Coronary artery calcium (CAC) is a frequent finding in smokers, and it is a marker of accelerated atherosclerosis in this population.1 Prior research has demonstrated a higher rate of five to ten year estimated all-cause mortality in smokers with CAC as compared to smokers without CAC.2,3 However, previous studies have produced limited insight regarding the long-term efficacy of CAC for risk stratification in smokers. This study therefore sought to examine the association between smoking, CAC, and all-cause mortality over a 15-year period.
The study population was a cohort of 4,143 consecutive asymptomatic patients aged 55 and older (mean 63.2±6.6 years, range 55–99) without known coronary artery disease (CAD) who had been referred by their physician for CAC testing between 1991 and 2004. All study participants completed a baseline questionnaire of demographic characteristics and baseline cardiovascular risk factors. Cigarette smoking was considered present if a subject was an active smoker at the time of CAC scanning. CAC measurement was performed by electron beam computed tomography (EBCT) at three different centers in the United States using standard methods as previously described.3 Each calcified lesion was scored using the method developed by Agatston et al.4 All individuals provided informed consent for a pre-test interview, CAC testing, and follow-up. The study received approval from the appropriate Human Investigations Committee and conforms to the 1975 Declaration of Helsinki.
The primary endpoint was all-cause mortality. Individuals masked to baseline data ascertained mortality status using the Social Security Death Index with 100% mortality ascertainment among study participants.
For statistical analyses, the chi-square test was employed for comparison of categorical variables. Between-group comparisons for continuous variables were computed using an independent samples t-test or Mann-Whitney U test as appropriate. A Kaplan-Meier survival curve with log-rank test compared survival rates for smokers versus nonsmokers, according to the presence and severity of CAC. Cox proportional hazard regression reporting hazard ratios (HR) with 95% confidence intervals (95% CI) were used to estimate all-cause mortality adjusting for age, sex, diabetes, hypertension, dyslipidemia, and family history of premature CAD. All Cox models were stratified according to smoking status as well as the presence or absence (Model 1) or severity (Model 2) of CAC. As there was no significant interaction effect between sex and CAC, analyses stratified by sex were not performed. Assumption of proportional hazards was evaluated using Schoenfeld residuals. Statistical analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC). A two-tailed p-value <0.05 was considered statistically significant.
The patients were followed on average for 14.5 years (interquartile range 13.5–15.3). At the time of CAC assessment, 39% were self-reported active smokers. Of 553 deaths that occurred, 270 (16.6%) and 283 (11.3%) were smokers and nonsmokers at the time of CAC scan, respectively. Smokers were more prone to a family history of premature CAD (70.7% vs 65.3%, p<0.001) and diabetes (10.4% vs 8.5%, p=0.04) as compared with nonsmokers (Table 1). Smokers had higher median CAC scores (19 vs 3, interquartile range 0–195, p<0.001) and increased CAC severity (p<0.001 for trend), while nonsmokers were more likely to have a CAC of 0 than smokers (47.8 vs. 38.7%, p<0.001).
Table 1.
Clinical Characteristics of Subjects
| Nonsmokers (N=2,515) | Smokers (N=1,628) | p-Value | |
|---|---|---|---|
| Mean follow-up | 14.6 ± 1.0 | 14.4 ± 1.1 | <0.001 |
| Death events (%) | 283 (11.3) | 270 (16.6) | <0.001 |
| Age (yrs) | 63.4 ± 6.9 | 62.8 ± 6.2 | 0.07 |
| Female n(%) | 1,224 (48.7) | 750 (46.1) | 0.1 |
| Hypertension n(%) | 1,157 (46.0) | 755 (46.4) | 0.82 |
| Diabetes n(%) | 214 (8.5) | 170 (10.4) | 0.04 |
| Dyslipidemia n(%) | 1,599 (63.6) | 1,044 (64.1) | 0.72 |
| Family History of CAD n(%) | 1,643 (65.3) | 1,151 (70.7) | <0.001 |
| Median CAC score (IQR) | 3 (0–85) | 19 (0–195) | <0.001 |
CAC = coronary artery calcium (in Agatston units)
Irrespective of smoking status, higher CAC severity was associated with heightened mortality risk over the course of this study (p<0.001 by log-rank) (Figure). In multivariable Cox hazard regression models, smokers with a CAC of zero had a nearly two-fold (HR 1.73, 95% CI = 1.20–2.50, p=0.003) increased risk of mortality (Table 2, Model 1). In the presence of any CAC, the adjusted risk of mortality was more than three-fold (HR 3.07, 95% CI = 2.32–4.07, p<0.001) higher in nonsmokers, while the adjusted risk of mortality was almost five-fold (HR 4.67, 95% CI = 3.52–6.20, p<0.001) higher among smokers. Similar findings were observed in patients without additional cardiac risk factors (e.g. hypertension, diabetes, dyslipidemia, family history of premature CAD). In both smokers and nonsmokers, the adjusted risk of death appeared to increase incrementally according to the severity of CAC (Table 2, Model 2).
Figure. Cumulative survival among non-smokers and smokers stratified by CAC score.
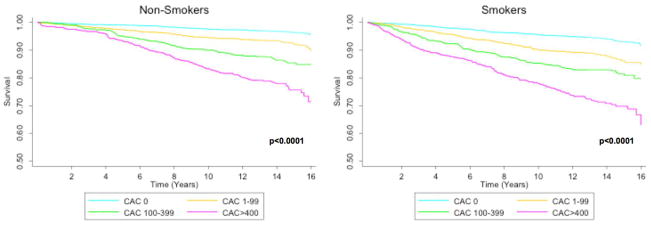
Legend: CAC = coronary artery calcium
Table 2.
Risk of all-cause death among non-smokers and smokers according to the presence and severity of coronary artery calcium
| Unadjusted HR | Adjusted HR* | ||||||
|---|---|---|---|---|---|---|---|
| n | Deaths | Death rate/1,000 person-yrs at Risk | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MODEL 1 | |||||||
| Nonsmokers | |||||||
| CAC=0 | 1202 | 64 | 3.77 | 1.00 (Ref) | 1.00 (Ref) | ||
| CAC>0 | 1313 | 219 | 12.43 | 3.30 (2.49–4.36) | <0.001 | 3.07 (2.32–4.07) | <0.001 |
| Smokers | |||||||
| CAC=0 | 630 | 52 | 6.02 | 1.61 (1.12–2.33) | 0.01 | 1.73 (1.20–2.50) | 0.003 |
| CAC>0 | 998 | 218 | 17.22 | 4.66 (3.53–6.17) | <0.001 | 4.67 (3.52–6.20) | <0.001 |
| MODEL 2 | |||||||
| Nonsmokers | |||||||
| CAC=0 | 1202 | 64 | 3.77 | 1.00 (Ref) | 1.00 (Ref) | ||
| CAC 1–99 | 740 | 81 | 7.95 | 2.11 (1.52–2.93) | <0.001 | 2.12 (1.53–2.95) | <0.001 |
| CAC 100–399 | 318 | 60 | 14.38 | 3.88 (2.73–5.52) | <0.001 | 3.71 (2.60–5.28) | <0.001 |
| CAC ≥400 | 255 | 78 | 24.03 | 6.25 (4.49–8.71) | <0.001 | 4.97 (3.55–6.96) | <0.001 |
| Smokers | |||||||
| CAC=0 | 630 | 52 | 6.02 | 1.61 (1.12–2.33) | 0.01 | 1.73 (1.20–2.49) | 0.004 |
| CAC 1–99 | 442 | 62 | 10.41 | 2.82 (1.99–4.01) | <0.001 | 3.08 (2.16–4.37) | <0.001 |
| CAC 100–399 | 299 | 68 | 18.17 | 4.89 (3.47–6.88) | <0.001 | 4.92 (3.48–6.94) | <0.001 |
| CAC ≥400 | 257 | 88 | 29.69 | 8.16 (5.91–11.27) | <0.001 | 7.42 (5.35–10.31) | <0.001 |
Adjusted for gender, age, hypertension, hyperlipidemia, diabetes, family history of CAD
Overall, we found that across nearly 15 years of follow-up, the presence of CAC remained strongly predictive of all-cause mortality in this cohort of older smokers, even in the absence of other cardiac risk factors. Our findings are consistent with prior studies of shorter duration demonstrating increased mortality in smokers with CAC.2,3 Furthermore, in contrast to the general population for which the absence of CAC (CAC=0) is associated with an excellent prognosis,5 in our study smokers with a CAC=0 remained at an elevated risk of death. As such, for smokers a CAC=0 should not be considered a “negative risk factor.”3
Our study was limited by its observational design. Prior smoking history and smoking intensity as measured by pack years were not obtained. Data were unavailable regarding cause-specific mortality, cardiovascular events, post-test changes in risk factors, downstream pharmacological therapy or smoking cessation. Future long-term prospective cohort studies are needed to address these limitations. However, this is the largest cohort of consecutive patients undergoing CAC screening for which outcome data are available.
Our findings are timely in that many smokers aged 55–80 are poised to undergo annual lung cancer screening by low dose computed tomography (CT).6–8 There is a high correlation between CAC discovered by CT and ECG-gated CAC screening protocols.9 This study proposes a potential benefit in highlighting the presence of any CAC detected by CT, rather than considering it as an “incidental” finding. While further research regarding CAC in lung cancer screening cohorts is clearly needed, our findings indicate that smokers with CAC detected by CT are at elevated risk and warrant early and aggressive cardiac risk factor reduction.
Acknowledgments
FUNDING
Dr Min is the guarantor of the content of this study, including data and analysis. All coauthors contributed to the design, data collection, data analysis, preparation and/or revision of the draft, and approval of the final manuscript.
Research reported in this publication was supported by the Heart Lung and Blood Institute of the National institutes of Health (Bethesda, Maryland) under award number R01 HL115150. Dr. Truong was supported by the NIH (K23HL098370 and L30HL093896). This study was also funded, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging (New York, NY) and the Michael Wolk Foundation (New York, NY).
Footnotes
Relationships with industry. Dr. Min has served on the medical advisory boards of GE Healthcare, Arineta, Astra Zeneca, and Bristol-Myers Squibb; Speakers’ Bureau of GE Healthcare; and received research support from GE Healthcare, Vital Images, and Phillips Healthcare. Dr. Truong receives grant support from St. Jude Medical, American College of Radiology Imaging Network, and Duke Clinical Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howard G, Wagenknecht LE, Burke GL, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA [Internet] 1998;279(2):119–24. doi: 10.1001/jama.279.2.119. [cited 2014 Mar 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9440661. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Raggi P, Callister TQ, Berman DS. Prognostic value of coronary artery calcium screening in asymptomatic smokers and non-smokers. Eur Heart J. 2006;27:968–75. doi: 10.1093/eurheartj/ehi750. [DOI] [PubMed] [Google Scholar]
- 3.McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging [Internet] 2012;5(10):1037–45. doi: 10.1016/j.jcmg.2012.02.017. [cited 2014 Jan 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23058072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol [Internet] 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2407762. [DOI] [PubMed] [Google Scholar]
- 5.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging [Internet] 2009;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [cited 2014 Feb 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19520338. [DOI] [PubMed] [Google Scholar]
- 6.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med [Internet] 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [cited 2014 Feb 21] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21714641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest [Internet] 2013;143(5 Suppl):e78S–92S. doi: 10.1378/chest.12-2350. [cited 2014 Mar 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23649455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyer VA. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med [Internet] 2013 doi: 10.7326/M13-2771. [cited 2014 Feb 13];Available from: http://www.ncbi.nlm.nih.gov/pubmed/24378917. [DOI] [PubMed]
- 9.Xie X, Zhao Y, de Bock GH, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging [Internet] 2013;6(4):514–21. doi: 10.1161/CIRCIMAGING.113.000092. [cited 2014 Feb 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23756678. [DOI] [PubMed] [Google Scholar]


