Abstract
Aims
Diabetic retinopathy (DR) is associated with a higher risk of renal and cardiovascular events. We sought to compare the risk for renal versus cardiovascular (CV) outcomes, stratified by retinopathy severity.
Methods
ACCORD was a randomized trial of people with type 2 diabetes, at high-risk for CV disease. A subgroup (n=3,369 from 71 clinics) had stereoscopic fundus photographs graded centrally. Participants were stratified at baseline to moderate/severe DR or no/mild DR and were monitored for renal and CV outcomes at follow-up visits over 4 years. The composite renal outcome was comprised of serum creatinine doubling, macroalbuminuria, or end-stage renal disease. The composite CV outcome was the ACCORD trial primary outcome. Competing risk techniques were used to estimate the relative risk (RR) of renal versus CV composite outcomes within each DR stratum.
Results
The hazards ratio for doubling of serum creatinine and incident CV event in the moderate/severe DR versus no/mild DR strata were: 2.31 (95%CI: 1.25–4.26) and 1.98 (95%CI: 1.49–2.62), respectively. The RR of the two composite outcomes was highly similar in the no/mild DR stratum (adjusted RR at 4 years for CV versus renal events=0.96, 95%CI:0.72–1.28) and the moderate/severe DR stratum (adjusted RR=0.92, 95%CI:0.64–1.31).
Conclusions
Thus, in people with type 2 diabetes at high risk for cardiovascular disease, incident CV versus renal events was similar, irrespective of the severity of the DR. Further evaluation of the specificity of DR for microvascular versus macrovascular events in other populations is warranted.
Keywords: retinopathy, nephropathy, macrovascular, albuminuria
INTRODUCTION
Diabetic microvascular complications of the eye and kidney are similar in their risk factor profiles and share some mechanisms in their pathogenesis.(Brownlee, 2005; Ravid et al., 1998; Yau et al., 2012) Numerous studies have examined the association of retinopathy with diabetic kidney disease, but the vast majority of these have been of cross sectional design. In type 2 diabetes, the presence versus absence of any diabetic retinopathy (DR) gives an adjusted odds ratio of between 2.5 and 3.3 for the presence of macroalbuminuria (defined as a urine albumin:creatinine ratio > 300ug/mg).(El-Asrar et al., 2001; Romero-Aroca et al., 2010) Similar results have been obtained in studies examining the association of DR with decreased estimated glomerular filtration rate (eGFR), defined as <60ml/min/1.73m2.(Mottl et al., 2012; Penno et al., 2012) In a step-wise fashion, more severe retinopathy is increasingly associated with diabetic kidney disease. The presence of proliferative retinopathy carries an odds ratio for concurrent macroalbuminuria or decreased eGFR as high as 17-fold.(El-Asrar et al., 2001; Mottl et al., 2012)
A strong relationship between DR and cardiovascular (CV) disease has also been well established. In prospective observational cohorts of people with type 2 diabetes, the presence of any DR versus no DR is associated with an adjusted hazard ratios of 2.3 for stroke,(Cheung, Rogers, et al., 2007) 2.5 for heart failure,(Cheung et al., 2008) and 2.2–3.3 for cardiovascular death.(Cheung, Wang, et al., 2007; Liew et al., 2009) Longitudinal studies have found more severe retinopathy to result in adjusted hazard ratios of 1.7–3.8 for combined fatal and nonfatal ischemic CV endpoints.(Cheung, Wang, et al., 2007; Gerstein et al., 2012; Gimeno-Orna et al., 2009; Targher et al., 2008)
The similarity in risk estimates for diabetic kidney and CV outcomes in the setting of retinopathy might suggest a similar or shared pathogenesis for micro- and macrovascular complications of diabetes. Although an overlap in their pathobiology likely exists, there are also data to suggest there is some distinction. The association between retinopathy and CV events has been noted to be of similar magnitude, regardless of diabetes type,(Kramer et al., 2011) however, the link between retinopathy and nephropathy is considered to be significantly greater in type 1 versus type 2 diabetes.(Wolf et al., 2007) It has been suggested that kidney disease in type 2 diabetes is more heterogeneous than in type 1 diabetes, with a greater prevalence of tubulointerstitial versus glomerular lesions,(Fioretto et al., 1996) and a higher prevalence of normoalbuminuric chronic kidney disease (NA-CKD).(Macisaac & Jerums, 2011) Whether these distinctions in the clinical and pathologic characteristics in type 2 diabetes explain the weaker association of retinopathy with kidney disease than in type 1 diabetes is unknown.
Given the uncertainty over the specificity of DR for renal versus CV disease, we sought to compare the relative incidence of these events according to the severity of retinopathy. Detailed analyses of the association of DR with incident CV events in the ACCORD trial have been previously published.(Gerstein et al., 2012) Given the assumption that DR has greater overlap in pathogenesis with other diabetic microvascular complications, such as kidney disease, we hypothesized that kidney outcomes should occur at a greater relative frequency compared to CV outcomes in participants with more severe retinopathy. A simplistic approach to this question could include fitting separate survival models for kidney and CV outcomes, using the resulting hazard ratios to compare associations with DR, however, such a comparison ignores the increased risk of CV events associated with the development of chronic kidney disease (CKD).(Hemmelgarn et al., 2010; Weiner et al., 2004) In circumstances where one event modifies the risk for another event, treating the outcomes as competing risks can be more appropriate.(Noordzij et al., 2013) Therefore in the present study, we used competing risks techniques to compare the relative incidence of CV and renal outcomes, focusing on which event occurs first in subgroups of participants from the ACCORD trial, stratified by severity of DR.
SUBJECTS, MATERIALS AND METHODS
Study Population
The design of the ACCORD trial and ACCORD Eye Study have been previously reported.(Buse et al., 2007; Chew et al., 2007) Briefly, middle-aged and elderly people with type 2 diabetes, hemoglobin A1c (HbA1c) levels ≥ 7.5% and known CV disease or additional CV risk factors were recruited from 77 clinical centers. Exclusion criteria included BMI > 45kg/m2 and SCr > 132.6 µmol/L (1.5mg/dL). Participants were randomized to the intensive glucose-lowering trial as well as an intensive blood pressure-lowering or fibrate trial. The primary trial outcome consisted of a composite of fatal and nonfatal CV events and all-cause mortality. Events were ascertained every 4 months. The mean follow-up time for cardiovascular events and mortality was 4.7 and 5.0 years, respectively. ACCORD participants who did not have a history of proliferative DR treated with laser photocoagulation or vitrectomy were eligible to also participate in the ACCORD Eye Study. All Eye Study participants provided written informed consent for both the ACCORD trial and the Eye Study.
Measurements
The baseline eye assessment consisted of standardized eye examination with fundus photography of seven standard stereoscopic fields. Fundus photographs were evaluated centrally by trained graders according to the modified version of the ETDRS Final Diabetic Retinopathy Severity Scale, which combines the severity levels from both eyes.(Early Treatment Diabetic Retinopathy Study (ETDRS) criteria, 1991) The severity was classified as no retinopathy, mild nonproliferative diabetic retinopathy (NPDR), moderate NPDR and severe retinopathy (severe NPDR or proliferative retinopathy).
Participants were evaluated every four months to obtain information on study outcomes, perform clinical examination and to collect fasting blood samples.(Buse et al., 2007) Single, random urine specimens were collected every 24 months for urine albumin and creatinine measurements.
Statistical Analysis
Statistical analyses were performed using SAS software (Version 9.2 for Windows) and the R Statistical Computing Environment.(R Core Team, 2012) Descriptive statistics were calculated and compared according to the severity of baseline retinopathy using Analysis of Variance (ANOVA), Kruskal-Wallis tests, or Chi-squared tests, as appropriate. Microalbuminuria was defined as a urine albumin creatinine ratio (UACR) of ≥ 30µg/mg and macroalbuminuria as a UACR of ≥ 300 µg/mg.("Standards of medical care in diabetes--2011," 2011) Estimated GFR (eGFR) was calculated using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation.(Levey et al., 2009) ESRD was defined as an eGFR <15ml/min/1.73m2 or if a participant was on dialysis or received renal transplantation.
Cox proportional hazards (PH) regression models were used to examine the effect of retinopathy (classified as no/mild DR versus moderate/severe DR) on 1) the composite renal outcome: doubling of SCr or incident macroalbuminuria (≥300µg/mg) or ESRD or 2) the CV composite outcome (which was the same as the primary outcome of the ACCORD clinical trial): incident myocardial infarct or stroke and CV death. Secondary outcomes included each of the individual outcomes which comprised the composite outcomes.
Given the current debate concerning the suitability of doubling of SCr and incident macroalbuminuria as surrogate endpoints for ESRD,(Lambers Heerspink et al., 2011) we defined these outcomes to exclude transient changes in kidney function. We required that a doubling of SCr was observed at two consecutive study visits, with SCr levels remaining above a 100% increase for the remainder of available follow-up. We imposed a similar requirement for incident macroalbuminuria, only considering outcomes where the UACR remained above 300 µg/g at all subsequent follow-up visits. Because the UACR was only measured at yearly follow-up visits in ACCORD, a significant proportion of incident macroalbuminuria cases were only observed at the final study visit (72/129=55.8%). Because of the relatively low overall incidence of macroalbuminuria in ACCORD, we did not remove such cases from this revised definition of incident macroalbuminuria.
For the composite outcomes, we included the following factors as covariates in the Cox regression models: age (modeled as a quadratic polynomial), gender, non-white ethnicity, BMI, SBP, hemoglobin A1C, diabetes duration, history of a prior CV event, participation in the lipid trial, allocation to the intensive glycemia group, allocation to the intensive blood pressure group, allocation to fenofibrate, and indicators to adjust for effects across the 7 clinical center networks in ACCORD. We evaluated the PH assumption using hypothesis tests based on the scaled Schoenfeld residuals.(Grambsch & Therneau, 1994) We did not observe any strong deviations from PH for either retinopathy status or any of the covariates considered.
We used the competing risk regression framework of Fine and Gray(Fine & Gray, 1999) to compare the cumulative incidence of kidney disease outcomes versus CV outcomes within each DR stratum. In the competing risk regression framework, we utilized a stratified version of the Fine and Gray model so as not to assume that the differences in cumulative incidence functions across DR strata were time invariant. Comparisons between cumulative incidence functions at a specific point in time (4 years of follow-up) are presented as relative risks, as described in Zhang and Fine. We used the approach of Zhang and Zhang(Zhang & Zhang, 2011) to produce direct adjusted estimates of the respective cumulative incidence functions, using the same set of covariates included in the Cox regression models described above.
RESULTS
Of the 3,472 participants recruited for the ACCORD Eye Study, 3,210 had complete baseline covariate data and complete follow-up for both kidney and cardiovascular outcomes. At baseline, 1,628, 587, 955 and 40 participants had no, mild, moderate, and severe DR, respectively. Sociodemographic and clinical characteristics of the participants, stratified by category of DR severity are displayed in Table 1. The prevalence of macular edema did not differ amongst the four categories of DR. The majority of participants were male and in their sixth decade of age. The mean duration of diabetes increased with worsening severity of DR from 6 years in those with no DR to 15 years in those with severe DR. Similarly, both systolic and diastolic blood pressures, HbA1c, BMI and UACR tended to be increasingly higher with worse categories of retinopathy severity. ACEI/ARB use increased with increasing severity of DR from 63.4% in those without DR to 80% in those with severe DR. A similar trend is observed for other clinical characteristics such as the prevalence of micro and macroalbuminuria and previous CV event. Estimated GFR (eGFR) was not statistically different between DR categories, nor were randomization to the three treatment arms of the trial. For individual renal and CV outcomes, participants in the moderate/severe DR status had unadjusted hazard ratios (HR) of 2.58, 2.31, and 1.98 for incident macroalbuminuria, doubling of SCr, and nonfatal CV events, respectively (Table 2). We also examined the relationship of severity of DR with incidence of non-CV-related deaths. There was a trend for a greater HR for cardiovascular and nonvascular death with worse DR, but these did not reach statistical significance.
Table 1.
Baseline sociodemographic and clinical characteristics of 3,210 participants of the ACCORD Eye substudy, according to retinopathy status.
| Baseline retinopathy status | |||||
|---|---|---|---|---|---|
| Baseline characteristics mean(95% CI) |
None n=1628 |
Mild n=587 |
Moderate n=955 |
Severe n=40 |
p-value |
| Age, years | 61(57–65) | 61(57–65) | 62(57–66) | 60(56–63) | 0.04 |
| Female gender* | 632(38.8) | 216(36.8) | 369(38.6) | 10(25.0) | 0.3 |
| Non-white ethnicity* | 445(27.3) | 156(26.6) | 351(36.8) | 18(45.0) | <0.0001 |
| Diabetes duration, years yyryears | 6(4–10) | 9(5–14) | 13(9–18) | 15(11–21) | <0.0001 |
| Hemoglobin A1C, % | 8.2 ± 1.0 | 8.2 ± 1.0 | 8.4 ± 1.1 | 8.7 ± 1.3 | <0.0001 |
| Blood pressure, mmHg | |||||
| Systolic | 133 ± 16.3 | 134.9 ± 17.1 | 137.5 ± 17.4 | 140.7 ± 17.7 | <0.0001 |
| Diastolic | 75.7 ± 10.3 | 74.9 ± 10.5 | 73.8 ± 10.9 | 74.8 ± 12.4 | 0.0003 |
| BMI, kg/m2 | 32.8 ± 5.4 | 32.5 ± 5.5 | 32.0 ± 5.5 | 32.4 ± 5.9 | 0.0070 |
| Macular edema* † | 117(7.9) | 45(8.2) | 68(7.8) | 5(12.8) | 0.7 |
| Previous CV event* cccardiovascular event | 495(30.4) | 186(31.7) | 331(34.7) | 19(47.5) | 0.02 |
| Serum creatinine(SCr), mg/dl | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.0005 |
| eGFR, ml/min/1.73m2 | 90.9(77.3–97.8) | 90.5(74.9–98.5) | 90.2(72.5–97.5) | 85.6(68.6–98.3) | 0.2 |
| UACR, µg/mg | 10.2(5.9–26.3) | 10.5(5.9–27) | 17(7.7–64.3) | 41(12.9–202.9) | <0.0001 |
| Albuminuria* | <0.0001 | ||||
| None | 1264(77.6) | 449(76.5) | 579(60.6) | 15(37.5) | |
| Microalbuminuria | 307(18.9) | 108(18.4) | 289(30.3) | 18(45) | |
| Macroalbuminuria | 57(3.5) | 30(5.1) | 87(9.1) | 7(17.5) | |
| ACEI/ARB therapy* | 1025(63.4) | 406(69.4) | 675(71) | 32(80) | 0.0001 |
| Intensive glycemia* control | 820(50.4) | 267(45.5) | 490(51.3) | 16(40) | 0.07 |
| BP tria*l | 713(43.8) | 256(43.6) | 455(47.6) | 24(60) | 0.05 |
| Intensive | 378(53) | 125(48.8) | 224(49.2) | 16(66.7) | 0.2 |
| Lipid trial* | 915(56.2) | 331(56.4) | 500(52.4) | 16(40) | 0.05 |
| Fenofibrate | 472(51.6) | 158(47.7) | 253(50.6) | 5(31.2) | 0.3 |
Results displaced as n(%);
According to number of participants with at least one affected eye - denominator excludes participants with missing data
eGFR=estimated glomerular filtration rate calculated by the CKD-Epi equation; UACR=urine albumin:creatinine ratio; ACEI=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; microalbuminuria ≥ 30µg/mg; macroalbuminuria ≥300µg/mg
Table 2.
Renal, macrovascular and composite outcomes stratified by baseline severity of retinopathy in 3,210 participants of the ACCORD Eye substudy.
| Outcome | No/Mild Retinopathy Frequency/n(%) |
Moderate/Severe Retinopathy Frequency/n(%) |
Unadjusted HR(95% CI) |
|---|---|---|---|
| Sustained incident macroalbuminuriaa | 61/2128(2.9) | 68/901(7.5) | 2.58(1.83–3.65) |
| Sustained doubling of Baseline serum creatinine | 20/2215(0.9) | 21/995(2.1) | 2.31(1.25–4.26) |
| End stage renal diseaseb | 48/2215(2.2) | 23/995(2.3) | 1.05(0.64–1.73) |
| Incident cardiovascular eventc | 103/2215(4.7) | 91/995(9.1) | 1.98(1.49–2.62) |
| Cardiovascular death | 24/2215(1.1) | 14/995(1.4) | 1.24(0.64–2.39) |
| Nonvascular death | 36/2215(1.6) | 19/995(1.9) | 1.15(0.66–2.00) |
Urine albumin:creatinine ≥ 300µg/mg, excluding individuals with baseline macroalbuminuria
Estimated glomerular filtration rate < 15ml/min/1.73m2 using the Chronic Kidney Disease-Epi equation or requiring dialysis or renal transplantation
Nonfatal myocardial infarction or stroke
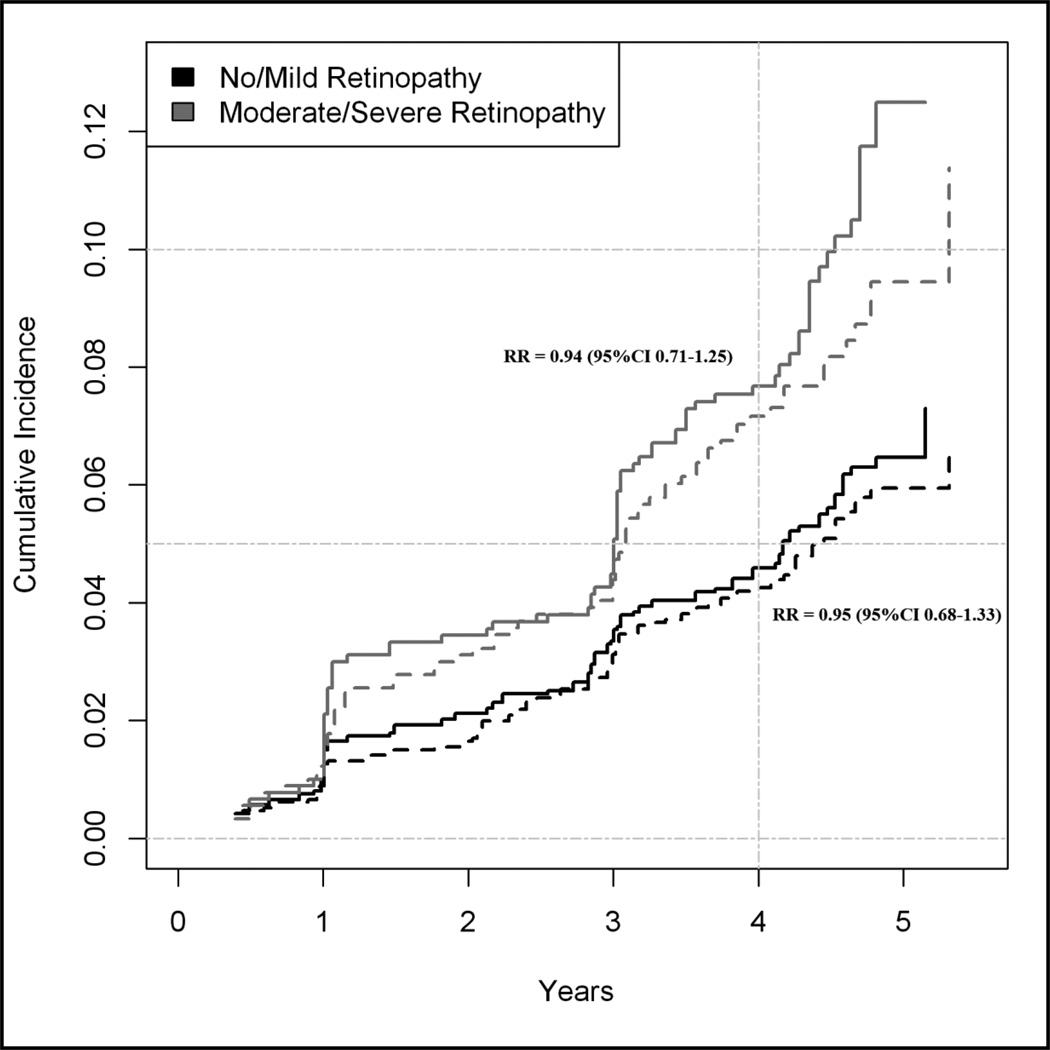
Figure 1 displays estimates of the cumulative incidence curves for the competing composite renal and CV outcomes, stratified by retinopathy status. Within each DR strata, the adjusted RRs of the CV versus renal composite outcome were not statistically different: 0.96 (95% CI: 0.72–1.28) for no/mild DR and 0.92 (95% CI: 0.64–1.31) for moderate/severe DR (Table 3). Results from competing risks modeling of the individual components of the composite CV and renal outcomes are also listed in table 3.
Figure 1.
Cumulative incidence curves for the competing risks of the renal composite outcome* (solid lines) and ACCORD primary outcome† (dashed lines) during follow-up according to baseline retinopathy status. Plot excludes individuals with macroalbuminuria at baseline.*Renal composite outcome includes sustained doubling of serum creatinine, incident macroalbuminuria (≥300µg/mg), or ESRD. †ACCORD primary outcome includes incident myocardial infarct or stroke or CV death.
Table 3.
Cumulative incidence at 4 years for sustained incident macroalbuminuria or sustained doubling of serum creatinine versus the risk for competing cardiovascular events, stratified by retinopathy severity
| No/Mild Retinopathy | Moderate/Severe Retinopathy | |||||
|---|---|---|---|---|---|---|
| Outcome | N | CIF(95% CI) | RR(95% CI) | N | CIF(95% CI) | RR(95% CI) |
| Incident macroalbuminuria | 2,128* | 0.020(0.014–0.026) | REF | 901* | 0.052(0.039–0.068) | REF |
| Incident cardiovascular event | 0.039(0.032–0.049) | 2.01(1.39–2.91) | 0.067(0.052–0.085) | 1.29(0.90–1.88) | ||
| Cardiovascular death | 0.004(0.002–0.008) | 0.20(0.09–0.42) | 0.005(0.002–0.012) | 0.10(0.03–0.26) | ||
| Nonvascular death | 0.012(0.008–0.017) | 0.61(0.37–1.00) | 0.015(0.008–0.025) | 0.29(0.16–0.54) | ||
| Doubling of serum creatinine | 2,215 | 0.008(0.005–0.012) | REF | 995 | 0.017(0.010–0.027) | REF |
| Incident cardiovascular event | 0.043(0.035–0.052) | 5.40(3.23–9.04) | 0.080(0.063–0.099) | 4.70(2.76–8.00) | ||
| Cardiovascular death | 0.006(0.003–0.010) | 0.77(0.37–1.58) | 0.005(0.002–0.012) | 0.31(0.12–0.86) | ||
| Nonvascular death | 0.012(0.008–0.017) | 1.49(0.80–2.75) | 0.016(0.009–0.025) | 0.92(0.46–1.86) | ||
| Composite renal outcome | 2,128* | 0.046(0.037–0.056) | REF | 901* | 0.076(0.060–0.095) | REF |
| Composite macrovascular outcome | 0.043(0.035–0.053) | 0.94(0.71–1.25) | 0.073(0.057–0.091) | 0.95(0.68–1.33) | ||
| Adjusted composite renal outcome† | 2,128* | 0.047(0.037–0.056) | REF | 901* | 0.075(0.057–0.094) | REF |
| Adjusted composite macrovascular outcome† | 0.045(0.035–0.054) | 0.96(0.72–1.28) | 0.069(0.051–0.087) | 0.92(0.64–1.31) | ||
Excluding individuals with macroalbuminuria at baseline
(CIF) Cause-specific cumulative incidence function
(RR) Relative-risk of competing events within each DR stratum
Adjusted for age, gender, non-white ethnicity, BMI, SBP, hemoglobin A1C, diabetes duration, history of a prior CV event, participation in the lipid trial, allocation to the intensive glycemia group, allocation to the intensive blood pressure group, allocation to fenofibrate, and clinical center networks
DISCUSSION
The novel finding in this study is that the incidence of CV versus renal endpoints was not different within strata of retinopathy status, indicating a similar strength in the association of retinopathy with renal versus CV outcomes. Comparison of kidney and CV outcomes is complex, as kidney disease is a strong risk factor for CV events(Hemmelgarn et al., 2010) and death often occurs prior to the progression of renal failure.(Adler et al., 2003) The use of a competing risk model takes both of these issues into consideration and although there did appear to be a trend toward greater kidney than CV outcomes in the more severe retinopathy stratum, this did not yield statistical significance. This may be due, in part, to the diabetic phenotype assembled by the inclusion criteria of ACCORD, which selected for older, type 2 diabetic persons at high risk for CV disease and low risk for severe kidney disease.(Buse et al., 2007) Whether this would hold true in type 1 diabetes and/or younger cohorts requires further investigation.
In type 2 diabetes, retinopathy and CV disease are more highly prevalent in those with low eGFR (<60ml/min/1.73m2) if they have the albuminuric rather than normoalbuminuric phenotype.(Penno et al., 2012; Rigalleau et al., 2007; Thomas et al., 2009) Macroalbuminuria without low eGFR also appears to be more tightly linked to retinopathy than NA-CKD, but the same may not hold true for CV events. In a cross sectional Japanese study of type 2 diabetes, participants with no albuminuria but decreased eGFR < 60ml/min/1.73m2 had a lower odds of having retinopathy than people with macroalbuminuria and eGFR > 60ml/min/1.73m2 (OR=0.51; 95%CI 0.27–0.97).(Ito et al., 2010) Conversely, there was no real difference in history of coronary or cerebrovascular disease between these two groups, OR=1.70 (95%CI 0.83–3.47) and OR=0.75 (95%CI 0.39–1.41), respectively. Extrapolating this information to the present study, one would have expected a greater RR for incident macroalbuminuria versus cardiovascular endpoints in the moderate/severe retinopathy stratum compared to the no/mild retinopathy stratum. The similar risk of incident macroalbuminuria versus cardiovascular events between the two DR strata in this study may be due to the inclusion criteria of ACCORD which selected for people with preexisting or at extremely high risk for CVD. Such high CVD risk may overpower any increased association between DR and macroalbuminuria in a more general population.
Another finding underscored by our analyses is that with use of a longitudinal study design and robust endpoints for diabetic kidney disease, more severe retinopathy remains significantly predictive of greater risk for subsequent renal outcomes. Multiple previous studies have demonstrated the association between diabetic complications of the eye and kidney, however, most have been cross sectional(Cruickshanks et al., 1993; Grunwald et al., 2012; Penno et al., 2012; Romero-Aroca et al., 2010) and longitudinal studies have rarely used hard clinical renal endpoints. Our hazard ratios of more severe retinopathy for renal endpoints was similar to those reached in cross sectional analyses and also those using less robust definitions for kidney outcomes. In agreement with our study, the Early Treatment for Diabetic Retinopathy Study (ETDRS) also found that over five years of follow-up, people with type 2 diabetes and baseline severe DR versus mild/moderate DR at baseline have a 60% higher risk for subsequent end-stage renal disease (ESRD) requiring renal replacement therapy.(Cusick et al., 2004)
It is possible that ACCORD’s very robust definition for CV events underestimated the association between retinopathy and CV disease. ACCORD did not include hospitalization for CHF or incident angina in the primary outcome. The same holds true with respect to our definition of renal outcomes, as we did not include incident microalbuminuria or sustained increases creatinine that were smaller than doubling. Hence, a number of design issues may be skewing our results towards a stronger association between DR and either CV or renal events.
Also limiting our analyses is that competing risks models approach the issue of specificity for renal and CV outcomes by asking which event happens first. An ideal analysis would have been the use of a multi-state model,(Putter et al., 2007) whereby renal and non-fatal CV events could be viewed as intermediate outcomes. This would permit evaluating how the risk of CV events changes if they are preceded by progression of kidney disease, or vice versa. However, over 90% of the renal and CV events observed during the primary follow-up period for the ACCORD trial were “first events”, and so there are an insufficient number of events with which to estimate such a multi-state model. The question of specificity of DR should likely be revisited once data from the extended follow-up of ACCORD participants is available.(" Action to control cardiovascular risk in diabetes (accord) follow-up study,")
The strengths of this study include its large number of participants, longitudinal study design, standardized central reading of fundus photographs, systematic ascertainment of CV events and robust renal outcomes. Although albuminuria measurements were taken from a single, random urine specimen, we attempted to impose criteria such that the macroalbuminuria threshold had to be persistent throughout subsequent measurements. However, we did not exclude events where a UACR > 300 µg/g was only observed at the final study visit (see METHODS). Thus while we attempted to restrict the definition of macroalbuminuria so that it reflected true pathologic kidney disease, we cannot rule out that a proportion of these events simply reflect a transient, reversible change in albuminuria. Similarly, doubling of baseline SCr had to be maintained throughout subsequent visits in order to qualify as a renal outcome, and hence fluctuations due to changes in diet, medications or volume status likely did not diminish the reliability of this endpoint.
To our knowledge, this is the first study to quantify and compare the strength of association of retinopathy with incident renal versus CV events. Our finding that more severe retinopathy is equally specific for incident macroalbuminuria, doubling of SCr, and cardiovascular endpoints suggests these three entities may have overlapping pathophysiologic mechanisms. Whether there is a distinction between micro and macrovascular diabetic complications in other populations requires further study.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23DK093804 and NHLBI ACCORDion and PAR, NCATS CTSA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to report.
AKM designed the analyses, interpreted the results and wrote the manuscript. NP designed the analyses, performed all statistical analyses, interpreted the results and reviewed and contributed to the writing of the manuscript. CVF, FIB, EC, WTA, CG, US, JB designed the study, collected the data, reviewed and contributed to the writing of the manuscript. AKM and NP are the guarantors of the manuscript.
Contents of this manuscript appeared in abstract/poster form at the 74th Scientific Sessions of the American Diabetes Association in June 2013, Chicago, IL.
REFERENCES
- Action to control cardiovascular risk in diabetes (accord) follow-up study. Retrieved Jan 28, 2014, http://www.wakehealth.edu/Research/Gerontology-and-Geriatrics/Kulynych-Center/ACCORDIAN.htm.
- Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: The united kingdom prospective diabetes study (ukpds 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Buse JB, Bigger JT, Byington RP, et al. Action to control cardiovascular risk in diabetes (accord) trial: Design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Cheung N, Rogers S, Couper DJ, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke. 2007;38(2):398–401. doi: 10.1161/01.STR.0000254547.91276.50. [DOI] [PubMed] [Google Scholar]
- Cheung N, Wang JJ, Klein R, et al. Diabetic retinopathy and the risk of coronary heart disease: The atherosclerosis risk in communities study. Diabetes Care. 2007;30(7):1742–1746. doi: 10.2337/dc07-0264. [DOI] [PubMed] [Google Scholar]
- Cheung N, Wang JJ, Rogers SL, et al. Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol. 2008;51(16):1573–1578. doi: 10.1016/j.jacc.2007.11.076. [DOI] [PubMed] [Google Scholar]
- Chew EY, Ambrosius WT, Howard LT, et al. Rationale, design, and methods of the action to control cardiovascular risk in diabetes eye study (accord-eye) Am J Cardiol. 2007;99(12A):103i–111i. doi: 10.1016/j.amjcard.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Ritter LL, Klein R, et al. The association of microalbuminuria with diabetic retinopathy. The wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 1993;100(6):862–867. doi: 10.1016/s0161-6420(93)31562-9. [DOI] [PubMed] [Google Scholar]
- Cusick M, Chew EY, Hoogwerf B, et al. Risk factors for renal replacement therapy in the early treatment diabetic retinopathy study (etdrs), early treatment diabetic retinopathy study report no. 26. Kidney Int. 2004;66(3):1173–1179. doi: 10.1111/j.1523-1755.2004.00869.x. [DOI] [PubMed] [Google Scholar]
- El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, et al. Retinopathy as a predictor of other diabetic complications. Int Ophthalmol. 2001;24(1):1–11. doi: 10.1023/a:1014409829614. [DOI] [PubMed] [Google Scholar]
- Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in niddm patients with microalbuminuria. Diabetologia. 1996;39(12):1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- Fundus photographic risk factors for progression of diabetic retinopathy. Etdrs report number 12. Early treatment diabetic retinopathy study research group. Ophthalmology. 1991;98(5 Suppl):823–833. [PubMed] [Google Scholar]
- Gerstein HC, Ambrosius WT, Danis R, et al. Diabetic retinopathy, its progression and incident cardiovascular events in the accord trial. Diabetes Care. 2012 doi: 10.2337/dc12-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno-Orna JA, Faure-Nogueras E, Castro-Alonso FJ, et al. Ability of retinopathy to predict cardiovascular disease in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103(10):1364–1367. doi: 10.1016/j.amjcard.2009.01.345. [DOI] [PubMed] [Google Scholar]
- Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- Grunwald JE, Ying GS, Maguire M, et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the chronic renal insufficiency cohort [cric] study) Am J Cardiol. 2012;110(2):246–253. doi: 10.1016/j.amjcard.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- Ito H, Takeuchi Y, Ishida H, et al. High frequencies of diabetic micro- and macroangiopathies in patients with type 2 diabetes mellitus with decreased estimated glomerular filtration rate and normoalbuminuria. Nephrol Dial Transplant. 2010;25(4):1161–1167. doi: 10.1093/ndt/gfp579. [DOI] [PubMed] [Google Scholar]
- Kramer CK, Rodrigues TC, Canani LH, et al. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: Meta-analysis of observational studies. Diabetes Care. 2011;34(5):1238–1244. doi: 10.2337/dc11-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers Heerspink H, Perkovic V, De Zeeuw D. Is doubling of serum creatinine a valid clinical 'hard' endpoint in clinical nephrology trials? Nephron Clin Pract. 2011;119(3):195–199. doi: 10.1159/000327614. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew G, Wong TY, Mitchell P, et al. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95(5):391–394. doi: 10.1136/hrt.2008.146670. [DOI] [PubMed] [Google Scholar]
- Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens. 2011;20(3):246–257. doi: 10.1097/MNH.0b013e3283456546. [DOI] [PubMed] [Google Scholar]
- Mottl AK, Kwon KS, Garg S, et al. The association of retinopathy and low gfr in type 2 diabetes. Diabetes Res Clin Pract. 2012;98(3):487–493. doi: 10.1016/j.diabres.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordzij M, Leffondre K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- Penno G, Solini A, Zoppini G, et al. Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: The renal insufficiency and cardiovascular events (riace) italian multicenter study. Diabetes Care. 2012;35(11):2317–2323. doi: 10.2337/dc12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2012 Retrieved May 1, 2013, from http://www.R-project.org/
- Ravid M, Brosh D, Ravid-Safran D, et al. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158(9):998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- Rigalleau V, Lasseur C, Raffaitin C, et al. Normoalbuminuric renal-insufficient diabetic patients: A lower-risk group. Diabetes Care. 2007;30(8):2034–2039. doi: 10.2337/dc07-0140. [DOI] [PubMed] [Google Scholar]
- Romero-Aroca P, Sagarra-Alamo R, Baget-Bernaldiz M, et al. Prevalence and relationship between diabetic retinopathy and nephropathy and its risk factors in the north-east of spain, a population-based study. Opthalmic Epidemiology. 2010;17(4):251–265. doi: 10.3109/09286586.2010.498661. [DOI] [PubMed] [Google Scholar]
- Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabet Med. 2008;25(1):45–50. doi: 10.1111/j.1464-5491.2007.02327.x. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Macisaac RJ, Jerums G, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment co-existing with niddm [nefron] 11) Diabetes Care. 2009;32(8):1497–1502. doi: 10.2337/dc08-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- Wolf G, Muller N, Mandecka A, et al. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin Nephrol. 2007;68(2):81–86. doi: 10.5414/cnp68081. [DOI] [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang M. Sas macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Computer Methods and Programs in Biomedicine. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]