependent endocytic process designated as MEND 48, 49 might not involve the lysosomal sphingomyelinase ASM. However, it remains unclear whether full inhibition of ASM activity was achieved under those experimental conditions, and whether the large intracellular Ca2+ transients generated by reverse Na/Ca exchange are equivalent to what occurs during physiological membrane wounding 48. Interestingly, it was suggested that MEND might be facilitated by palmitoylation of plasma membrane proteins facing the cytosol, as a consequence of Ca2+-induced coenzyme A release from mitochondria 49. These are intriguing findings, given that proteomic analysis identified mitochondrial proteins associated to the sarcolemma of injured, but not intact myofibers 51. In future studies, it will be interesting to determine if remodeling of the cytoplasmic leaflet of the plasma membrane triggered by mitochondrial stress is a process that acts in concert with lysosomal exocytosis and ASM release, in order to achieve more efficient lesion endocytosis and cell resealing.
A role for caveolae internalization in plasma membrane repair
Cholesterol extraction from the plasma membrane inhibits injury-induced endocytosis and plasma membrane repair 22. Conversely, cholesterol enrichment was reported to potentiate the MEND massive endocytic process triggered by Ca2+ entry or exposure to sphingomyelinase 48. The large fractions of plasma membrane internalized by MEND were found to bind amphipathic molecules less efficiently than membrane remaining at the cell surface, leading to the suggestion that this form of endocytosis involves more highly ordered lipid microdomains 52. These observations support the involvement of ASM-generated ceramide microdomains, formed within ordered cholesterol/sphingolipid-enriched lipid rafts, in the Ca2+-dependent endocytic process that mediates plasma membrane repair. Supporting this view, immunolabeling revealed a marked increase in ceramide levels at the cell periphery, a few seconds after permeabilization with the pore-forming toxin SLO 16.
Strikingly, electron microscopy (EM) analysis performed a few seconds after wounding did not show the >300 nm endocytic vesicles that are normally observed several minutes after cells are wounded 13, 22. Rather, at these early time points injured cells contained a large number of highly homogenous, <100 nm vesicular structures that were also present in cells treated with ASM or bacterial sphingomyelinase 16. These intracellular vesicles strongly resembled caveolae, ~80 nm flask-shaped invaginations associated with the plasma membrane of many cell types and are particularly abundant in cells under mechanical stress 53 (Box 3). A hallmark of caveolae is their enrichment in cholesterol and sphingomyelin, membrane lipids used to define lipid rafts. In addition, ceramide has been detected in caveolae undergoing endocytosis 43. The increased number of caveolae-like vesicles in cells treated with ASM or bacterial sphingomyelinase raises the intriguing possibility that ceramide generated by sphingomyelin cleavage may give rise to caveolae, which may then remove lesions by pinching off from the plasma membrane (see Figure 1A). Supporting this view, siRNA-mediated depletion of the caveolae-specific protein caveolin inhibited plasma membrane repair. Furthermore, live imaging, immunogold EM and quench protection experiments suggested that plasma membrane-associated SLO pores enter cells in vesicles containing the caveolae-specific protein caveolin 16. Ultrastructural studies showed that once inside cells, caveolae-like vesicles merge with each other, forming larger compartments that gradually acquire endosomal and lysosomal markers, followed by sorting of the internalized toxin into the lumen of lysosomes for degradation 16, 26 (Figure 2). Several bacterial proteins with membrane-damaging activity have affinity for raft lipids (enriched in cholesterol and sphingomyelin) 54, raising the possibility that rapid internalization of these domains via caveolae may play an important role in cellular survival after pathogen attack.
Box 3. Caveolae.
Lipid rafts are lateral assemblies of sphingolipids and cholesterol that form discrete membrane microdomains. Lipid rafts have been proposed to have numerous cellular functions, such as regulation of membrane protein clustering, signaling and vesicular traffic. Caveolae are specialized lipid raft domains that appear as flask-like invaginations of 60-80 nm in diameter along the plasma membrane of many cell types. Caveolae are enriched in sphingomyelin, gangliosides GD3, GM1 and GM3, cholesterol, phosphatidylinositol (4,5)-bisphosphate, and a family of integral membrane proteins known as caveolins. Caveolin-1 and caveolin-2 are present in a wide range of tissues, while caveolin-3 is exclusively expressed in skeletal and cardiac muscle. Caveolins are essential for caveolae formation, with approximately 144 caveolin molecules estimated to participate in the formation of each caveolar structure. Recent studies suggest that a scaffold formed by caveolin on the cytosolic face of caveolae is stabilized by members of another protein family, the cavins 75.
Caveolae are particularly abundant in muscle fibers, adipocytes, endothelial cells, type I pneumocytes and fibroblasts, which are frequently under mechanical stress. Interestingly, grape-like clusters or rosettes of caveolae are frequently observed in muscle fibers and adipocytes, while caveolar vesicles that appear detached from the plasma membrane are more common in endothelial cells 53. While the mechanism underlying these tissue differences in the morphology and arrangement of caveolae are still poorly understood, there is evidence that caveolae play an important role providing cells with protection against mechanical stress. Such roles includes caveolae flattening upon cell stretching as a mechanism to prevent injury 64, and removal of plasma membrane wounds through caveolae internalization 16. Ceramide, the product of sphingomyelin hydrolysis by sphingomyelin, is known to segregate laterally into lipid rafts/caveolae 16, providing a potential mechanism for ceramide-driven caveolae internalization during plasma membrane repair. Consistent with an important role of caveolae in the prevention and restoration of plasma membrane integrity, mutations in caveolins and cavins have been linked to various forms of pathology affecting the function of skeletal and cardiac muscle, tissues under significant mechanical stress 76.
Caveolae are known to be endocytosed under certain conditions, such as after binding of SV40 viruses, or cross-linking of surface proteins 55. Interestingly, conditions that can cause cell injury, such as exposure to hyperosmotic shock 56 or detachment from the substrate 57 were also reported to induce caveolae endocytosis. However, how caveolar internalization and scission from the plasma membrane is triggered has remained poorly understood. The recently uncovered correlation between sphingomyelinase-mediated ceramide production and the accumulation of caveolar vesicles within injured cells suggests that Ca2+-triggered exocytosis of lysosomal ASM may represent a major trigger for the internalization, and possibly de novo formation of caveolae. A role for caveolae internalization in plasma membrane repair is consistent with the higher abundance of caveolar profiles on the plasmalemma of cells from tissues under mechanical stress, such as endothelial cells, adipocytes and muscle fibers 53. Furthermore, numerous studies showed that mutations in the caveolar proteins caveolin-3 and PTRF/cavin 58-60 cause serious forms of pathology in muscle, a tissue that is often injured in vivo. A role for caveolae in resealing the sarcolemma is also consistent with the intracellular accumulation of caveolae observed in muscle fibers carrying dystrophin mutations, which increase sarcolemma fragility 61, 62. The abundance of caveolae in dystrophic muscle fibers may be related to elevated levels of caveolin-3 observed in these cells 62, or to disruptions in caveolin-3 interactions with the dystrophin glycoprotein complex 63. However, recent ultrastructural analysis revealed a marked accumulation of sub-sarcolemmal vesicles in mechanically injured muscle fibers, and the size and morphology of these vesicles resembled caveolae-derived vesicles in the process of merging with each other (Figure 3B). Thus, frequent cycles of injury and repair in dystrophic muscle fibers may trigger de novo formation of caveolae, a process required for resealing. In this context, the upregulation of caveolin-3 expression observed in dystrophin-deficient muscle could reflect a cellular adaptation to the need for assembling additional caveolae. Remarkably, treatment of intact muscle fibers with purified sphingomyelinase for a few seconds was sufficient to cause the peripheral accumulation of caveolae-like vesicles similar to that observed after injury 16 (Figure 3A), suggesting the presence of an intracellular pool of caveolar structural proteins that can be rapidly mobilized.
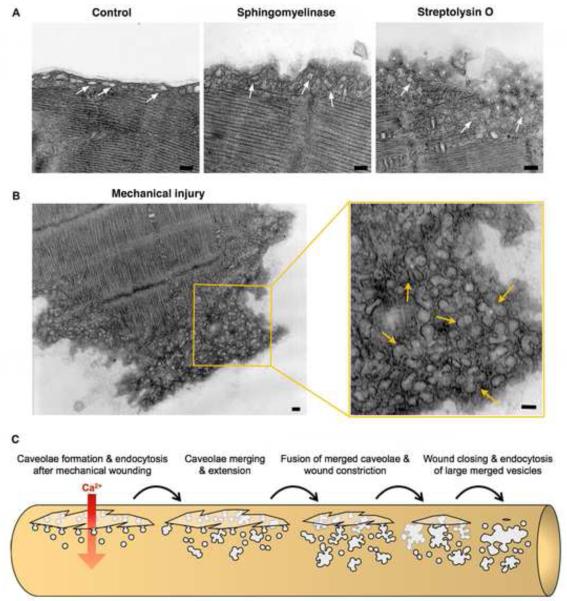
Figure 3. Caveolae-like vesicles accumulate below the sarcolemma of primary muscle fibers in response to injury or exposure to purified sphingomyelinase.
(A) Transmission EM images of mouse Flexor digitorum brevis muscle fibers untreated (Control), exposed to 50 mU/ml B. cereus sphingomyelinase for 5 min, or treated with 400 ng/ml SLO for 30 seconds. (B) Transmission EM image of the mechanically severed tip of a Flexor digitorum brevis muscle fiber. Arrows in the enlarged section to the right point to merged caveolae-derived compartments below the sarcolemma. Bars= 100 nm. (C) Proposed caveolae-mediated mechanism for the resealing of mechanical wounds on a muscle fiber. Ca2+ flowing through a wound would induce internalization and intracellular merging of sarcolemma-associated caveolae, leading to the formation of larger compartments tethered to the sarcolemma that constrict and ultimately reseal the wound.
22, 3116, 22While the pore-forming toxin SLO has been directly visualized entering cells in caveolin-positive caveolar vesicles 16, it remains unclear how caveolae internalization could promote the repair of larger mechanical wounds. The highly branched vesicular profiles observed under the sarcolemma in mechanically injured muscle fibers (Figure 3) suggest that these structures may participate in a process of wound constriction, which may ultimately reseal the wound generating large intracellular vesicles 16 (see Figure 1B). Interestingly, caveolae can also diffuse surface tension by flattening out when membranes undergo mechanical stress 64, suggesting that an expanded caveolae reservoir may also protect plasma membrane integrity through this mechanism.
It remains to be determined if caveolae are necessary for plasma membrane repair in all cell types, or if functionally equivalent lipid raft-like membrane domains can play a similar role. Muscle-specific proteins such as dysferlin and mitsugumin 53 (MG53) have been implicated in sarcolemma resealing 65, suggesting that cells at a high risk of mechanical damage have a specialized machinery to facilitate repair. 531616Nonetheless, Ca2+ influx induces exocytosis of lysosomes and extracellular release of ASM in muscle fibers 15, 16, indicating that resealing in muscle may share a similar mechanism to other cell types that is based on sphingomyelinase-dependent generation of ceramide on the outer leaflet of the sarcolemma, followed by lesion internalization. Interestingly, studies in mouse cardiomyocytes revealed a close interaction between caveolin-3 and the mitochondrial marker cytochrome c, and suggested a role for caveolae in the modulation of mitochondrial function during adaptation to stress in muscle cells 66. Future studies should clarify the basis of the frequently reported interactions between muscle-specific proteins such as dysferlin and MG53 and caveolar components 67, and whether these interactions facilitate ASM-dependent, endocytosis-mediated resealing.
Concluding remarks
Surprising recent developments revealed that nucleated cells repair wounds on their plasma membrane by mechanisms involving lesion removal, which differ significantly from the previously proposed “patch” model. The role of Ca2+-induced exocytosis of lysosomal ASM in triggering endocytosis and lesion internalization suggests that other lysosomal hydrolases could also be involved in remodeling the cell surface to facilitate resealing. While many questions remain (See Box 4), it will be interesting to determine if lysosomal proteases participate in this process, perhaps by cleaving cell surface proteins to facilitate the access of lipases to the lipid bilayer, or by activating other enzymes through proteolytic processing. It will also be of great interest to determine if only pre-existing caveolae are internalized after plasma membrane wounding, or whether ceramide generated on the outer cell surface by sphingomyelin hydrolysis leads to de novo caveolae formation. Another important topic for further investigation should be the identification of early events in the repair process. Injured cells reseal within 30 seconds of injury 5, and increasing evidence indicates that mechanisms may be in place to prevent loss of cytosol before integrity of the plasma membrane can be fully restored. Previous reports suggesting the involvement of calpain, annexins and/or transglutaminases in plasma membrane repair 22, 29, 31, 68 propose that these Ca2+-dependent cytosolic proteins generate a barrier to cytosolic loss at wound sites, an intriguing possibility that should be investigated. Finally, recent evidence for the involvement of the ESCRT membrane sculpting machinery in the shedding of small lesions from cells will certainly encourage additional investigations, which are likely to be facilitated by established protocols for cell permeabilization using pore-forming toxins. The pace of discoveries in this field will certainly continue to accelerate, gradually uncovering more details of the fascinating cellular machinery dedicated to restoration of plasma membrane integrity.
Box 4. Outstanding questions.
Does ceramide generation on the outer leaflet of the plasma membrane induce de novo caveolae formation?
How does caveolar endocytosis mediate the repair of large wounds on the plasma membrane?
In addition to acid sphingomyelinase, do other lysosomal hydrolases released from wounded cells have a role in plasma membrane repair?
What is the mechanism of action of muscle-specific proteins involved in plasma membrane repair, such as dysferlin and MG53?
How do Ca2+-dependent cytosolic proteins and the ESCRT machinery function in plasma membrane repair?
Highlights.
*Plasma membrane wounding triggers exocytosis of lysosomal acid sphingomyelinase.
*Secreted acid sphingomyelinase generates ceramide and promotes endocytosis.
*Plasma membrane wounds are removed by caveolar endocytosis.
*Caveolae-mediated repair provides insight into why mutations in caveolar proteins cause muscle pathology.
Figure I.
Proposed mechanism for ceramide-mediate membrane invagination
Acknowledgements
The authors thank members of the Andrews laboratory for helpful discussions. Work in the Andrews laboratory discussed in this review was supported by the grant R01 GM064625 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heilbrunn L. The dynamics of living protoplasm. Academic Press; 1956. [Google Scholar]
- 2.Chambers R, Chambers E. Explorations into the nature of the living cell. Harvard University Press; 1961. [Google Scholar]
- 3.Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi GQ, et al. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeil PL, et al. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- 6.Reddy A, et al. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 7.Roy D, et al. A process for controlling intracellular bacterial infections induced by membrane injury. Science. 2004;304:1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- 8.Forestier CL, et al. Imaging host cell-Leishmania interaction dynamics implicates parasite motility, lysosome recruitment, and host cell wounding in the infection process. Cell Host Microbe. 2011;9:319–330. doi: 10.1016/j.chom.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Tam C, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189:1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez A, et al. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths GM. Secretory lysosomes - a special mechanism of regulated secretion in haemopoietic cells. Trends Cell Biol. 1996;6:329–332. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- 12.Andrews NW. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- 13.Keefe D, et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Bergsbaken T, et al. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J Immunol. 2011;187:2748–2754. doi: 10.4049/jimmunol.1100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon NJ, et al. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 16.Corrotte M, et al. Caveolae internalization repairs wounded cells and muscle fibers. Elife. 2013;2:e00926. doi: 10.7554/eLife.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal JK, et al. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal JK, et al. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:E233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Togo T, et al. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell. 2000;11:4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walev I, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci U S A. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan BP, Campbell AK. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985;231:205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idone V, et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddleman CS, et al. Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axolemmal injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4029–4041. doi: 10.1523/JNEUROSCI.18-11-04029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Los FC, et al. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe. 2011;9:147–157. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 26.Corrotte M, et al. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic. 2012;13:483–494. doi: 10.1111/j.1600-0854.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez AJ, et al. ESCRT machinery is required for plasma membrane repair. Science. 2014;343:1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 28.Charras GT, et al. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477–490. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potez S, et al. Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J Biol Chem. 2011;286:17982–17991. doi: 10.1074/jbc.M110.187625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keyel PA, et al. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J Cell Sci. 2011;124:2414–2423. doi: 10.1242/jcs.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinhardt RA, et al. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 32.Henne WM, et al. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murk JL, et al. Endosomal compartmentalization in three dimensions: implications for membrane fusion. Proc Natl Acad Sci U S A. 2003;100:13332–13337. doi: 10.1073/pnas.2232379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cashikar AG, et al. Structure of cellular ESCRT-III spirals and their relationship to HIV budding. Elife (Cambridge) 2014:e02184. doi: 10.7554/eLife.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabhan JF, et al. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zha X, et al. Sphingomyelinase treatment induces ATP-independent endocytosis. J Cell Biol. 1998;140:39–47. doi: 10.1083/jcb.140.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 38.Holopainen JM, et al. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys J. 2000;78:830–838. doi: 10.1016/S0006-3495(00)76640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 40.Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol Res. 2003;47:393–399. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 41.Schissel SL, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 42.Deaglio S, et al. CD38/CD19: a lipid raft-dependent signaling complex in human B cells. Blood. 2007;109:5390–5398. doi: 10.1182/blood-2006-12-061812. [DOI] [PubMed] [Google Scholar]
- 43.van Blitterswijk WJ, et al. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grassme H, et al. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 45.Babiychuk EB, et al. Fluorescent annexin A1 reveals dynamics of ceramide platforms in living cells. Traffic. 2008;9:1757–1775. doi: 10.1111/j.1600-0854.2008.00800.x. [DOI] [PubMed] [Google Scholar]
- 46.Xu M, et al. Requirement of translocated lysosomal V1 H(+)-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary endothelial cells. Mol Biol Cell. 2012;23:1546–1557. doi: 10.1091/mbc.E11-09-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam C, et al. Live imaging assay for assessing the roles of Ca2+ and sphingomyelinase in the repair of pore-forming toxin wounds. Journal of visualized experiments : JoVE. 2013:e50531. doi: 10.3791/50531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lariccia V, et al. Massive calcium-activated endocytosis without involvement of classical endocytic proteins. The Journal of general physiology. 2011;137:111–132. doi: 10.1085/jgp.201010468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilgemann DW, et al. Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts. Elife. 2013;2:e01293. doi: 10.7554/eLife.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang M, Chen G. Ca2+ regulation of dynamin-independent endocytosis in cortical astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8063–8074. doi: 10.1523/JNEUROSCI.6139-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma N, et al. Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles. J Biol Chem. 2012;287:30455–30467. doi: 10.1074/jbc.M112.354415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hilgemann DW, Fine M. Mechanistic analysis of massive endocytosis in relation to functionally defined surface membrane domains. The Journal of general physiology. 2011;137:155–172. doi: 10.1085/jgp.201010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 54.Hommelgaard AM, et al. Caveolae: stable membrane domains with a potential for internalization. Traffic. 2005;6:720–724. doi: 10.1111/j.1600-0854.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 55.Parton RG, et al. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gazzerro E, et al. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet. 2010;18:137–145. doi: 10.1038/ejhg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajab A, et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6:e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu H, et al. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem. 2011;286:12820–12824. doi: 10.1074/jbc.C111.221440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonilla E, et al. Freeze-fracture studies of muscle caveolae in human muscular dystrophy. Am J Pathol. 1981;104:167–173. [PMC free article] [PubMed] [Google Scholar]
- 61.Repetto S, et al. Increased number of caveolae and caveolin-3 overexpression in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1999;261:547–550. doi: 10.1006/bbrc.1999.1055. [DOI] [PubMed] [Google Scholar]
- 62.Song KS, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 63.Sinha B, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han R. Muscle membrane repair and inflammatory attack in dysferlinopathy. Skeletal muscle. 2011;1:10. doi: 10.1186/2044-5040-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fridolfsson HN, et al. Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J. 2012;26:4637–4649. doi: 10.1096/fj.12-215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai C, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouter A, et al. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nature communications. 2011;2:270. doi: 10.1038/ncomms1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Testi R. Sphingomyelin breakdown and cell fate. Trends Biochem Sci. 1996;21:468–471. doi: 10.1016/s0968-0004(96)10056-6. [DOI] [PubMed] [Google Scholar]
- 69.Li R, et al. Induction of endocytic vesicles by exogenous C(6)-ceramide. J Biol Chem. 1999;274:21121–21127. doi: 10.1074/jbc.274.30.21121. [DOI] [PubMed] [Google Scholar]
- 70.Bai J, Pagano RE. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 71.Schuchman EH. Acid sphingomyelinase, cell membranes and human disease: Lessons from Niemann-Pick disease. FEBS Lett. 2009;584:1895–1900. doi: 10.1016/j.febslet.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 72.Jenkins RW, et al. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21:836–846. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGovern MM, et al. Natural history of Type A Niemann-Pick disease: possible endpoints for therapeutic trials. Neurology. 2006;66:228–232. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- 74.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 75.Cohen AW, et al. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]