Abstract
This review summarizes the endocrine and neurodevelopmental effects of two current-use additive flame retardants (FRs), tris (1,3-dichloro-isopropyl) phosphate (TDCPP) and Firemaster® 550 (FM 550), and the recently phased-out polybrominated diphenyl ethers (PBDEs), all of which were historically or are currently used in polyurethane foam applications. Use of these chemicals in consumer products has led to widespread exposure in indoor environments. PBDEs and their hydroxylated metabolites appear to primarily target the thyroid system, likely due to their structural similarity to endogenous thyroid hormones. In contrast, much less is known about the toxicity of TDCPP and FM550. However, recent in vitro and in vivo studies suggest that both should be considered endocrine disruptors as studies have linked TDCPP exposure with changes in circulating hormone levels, and FM 550 exposure with changes in adipogenic and osteogenic pathways.
Keywords: flame retardant, PBDE, endocrine disruption, thyroid, metabolite, OH-BDE, neurodevelopment, organophosphate
Introduction
Flame retardants (FRs) are chemicals applied to a variety of consumer products, including upholstered furniture, electronic casings, building materials, and baby products, to meet flammability standards [1,2]. California technical bulletin 117 (TB 117) is flammability standard for residential furniture that requires products to meet an open flame test. Although TB117 applies only to items sold in California, it has led to the widespread use of FRs in residential furniture sold throughout the US.
This review will focus on halogenated and organophosphate FRs (OPFRs), which interrupt the fire propagation cycle through two mechanisms (for physicochemical properties and structural information see Figure 1). Halogenated FRs, (i.e., those containing bromine and chlorine), are particularly effective at trapping free radicals and slowing fire spread in the gas phase. In contrast, the phosphate backbone in OPFRs increases char formation, creating a physical barrier between the ignition source and the material [3]. Additive FRs are applied after polymerization and are not covalently bound while reactive FRs are added during polymerization and are chemically bound to the material. Most FRs associated with human health concerns are additive and can be released from treated products over time, accumulating in indoor environments.
Figure 1.
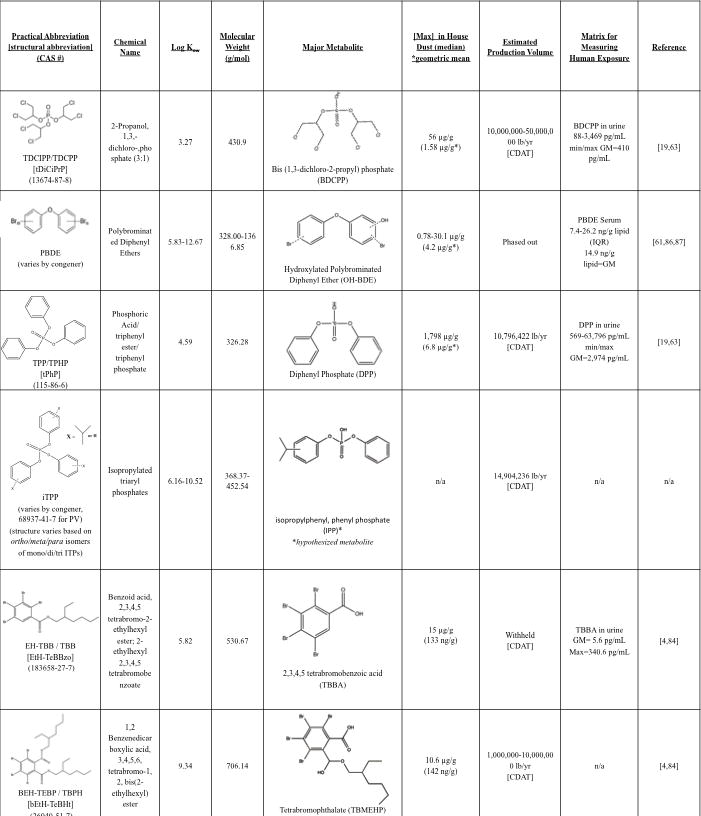
Structures, Physical Properties and Dust Concentrations of Selected Flame Retardants. Log Kow values were taken from EPA’s modeling software EPISuite and from a recent review [85] The Production Volume estimates were taken from EPA’s Chemical Data Access Tool (CDAT), which contains the 2012 Chemical Data Reporting (CDR) information. All dust, serum, and urine measurements are reported from US cohorts.
Halogenated FRs, such as polybrominated diphenyl ethers (PBDEs) were a dominant class of FRs in North America for several decades. PBDEs were sold as three commercial mixtures, referred to as PentaBDE, OctaBDE, and DecaBDE, in reference to the degree of bromination of the major BDE congener in each mixture. In the United States, the Penta- and OctaBDE mixtures were voluntarily phased out starting in 2005 after reports of persistence, bioaccumulation, and toxicity. The DecaBDE mixture was phased out at the end of 2013. While the use of PBDEs in products has ceased, human exposure will continue for decades due to the reservoir of existing products containing PBDEs.
Tris (1,3 dichloro-2-propyl) phosphate (TDCPP), was applied to children’s pajamas during the late 1970s. Although TDCPP use in children’s sleepwear was discontinued in the 1970s due to concerns of potential carcinogenicity, it became the primary FR replacement in polyurethane foam (PUF) following the phase-out of PBDEs. Firemaster® 550 (FM 550), a mixture of brominated and organophosphate flame retardants, was marketed as a less toxic and less bioaccumulative alternative to PentaBDE and was intended to be used in polyurethane foam [4]. The brominated components of FM 550 have been identified as 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB or TBB) and bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (BEH-TEBP or TBPH), the latter of which is a brominated analog of the phthalate plasticizer di-2-ethylhexylphthalate (DEHP) [5]. The organophosphate components of FM 550 include triphenylphosphate (TPP) and numerous TPP analogs with various degrees of aryl isopropylation (referred to collectively as iTPP) [3]. Figure 1 contains a summary of these chemicals, their properties, and their structures.
Exposure to Flame Retardants
Over the past few decades, increasing attention has focused on FR additives used in polyurethane foam (PUF) found in furniture (e.g., sofas, chairs, mattresses, etc.) and electronics (e.g., televisions) [1,2,6]. The typical application rates for FRs in PUF in furniture are approximately 4-5% by weight of the foam, and FR application in plastic casings of TVs has been measured at around 10-15% by weight [7,8]. Over the product lifetime, FRs slowly volatilize or escape from the treated materials and accumulate in indoor air and dust particles or escape to the outdoor environment (for dust measurements, see Figure 1).
Human exposure to FRs occurs through three pathways: inhalation, diet, and exposure to dust particles either through inadvertent ingestion or dermal absorption [9**,10]. Exposure to FRs is typically greater in children compared with adults [11]. FRs accumulate in indoor dust, and children, particularly toddlers, ingest more dust particles on a daily basis due to higher hand-to-mouth and object-to-mouth activities [9**,12]. In a children’s exposure study, Stapleton et al. (2012b) found that PBDE residues measured on handwipes and indoor dust were the strongest predictor of PBDE levels measured in blood, supporting dust ingestion as a pathway of exposure [9**]. In contrast, studies conducted in Europe have found that diet is a primary route of exposure to PBDEs among the general population [13,14]. These regional differences in exposure pathways are likely driven by differences in flammability standards. The concentrations of the FRs most commonly used in PUF are higher in dust samples collected in the US compared with the concentrations measured in dust from some European countries that do not have residential flammability standards [15]. This likely explains why body burdens of PBDEs commonly used in PUF are higher in the US compared with European countries [16].
A few recent studies have focused on potential human exposure to TDCPP and FM 550. Similar to PBDEs, TDCPP and FM550 components (e.g. TPP), are nearly ubiquitous in dust samples, however concentrations vary widely [3]. Based on measurements of TDCPP and EH-TBB in dust collected from several European countries [17,18] it appears that exposure levels of both flame retardants would be lower in Europe relative to the US, similar to trends observed for PBDEs. This again may be a reflection of the higher use of these FRs in PUF containing furniture in the US to meet residential flammability standards. However, more studies are really needed to understand regional differences in exposure. For biomonitoring of TDCPP exposure, most studies rely on measurements of a urinary metabolite, bis (1,3-dichloro-isopropyl) phosphate (BDCPP). A few recent studies have observed some weak but significant associations between TDCPP levels in indoor dust with urinary BDCPP levels in the US populations; however, the relationships are not as strong as those observed for PBDEs, suggesting that exposure to indoor dust may not be the leading source of exposure [19**,20]. As FM 550 is a mixture of several types of FRs, some of which are used separately as FRs, measuring exposure is a bit more challenging. A recent study measured tetrabromobenzoic acid (TBBA), a metabolite of EH-TBB, in urine and suggested it would be a good biomarker of exposure to FM 550 [21]. TBBA was detected in >70% of urine samples and levels were significantly associated with EH-TBB levels measured in handwipes. These studies demonstrate that use of FRs in PUF leads to widespread exposure, particularly for children, highlighting a need to understand potential health impacts.
Endocrine Related Effects of FRs
1. PBDEs
Epidemiology studies have shown that children of mothers with higher pregnancy PBDE burdens had greater risk for IQ deficits and impaired learning behaviors later in life [22-24*]. Additionally, in a series of rodent studies Viberg et al. found that exposure to PBDEs impaired spatial memory, learning, and altered spontaneous behavior in adult and developing rats [25-30], supporting the human observations. In addition, in a series of in vitro experiments using a rat neuronal cell line (PC12 cells), PBDEs and their metabolites caused increased calcium release and altered neurotransmitter release suggesting disrupted neurodevelopment [31,32].
Potential mechanisms of PBDE-induced neurotoxicity have been reviewed previously [33,34], and one hypothesized mechanism for the behavioral/neurodevelopmental effects is disruption of thyroid hormone regulation during critical developmental windows. PBDEs are biotransformed into hydroxylated polybrominated diphenyl ethers (OH-BDEs) and bromophenols through oxidative metabolism in mammals [35,36]. OH-BDEs are structurally more similar to endogenous thyroid hormones, and may be responsible for some of the observed PBDE toxicity [32]. Thyroid hormones (TH) are essential for cell migration and synaptogenesis in the brain, and proper neurodevelopment overall [37,38]. Multiple studies have documented the ability of OH-BDEs to bind to thyroid transporter proteins, the thyroid nuclear receptor, and even the estrogen receptor [39*-43]. PBDE/OH-BDE receptor binding differs by the congener and by the species/assay used [39*,43-46], and PBDE effects on other nuclear hormone receptors have been recently reviewed by Ren et al. [47]. In rodent studies, PBDE exposure often causes a reduction in serum thyroxine (T4) or triiodothyronine (T3) [48,52], which is attributed to increased clearance of TH by metabolizing enzymes [53]. Multiple proteins, including deiodinase (DI), sulfotransferase (SULT), and uridine diphosphate-glucuronosyltransferases (UGT) enzymes, along with membrane transporters mediate the activation, metabolism, and uptake of thyroid hormones in peripheral tissues. In support of this hypothesis, rodent studies have reported enhanced expression of UGTs in animals exposed to PBDEs [53,54]. Other in vitro studies have reported that PBDEs and OH-BDEs alter DI and SULT activity, suggesting that there are multiple endpoints by which thyroid hormone regulation may be impacted by PBDE exposure [55,56]. Similar thyroid disruption has also been observed in fish species exposed to PBDEs, which has been recently reviewed by Noyes et al [57]. Human studies have observed conflicting associations between PBDE exposure and TH status, with some reporting increases in T4 and TSH, and others finding no significant associations [58,60].
In addition to PBDE effects on neurodevelopment and thyroid hormone regulation, there have also been studies observing effects between PBDE exposure and reproductive endpoints. Serum PBDE levels in US women were associated with longer time to pregnancy and reduced fecundability [61]. Dust PBDE concentrations were inversely associated with levels of free androgen, luteinizing hormone (LH), and follicle stimulating hormone (FSH), and positively associated with inhibin B and sex hormone binding globulin (SHBG) in a study of US men [62]. The summarized effects suggest that PBDEs are endocrine active (Table 1).
Table 1.
Reproductive, Thyroid, and Neurodevelopmental Effects of Selected Flame Retardants.
| Chemical | Effects | References |
|---|---|---|
| PBDEs OH-BDEs |
Reproduction
|
[22,23,61,85] |
Thyroid
|
[39*,42,48,53,86-88] | |
Neurodevelopment
|
[22,24*,26,31,32,89,90] | |
| TDCPP | Reproduction/Embryogenesis
|
[64**-66,91] |
Thyroid
|
[66-69] | |
Neurodevelopment
|
[70,71*] | |
| TPP iTPPs |
Reproduction/Embryogenesis
|
[65-67,69,72,73] |
Thyroid
|
[69] | |
| TBB TBPH |
Development
|
[75,76**,77*,78*] |
Thyroid
|
[76**,93] | |
Reproduction
|
[79,80] |
2. TDCPP
TDCPP has a much shorter half-life in tissues compared with PBDEs. In human and rodents, it is rapidly metabolized to the dialkyl phosphate, BDCPP and excreted in urine [19**,20,63]. Until recently, few studies have evaluated the potential adverse effects of TDCPP, however, there is mounting evidence to suggest that OPFRs also affect endocrine systems (Table 1). Several studies have reported sex-dependent effects of TDCPP exposure on the hypothalamic-pituitary-gonad (HPG) axis. Adult zebrafish exposed to TDCPP for 14 days showed elevated serum levels of estradiol (E2) and testosterone (T) in both males and females. In males, the E2/T ratio was slightly elevated, while females showed an E2/T ratio decrease. Similarly, mRNA expression of vitellogenin (VTG) was increased in males and decreased in females. Changes in serum hormone levels corresponded with increased mRNA expression of CYP17 and CYP19A, enzymes involved in sex steroid synthesis. Nearly identical results were observed in two human cell lines, MVLN and H295R, suggesting that these results are likely to be conserved across vertebrate species [64**,65]. In a similar study, a 21 day exposure in adult zebrafish decreased fecundity, egg production, the number of spawning events, fertilization, and hatching success in addition to altering serum hormone levels and mRNA expression of numerous HPG axis genes [65]. The mechanisms driving these HPG axis effects have yet to be fully elucidated, however one study reported that TDCPP and other OPFRs acted as ER antagonists, decreasing the binding of E2 to the ER in a human cell line (MVLN) [64**].
In addition to reproductive end points, TDCPP has also been implicated in dysregulation of the thyroid hormone system. In one human epidemiological study, high concentrations of TDCPP in house dust were associated with decreased T4 levels in a cohort of men [66]. Similarly, thyroid hormone levels were altered in TDCPP exposed zebrafish and chicken embryos: T3 levels increased in exposed zebrafish while T4 levels decreased in zebrafish and chicken embryos [67,68]. Early life TDCPP exposure in zebrafish was also found to alter mRNA expression of several genes that regulate thyroid function [67,69]. However, the current mechanisms responsible for these changes in circulating thyroid hormone levels are unknown.
In PC12 cells, TDCPP altered several neurodevelopmental processes, including cell replication, growth, and phenotypic differentiation [70]. These effects were similar to the effects of chlorpyrifos, an organophosphate pesticide and established developmental neurotoxicant. Importantly, TDCPP effects were equivalent to or greater than chlorpyrifos at equimolar concentrations [70]. A more recent study in PC12 cells found that TDCPP exposure altered expression (mRMA and protein) of several genes that regulate several important neurodevelopmental processes, including apoptosis, synaptogenesis, and neurite outgrowth [71*]. Notably, due to the importance of the HPT axis in neurodevelopment these two endpoints may be causally linked.
3. FM 550
Because FM 550 is a more recently introduced chemical product, little is known about the potential toxicity of this mixture. However, several recent studies have examined the specific toxicity of TPP, one of the organophosphates in FM 550. For example, TPP appears to elicit effects very similar to that of TDCPP. Adult zebrafish exposed to TPP for 14 or 21 days exhibited reduced fecundity, altered sex steroid levels (E2, T, and VTG) and changes in mRNA expression of HPG axis related genes [65]. In vitro studies reported similar effects in human cell lines [64**]. Importantly, TPP and iTPPs interact with several nuclear receptors, including the androgen receptor (AR), in vitro. TPP inhibited AR activity, while the effects of iTPP congeners varied [72]. In one epidemiological study, high levels of TPP in indoor dust were associated with decreased sperm counts in men, but had no effects on T4 serum levels [66]. Although less pronounced than those for TDCPP, increased expression of thyroid receptors have also been reported with TPP exposure in zebrafish [69]. FM 550 elicited severe cardiotoxicity and heart deformities in embryonic zebrafish. These phenotypes were found to be driven by TPP and mono-iTPPs acting as arylhydrocarbon receptor 2 (AHR2) agonists [73].
Less is known about the potential toxicity of the brominated components of FM 550, EH-TBB and BEH-TEBP. BEH-TEBP is the brominated analog of bis (2-ethylhexyl) phthalate (DEHP), which is considered a known reproductive toxicant and also impairs fatty acid metabolism and thyroid hormone levels [74]. In addition, EH-TBB exhibits a similar structure to the toxic metabolite of DEHP, mono-2-ethylhexyl phthalate (MEHP). Several studies have shown that EH-TBB and BEH-TEBP can be absorbed and elicit various toxicological effects, including behavioral and endocrine-disrupting effects. In a laboratory study, fish accumulated EH-TBB and EH-TBPH via dietary exposure and demonstrated increased DNA damage in blood and liver tissue [75]. Perinatal exposure to FM 550 in rats led to accumulation of EH-TBB and BEH-TEBP in both maternal and pup tissues. Male pups in this study exhibited a significant weight increase, increased thickness of the left ventricular wall, and decreased performance in several behavioral tests compared with pups born from unexposed dams [76**]. Female pups also exhibited a significant weight increase and entered puberty earlier than pups from unexposed dams. While limited to only one study, this perinatal exposure suggests that exposure to FM 550 can alter adipogenic and developmental pathways. As a follow-up to this study, researchers also investigated the effects of FM 550 and its individual components on nuclear receptor activation [77*] and adipogenic and osteogenic pathways in cell culture [78*]. Combined results from these studies suggest that FM 550, and particularly TPP, may be eliciting an obesogenic phenotype due to activation of the nuclear receptor PPARγ and upregulation of adipogenesis, which may occur at the expense of osteogenesis. Further studies are warranted to determine if the phenotype observed in rodents is due to effects from TPP alone or from the mixture of chemicals present in FM 550.
In vitro, EH-TBB and BEH-TEBP have been shown to alter the production of sex hormones in human testicular cells and in a mammalian steroidogenesis assay [79,80]. While the mechanism of toxicity has not been fully elucidated, evidence indicates that EH-TBB and BEH-TEBP may affect the reproductive and thyroid systems in a manner similar to DEHP. It is important to note that EH-TBB and BEH-TEBP are likely rapidly biotransformed following absorption. Metabolism studies are limited, but in fish, rats, and human tissue preparations, EH-TBB was metabolized to form 2,3,4,5-tetrabromobenzoic acid (TBBA) and its methyl-ester analog (2,3,4,5-tetrabromomethyl benzoate) [76**,81,82]. Less is known about the potential toxicity of these metabolites, but they may be useful urinary biomarkers of exposure [83]. BEH-TEBP can be metabolized to form mono (2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBMEHP), but it appears to occur at a much slower rate than EH-TBB [81,84]. Due to biotransformation, EH-TBB will be less persistent and exhibit shorter half-lives in humans than PBDEs. However, chronic exposure to the components of FM 550 is occurring in the indoor environment, particularly to children, and the potential health implications should be further investigated.
Conclusions
Through increased biomonitoring research, it is quite clear that the most common flame retardants used in polyurethane foam are now ubiquitous in indoor environments, leading to chronic exposure to large populations, particularly children. With PBDEs, inadvertent ingestion of dust particles in the home environment has been established as the primary exposure pathway in the US population; however, the differences in vapor pressure and partitioning behavior of these new FRs may lead to differences in the exposure pathways, which requires further investigation. For example, given the higher vapor pressure of TDCPP relative to PBDEs, inhalation may be a more important exposure pathway than dust ingestion, yet no studies have investigated the relative contributions of each to total exposure. While PBDEs have been primarily phased out of use in consumer products, exposures to new types of FRs are increasing, and recent data suggest that both TDCPP and FM 550 are likely endocrine disruptors. Based on the studies described here, it appears that several FRs have the ability to interfere with the HPT and HPG axes. Future studies should examine health effects of these non-PBDE flame retardant chemicals during key developmental stages regulated by HPT and HPG. In addition, more research is needed on effects of exposure to environmentally relevant mixtures of these FRs, particularly during perinatal periods, to better understand the risks for human health.
Highlights.
Widespread exposure to flame retardants occurs in indoor environments
Indoor exposure is associated with body burdens
Highest human exposures occur in early development, a sensitive window
PBDEs elicit neurodevelopmental and thyroid hormone disrupting effects
OPFRs also appear to be endocrine active
Acknowledgments
The authors acknowledge grant support from the National Institute of Environmental Health Sciences research grants (R01-ES016099 and P42-ES010356) and U.S. EPA STAR graduate fellowships FP917496 (SR) and FP91728801 (LD).
Abbreviations
- FR
Flame Retardant
- PBDE
Polybrominated Diphenyl Ether
- OPFR
Organophosphate Flame Retardant
- TDCPP
Tris (1,3 dichloro-2-propyl) phosphate
- OH-BDE
Hydroxylated Polybrominated Diphenyl Ether
- EH-TBB
2-ethylhexyl-2,3,4,5 tetrabromobenzoate
- BEH-TEBP
bis (2-ethylhexyl) tetrabromophthalate
- TH
Thyroid Hormone
- DI
deiodinase enzyme
- T4
Thyroxine
- T3
Triiodothyronine
- TR
Thyroid receptor
- PUF
Polyurethane foam
- TPP
Triphenyl phosphate
- ITP
isopropylated triphenyl phosphate
- FM 550
Firemaster® 550
- LH
Leutenizing hormone
- FSH
Follicle stimulating hormone
- TBBA
Tetrabromobenzoic acid
- DEHP
Di-2-ethylhexylphthalate
- SHBG
Sex hormone binding globulin
- UGT
Uridine diphosphate-glucuronosyltransferases
- SULT
Sulfotransferase
- BDCPP
Bis (1,3-dichloropropyl) phosphate
- HPT
Hypothalamic-pituitary-thyroid
- HPG
Hypothalamic-pituitary-gonad
- E2
Estradiol
- T
Testosterone
- VTG
Vitellogenin
- AR
Androgen receptor
- MEHP
Mono-2-ethylhexyl phthalate
- TBMEHP
Mono (2-ethylhexyl)-2,3,4,5-tetrabromophthalate
- DPP
Diphenyl phosphate
- AHR2
Arylhydrocarbon receptor 2
Footnotes
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46:13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45:5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42:6910–6. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Z, Bi X, Huang B, Liu M, Sheng G, Fu J. Hydroxylated PBDEs and brominated phenolic compounds in particulate matters emitted during recycling of waste printed circuit boards in a typical e-waste workshop of South China. Environ Pollut. 2013;177:71–7. doi: 10.1016/j.envpol.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Kajiwara N, Noma Y, Takigami H. Brominated and organophosphate flame retardants in selected consumer products on the Japanese market in 2008. J Hazard Mater. 2011;192:1250–9. doi: 10.1016/j.jhazmat.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ Int. 2008;34:1085–91. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- **9.Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina Toddler Cohort: Associations with Handwipes, House Dust, and Socioeconomic Variables. Environ Health Perspect. 2012;120:1049–1054. doi: 10.1289/ehp.1104802. Stapleton et al. present evidence that hand-to-mouth contact is an important source of PBDE exposure in toddlers using measurements of PBDEs in serum and handwipes. Age, socioeconomic status, and breast-feeding were all significant predictors of PBDE exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 11.Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol. 2010;44:5256–62. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- 12.Sharp R, Lunder S. In the dust. Toxic Fire Retardants in American Homes. 2004 [Google Scholar]
- 13.Roosens L, Abdallah MA-E, Harrad S, Neels H, Covaci A. Factors Influencing Concentrations of Polybrominated Diphenyl Ethers (PBDEs) in Students from Antwerp, Belgium. Environ Sci Technol. 2009;43:3535–3541. doi: 10.1021/es900571h. [DOI] [PubMed] [Google Scholar]
- 14.Domingo JL. Human exposure to polybrominated diphenyl ethers through the diet. J Chromatogr A. 2004;1054:321–326. doi: 10.1016/j.chroma.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Sjödin A, Päpke O, McGahee E, Focant J-F, Jones RS, Pless-Mulloli T, Toms L-ML, Herrmann T, Müller J, Needham LL, et al. Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008;73:S131–6. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 16.Hites RA. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 17.Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta. 2012;89:292–300. doi: 10.1016/j.talanta.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Marklund A, Andersson B, Haglund P. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere. 2003;53:1137–46. doi: 10.1016/S0045-6535(03)00666-0. [DOI] [PubMed] [Google Scholar]
- **19.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect. 2013;121:580–5. doi: 10.1289/ehp.1205907. Meeker et al. identified several urinary metabolites of OPFRs as potential biomarkers of exposure. The concentrations of TDCPP in house dust correlated with the concentrations of the urinary metabolites and were used to investigate the relative importance of different exposure pathways for different flame retardants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbstman JB, Sjödin A, Kurzon M, Lederman Sa, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. Eskenazi et al. present evidence of impaired IQ, attention, and coordination resulting from prenatal exposure to PBDEs. The study indicates that exposure to PBDEs during pregnancy causes long-term detrimental effects on neurobehavioral development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2’,4,4’,5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol Sci. 2002;67:104–7. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2,2’,4,4’,5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci. 2002;67:98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- 27.Viberg H, Fredriksson A, Jakobsson E, Orn U, Eriksson P. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol Sci. 2003;76:112–20. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- 28.Viberg H. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 29.Viberg H, Fredriksson A, Eriksson P. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209) Neurotoxicology. 2007;28:136–42. doi: 10.1016/j.neuro.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Viberg H. Exposure to polybrominated diphenyl ethers 203 and 206 during the neonatal brain growth spurt affects proteins important for normal neurodevelopment in mice. Toxicol Sci. 2009;109:306–11. doi: 10.1093/toxsci/kfp074. [DOI] [PubMed] [Google Scholar]
- 31.Dingemans MML, de Groot A, van Kleef RGDM, Bergman A, van den Berg M, Vijverberg HPM, Westerink RHS. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ Health Perspect. 2008;116:637–43. doi: 10.1289/ehp.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dingemans MML, van den Berg M, Westerink RHS. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–7. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett. 2013 doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu X, Mercado-Feliciano M, Bigsby RM, Hites Ra. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromodiphenyl ether mixture. Environ Health Perspect. 2007;115:1052–8. doi: 10.1289/ehp.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erratico CA, Szeitz A, Bandiera SM. Oxidative metabolism of BDE-99 by human liver microsomes: predominant role of CYP2B6. Toxicol Sci. 2012;129:280–92. doi: 10.1093/toxsci/kfs215. [DOI] [PubMed] [Google Scholar]
- 37.Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110(Suppl):337–48. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- *39.Ren X-M, Guo L-H, Gao Y, Zhang B-T, Wan B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol Appl Pharmacol. 2013;268:256–263. doi: 10.1016/j.taap.2013.01.026. Ren and Guo report a detailed analysis of studies the toxicological mechanisms of PBDEs with a specific focus on the interactions between PBDEs and thyroid nuclear receptors. The authors present evidence that PBDEs interact directly with nuclear hormone receptors and the binding of corepressors and coactivators. [DOI] [PubMed] [Google Scholar]
- 40.Cao J, Lin Y, Guo L-H, Zhang A-Q, Wei Y, Yang Y. Structure-based investigation on the binding interaction of hydroxylated polybrominated diphenyl ethers with thyroxine transport proteins. Toxicology. 2010;277:20–8. doi: 10.1016/j.tox.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ Health Perspect. 2013;121:1194–9. doi: 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 43.Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl. Environ Health Perspect. 2011;119:168–175. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117:1210–8. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeuchi S, Shiraishi F, Kitamura S, Kuroki H, Jin K, Kojima H. Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans. Toxicology. 2011;289:112–21. doi: 10.1016/j.tox.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Xie Q, Li X, Li N, Chi P, Chen J, Wang Z, Hao C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: in vitro and in silico investigations. Environ Health Perspect. 2010;118:602–6. doi: 10.1289/ehp.0901457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren X-M, Guo L-H. Molecular toxicology of polybrominated diphenyl ethers: nuclear hormone receptor mediated pathways. Environ Sci Process Impacts. 2013;15:702–8. doi: 10.1039/c3em00023k. [DOI] [PubMed] [Google Scholar]
- 48.Zhou T, Taylor MM, DeVito MJ, Crofton KMA. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–16. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- 49.Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78:144–55. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- 50.Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environ Sci Technol. 2004;38:1496–504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- 51.Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2’,4,4’-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Perspect. 2008;116:1694–9. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noyes P, Lema S, Macaulay L, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol. 2013 doi: 10.1021/es402650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicol Sci. 2010;113:367–79. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci. 2011;124:339–47. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butt C, Stapleton HM. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem Res Toxicol 2013. doi: 10.1021/tx400342k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noyes PD, Stapleton HM. PBDE flame retardants: Toxicokinetics and thyroid hormone endocrine disruption in fish. Endocr Disruptors. 2014;2:e29430. [Google Scholar]
- 58.Zota AR, Park J-S, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119:1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roze E, Meijer L, Bakker A, Van Braeckel KNJa, Sauer PJJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–8. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harley KG, Marks AR, Chevrier J, Bradman A, Sjödin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ. 2009;407:3425–9. doi: 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper EM, Covaci A, van Nuijs ALN, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:2123–32. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 2012;114-115:173–81. doi: 10.1016/j.aquatox.2012.02.019. Liu, Ji, and Choi present evidence that TDCPP and other OPFRs alter the HPG axis in zebrafish and human cell lines. They observed in vivo effects on sex hormone homeostasis observed after a 14-day exposure. Cell culture experiments suggest some TDCPP acts as an estrogen receptor antagonist. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Ji K, Jo A, Moon H-B, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio) Aquat Toxicol. 2013;134-135:104–11. doi: 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect. 2010;118:318–23. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, Zhou B. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol. 2013;126:207–13. doi: 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, Kennedy SW. In Ovo effects of two organophosphate flame retardants--TCPP and TDCPP--on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. 2013;134:92–102. doi: 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- 69.Liu C, Wang Q, Liang K, Liu J, Zhou B, Zhang X, Liu H, Giesy JP, Yu H. Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat Toxicol. 2013;128-129:147–57. doi: 10.1016/j.aquatox.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 70.Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin Ta, Stapleton HM. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol. 2011;256:281–9. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *71.Ta N, Li C, Fang Y, Liu H, Lin B, Jin H, Tian L, Zhang H, Zhang W, Xi Z. Toxicity of TDCPP and TCEP on PC12 cell: Changes in CAMKII, GAP43, tubulin and NF-H gene and protein levels. Toxicol Lett. 2014;227:164–171. doi: 10.1016/j.toxlet.2014.03.023. Ta et al. show that TDCPP exposure in neuronotypic PC12 cells alters expression of several genes that are critical to neurodevelopment. These genes are involved in regulation of apoptosis, neurite optgrowth, and synapse formation. [DOI] [PubMed] [Google Scholar]
- 72.Honkakoski P, Palvimo JJ, Penttilä L, Vepsäläinen J, Auriola S. Effects of triaryl phosphates on mouse and human nuclear receptors. Biochem Pharmacol. 2004;67:97–106. doi: 10.1016/j.bcp.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 73.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci. 2013;133:144–56. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- 74.Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–34. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster 550 and Firemaster BZ-54. Environ Toxicol Chem. 2010;29:722–9. doi: 10.1002/etc.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **76.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and Endocrine Disrupting Effects of the Flame Retardant Mixture Firemaster® 550 in Rats: An Exploratory Assessment. J Biochem Mol Toxicol. 2013;27:124–136. doi: 10.1002/jbt.21439. Patisaul et al. exposed pregnant rats to FM550 and demonstrated evidence of increased body weight and decreased performance in behavioral tests in pups. This represents the only peer-reviewed, in vivo, mammalian study addressing the potential toxicity of FM550 and needs to be confirmed in further studies with larger sample sizes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *77.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett. 2014;228:93–102. doi: 10.1016/j.toxlet.2014.04.017. Belcher et al. present evidence that FM550 activates PPARγ using human luciferase reporter assays. TPP appeared to be the most potent ingredient in the FM550 commercial mixture. This study supports other evidence that FM550 and especially the OP components of FM550 are potential obesogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *78.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand Binding and Activation of PPARγ by Firemaster® 550: Effects on Adipogenesis and Osteogenesis in Vitro. Environ Health Perspect. 2014 doi: 10.1289/ehp.1408111. Pillai et al. showed that FM550 components, particularly TPP, bound to and activated PPARγ. FM550 also induced adipocyte differentiation in BMS2 cells and inhibited osteogenesis in primary mouse bone marrow cultures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mankidy R, Ranjan B, Honaramooz A, Giesy JP. Effects of Novel Brominated Flame Retardants on Steroidogenesis in Primary Porcine Testicular Cells. Toxicol Lett. 2013;224:141–146. [PubMed] [Google Scholar]
- 80.Saunders DMV, Higley EB, Hecker M, Mankidy R, Giesy JP. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol Lett. 2013;223:252–9. doi: 10.1016/j.toxlet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 81.Roberts SC, Macaulay LJ, Stapleton HM. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem Res Toxicol. 2012;25:1435–41. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bearr JS, Mitchelmore CL, Roberts SC, Stapleton HM. Species specific differences in the in vitro metabolism of the flame retardant mixture, Firemaster® BZ-54. Aquat Toxicol. 2012;124-125:41–7. doi: 10.1016/j.aquatox.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM. Environmental health perspectives. 2014 doi: 10.1289/ehp.1308028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffman K, Fang M, Horman B. Urinary Tetrabromobenzoic Acid (TBBA) as a Biomarker of Exposure to the Flame Retardant Mixture Firemaster® 550. Env Heal …. 2014 doi: 10.1289/ehp.1308028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115:1519–26. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noyes PD, Hinton DE, Stapleton HM. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol Sci. 2011;122:265–74. doi: 10.1093/toxsci/kfr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119:1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdelouahab N, Langlois M-F, Lavoie L, Corbin F, Pasquier J-C, Takser L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013;178:701–13. doi: 10.1093/aje/kwt141. [DOI] [PubMed] [Google Scholar]
- 89.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chevrier J, Harley KG, Bradman A, Sjödin A, Eskenazi B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol. 2011;174:1166–74. doi: 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 92.McGee SP, Cooper EM, Stapleton HM, Volz DC. Early Zebrafish Embryogenesis Is Susceptible to Developmental TDCPP Exposure. Environ Health Perspect. 2012;120:1585–1591. doi: 10.1289/ehp.1205316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Springer C, Dere E, Hall SJ, McDonnell EV, Roberts SC, Butt CM, Stapleton HM, Watkins DJ, McClean MD, Webster TF, et al. Rodent Thyroid, Liver, and Fetal Testis Toxicity of the Monoester Metabolite of Bis-(2-ethylhexyl) Tetrabromophthalate (TBPH), a Novel Brominated Flame Retardant Present in Indoor Dust. Environ Health Perspect. 2012;120:1711–1719. doi: 10.1289/ehp.1204932. [DOI] [PMC free article] [PubMed] [Google Scholar]


