Abstract
Purpose
Mechanisms of immune dysregulation associated with advanced tumors are relatively well understood. Much less is known about the role of immune effectors against cancer precursor lesions. Endometrioid and clear cell ovarian tumors partly derive from endometriosis, a commonly diagnosed chronic inflammatory disease. We performed here a comprehensive immune gene expression analysis of pelvic inflammation in endometriosis and endometriosis-associated ovarian cancer (EAOC).
Experimental design
RNA was extracted from 120 paraffin tissue blocks comprising of normal endometrium (n=32), benign endometriosis (n=30), atypical endometriosis (n=15) and EAOC (n=43). Serous tumors (n=15) were included as non-endometriosis associated controls. The immune microenvironment was profiled using Nanostring and the nCounter® GX Human Immunology Kit, comprising probes for a total of 511 immune genes.
Results
One third of the endometriosis patients revealed a tumor-like inflammation profile, suggesting that cancer–like immune signatures may develop earlier, in patients classified as clinically benign. Gene expression analyses revealed the complement pathway as most prominently involved in both endometriosis and EAOC. Complement proteins are abundantly present in epithelial cells in both benign and malignant lesions. Mechanistic studies in ovarian surface epithelial (OSE) cells from mice with conditional (Cre-loxP) mutations show intrinsic production of complement in epithelia and demonstrate an early link between Kras- and Pten-driven pathways and complement upregulation. Downregulation of complement in these cells interferes with cell proliferation.
Conclusions
These findings reveal new characteristics of inflammation in precursor lesions and point to previously unknown roles of complement in endometriosis and EAOC.
Keywords: endometriosis, endometriosis associated ovarian cancer, immune genes, complement, Nanostring
Introduction
The host’s immunological makeup can protect against cancer development via complex mechanisms commonly referred to as “immune surveillance” (1). For certain tumors, transition to malignancy occurs via precursor lesions, where complex changes in immune surveillance lead to chronic inflammation (2). While tumor-immune cell interactions have been long studied and are now well deciphered, much less known are the roles of immune effectors against cancer precursor lesions in general and ovarian cancer precursors in particular. Ovarian epithelial tumors are highly heterogeneous and may arise from different premalignant lesions, according to their histology (3). High grade serous ovarian tumors represent the most common histologic subtype and to some extent, they are considered to originate in the fallopian tubes (4, 5). Endometrioid, clear cell and low grade serous tumors may derive, at least in part, from endometriosis, a chronic inflammatory disease that can affect approximately 10–15% of women in the reproductive age group and 20–50% of women with infertility (6, 7). Endometriosis consists of endometrial-like tissue (consisting of glandular epithelia and stromal cells) outside the uterine cavity and is often treated through a combination of hormone therapy and surgical removal of lesions (8).
Although endometriosis remains largely benign, malignant transformation may occur in up to 1% of cases, most commonly from ovarian lesions (7, 9, 10). Gene profiling studies recently revealed that clear-cell and low-grade endometrioid carcinomas share a similar gene expression pattern, consistent with a common origin. As a prevailing trait, inactivating mutations of ARID1A, a chromatin-remodeling gene, have been found in 49% of clear-cell carcinomas and 30% of endometrioid ovarian cancers (11–13).
It is estimated that 60–80% of all endometriosis associated ovarian cancers (EAOC) occur in the presence of atypical endometriosis (AE), often found in direct continuity with the tumor, suggesting AE as the transitioning entity from benign lesions to malignant variants. The intermediate, “atypical” lesions, are defined by several histological criteria, including large nuclei with moderate to marked pleomorphism, increased nuclear-to-cytoplasmic ratio, cellular crowding, stratification, or tufting (14, 15).
Endometriosis-associated ovarian carcinogenesis is believed to be a culmination of a multifaceted complex of pathogenic factors (16). In conjunction with endocrine imbalance and oxidative stress, immune dysregulation is a major factor that contributes to disease pathogenesis (17). In women with endometriosis, the peritoneal fluid often shows increased levels of pro-inflammatory mediators like TNF-α, IL-1β, IL-6 (18). In addition, the patients show dysfunctional macrophages, depressed killing capacity of NK cells, increased accumulation of regulatory T suppressor cells all of which may favor chronic inflammation and promote the initiation and progression of EAOC (18, 19). Notably, the vast majority of studies have previously focused on profiling immunity in endometriosis patients by either measuring cellular phenotypes or soluble mediators in the peritoneal fluid or peripheral blood (20).
Here, we focused on the tissue immune microenvironment and provide the first comprehensive immune transcriptome profiling of tissues from 120 cases comprising normal controls, benign endometriosis, atypical endometriosis and EAOC cases. With a broad collection of 511 immune gene probes, we identified gene expression changes associated with each disease state. One of the immune pathways enriched for differentially expressed genes in all disease categories was the complement pathway. Complement proteins were significantly upregulated in epithelial cells. Using a novel, ovarian surface epithelium (OSE) - derived cell line from genetically engineered mice with conditional mutations that trigger EAOC, we demonstrate that activation of Kras and Pten tumor driving pathways leads to upregulation of complement in epithelial cells. These results reveal for the first time the link between tumor initiating events and immune surveillance via complement, and point to this pathway as a potential target for therapy and early prevention in EAOC.
Materials and Methods
Ethics statement
This research study protocol was approved by the Institutional Review Board (IRB) at the University of Pittsburgh, (Pittsburgh, PA).
Patients
We accessed n=120 formalin fixed paraffin embedded (FFPE) tissue samples, through the Health Science Tissue Databank at the University of Pittsburgh Medical Center (UPMC). The FFPE tissue blocks comprised of normal endometrium (n=32, equally distributed between secretory and proliferative stage), benign endometriosis (n=30) consisting of ovarian endometriosis (n=11) and extra ovarian endometriosis cases (n=19), atypical endometriosis (n=15), clear cell tumors (n=12), endometrioid ovarian cancer (n=16). Serous tumors (n=15) were included as non-endometriosis associated controls.
RNA extraction and Nanostring
The RNeasy FFPE Kit (Qiagen) was used to isolate total RNA from FFPE tissues. The nCounter® GX Human Immunology Kit (Nanostring) was used to profile 511 immune genes. Genes in this kit belong to several pathways such as inflammatory disease, cell to cell signaling, cellular development, cell death and hematological system development. Additional details are included in Supplementary Materials and Methods.
Immunohistochemistry and immunocytochemistry
Immunohistochemical studies were performed in FFPE tissue with the following antibodies: anti-human C7, C3, C4b, MASP1, CFH, CFD, membrane attack (MAC). Detailed protocols, clone identification, antibody dilutions and image acquisition are included in Supplementary Materials and Methods.
Reverse transcriptase quantitative PCR (RT-qPCR)
Two microgram of purified RNA was used for RT using the iScript cDNA Synthesis Kit (Bio-Rad). RT-qPCR was carried out with the CFX96 Touch Real-time PCR Detection System (Bio-Rad) using the SsoAdvanced SSO SYBR Green Supermix (Bio-Rad). Detailed protocols are provided in Supplementary Materials and Methods.
Establishment of the murine MUC1+/-KrasG12D/+PtenloxP/loxP (MKP) OSE cell line
MKPOSE cells were derived in Anda Vlad’s laboratory at Magee Womens Research Institute, from primary ovarian surface epithelial (OSE) cells isolated from healthy MKP mice (21) via gentle trypsinization of six ovaries collected at necropsy from three healthy female mice according to previously published protocols (22) and as further detailed in Supplementary Materials and Methods. Authentication was performed through genomic PCR of MKPOSE cells for the presence of loxP cassettes at the (murine-specific) Kras and Pten loci before and after AdCre infection, as shown in Supplementary Fig. S2. This verification approach was performed for each experiment described herein.
Complement-mediated cell lysis
A total of 5 × 105 AdCre infected and uninfected MKPOSE cells were incubated with 90 μl mouse ascites and 5 μl of anti-MUC1 antibody (50 μg/ml) and 15 μl of DMEM medium. Either reagents alone or cells in serum-free medium served as controls. As positive controls for lysed cells, we incubated MKPOSE cells with 0.2% triton for 15 min at 37°C. Number of live and dead cell were measured post-staining with either 7-AAD or propidium iodide (BD Biosciences), via flow cytometry. Similarly treated, normal mouse splenocytes served as control for complement-induced cell lysis.
siRNA transfection
Commercially available ON-TARGETplus siRNA consisting of mixture of four mRNA regions directed against mouse C7 or mouse GAPDH were used (Thermo Scientific Dharmacon RNAi Technologies, Lafayette, CO, USA). Transfection methods were according to the manufacturer’s instructions and are further detailed in Supplementary Materials and Methods.
nCounter data preprocessing
Experiments were compliant with nCounter mRNA Expression Assay (http://www.genetics.pitt.edu/forms/nCounter_Gene_Expression_Data_Analysis_Guidelines.pdf) and the complete data sets are available in the Gene Expression Omnibus database under accession number GSE57545. To minimize the impact of detection anomalies, we normalized the dataset to the positive control count values (with sum of positive control counts). Subsequently, we applied negative control normalization (negative control mean plus two standard deviations). Lastly, we filtered out samples with a positive control normalization factor outside the recommended range of 0.3 to 3 or with an estimated background > 3 standard deviations from the mean.
Statistical analyses
To identify differentially expressed (DE) genes between any two patient categories, we used the generalized linear model with negative binomial distribution family for count response data. Once negative binomial models were fitted and dispersion estimates were obtained, we performed testing procedures for determining differential expression using an exact test with the quantile-adjusted conditional maximum likelihood (qCML) method. For a paired group we evaluated the average effect of disease type over involving patients. All statistical programming was implemented in R, using the edgeR package (23).
For hierarchical clustering, we first filtered out features with means or standard deviations under the 50th-quantile of all the features. The filtering procedure left 100 expressed (large means) and informative (large standard deviations) features, based on which hierarchical clustering with complete linkage was then applied.
Results
Immune gene expression profiles can distinguish different disease categories
The clinical demographics of all patients and controls used in this study are summarized in Table 1. Most of the individuals self-identified as Caucasians, regardless of disease category. The majority of patients in control group (18 of 32, 56%) underwent hysterectomy due to leiomyomas. The remaining of cases were treated due to ovarian cysts (7 cases), adenomyosis, pelvic organ prolapsed and endometrial polyps (2 cases of each). One patient underwent prophylactic procedure due to risk for Lynch syndrome.
Table 1.
Demographic and clinical characteristics of patients
| Characteristic | Normal n=32 (26.7%) |
Benign Endo n=30 (25%) |
Atypical Endo n=15 (12.5%) |
EAOCa n=28 (23.3%) |
SOC n=15 (12.5%) |
Pb |
|---|---|---|---|---|---|---|
| Age at presentation (years), mean (SD) | 46.5 (6) | 40.1 (10.9) | 48.01 (6.5) | 54.8 (11.6) | 65.4 (12.4) | 1.135e-09 |
| Gravidity, median (IQR)c | 2 (1) | 1 (2.5) | 1 (2) | 1.5 (2.25) | 2 (2) | 0.0155 |
| Parity, median (IQR)c | 2 (2) | 0.5 (1.25) | 1 (1.75) | 0.5 (2) | 2 (2) | 0.002145 |
| Body mass index (kg/m2), mean (SD) | 30.8 (9.9) | 28 (7.3) | 31.5 (12.7) | 28.7 (8.1) | 28.4 (6.6) | 0.8493 |
| Race (White), n (%) | 28 (93%) | 28 (93%) | 15 (100%) | 27 (100%) | 13 (93%) | 0.548 |
| History of Tobacco use, n (%) | 9 (33%) | 10 (36%) | 3 (21%) | 9 (36%) | 6 (50%) | 0.6675 |
| History of alcohol use, n (%) | 14 (54%) | 13 (46%) | 8 (57%) | 11 (46%) | 4 (33%) | 0.7484 |
| Pre-op CA125 level (U/ml) | 27 | 79.1 | 18.8 | 736.3 | 1920 | 0.004564 |
| History of diabetes, n (%) | 0 (0%) | 1 (3%) | 1 (7%) | 5 (22%) | 2 (17%) | 0.03533 |
| History of hypertension, n (%) | 10 (35%) | 5 (17%) | 6 (46%) | 12 (50%) | 9 (75%) | 0.007224 |
| Stage of diseased, n (%)Early Late | N/A | N/A | N/A | 15 (53%) 12 (42%) |
3 (20%) 11 (73%) |
0.07905 |
EAOC, endometriosis-associated ovarian cancer group comprises endometrioid (n=16, 57%) and clear cell (n=12, 43% ) tumors
ANOVA or Kruskal-Wallis test for continuous variables; Chi-square test or Cochran-Mantel-Haenszel Chi-square test for categorical variables
IQR, Interquartile Range (25th percentile, 75th percentile)
Tumor stage was available in 27 (96%) and 14 (93%) of EAOC and SOC cases, respectively.
Note: Statistically significant values are in boldface type.
Abbreviations: Endo, endometriosis; EAOC, endometriosis-associated ovarian cancer; SOC, serous ovarian cancer; N/A, not applicable.
Endometriosis patients were younger than patients with ovarian cancer, as expected (p =4.635e-11). Gravidity and parity scores were also lower in endometriosis and EAOC patients as compared to healthy individuals (p<0.05), in line with findings pointing to hormonal imbalances that accompany these conditions (24). Although disease staging was not consistently performed for the endometriosis cases in this retrospective study, we classified the subjects according to the type of lesions, using predefined criteria (25–27). Of all endometriosis cases, nine cases (47%) were classified as “peritoneal” disease and nine as “deeply infiltrating lesions” (DIE). One case presented with abdominal wall disease. The vast majority (13 of 15 cases, 87%) of all atypical endometriosis (AE) lesions presented in the context of ovarian endometrioma. The remaining two cases had peritoneal lesions.
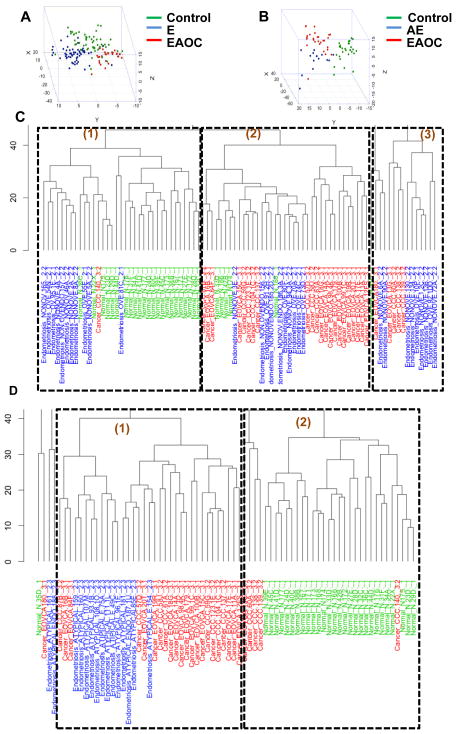
To profile the immune microenvironment in endometriosis and EAOC, we extracted mRNA from formalin-fixed and paraffin embedded (FFPE) tissue and performed Nanostring measurements of 511 immune gene transcripts, using the nCounter® GX Human Immunology Kit. Multi-dimensional scaling (MDS), constructed using 65% cutoff threshold for DE genes, shows a good level of separation of control individuals, endometriosis and EAOC patients (Fig. 1A). Unsupervised clustering of 100 filtered genes (as described in statistical analyses section in materials and methods) also distinguished the three disease categories well (Fig. 1C). Twenty-two of 28 (79%, after removal of outliers as described in Materials and Methods) healthy control samples and 22 of 28 (79%) of cancer cases cluster within their disease category and separate from each other (Fig. 1C clusters 1 and 2, respectively). Endometriosis shows a mixed immune gene profile with some (n=11, 37% of cases) clustering with healthy controls (cluster 1) while others (n=10, 33%) display profiles more similar to cancer (cluster 2). The remaining 30% were also more similar to cancer, albeit in the most distant cluster 3. These results suggest that in some endometriosis patients, endometriosis-associated inflammation carries features typically associated with cancer immune environments. Additionally, similar comparisons using the AE cohort show that the vast majority (n=13, 85%) of cases in this disease category have a cancer-like immune environment as they homogeneously cluster closer to the EAOC cases than to the control group (Fig 1 B and D), providing further support for AE as a tumor precursor lesion.
Fig. 1.
Global gene expression across disease categories. A–B. Multidimensional scaling plot showing separation of disease categories with for differentially expressed (DE) genes filtered with 50-quantile cutoff of mean and standard deviation. Endometriosis-associated ovarian cancer (EAOC) cases are shown in red and controls in green. Endometriosis (E) and atypical endometriosis (AE) are shown in blue (panel A and B, respectively). C–D. Unsupervised clustering of 100 filtered genes. EAOC cases are listed in red and controls in green; E and AE are listed in blue (panel C and D, respectively).
Differential expression of immune genes that differentiate between controls, endometriosis and EAOC reveal complement pathway
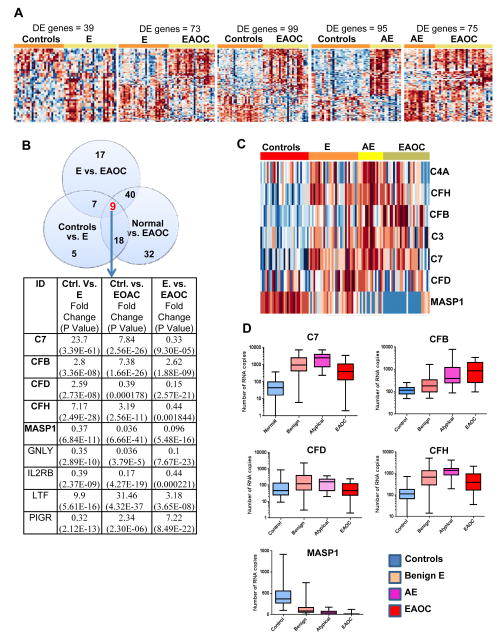
Analyses of differentially expressed (DE) genes (using a false discovery rate-FDR-5%, and log2 fold change in expression of greater than or equal to 1) identified 39 immune genes that distinguish normal from endometriosis, 73 immune genes that differentiate between endometriosis and EAOC, and 99 immune genes that are different in EAOC compared to controls (Fig. 2A). Analyses of AE reveal 95.and 75 DE genes when compared to controls and EAOC, respectively (Fig. 2A). The complete list of all DE genes and their p values is shown in Supplementary Table S1. Analysis of the DE gene lists comparing endometriosis to control or EAOC revealed that a total of 74 genes were present in at least two of the three signatures (Fig. 2B). Nine of the 74 genes were present in all three datasets and of these nine, five genes belong to the complement pathway: complement factors 7, D, B. H (C7, CFD, CFB, CFH), and mannose-associated serine protease 1 (MASP1) (Fig. 2B). Two other complement genes encoding for complement factors 3 and 4a (C3, C4A) were also revealed in other 2 way intersections such as normal vs. endometriosis, endometriosis vs. EAOC and normal vs. EAOC (Supplementary Table S1). Additionally, C3, C7 and CFH remain dysregulated in AE (Supplementary Fig. S1) and when analyzed together, a differentiating pattern of the seven complement genes (C3, C4A, C7, CFH, CFD, CFB, MASP1) was detected among all four disease categories (control, endometriosis, AE and EAOC, Fig. 2C). Notably, changes in expression for most of the complement genes displayed a consistent trend, from low levels seen in control endometrium to higher expression in endometriosis, AE and EAOC (Fig. 2D). In contrast, MASP1, which encodes for mannan-binding lectin serine protease 1, follows the opposite pattern, suggesting that lectin pathway of complement activation may be not involved in endometriosis and EAOC-associated inflammation. In addition, Ingenuity Pathway Analyses (IPA) confirms that complement is the most significantly dysregulated immune pathway in endometriosis and EAOC (Supplementary Table S2). Importantly, complement was not among the top pathways in serous ovarian cancer, further demonstrating heterogeneity among ovarian cancer histotypes and suggesting that complement involvement may be specific to EAOC. Humoral immunity is also included in the top networks in endometriosis and EAOC (Supplementary Table S3 A and B), but not in serous ovarian cancer (Supplementary Table S3D) although both EAOC and serous cancer seems to trigger changes predominantly in cellular immunity.
Fig. 2.
Differentially expressed genes across disease categories. A. Heatmaps of DE genes in five different two-group comparisons: control vs. E (n=39), endometriosis vs. EAOC (n=73), normal vs. EAOC (n=99), control vs. AE (n=95), AE vs. EAOC (n=75). B. Venn diagram showing number DE genes that are differentially expressed in controls, E and EAOC. Accompanying table lists the nine common DE genes (common intersection, arrow), fold change and P value. Complement genes are in bold. C. Heat map of 7 complement genes (C4A, CFH, CFB, C3, C7, CFD, MASP1) shows a differentiating pattern among the four different disease categories (controls, E, AE and EAOC). D. Individual gene expression profile of five common complement DE genes (C7, CFB, CFD, CFH, MASP1) across the four cohorts (controls, E, AE and EAOC). All genes are significantly different (false discovery rate FDR < 0.05 and absolute log FC greater than 1) for any of the two group comparisons (normal, endometriosis and EAOC).
Taken together, these results suggest that gene expression of several complement components are over-expressed and that complement activation may be a common biologic process involved in immune surveillance in endometriosis and EAOC (Supplementary Table S3 C and D). Furthermore, presence of humoral immunity points towards complement as a possible mediator of epithelium-immune stoma interactions, potentially via antibody-dependent, complement-mediated immunity.
Complement proteins are abundantly present in epithelial cells endometriosis and EAOC
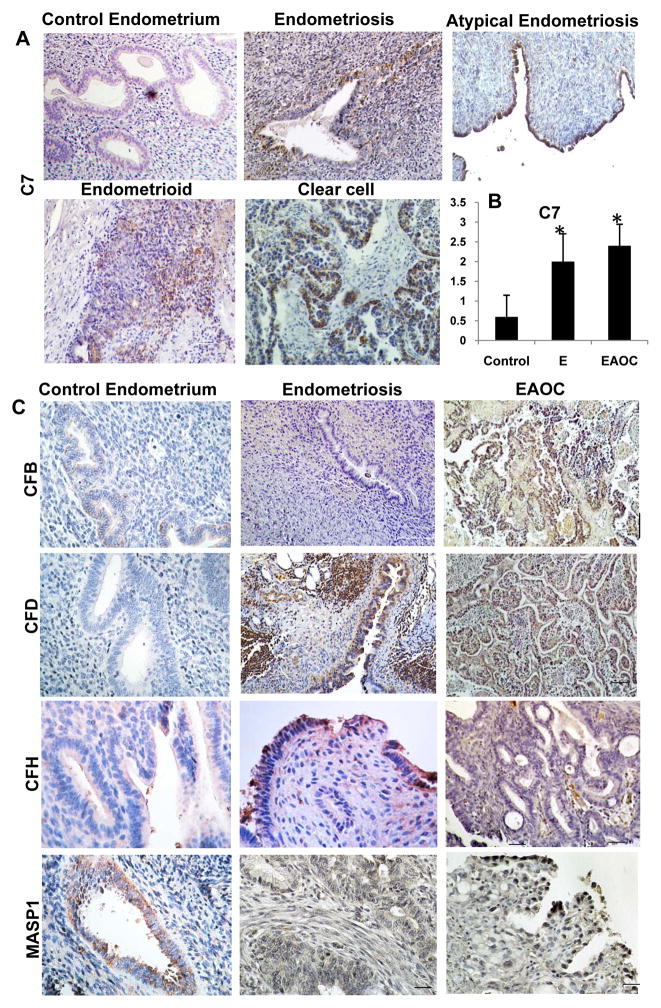
Using immunohistochemistry (IHC), we confirmed protein expression for all 5 complement genes (C7, CFD, CFB, CFH, MASP1, differentially expressed in endometriosis, control and EAOC, Figure 2B). Complement factor 7 (C7) is highly expressed in endometriosis and ovarian cancer while normal endometrium shows little to no mRNA expression (Fig 2D). Accordingly, expression of C7 protein, a member of the membrane attack complex (MAC), is minimal in eutopic (control) endometrial glands and stroma while epithelial cells in endometriosis, atypical endometriosis and EAOC show a clearly increased protein production (Fig. 3A). Scoring of C7 protein shows that at least for this complement component, its expression levels are in line with the C7 gene expression data (Fig 2D).
Fig. 3.
Tissue validation of complement proteins via IHC. A. Tissue deposition of complement C7 protein. B. Intensity staining for C7 was scored as follows: 0-no staining, 1-weak staining, 2-moderate staining and 3-strong staining. Y-axis: average score, plus standard deviation. Each of the three groups (controls, endometriosis and EAOC) contained five representative samples. One way ANOVA, p=0.001. C. Tissue expression of CFB, CFD, CFH and MASP1 proteins in endometriosis and EAOC FFPE tissues. At least five cases in each disease categories were stained for each marker. Representative images for each marker are shown.
Expression of other complement proteins such as CFB, CFD, CFH and MASP1 was confirmed by IHC (Fig. 3C). Similarly to C7, most of the complement proteins were present in epithelial cells lining endometriotic glands or endometrioma and in epithelial tumor cells (in EAOC). Staining pattern reveals complement proteins are distributed both intracellular and on the cell surface, suggesting an endogenous production and consumption in epithelial lesions of endometriosis and cancer.
Complement genes in a murine model for EAOC that mirrors expression seen in human tumors
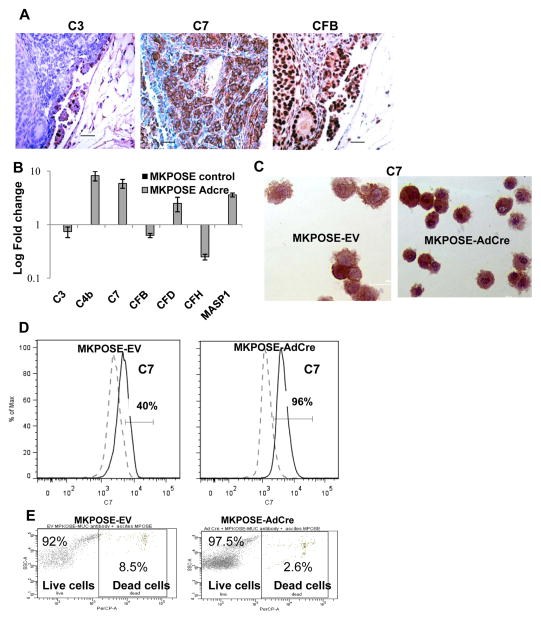
Based on the above ex vivo findings that complement proteins are abundant in epithelial cells in endometriosis, premalignant and malignant lesions, we proposed next to investigate in vitro the link between the complement pathway and early carcinogenic events in ovarian epithelial cells. To accomplish this, we employed ovarian surface epithelial cells derived from triple transgenic mice that progress to human mucin 1 (MUC1) - expressing endometrioid ovarian cancer that closely mirrors the human disease (21). The mice heterozygously express human MUC1 as a transgene, and simultaneously carry the conditional LoxP-Stop-LoxP K-rasG12D oncoallele and the floxed PtenloxP/loxP gene (28). In this Cre-loxP in vivo system, injection of Cre recombinase encoding adenovirus (AdCre) under the ovarian bursa of female MKP mice triggers progression to endometrioid ovarian tumors in about 7–8 weeks (21). The mouse tumors show similarly increased epithelial cell expression of complement proteins (Fig. 4A), further demonstrating that the MUC1KrasPten mouse model, which replicates with high fidelity the histopathology of human EAOC (21, 28), may also provide an useful preclinical tool for exploring the complement biology in the ovarian tumor microenvironment.
Fig. 4.
Complement biology in an EAOC preclinical mouse model. A. Tissue deposition of complement in mouse ovarian tumors from MUC1KrasPten mice. B. Changes in complement gene expression measured by qPCR in MKPOSE cells treated with either empty vector (MKPOSE-EV, control) or Cre-encoding adenovirus (MKPOSE-AdCre). C. MKPOSE intracellular staining for C7 by immunocytochemistry. D. Quantitation of C7 by flow cytometry. Positive cells (percentages) were gated outside of the negative control gate. E. Antibody-mediated complement mediated cell killing assay using MKPOSE-EV and MKPOSE-AdCre cells. Cell death was assessed via flow cytometry, using propidium iodide staining. Positive staining measures cells death.
Conditional activation of tumor driving pathways leads to complement gene upregulation
Using conditional mice with a Kras activating mutation and Pten deletion we studied next how engagement of these classical oncogenic and tumor suppressor pathway respectively, affects complement activation in primary epithelial cells. To accomplish this, we generated a novel cell line (MKPOSE) from primary ovarian surface epithelial (OSE) cells of healthy MUC1KrasPten mice (21), using previously established protocols (22). At baseline, Kras and Pten/PI3k pathways are unaffected in these mice (21, 28). However, as with our in vivo experiment, in vitro exposure of the MKPOSE cells to AdCre floxes out loxP sites and triggers Pten deletion and oncogenic Kras activation (Supplementary Fig. S2A), similarly to levels seen in vivo in tumors (21).
We postulated that MKPOSE cells exposed to AdCre create a surrogate in vitro system for early transformation and tumor formation and offer an experimental setting in which we can monitor early changes in complement gene expression before and after Cre-induced genetic events that effectively trigger in vivo tumorigenesis. Focusing on C7 as the prototype member of the MAC, the end-product of complement activation, we show that in MKPOSE cells, C7 is present at detectable levels at baseline and is further upregulated (alongside other complement genes) following exposure to AdCre (Fig. 4 B–D). In addition, treatment of cells with small molecule inhibitors that inhibit the AdCre-induced Kras and PI3K pathways (AZD 6422 or BEZ235, respectively) reverses C7 upregulation (Supplementary Fig S2B). These drugs are in preclinical development as cancer therapeutics, AZD6244 as a highly selective inhibitor of ERK1 and ERK2 (29) and BEZ235 as a pan-PI3K-mTOR inhibitor (30).
One of the consequences for complement upregulation is mediation of antibody-induced, complement-mediated cell death. Given that humoral immunity was among the top five networks in both endometriosis and EAOC (Supplementary Table S3 A and B), we explored next whether complement upregulation triggered by pathways downstream of Kras and Pten/PI3K modifies antibody-induced, complement-mediated cell death of MKPOSE cells. AdCre-treated MKPOSE cells were exposed to ovarian cancer ascites fluid, collected from mice challenged intraperitoneally (IP) with syngeneic, human MUC1-expressing ovarian tumors. These mice produce anti-MUC1 antibodies at high levels in both serum and ascites (Supplementary Fig. S3A). The MUC1-specific antibodies in ascites have the ability to bind to MUC1 molecules present on tumor cell surface (Supplementary Fig. S3B). We postulated that if complement engagement would be effective, AdCre MKPOSE cells exposed to antibody-containing ascites would undergo antibody-induced, complement-mediated cell death. However, we observed either no effect or slight decrease rather than increase in cell death (compared to EV control cells, Fig. 4E). In contrast, control cells (consisting of similarly treated splenocytes exposed to same ascites, as well as mouse serum as control) are successfully lysed (Supplementary Fig. S3C). This suggests that despite upregulation in transformed OSE, complement engagement in the predicted lytic pathways is rather ineffective.
Complement C7 knockdown inhibits ovarian cell proliferation
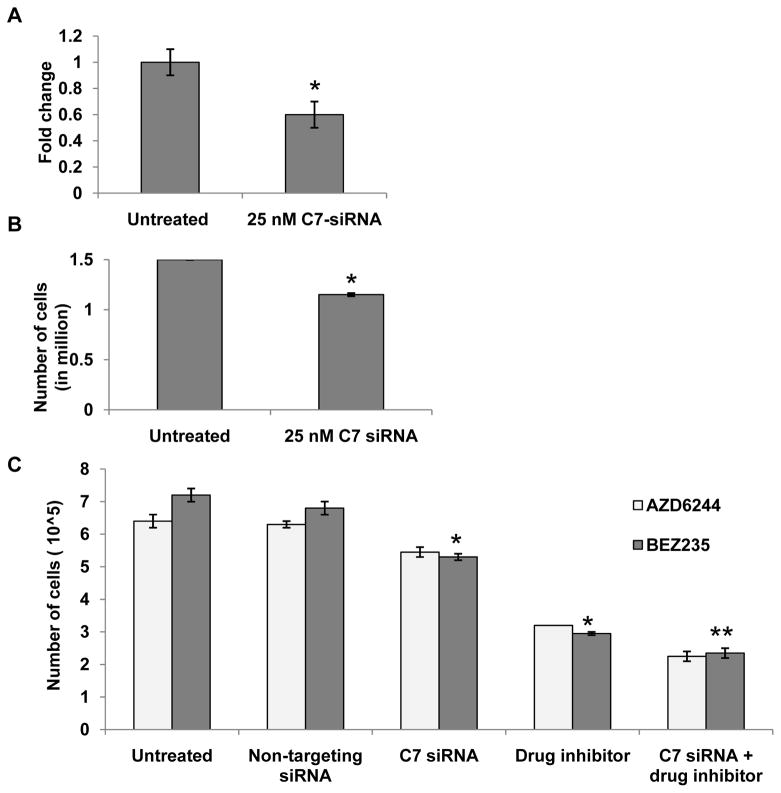
Complement inhibition has been recently reported as having a beneficial effect against tumor growth (31). To investigate roles of C7 expression on proliferation of ovarian epithelial cells in vitro, we knocked down C7 expression in MKPOSE AdCre infected and control cells, using a mixture of siRNAs targeting four regions of mouse C7 mRNA (Fig. 5A). Mouse GAPDH was used as positive control for knock-down efficacy (Supplementary Fig. S4A) while non-target siRNA, which does not affect C7 expression was used to control for target specificity (Supplementary Fig. S4B). Our results show that inhibition of C7 gene expression in MKPOSE exposed to AdCre inhibits growth curve (p=0.03, Fig. 5B). Non-target siRNA at increasing concentrations does not interfere with cell growth (Supplementary Fig. S4C). We further tested complement inhibition in combination with AZD 6422 (80 nM) or BEZ235 (25 nM). As expected, when cells were treated with C7 siRNA and either of the inhibitor drugs, growth rate of cells was significantly inhibited (p<0.05). Combination of complement knock-down and either drug showed additional inhibitory effect (Fig. 5C, p<0.005).
Fig. 5.
Complement inhibition and tumor cell growth. A. Changes in C7 gene expression in C7 siRNA (25 nM) treated cells as compared to untreated cells. B. C7 siRNA treated cells show decreased cell growth as compared to untreated cells (*, p<0.01). C. Survival curve of MKPOSE AdCre cells treated with Kras and Pten pathway inhibitor drugs AZD6244 (80 nM) or BEZ235 (25 nM) respectively, along with C7 siRNA. Assays were run in triplicate. Average values and standard errors are shown. Results from one of two experiments shown. Student t test. * p<0.05; **p<0.005.
Overall, these in vitro results link Kras and Pten/Pi3k pathways to increased complement gene expression and suggest that complement inhibition reduces tumor cell proliferation an effect further increased, albeit moderately, by specific inhibitors of the above tumor pathways.
Discussion
Several inflammatory phenotypes have been associated with endometriosis and efforts are under way to incorporate these into noninvasive diagnostic tests (32). However, most of the studies to date have focused primarily on secreted chemokine profiles (20) or immune cells resident in the peritoneal cavity (macrophages, NK cells) or isolated from circulation (T lymphocytes, NK cells) (33, 34). We performed here the first comprehensive immune gene expression analysis of pelvic inflammation to date, using a collection of 511 Nanostring probes and RNA extracted from affected tissues. Our results revealed immune gene profiles that can differentiate endometriosis from both healthy and cancer cases. Surprisingly however, unsupervised clustering shows that several of the endometriosis patients may have an inflammation profile similar to those with EAOC (Fig. 1). This intriguing finding further confirms heterogeneity of inflammatory milieu in endometriosis and suggests that cancer–like immune signatures may develop earlier, in patients with lesions that are classified as clinically benign. Given the sensitivity and specificity of immune effectors that can sense early molecular changes of cells undergoing transformation, it is conceivable that profiling the immune response in tissue may provide molecular clues for patients with EAOC risk. This hypothesis stimulates further studies, with significantly larger sample sizes and additional controls, to validate and test the efficacy of cancer-like immune signatures as potential predictors of progression to EAOC. We also acknowledge that all the cases used here as controls consisted of a separate cohort of endometrial tissue, due to challenges in obtaining matching eutopic endometrium /ectopic glands from same patients. Inclusion of healthy ovaries, as additional reference, is equally problematic due to the fact that ovaries typically display only a delicate epithelial component (the OSE monolayer, which is often easily detached during surgical removal) and that little/no immune stroma is typically present in disease-free ovaries.
Analyses of DE gene sets revealed that complement is the top pathway in endometriosis and EAOC cases. Although this is the first tissue profiling study on complement, changes in peritoneal fluid levels of complement have been recently reported in endometriosis. Using qPCR gene profiling of 84 genes in a cohort of 20 endometriosis cases, Aslan et al recently reported that most significant upregulation was in C5 gene expression, although no other complement genes were detected, possibly due to the limited breadth of the profiler employed (35). We utilized here a comprehensive immune gene set and focused not only on endometriosis but also on EAOC. Our finding that 5 out of the total of 9 genes (56%) differentially expressed in both endometriosis and EAOC are complement genes strongly supports the importance of complement cascade in these diseases. In addition, the lower prevalence of complement genes in serous ovarian cancer suggests a potential specificity for complement in EAOC.
Primarily classified as a powerful innate immune effector, the complement system and its engagement in both acute and chronic inflammation are well defined (36). However, emerging literature during the past decade has revealed that complement activation may support tumor growth, thus creating a paradigm shift in complement cascade in cancer (37). It is now postulated that complement pathway may act via several mechanisms that co-exist in the tumor environment: directly, by playing an active role in stimulating proliferation of tumor cells or indirectly, via immune suppression (38, 39) and neovascularization (40). Nevertheless, the initiating steps that link epithelial cell biology and complement cascade are not well defined. Using our recently reported triple transgenic MUC1KrsPten mice with conditional mutations in Kras and Pten genes (21), we generated a novel primary OSE-derived murine ovarian cancer line (MKPOSE) and a versatile in vitro system that served as a surrogate for early transformation in ovarian epithelial cells. By turning on Kras and deleting Pten in MKPOSE cells we showed for the first time that activation through Kras and Pten/Pi3k leads to complement upregulation, an effect reversed when small drug inhibitors acting downstream of Kras and/or Pten are added. Inhibitors of tumor-driving pathways (including AZD6244 and BEZ325 used here) are currently tested in clinical trials for several types of tumors (41, 42). Although complement genes are likely controlled via several pathways, molecular profiling of tumor responses in these trials may provide further molecular evidence on modulation of complement pathways by targeted therapies.
Lastly, we report here that one other major biological pathway active in endometriosis is humoral immunity. In addition to changes in immune cells and cytokines, mostly detected via peripheral blood or peritoneal fluid measurements, women with endometriosis often display increased B cell activation (43–45). Systemic antibody production and deposition of IgG and complement in tissue has been previously reported in patients with endometriosis, showing humoral responses to various autoantigens (43–46).
Antibody-induced, complement mediated cell death is very effective in clearing dead cells, mostly via the classical pathway, activated by the Fc portion of immunoglobulins bound to various antigens on either infectious agents or apoptotic cells. The alternative pathway can be triggered via continuous, low level cleavage of C3 and can be activated by bacteria, viruses or fungi, as well as neoplastic cells. The third complement activation pathway, called the MBL pathway is activated by pathogens, via pathogen-associated molecular patterns (PAMPs) (36). The expression levels for the DE complement genes detected here suggest that MBL pathway was not involved in these cases (hence ruling out potential infectious causes for pelvic inflammation) and that classical pathway was likely most prominently used.
In summary, our findings reveal that chronic inflammation in endometriosis is dominated by complement, which remains active in EAOC but not tumors with serous histology, further demonstrating heterogeneity in the inflammatory milieu within this category commonly referred to as ovarian cancer. Pharmacologic inhibition of complement is currently tested in clinical trials (36) and results from these studies will provide much needed clinical evidence to support (or refute) the recent paradigm shift on pro-tumor roles of complement in cancer. Profiling studies like the one presented here might aid in patient selection for a personalized approach.
Supplementary Material
Translational Relevance.
Endometrioid and clear cell ovarian cancers are partly derived from endometriosis, a chronic inflammatory disease that often shows signs of local invasion, treatment resistance and recurrence. Majority of studies have previously focused on profiling immunity in endometriosis patients by either measuring cellular phenotypes or soluble mediators in the peritoneal fluid or peripheral blood. We performed here a comprehensive tissue immune gene expression analysis in lesions of endometriosis and endometriosis-associated ovarian cancer (EAOC). Some of the endometriosis patients revealed a cancer-like inflammation profile, suggesting that cancer–like immune signatures may develop earlier, in patients with lesions that are classified as clinically benign. Gene expression analyses revealed the complement pathway as most prominently involved in both endometriosis and EAOC. Our studies provide a novel insight into immune dysregulation in endometriosis and EAOC and reveal complement as a potential target for prevention and treatment.
Acknowledgments
Financial support: This study was supported by the UPMC Research Fund (to RPE, XH and AMV), Department of Defense (DOD) Ovarian Cancer Academy Award W81XWH-10-1-0525 and NIH/NCI R01 CA163462 (to AMV) and P50 CA159981 (to RPE). XH is Ovarian Cancer Research Fund Liz Tilberis Scholar.
The authors would like to thank Dr Adrian Lee for access to the Nanostring platform and Lindsay Mock and Julia Thaller for technical assistance with specimen retrieval. This study was supported by the UPMC Research Fund (to RPE, XH and AMV), Department of Defense (DOD) Ovarian Cancer Academy Award W81XWH-10-1-0525 and NIH/NCI R01 CA163462 (to AMV) and P50 CA159981 (to RPE). XH is Ovarian Cancer Research Fund Liz Tilberis Scholar.
Footnotes
Authors have no conflicts to declare.
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164–73. doi: 10.1016/j.canlet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014 doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 5.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca; Tp53; Pten models. Cancer Cell. 2013;24:751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 362:2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2014;3:CD009590. doi: 10.1002/14651858.CD009590.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida M, Watanabe K, Sato N, Ichikawa Y. Malignant transformation of ovarian endometriosis. Gynecol Obstet Invest. 2000;50 (Suppl 1):18–25. doi: 10.1159/000052874. [DOI] [PubMed] [Google Scholar]
- 10.Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15:1–9. doi: 10.1097/00004347-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 330:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samartzis EP, Samartzis N, Noske A, Fedier A, Caduff R, Dedes KJ, et al. Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol. doi: 10.1038/modpathol.2011.217. [DOI] [PubMed] [Google Scholar]
- 13.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–55. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 15.Varma R, Rollason T, Gupta JK, Maher ER. Endometriosis and the neoplastic process. Reproduction. 2004;127:293–304. doi: 10.1530/rep.1.00020. [DOI] [PubMed] [Google Scholar]
- 16.Vlahos NF, Economopoulos KP, Fotiou S. Endometriosis, in vitro fertilisation and the risk of gynaecological malignancies, including ovarian and breast cancer. Best Pract Res Clin Obstet Gynaecol. 2010;24:39–50. doi: 10.1016/j.bpobgyn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, et al. Toll-like receptors in innate immunity: role of bacterial endotoxin and toll-like receptor 4 in endometrium and endometriosis. Gynecol Obstet Invest. 2009;68:40–52. doi: 10.1159/000212061. [DOI] [PubMed] [Google Scholar]
- 18.Hou Z, Sun L, Gao L, Liao L, Mao Y, Liu J. Cytokine array analysis of peritoneal fluid between women with endometriosis of different stages and those without endometriosis. Biomarkers. 2009;14:604–18. doi: 10.3109/13547500903183970. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Agarwal A, Sekhon L, Krajcir N, Cocuzza M, Falcone T. Serum and peritoneal abnormalities in endometriosis: potential use as diagnostic markers. Minerva Ginecol. 2006;58:527–51. [PubMed] [Google Scholar]
- 20.Borrelli GM, Abrao MS, Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum Reprod. 2014;29:253–66. doi: 10.1093/humrep/det401. [DOI] [PubMed] [Google Scholar]
- 21.Budiu RA, Elishaev E, Brozick J, Lee M, Edwards RP, Kalinski P, et al. Immunobiology of human mucin 1 in a preclinical ovarian tumor model. Oncogene. 2013;32:3664–75. doi: 10.1038/onc.2012.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–91. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 23.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ness RB, Modugno F. Endometriosis as a model for inflammation-hormone interactions in ovarian and breast cancers. Eur J Cancer. 2006;42:691–703. doi: 10.1016/j.ejca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Alcazar JL, Guerriero S, Ajossa S, Parodo G, Piras B, Peiretti M, et al. Extragenital endometrial stromal sarcoma arising in endometriosis. Gynecol Obstet Invest. 2012;73:265–71. doi: 10.1159/000336522. [DOI] [PubMed] [Google Scholar]
- 26.Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759–65. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Wang H, Cho HY, Jung SJ, Kim KR, Ro JY, et al. Carcinoma of mullerian origin presenting as colorectal cancer: a clinicopathologic study of 13 Cases. Ann Diagn Pathol. 2011;15:12–8. doi: 10.1016/j.anndiagpath.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 29.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Molecular cancer therapeutics. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 31.Pio R, Ajona D, Lambris JD. Complement inhibition in cancer therapy. Semin Immunol. 2013;25:54–64. doi: 10.1016/j.smim.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, Chittams J, et al. Proteomic analysis of serum yields six candidate proteins that are differentially regulated in a subset of women with endometriosis. Fertil Steril. 2010;93:2137–44. doi: 10.1016/j.fertnstert.2008.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funamizu A, Fukui A, Kamoi M, Fuchinoue K, Yokota M, Fukuhara R, et al. Expression of natural cytotoxicity receptors on peritoneal fluid natural killer cell and cytokine production by peritoneal fluid natural killer cell in women with endometriosis. Am J Reprod Immunol. 2014;71:359–67. doi: 10.1111/aji.12206. [DOI] [PubMed] [Google Scholar]
- 34.Itoh F, Komohara Y, Takaishi K, Honda R, Tashiro H, Kyo S, et al. Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril. 2013;99:1705–13. doi: 10.1016/j.fertnstert.2013.01.133. [DOI] [PubMed] [Google Scholar]
- 35.Aslan C, Ak H, Askar N, Ozkaya AB, Ergenoglu AM, Yeniel AO, et al. Overexpression of complement C5 in endometriosis. Clin Biochem. 2014;47:496–8. doi: 10.1016/j.clinbiochem.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11:785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res. 8:1453–65. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- 38.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–35. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. 59:897–905. doi: 10.1007/s00011-010-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunez-Cruz S, Gimotty PA, Guerra MW, Connolly DC, Wu YQ, DeAngelis RA, et al. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 14:994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Britten CD. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother Pharmacol. 2013;71:1395–409. doi: 10.1007/s00280-013-2121-1. [DOI] [PubMed] [Google Scholar]
- 42.Patel SP, Kim KB. Selumetinib (AZD6244; ARRY-142886) in the treatment of metastatic melanoma. Expert Opin Investig Drugs. 2012;21:531–9. doi: 10.1517/13543784.2012.665871. [DOI] [PubMed] [Google Scholar]
- 43.Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun Rev. 2012;11:806–14. doi: 10.1016/j.autrev.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 45.Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–96. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 46.Badawy SZ, Cuenca V, Stitzel A, Jacobs RD, Tomar RH. Autoimmune phenomena in infertile patients with endometriosis. Obstet Gynecol. 1984;63:271–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.