Abstract
Sleep apnea and obesity are strongly associated and both increase the risk for coronary artery disease. Several cross-sectional studies have reported discrepant results regarding the role that obesity plays in the relation between sleep apnea and coronary artery calcium (CAC), a marker of subclinical coronary disease. The present study investigated the association between sleep apnea and presence of CAC in a community cohort of middle-aged men and women without preexisting cardiovascular disease, stratified by body mass index (BMI) (BMI < 30 versus ≥ BMI 30). Participants underwent electron beam computed tomography to measure CAC and underwent home sleep testing for sleep apnea. The presence of CAC was defined as an Agatston score > 0. Sleep apnea was analyzed categorically using apnea hypopnea index (AHI). The sample was comprised of primarily males (61%) and Caucasians (56%), with a mean age of 61 years. The prevalence of CAC was 76%. Among participants with a BMI < 30 (n = 139), AHI ≥ 15 (compared to AHI < 5) was associated with a 2.7-fold odds of having CAC, but the effect only approached significance. Conversely, in participants with a BMI ≥ 30, sleep apnea was not independently associated CAC. In conclusion, sleep apnea is independently associated with early atherosclerotic plaque burden in non-obese individuals.
Keywords: sleep apnea, coronary artery calcium, obesity
The present study investigated the association of sleep apnea and subclinical coronary atherosclerosis as defined by coronary artery calcium (CAC) scoring in a community cohort of middle-aged men and women without preexisting cardiovascular disease. We anticipate that the association between sleep apnea and CAC will be masked or minimized by the presence of obesity based on the known effects of obesity on coronary atherosclerotic burden1,2. Data were stratified by body mass index (BMI) (BMI < 30 versus BMI ≥ 30). We hypothesized that the effect of sleep apnea on CAC presence would be stronger among non-obese relative to obese participants.
METHODS
Participants were recruited from a prospective, community-based cohort study, Heart Strategies Concentrating On Risk Evaluation (Heart SCORE), designed to investigate racial disparities in cardiovascular risk in 2000 participants3. Heart SCORE eligibility criteria included age 45–75 years, residence in the greater Pittsburgh metropolitan area, ability to undergo baseline and annual follow-up visits, and absence of a known comorbidity expected to limit life expectancy to < 5 years. Participants were classified into 1 of 3 groups: preexisting cardiovascular disease (prior myocardial infarction, coronary revascularization, or stroke); moderate/high (>10%) probability of cardiovascular disease event in next 10 years; or low probability of cardiovascular disease events, based on Framingham risk score profiles4. For the present analysis, we examined baseline data and excluded participants with preexisting cardiovascular disease and/or missing data on cardiovascular risk factors. Electron beam computed tomography (EBCT) scans and home sleep testing for sleep apnea were not performed on all participants, thus CAC and sleep apnea data were available for a total of 324 participants. We included participants who underwent EBCT and home sleep testing within 24 months of each other (n = 276) and excluded participants who had a change in BMI ± 2 within the period between EBCT and home sleep testing assessments (n = 24), in order to account for potentially altered CAC profiles. The final sample for the present analysis included 252 participants. Participants included in the present analysis had a greater proportion of males and those with diabetes and/or hypertension as compared to the Heart SCORE population. The Heart SCORE protocol was approved by the institutional review board at the University of Pittsburgh Medical Center, and all participants provided written informed consent.
During the baseline visit, detailed demographic and medical histories were collected. Height and weight were measured to calculate BMI. The medical history inquired about a history of previously diagnosed hypertension, hyperlipidemia, and diabetes mellitus, as well as current medications. Resting blood pressure measurement was based on the average of 2 seated blood pressures by trained nurses. Laboratory assessment of lipoprotein levels were performed on venous blood draw in the fasting state. Age, gender, race/ethnicity, and smoking status (current/past smoker: yes/no) were self-reported.
Dyslipidemia was defined as having blood lipid concentrations within the following parameters, HDL cholesterol ≤ 40 mg/dL, total cholesterol ≥ 200 mg/dL, or self-reported treatment for dyslipidemia. Hypertension was defined as diastolic blood pressure (DBP) ≥ 90 mm Hg and/or systolic blood pressure (SBP) ≥ 140 mm Hg, or self-reported usage of antihypertensive medication. Diabetes was defined as a self-reported diagnosis, current use of anti-diabetic medication, or fasting glucose ≥ 110 mg/dL.
Sleep apnea was assessed with a previously validated portable monitor that measures airflow and snoring via a nasal pressure signal (ApneaLink, ResMed Corp)5. An apnea was defined as a decrease in airflow of ≥ 80% from baseline for ≥ 10 seconds. A hypopnea was defined as a decrease in airflow between > 30% and < 80% from baseline for ≥ 10 seconds. Sleep apnea was analyzed categorically using the apnea-hypopnea index (AHI): 0–4, 5–14, and ≥ 15 events/hour.
Electron beam computed tomography image acquisition was obtained with an Imatron C150 scanner (GE Imatron Inc, South San Francisco, CA). To evaluate the coronary arteries, 30–40 contiguous 3-mm thick transverse images were obtained from the level of the aortic root to the apex of the heart during maximal breath holding. Images were acquired by using electrocardiogram triggering (80% of the RR interval) of 100 millisecond exposure during the same phase of the cardiac cycle. Calcium scores were calculated by the Agatston method, based on the detection of ≥ 3 contiguous pixels > 130 Hounsfield units6. Presence of CAC was defined as an Agatston score > 0.
Statistical Analysis
Differences in demographic characteristics were compared between the 3 sleep apnea groups (AHI < 5, AHI 5–14, AHI ≥ 15) using χ2 test for categorical variables and analysis of variance with post hoc comparison for continuous variable. The non-parametric Kruskall-Wallis test was used to compare the median value of CAC scores among the sleep apnea groups. Frequencies of the presence of CAC were calculated among the 3 groups in the overall population and within non-obese and obese (BMI < 30 versus BMI ≥ 30). Multivariate logistic regression controlling for age, gender, race/ethnicity (Caucasian and other versus African American), smoking status, diabetes, hypertension, dyslipidemia, and BMI was used to calculate the odds ratio of the presence of CAC among individuals with no sleep apnea (AHI <5: reference group), mild sleep apnea (AHI 5–14), and moderate to severe sleep apnea (AHI ≥ 15). SPSS statistical software (SPSS Inc, Chicago, IL) for Windows 20 was used for all statistical analyses.
RESULTS
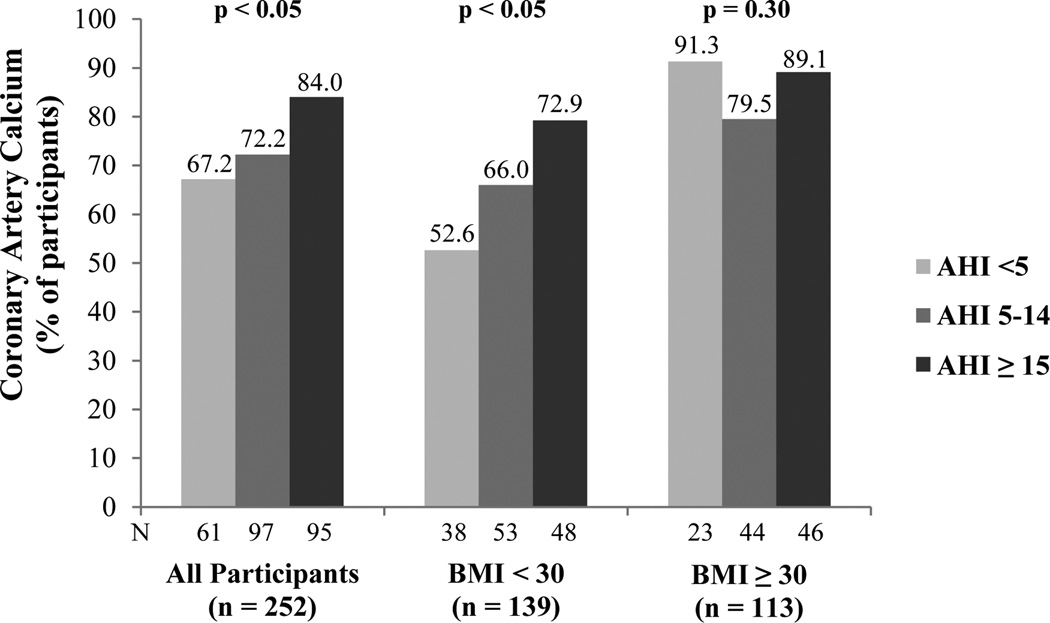
Demographic and clinical characteristics of the sample are presented in Table 1. The prevalence of CAC in the total sample was 75%. The frequency of CAC presence increased with severity of sleep apnea (χ2 = 6.54, p < .05) (Figure 1). In age, gender, race/ethnicity, smoking status, diabetes, hypertension, and dyslipidemia adjusted analysis, the odds ratio (95% confidence interval) for the presence of CAC was 2.33 (1.01 – 5.38) for participants with AHI ≥ 15 compared to those with AHI < 5 (p < .05) (Table 2). The association was no longer significant after adjusting for BMI. No significant difference in presence of CAC was found between those with AHI 5–14 and AHI < 5.
Table 1.
Demographic and clinical characteristics by apnea hypopnea index and in overall sample
| Apnea Hypopnea Index | |||||
|---|---|---|---|---|---|
| Variable | < 5 (n = 61) |
5–14 (n = 97) |
≥ 15 (n = 94) |
Total sample (N = 252) |
p-valuea |
| Age, mean (SD) (years) | 59.1 ± 7.8 | 60.9 ± 7.6 | 62.0 ± 6.5 | 60.9 ± 7.3 | 0.05 |
| Male | 29 (48%) | 48 (49%) | 64 (68%) | 141 (56%) | 0.008 |
| Female | 32 (52%) | 49 (51%) | 30 (32%) | 111 (44%) | |
| European American | 29 (52%) | 66 (68%) | 59 (63%) | 156 (62%) | 0.84 |
| African American | 32 (48%) | 31 (32%) | 35 (37%) | 96 (38%) | |
| BMI, mean (SD) (kg/m2) | 29.0 ± 5.8 | 29.9 ± 4.5 | 30.4 ± 5.0 | 29.9 ± 5.0 | 0.26 |
| AHI, mean (SD) | 2.7 ± 1.1 | 9.0 ± 2.7 | 26.9 ± 12.3 | 14.1 ± 12.7 | |
| Current/past smoker | 33 (54%) | 60 (62%) | 46 (49%) | 137 (55%) | 0.19 |
| Diabetes Mellitus | 10 (16%) | 22 (23%) | 19 (20%) | 50 (20%) | 0.63 |
| Prehypertension | 13 (21%) | 19 (20%) | 18 (19%) | 50 (20%) | 0.94 |
| Hypertensionc | 43 (71%) | 65 (67%) | 71 (75%) | 179 (71%) | 0.53 |
| Dyslipidemiad | 45 (77%) | 81 (83%) | 65 (69%) | 192 (76%) | 0.10 |
| CAC score, median (range) | 18.3 (0 – 786) | 18.1 (0 – 1359) | 64.2 (0 – 1115) | 38.5 (0 – 1359) | 0.01b |
P values based on Pearson χ2 test and analysis of variance.
P value based on Kruskall-Wallis test. BMI, body mass index; AHI, apnea hypopnea-index; CAC, coronary artery calcium.
Hypertension was defined as diastolic blood pressure ≥ 90 mm Hg and/or systolic blood pressure ≥ 140 mm Hg, or self-reported usage of antihypertensive medication.
Dyslipidemia was defined as having blood lipid concentrations within the following parameters, HDL cholesterol ≤ 40 mg/dL, total cholesterol ≥ 200 mg/dL, or self-reported treatment for dyslipidemia.
Figure 1.
Percentage of participants with coronary artery calcium by apnea hypopnea index in the overall sample and stratified by body mass index. AHI, apnea hypopnea index; BMI, body mass index.
Table 2.
Logistic regression analysis of adjusted odds ratios of coronary artery calcium in relation to apnea hypopnea index in the total sample and stratified by body mass index
| AHI Score (compared to < 5) | OR | 95% CI | p-value | |
| Adjusted for age, gender, race/ethnicity, smoking status, diabetes, hypertension, and dyslipidemia | ||||
| Total Sample (n = 252) | 5–14 | 0.97 | (0.46 – 2.06) | 0.94 |
| ≥ 15 | 2.33 | (1.01 – 5.38) | 0.04 | |
| AHI Score (compared to < 5) | OR | 95% CI | p-value | |
| Adjusted for above covariates + BMI | ||||
| Total Sample (n = 252) | 5–14 | 0.72 | (0.33 – 1.61) | 0.43 |
| ≥ 15 | 1.71 | (0.71 – 4.13) | 0.23 | |
| AHI Score (compared to <5) | ORa | 95% CI | p-value | |
| BMI < 30 (n = 139) | 5–14 | 1.12 | (0.40 – 3.14) | 0.83 |
| ≥ 15 | 2.70 | (0.88 – 8.33) | 0.08 | |
| AHI Score (compared to <5) | ORa | 95% CI | p-value | |
| BMI ≥ 30 (n = 113) | 5–14 | 0.29 | (0.05 – 1.62) | 0.16 |
| ≥ 15 | 0.73 | (0.12 – 4.48) | 0.74 | |
Adjusted for age, gender, race/ethnicity, smoking status, diabetes, hypertension, and dyslipidemia. AHI, apnea-hypopnea index; OR, odds ratio; CI, confidence interval; BMI, body mass index.
The % of participants with CAC increased with sleep apnea severity among those with a BMI < 30 (χ2 = 6.77, p < .05) (Figure 1). Specifically, among those with a BMI < 30, 53% of participants with AHI < 5, 66% with AHI 5–14, and 79% with AHI ≥ 30 had evidence of CAC. Among those with a BMI ≥ 30, there was no significant difference in % of participants with CAC among the 3 AHI groups (< 5, 5–14, ≥ 30) (Figure 1). There were similar trends among males and females (data not shown). Consistent results were observed in logistic regression analyses adjusted for age, gender, race/ethnicity, smoking status, diabetes, hypertension, and dyslipidemia. Among 113 participants with a BMI ≥ 30, AHI was not independently associated with CAC (Table 2). Conversely, among 139 participants with a BMI < 30, AHI ≥ 15 (compared to AHI < 5) was associated with a 2.7-fold odds of having CAC (adjusted odds ratio = 2.70, 95% confidence interval: 0.88–8.33, p =.08), but the effect only approached conventional level of statistical significance. The formal test of interaction between BMI (dichotomous) × AHI ≥ 15 in relation to CAC presence was not statistically significant (p = 0.13).
Discussion
In this community cohort of middle aged adults without preexisting cardiovascular disease, we found an AHI ≥ 15 to be associated with a 2.3-fold odds of having CAC compared to an AHI < 5; however adjustment for cardiovascular risk factors and BMI rendered the association to non-significant levels. No difference in CAC presence was found between AHI 5–14 and AHI < 5. In analyses stratified by BMI, no significant association between AHI severity and presence of CAC was found among obese (BMI ≥ 30) individuals. Conversely, among non-obese adults, those with an AHI ≥ 15 had a 2.7-fold odds of having CAC compared to no sleep apnea after adjustment for cardiovascular risk factors and BMI, but the effect only approached statistical significance. Our sample was primarily comprised of participants with mild sleep apnea, thus a sample enriched with more severe sleep apnea may have revealed a stronger effect. Nevertheless, these findings suggest that sleep apnea, in particular moderate to severe sleep apnea (AHI ≥ 15), is independently associated with subclinical coronary atherosclerosis among non-obese individuals.
Sleep apnea and obesity often coexist7,8 and each are strongly associated with cardiovascular disease including the development of coronary artery disease9,10. Obesity has been identified as an independent risk factor for atherosclerosis1,11,12. A randomized trial evaluating the effects of 4 months of continuous positive airway pressure (CPAP) therapy on early markers of atherosclerosis found a decrease in intima-media thickness which was associated with reductions in C-reactive protein and catecholamines suggesting that sleep apnea is an independent risk factor for atherosclerosis13. Given that both obesity and sleep apnea have similar cardiovascular consequences, it is important to explore whether the effect of sleep apnea on atherosclerotic burden may be masked or minimized by obesity. Results from previous community-based studies examining the association between sleep apnea and subclinical coronary atherosclerosis, as measured by CAC, are similar to our findings showing no association between AHI severity and CAC after adjustment for BMI14,15. In a population-based cohort of 258 Korean men and a community sample of 224 middle-aged men and women, higher AHI was associated with having any measureable CAC; however adjustment for BMI rendered the associations to non-significant levels14,15. Conversely, in patients with suspected sleep disorders, more severe sleep apnea was associated with CAC after controlling for BMI16,17. We extended prior investigations by stratifying our sample by BMI (BMI < 30 vs BMI ≥ 30). Moderate to severe sleep apnea was independently associated with greater odds of having CAC but only among non-obese participants suggesting that obesity is a confounder of the association between sleep apnea and subclinical coronary atherosclerosis.
Pathogenic mechanisms linking sleep apnea to atherosclerosis have been proposed18. Inflammation is one possible mechanism underlying the pro-atherogenic effects of sleep apnea. More specifically, C-reactive protein, a serum maker of systemic inflammation, plays a direct role in the manifestation of atherosclerosis19,20 and is elevated in those with sleep apnea independent of BMI21 and visceral obesity22. Other possible mechanisms associated with sleep apnea that may contribute to the progression of atherosclerosis include increased oxidative stress23, endothelial dysfunction24,25, and sustained sympathetic nerve activity due to decreased baroreflex-mediated suppression of chemoreceptor-mediated sympathoexcitation26. Sleep apnea could also contribute to atherosclerosis indirectly, by causing insulin resistance due to elevated leptin levels27 and dyslipidemia28.
Study limitations include the cross-sectional study design, which precludes causal relationships between sleep apnea and subclinical coronary atherosclerosis and slight differences in gender distribution and rates of diabetes and hypertension between the current study population and the Heart SCORE cohort. AHI was assessed using a single-channel portable monitor (ApneaLink, ResMed Corp) instead of polysomnography. However, the portable monitor that we used has been previously validated with acceptable performance to identify obstructive sleep apnea5,29,30. There was a low prevalence of severe sleep apnea (AHI ≥ 30: n = 25) in our sample.
Greater sleep apnea severity is associated with coronary artery calcification.
This association may exist primarily among non-obese individuals.
Treatment of sleep apnea may be important for attenuating atherosclerotic progression.
Acknowledgements
This study was funded by the Pennsylvania Department of Health (ME-02-384) and National Institutes of Health (R01HL089292). The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Support for the first author was provided by the National Heart Lung Blood Institute (NHLBI) K23HL105887.
This was not an industry supported study.
Dr. Strollo has received research support from ResMed, Philips-Respironics, ResMed Foundation, Inspire Medical, and the National Football League. Dr. Strollo has served as a paid consultant for Apnicure and PimMed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Drs. Luyster, Kip, Aiyer, and Reis have indicated no financial conflicts of interest.
References
- 1.Kramer CK, von Mühlen D, Gross JL, Barrett-Connor E. A prospective study of abdominal obesity and coronary artery calcium progression in older adults. J Clin Endocrinol Metab. 2009;94:5039–5044. doi: 10.1210/jc.2009-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, Terry JG, Liu K. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiyer AN, Kip KE, Marroquin OC, Mulukutla SR, Edmundowicz D, Reis SE. Racial differences in coronary artery calcification are not attributed to differences in lipoprotein particle sizes: the Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) Study. Am Heart J. 2007;153:328–334. doi: 10.1016/j.ahj.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KM, Wolson P, Odell PM, Kannel WB. An updated coronary risk profile: a statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 5.Oktay B, Rice TB, Atwood CW, Jr, Passero M, Jr, Gupta N, Givelber R, Drumheller OJ, Houck P, Gordon N, Strollo PJ. Evaluation of a single-channel portable monitor for the diagnosis of obstructive sleep apnea. J Clin Sleep Med. 2011;7:384–390. doi: 10.5664/JCSM.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 7.Daltro C, Gregorio PB, Alves E, Abreu M, Bomfim D, Chicourel MH, Araújo L, Cotrim HP. Prevalence and severity of sleep apnea in a group of morbidly obese patients. Obes Surg. 2007;17:809–814. doi: 10.1007/s11695-007-9147-6. [DOI] [PubMed] [Google Scholar]
- 8.Resta O, Foschino-Barbaro M, Legari G, Talamo S, Bonfitto P, Palumbo A, Minenna A, Giorgino R, De Pergola G. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–675. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 9.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 10.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 11.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 12.Lee CD, Jacobs DR, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 13.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Critical Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Cho G-Y, Baik I, Kim J, Kim S, Lee J, Lim H, Lim S, Park J, Shin C. Association of coronary artery calcification with obstructive sleep apnea and obesity in middle-aged men. Nutr Metab Cardiovasc Dis. 2010;20:575–582. doi: 10.1016/j.numecd.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Strollo PJ, Hall M, Mezick EJ, Kamarck TW, Owens JF, Buysse DJ, Reis SE. Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle-aged men and women: Pittsburgh SleepSCORE study. Sleep. 2011;34:711–716. doi: 10.5665/SLEEP.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arik B, Inci MF, Gumus C, Varol K, Ege MR, Dogan OT, Zorlu A. Advanced age and apnea-hypopnea index predict subclinical atherosclerosis in patients with obstructive sleep apnea syndrome. Multidiscip Respir Med. 2013;8:9. doi: 10.1186/2049-6958-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140:534–542. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–2091. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- 20.Zwaka TP, Hombach V, Torzewski J. C-reactive protein–mediated low density lipoprotein uptake by macrophages implications for atherosclerosis. Circulation. 2001;103:1194–1197. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 21.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 22.Lui MM, Lam JC, Mak HK, Xu A, Ooi C, Lam DC, Mak JC, Khong PL, Ip MS. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–956. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 23.Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 24.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 26.Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic over activity. J Clin Invest. 2014;124:1454–1457. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 28.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17:161–165. doi: 10.1097/MED.0b013e3283373624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayappa I, Norman RG, Suryadevara M, Rapoport DM. Comparison of limited monitoring using a nasal-cannula flow signal to full polysomnography in sleep-disordered breathing. Sleep. 2004;27:1171–1179. doi: 10.1093/sleep/27.6.1171. [DOI] [PubMed] [Google Scholar]
- 30.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink™ for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–392. [PMC free article] [PubMed] [Google Scholar]