Abstract
Background
T1 melanoma staging is significantly affected by tissue sampling approaches, which have not been well characterize.
Objective
Characterize presence of mitotic figures across a minimum of five sequential sections of T1 melanomas.
Methods
A cohort of T1 melanomas with either five (single section per slide) or ten (two sections per slide) sequential sections (5-μm thickness) per case were prepared and examined for mitotic figures.
Results
44 of 82 T1 melanomas (54%) were classified as T1b. The number of sections with a mitotic figure present ranged from only one of five sections (n=5 of 44 cases, 11.4%) to all five (n = 20 of 44 cases, 45.5%). A sequential approach versus a non sequential approach did not appear to matter.
Limitation
Cases were taken from a single pathology practice in the Pacific Northwest, which may not generalize to other populations in the U.S.
Conclusion
The variation in the presence of mitotic figures within sequential sections supports reviewing 3-5 sections to fulfill AJCC recommendations. The prognostic significance of a T1b melanoma with a rare mitotic figure on a single section versus a T1b melanoma with mitotic figures on multiple sections deserves more attention to see if further sub-classification is possible or even necessary.
Introduction
Correct melanoma staging is significantly affected by tissue sampling approaches, as the particular approach one laboratory takes compared to another could influence the prognostic determinants (Breslow depth, mitoses, and epidermal ulceration) (1-6). Mitotic figures can be focal (e.g., hot spots) and accurate documentation of their presence then becomes a function of adequate sampling; however, thorough sampling is discouraged because of the need to preserve tissue for future molecular testing and because the studies demonstrating the importance of mitogenicity did not use an exhaustive sampling technique.
While prior studies have shown interobserver variability on what constitutes a mitotic figure (7), we are unaware of any studies that have examined the equivalence of serial sections in noting the presence or absence of a mitotic figure. Without evidence supporting the assumption that mitotic figures are consistently present within sections of T1b melanomas, clinicians cannot safely presume that the biopsy was adequately sampled.
As part of a national study of pathologists' diagnostic interpretation that involved developing a test set of melanocytic lesions, we noted significant variability in the presence of mitotic figures within sequential sections of thin invasive melanomas. Given the importance of mitoses in T1 melanoma classification, we evaluated sequential tissue sections from all thin invasive melanomas specifically to characterize variability in the presence of mitotic figures across sequential sections of thin melanomas. This would allow us to consider implications of sampling on staging criteria.
Materials and Methods
Patient Cohort
Skin lesions biopsied in 2010-2011 were identified from a private dermatopathology practice in Washington State utilizing their in-house database of patient records. New slides were made for each case, with five micron sequential sections transferred onto new slides for each case. All procedures were HIPAA compliant, and approval was obtained from the University of Washington Institutional Review Board (#41700).
Identification of Thin Melanomas –Panel Dermatopathologists' Independent Reviews and Consensus Panel Review
Development of study materials, including a histology form designed for independent and consensus panel reviews, for the primary NIH-funded study of pathologists is published elsewhere (8). Briefly, three internationally recognized dermatopathologists (the “Panel”) each independently reviewed one of the first three of five sequential sections made for each case, blinded to others' interpretations. The Panel subsequently met over the course of six days to review each patient case together under a multi-headed microscope with the assistance of a fourth pathologist. During these meetings, agreement was obtained on Breslow depth, mitotic rate, and presence of epidermal ulceration for each case. All T1b melanomas showed no epidermal ulceration.
Detailed Re-evaluation of All Thin Melanomas and Validation of Mitotic Figures
All thin invasive melanomas (Breslow depth of ≤1.00mm) (n=85) were pulled for corroboration of mitoses on every section by the lead author (SRK). Confirmation from the laboratory revealed that three of these cases were not sequentially cut due to technical difficulties and were thus eliminated from the current study for a final total of 82 T1 melanoma cases. The re-evaluation was not blinded to the Panel's consensus findings, since the goal was to supplement the consensus review by accurately identifying the presence or absence of mitotic figures in all sections for each case. Therefore, cases found to have no mitotic figures or only one mitotic figure on one of the three initially reviewed slides received more attention (up to 30 minutes per section) than those with a mitotic figure identified by all three panel members. In addition, the 4th and 5th slides, initially intended as back-ups for the test set, were included in this review.
When a discrepancy in mitotic rate was noted between the evaluation of cases for this study and the Consensus Panel review or if it was difficult to determine if a mitotic figure was melanocytic or stromal (valid versus invalid, respectively), these cases were reviewed with one of the Panel dermatopathologists (MP) through a multi-headed microscope for final determination. Twenty-three cases were thus re-evaluated and validated for this study, including 18 T1a cases where the presence of a mitotic figure was questioned and 5 T1b cases where the absence of a mitotic figure was questioned. This re-review found 11 T1a cases (19 total sections) and 5 T1b cases (6 total sections) discrepant with the original Panel's consensus diagnosis. Fifteen of these cases (10 T1a and 5 T1b) were part of the slides prepared with two sections per slide (ten total sequential sections per case), and during the validation review only one of the two sections on each slide was reviewed due to time constraints. The fluctuation in the presence of mitotic figures observed between sequential sections quickly explains the apparent discordance between the individual panel members. The remaining single section per slide cases (N=8, all T1a cases) had less discordance between the independent Panel experts' assessments, likely due to the thorough evaluation of every section during the consensus Panel conferences.
Analytic Plan
The primary analyses included data on the five sequential sections available for all 82 patients. If no mitotic figure was detected on any of the 5 tissue sections, the patient was classified as AJCC stage T1a. If a mitotic figure was detected on any of the 5 tissue sections, that patient was classified as AJCC stage T1b. Sensitivity for detection of T1b, according to number of tissue slices examined, was computed as the proportion of all T1b patients who would have been classified as such on the basis of each number of sections examined. Binomial exact 95% confidence intervals for the proportions were computed using Stata/SE 12.1 (StataCorp LP, College Station, Texas). The distribution of mitotic figures beyond the 5th tissue section is described for the 32 patients with more than 5 sequential sections, with 28 cases having a full 10-sections each.
Results
Patient Characteristics
The characteristics of 82 patients with thin primary melanomas are shown in Table 1, with 38 patients (46%) classified as T1a and 44 patients (54%) as T1b. Additional characteristics include anatomic site, type of biopsy performed, corresponding Breslow depth, and type of melanoma. None of the melanomas had epidermal ulceration.
Table 1. Characteristics of patients diagnosed with thin (T1) invasive melanoma (N = 82).
| Patient Characteristics | T1a (N = 38)(46%) | T1b (N = 44)(54%) | Total (N = 82) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | N | % | |
| Sex | ||||||
| Female | 19 | 50.0 | 24 | 54.6 | 43 | 52.4 |
| Male | 19 | 50.0 | 20 | 45.5 | 39 | 47.6 |
| Age (years) | ||||||
| 20-49 | 13 | 34.2 | 16 | 36.4 | 29 | 35.4 |
| 50-64 | 12 | 31.6 | 18 | 40.9 | 30 | 36.6 |
| ≥65 | 13 | 34.2 | 10 | 22.7 | 23 | 28.1 |
| Mean (SD) | 56.6 (15.5) 54 (44-69) |
53.4 (15.5) | 54.9 (15.5) | |||
| Median (IQR) | 55 (43-64) | 55 (43-66) | ||||
| Anatomic location | ||||||
| Head and neck | 7 | 18.4 | 5 | 11.4 | 12 | 14.6 |
| Upper extremities | 7 | 18.4 | 9 | 13.6 | 16 | 19.5 |
| Trunk | 12 | 31.6 | 24 | 54.6 | 36 | 43.9 |
| Lower extremities | 12 | 31.6 | 6 | 20.5 | 18 | 22.0 |
| Type of biopsy | ||||||
| Excision | 5 | 13.2 | 6 | 13.6 | 11 | 13.4 |
| Punch | 13 | 34.2 | 13 | 29.6 | 26 | 31.7 |
| Shave | 20 | 52.6 | 25 | 56.8 | 45 | 54.9 |
| Breslow depth (mm) | ||||||
| >0 to 0.25 | 7 | 18.4 | 1 | 2.3 | 8 | 9.8 |
| 0.25 to <0.50 | 23 | 60.5 | 10 | 22.7 | 33 | 40.2 |
| 0.50 to <0.75 | 7 | 18.4 | 21 | 47.7 | 28 | 34.2 |
| 0.75 to 1.00 | 1 | 2.6 | 12 | 27.3 | 13 | 15.9 |
| Mean (SD) | 0.39 (0.17) | 0.62 (0.18) | 0.51 (0.21) | |||
| Median (IQR) | 0.36 (0.25-0.48) | 0.62 (0.49-0.78) | 0.50 (0.35-0.65) | |||
| Melanoma subtype | ||||||
| Superficial spreading | 15 | 39.5 | 26 | 59.1 | 41 | 50.0 |
| Lentigo malignant | 12 | 31.6 | 4 | 9.1 | 16 | 19.5 |
| Nevoid | 1 | 2.6 | 2 | 4.6 | 3 | 3.7 |
| Spitz-like | 2 | 5.3 | 0 | 0.0 | 2 | 2.4 |
| Pigmented spindle cell (borderline with superficial spreading) | 1 | 2.6 | 0 | 0.0 | 1 | 1.2 |
| Unclassifiable | 7 | 18.4 | 12 | 27.3 | 19 | 23.2 |
SD = standard deviation; IQR, interquartile range (25th-75th percentile)
Mitotic Variability Across Sequential Sections of Thin Melanomas
A mitotic figure was noted on all five sections for 20 of the 44 T1b cases (45.5%), while the remainder had mitotic figures on four or fewer sections (Figure 1). The distribution of mitotic figures across five or ten sequential sections is shown in Tables 2a and 2b, respectively, with results listed according to Breslow Depth. As shown in Table 2b, two additional cases would have been classified as T1b if additional sections (6 through 10) were examined (Cases 19 and 25).
Figure 1. T1b Melanoma.
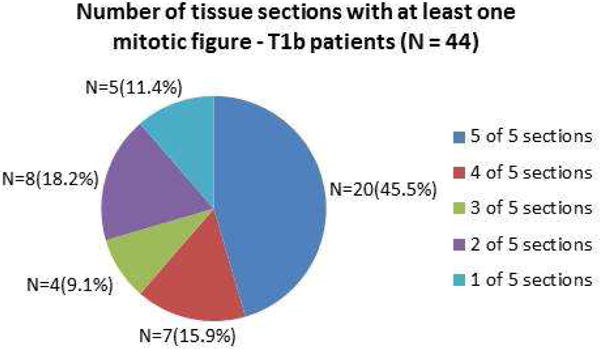
Number of Tissue Sections with at Least One Mitotic Figure Present Among AJCC Stage T1b Melanoma Patients.
Table 2a. Summary of T1 melanomas across five sequential sections.
| Cases | Breslow Depth (mm) | 1 | 2 | 3 | 4 | 5 | % Slides w Mitotic figure |
|---|---|---|---|---|---|---|---|
| 1-38^ | 0.15-0.75 | 0 | 0 | 0 | 0 | 0 | 0 |
| 39-60* | 0.55-0.95 | 1 | 1 | 1 | 1 | 1 | 100 |
| 61 | 0.97 | 0 | 1 | 0 | 1 | 0 | 40 |
| 62 | 0.79 | 1 | 1 | 0 | 1 | 1 | 80 |
| 63 | 0.78 | 1 | 1 | 0 | 0 | 0 | 40 |
| 64 | 0.63 | 1 | 1 | 0 | 1 | 1 | 80 |
| 65 | 0.62 | 1 | 1 | 1 | 0 | 0 | 60 |
| 66 | 0.58 | 1 | 1 | 0 | 0 | 1 | 60 |
| 67 | 0.54 | 0 | 0 | 1 | 0 | 1 | 40 |
| 68 | 0.54 | 1 | 1 | 1 | 1 | 0 | 80 |
| 69 | 0.53 | 0 | 0 | 0 | 1 | 1 | 40 |
| 70 | 0.52 | 0 | 1 | 0 | 0 | 0 | 20 |
| 71 | 0.52 | 1 | 1 | 0 | 1 | 1 | 80 |
| 72 | 0.50 | 1 | 0 | 0 | 0 | 0 | 20 |
| 73 | 0.48 | 1 | 1 | 1 | 0 | 0 | 60 |
| 74 | 0.48 | 0 | 1 | 1 | 1 | 1 | 80 |
| 75 | 0.48 | 0 | 0 | 0 | 1 | 1 | 40 |
| 76 | 0.47 | 0 | 0 | 1 | 0 | 1 | 40 |
| 77 | 0.46 | 1 | 1 | 0 | 1 | 1 | 80 |
| 78 | 0.45 | 1 | 0 | 1 | 1 | 1 | 80 |
| 79 | 0.45 | 1 | 0 | 0 | 0 | 1 | 40 |
| 80 | 0.40 | 0 | 1 | 1 | 0 | 0 | 40 |
| 81 | 0.37 | 0 | 1 | 0 | 0 | 0 | 20 |
| 82 | 0.12 | 1 | 0 | 0 | 0 | 0 | 20 |
Summary of all T1a melanomas
Summary of all T1b melanomas with mitotic figures on every slide
Table 2b. Summary of T1 melanomas across ten sequential sections.
| Cases | Breslow Depth (mm) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | % Slides w Mitotic figure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-12^ | 0.17-0.52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13-15* | 0.84-0.87 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 16 | 0.79 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 90 |
| 17 | 0.66 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 80 |
| 18 | 0.66 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 90 |
| 19 | 0.62 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 50 |
| 20 | 0.58 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 80 |
| 21 | 0.54 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 50 |
| 22 | 0.52 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 23 | 0.5 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 20 |
| 24 | 0.48 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 50 |
| 25 | 0.42 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 30 |
| 26 | 0.4 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 50 |
| 27 | 0.37 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 50 |
| 28 | 0.32 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 90 |
Summary of all T1a melanomas
Summary of all T1b melanomas with mitotic figures on every slide
Sensitivity for Detection of Mitoses
The sensitivity in diagnosing T1b melanoma increased with the number of sections examined per case (Table 3). Examination of any one of the 5 sections, on average, provided a sensitivity of 72.7% for accurately diagnosing T1b melanoma (95% confidence interval (CI), 57.2 to 85.0). When any two sections were examined the sensitivity was 86.4% (CI 72.6 to 94.8), any three sections 93.2% (CI 81.3 to 98.6), and any four sections 97.7% (CI 88.0 to 99.9) (Table 3). Examining sections sequentially or non-sequentially resulted in comparable sensitivities, indicating no particular advantage regarding the approach if five sequential sections were available for any particular case. For example, when any three sequential sections were examined, the sensitivity for accurately identifying a T1b melanoma ranged from 88.6% to 93.2% (mean=90.9%), while three non-sequential sections produced a comparable sensitivity ranging from 93.2% to 97.7% (mean = 93.2%).
Table 3.
Sensitivity for detection of T1b melanomas according to number of tissue sections examined (N=44 patients).
| Number of Sections Examined | Cases (n) with mitotic figure | Sensitivity for detection of T1b ([n/N]*100) | |
|---|---|---|---|
| % | 95% Confidence Interval | ||
| 1 Section Examined Only | |||
| First section | 35 | 79.5 | 64.7-90.2 |
| Second section | 34 | 77.3 | 62.2-88.5 |
| Third section | 31 | 70.5 | 54.8-83.2 |
| Fourth section | 29 | 65.9 | 50.1-79.5 |
| Fifth section | 32 | 72.7 | 57.2-85.0 |
| Mean | 32 | 72.7 | 57.2-85.0 |
| 2 Sections Examined | |||
| Sequential sections Examined | |||
| First and second sections | 38 | 86.4 | 72.6-94.8 |
| Second and third sections | 38 | 86.3 | 72.6-94.8 |
| Third and fourth sections | 36 | 81.8 | 67.3-91.8 |
| Fourth and fifth sections | 35 | 79.6 | 64.7-90.2 |
| Mean | 37 | 84.1 | 69.9-93.4 |
| All other combinations | |||
| First and third sections | 39 | 88.6 | 75.4-96.2 |
| First and fourth sections | 38 | 86.4 | 72.6-94.8 |
| First and fifth sections | 40 | 90.9 | 78.3-97.5 |
| Second and fourth sections | 38 | 86.4 | 72.6-94.8 |
| Second and fifth sections | 41 | 93.2 | 81.3-98.6 |
| Third and fifth sections | 39 | 88.6 | 75.4-96.2 |
| Mean | 39 | 88.6 | 75.4-96.2 |
| Total (mean) | 38 | 86.4 | 72.6-94.8 |
| 3 Sections Examined | |||
| Sequential sections | |||
| First, second, and third sections | 41 | 93.2 | 81.3-98.6 |
| Second, third, and fourth sections | 40 | 90.9 | 78.3-97.5 |
| Third, fourth, and fifth sections | 39 | 88.6 | 75.4-96.2 |
| Mean | 40 | 90.9 | 78.3-97.5 |
| All other combinations | |||
| First, second, and fourth sections | 41 | 93.2 | 81.3-98.6 |
| First, second, and fifth sections | 43 | 97.7 | 88.0-99.9 |
| First, third, and fourth sections | 41 | 93.2 | 81.3-98.6 |
| First, third, and fifth sections | 42 | 95.5 | 84.5-99.4 |
| First, fourth, and fifth sections | 41 | 93.2 | 81.3-98.6 |
| Second, third, and fifth sections | 42 | 95.5 | 84.5-99.4 |
| Second, fourth, and fifth sections | 42 | 95.5 | 84.5-99.4 |
| Mean | 42 | 95.5 | 84.5-99.4 |
| Total (mean) | 41 | 93.2 | 81.3-98.6 |
| 4 Sections Examined | |||
| Sequential sections | |||
| First, second, third, and fourth sections | 43 | 97.7 | 88.0-99.9 |
| Second, third, fourth, and fifth sections | 42 | 95.5 | 84.5-99.4 |
| Mean | 43 | 97.7 | 88.0-99.9 |
| All other combinations | |||
| First, second, third, and fifth sections | 44 | 100.0 | 92.0† |
| First, second, fourth, and fifth sections | 44 | 100.0 | 92.0† |
| First, third, fourth, and fifth sections | 42 | 95.5 | 84.5-99.4 |
| Mean | 43 | 97.7 | 88.0-99.9 |
| Total (mean) | 43 | 97.7 | 88.0-99.9 |
| Total number of patients with a mitotic figure on any of the 5 sections (N) | 44 | N/A | N/A |
one-sided 97.5% confidence interval
Distribution of T1 Melanomas by Breslow Depth
A Breslow depth of <0.5mm was noted for the majority of T1a melanoma cases (79%) while only 25% of the T1b cases had a Breslow depth of <0.5mm (Figure 2). Deeper Breslow depths showed an increasing probability of finding a mitotically active melanoma (Figure 3). This generalization, however, has significant outliers in both categories. 11 (25%) T1b melanomas were under 0.5mm in thickness while 8 (21%) of T1a melanomas were found to have Breslow depths greater than or equal to 0.5mm (Figures 2 and 3). Figure 3 examines the number of slides showing mitotic figures by Breslow depth with similar generalizations and outliers as in Figure 2.
Figure 2. T1 Melanoma.
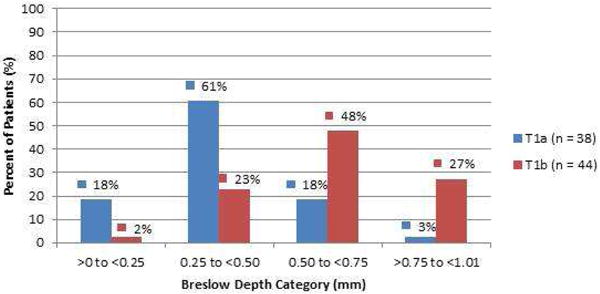
Percent of AJCC stage T1a and T1b melanomas by Breslow depth (mm).
Figure 3. T1 Melanoma.
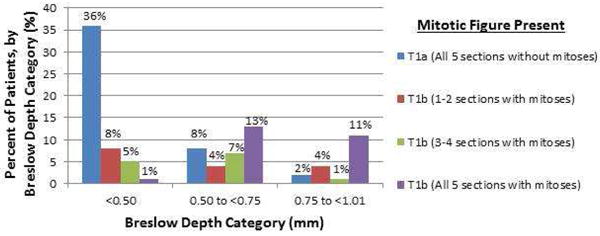
For each Breslow depth category, the percent of AJCC T1b melanoma patients according to the number of sections in which a mitotic figure was present (N = 44 patients).
Challenges Interpreting Mitotic Figures
During detailed re-review for mitotic figures, situations arose that required corroboration, which are highlighted in Figure 4. These include difficulty determining a junctional versus dermal mitotic figure (4A), small and easily missed mitotic figures (4B and 4C), and mitotic figures that may be missed because of obscuring inflammation or pigment dropout (4D). More obvious examples of what should not be counted as a dermal melanocytic mitotic figure (4E) and what should (4F) were also included.
Figure 4. T1 Melanoma.
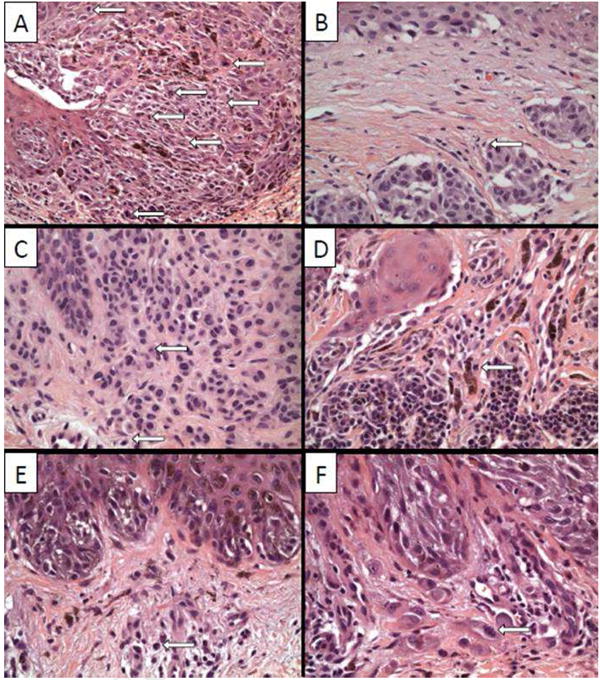
Photomicrographs (200x original (A) and 400x original (B-F)) highlighting various uncertain or difficult (A-E) and definite (F) mitotic figures. The distinction between a junctional and dermal mitotic figure can be difficult as seen in panel A where large, indistinct junctional nests blend with the dermis. Mitotic figures that can be easily missed due to their size, but would still qualify to upgrade a melanoma from T1a to T1b, are shown in panels B and C. A lymphohistiocytic infiltrate may make the distinction between a dermal melanocytic mitotic figure and an inflammatory cell difficult (D) unlike mitotic figures encountered in a purely lymphohistiocytic milieux (E).
Discussion
The objective of this study was to characterize variability in the presence of mitotic figures across a minimum of five sequential sections among T1 melanomas. Our review of T1b melanomas showed that approximately half (46%) had a mitotic figure on all five sequential sections. We note a general increase in likelihood of finding a mitotically active melanoma, in addition to finding mitoses on most sections, with increasing Breslow depth. This generalization is met with important outliers that preclude one from expecting a very thin Breslow depth (e.g., <0.4mm) to be mitotically inactive.
Review of only one of the five sections resulted in correct classification of T1b 66-80% of the time depending on which of the five sections was reviewed. However, most T1b cases (>95%) were identified when at least four of the five sequential sections were examined. Incomplete sampling therefore offers a possible explanation for why certain patients with T1a melanomas go on to develop metastases. Within the context of five sequential sections, we show similar sensitivities when one compares a review of sequential sections versus a nonsequential approach in finding mitoses. The variability in mitoses among sections identified in this study raises concerns over the ideal approach to sampling and review of a biopsy.
Assuming the average size of a biopsy is ∼5mm, then our results are extrapolated from a very focal point within the tissue (5 sequential sections, totaling 25-μm out of 5mm or <1% of the total sample). Furthermore, the tissue we studied was already used for a primary diagnosis and mitotic hot spots may have been cut through. These two factors could make our data appear as though finding T1b cases requires more effort than is necessary. Thorough block sampling would provide valuable information with regards to which approach (sequential versus non-sequential sampling) is truly the most sensitive in determining mitotic rate. However, this would result in available tissue being diminished for potential subsequent (e.g. molecular) testing (1, 4).
We did not assess the total number of mitoses per section or if the adjacent section represents the same or a novel mitosis. This latter point may also be associated with a change in prognosis since a different plane of section could result in an increased mitotic rate; however, such details are not clearly accounted for in the most recent AJCC edition.
The strengths of our study include the in-depth review and validation of mitoses per section in sequential tissue samples identified by an expert panel as T1 melanoma. Another strength is the inclusion of assessments of accuracy according to the number of sequential tissue cuts assessed. Weaknesses include that the cases were taken from a single pathology practice in the Pacific Northwest, which may not generalize to other populations in the U.S. Another limitation is the chance of misclassification bias related to tissue sampling techniques that may have affected identification of mitotic activity.
In conclusion, the current AJCC 2009 edition added another quantifiable, prognostic factor, namely mitotic rate ≥ 1 per square millimeter, to existing Breslow depth and epidermal ulceration in the classification of T1 melanomas with significant downstream consequences for clinical management. Our study findings raise questions regarding the ideal approach to sampling a biopsy so that Breslow depth, mitotic rate, and epidermal ulceration are accurately documented. We found a surprising fluctuation in the presence of mitotic figures across five sequential sections clearly illustrating the importance of reviewing more than one section per case. While our data show increasing sensitivity or interpretive accuracy with increasing number of sections reviewed and suggests a high sensitivity when examining at least four sections (sequential or non-sequential), this may be more than is necessary, as our data are extrapolated from of a very focal point within the tissue and may not be representative. Regardless, with three prognostic variables to consider, and their independent variation throughout a biopsy (9), multiple sections need to be examined as it is unlikely that a single section will provide an accurate representation of all three variables (e.g., In a T1b melanoma, the section with the deepest Breslow depth may not have any mitoses or epidermal ulceration).
While the ability to reduce interobserver variability in the assessment of Breslow depth through the use of a micrometer exists, there is no such tool to help reduce interobserver variability in assessment of mitotic rate, let alone help identify mitotic figures. The identification of mitoses then becomes a function of the number of sections reviewed and effort spent per slide. None of the thin melanomas in our study had epidermal ulceration, which could add another level of complexity in clinical care. Recent studies incorporating the use of immunohistochemical stains to aid in the assessment of mitotic rate, such as the use of anti-phosphohistone H3 (10-12), may help ease the problems in identifying and quantifying mitoses, however would require recalibration as current AJCC recommendations do not account for immunohistochemical approaches to mitotic rate and associated prognosis.
Additional studies may show differences between T1b melanomas with rare mitoses and those with readily identifiable mitotic figures, thus leading to finer sub-classification of T1 melanomas and improved patient care and subsequent outcomes. Should future studies fail to find a prognostic difference between such cases, our study suggests the review of 3-5 sections to increase the accuracy of mitotic rate interpretations. Future studies may benefit from incorporating data on how many sections were examined for each case and if they were sequential or random.
Acknowledgments
The authors are grateful to Karla Carlmas, Stacey Murdoch, Andrea Radick and Regina Liszanckie for their assistance with this study.
Funding source: National Cancer Institute (R01 CA151306 and K05 CA104699) supported this work.
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- HIPAA
Health Insurance Portability and Accountability Act
- CI
Confidence Interval
- SD
Standard Deviation
- IQR
Interquartile Range
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dyson SW, Bass J, Pomeranz J, Jaworsky C, Sigel J, Somach S. Impact of thorough block sampling in the histologic evaluation of melanomas. Arch Dermatol. 2005;141(6):734–6. doi: 10.1001/archderm.141.6.734. Epub 2005/06/22. doi:141/6/734 [pii] 10.1001/archderm.141.6.734. [DOI] [PubMed] [Google Scholar]
- 2.Ellis CN, Solomon AR. Histologic evaluation of melanomas. Arch Dermatol. 2005;141(11):1466. doi: 10.1001/archderm.141.11.1466-b. Epub 2005/11/23. doi:141/11/1466-a [pii] 10.1001/archderm.141.11.1466-b. [DOI] [PubMed] [Google Scholar]
- 3.Hurt MA, Santa Cruz DJ. Malignant melanoma microstaging. History, premises, methods, problems, and recommendations--a call for standardization. Pathol Annu. 1994;29(Pt 2):51–74. Epub 1994/01/01. [PubMed] [Google Scholar]
- 4.Patrick RJ, Corey S, Glass LF. The use of sequential serial sectioning of thin melanomas in determining maximum Breslow depth. J Am Acad Dermatol. 2007;57(5 Suppl):S127–8. doi: 10.1016/j.jaad.2006.02.007. Epub 2007/11/02. doi:S0190-9622(06)00386-0 [pii] 10.1016/j.jaad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz AD, Silvers DN. Dermatopathology standards. J Cutan Pathol. 1996;23(2):194–6. doi: 10.1111/j.1600-0560.1996.tb01295.x. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 6.Solomon AR, Ellis CN, Headington JT. An evaluation of vertical growth in thin superficial spreading melanomas by sequential serial microscopic sections. Cancer. 1983;52(12):2338–41. doi: 10.1002/1097-0142(19831215)52:12<2338::aid-cncr2820521229>3.0.co;2-t. Epub 1983/12/15. [DOI] [PubMed] [Google Scholar]
- 7.Scolyer RA, Shaw HM, Thompson JF, Li LX, Colman MH, Lo SK, et al. Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am J Surg Pathol. 2003;27(12):1571–6. doi: 10.1097/00000478-200312000-00011. Epub 2003/12/06. [DOI] [PubMed] [Google Scholar]
- 8.Piepkorn MW, Barnhill RL, Elder DE, Knezevich SR, Carney PA, Reisch LM, et al. The MPATH Reporting Schema for Melanocytic Proliferations and Melanoma. Journal of The American Academy of Dermatology. 2013 doi: 10.1016/j.jaad.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sondergaard K. The intra-lesional variation of type, level of invasion, and tumour thickness of primary cutaneous malignant melanoma. Acta Pathol Microbiol Scand A. 1980;88(5):269–74. doi: 10.1111/j.1699-0463.1980.tb02496.x. Epub 1980/09/01. [DOI] [PubMed] [Google Scholar]
- 10.Casper DJ, Ross KI, Messina JL, Sondak VK, Bodden CN, McCardle TW, et al. Useof anti-phosphohistone H3 immunohistochemistry to determine mitotic rate in thin melanoma. Am J Dermatopathol. 2010;32(7):650–4. doi: 10.1097/DAD.0b013e3181cf7cc1. Epub 2010/06/19. [DOI] [PubMed] [Google Scholar]
- 11.Ikenberg K, Pfaltz M, Rakozy C, Kempf W. Immunohistochemical dual staining as an adjunct in assessment of mitotic activity in melanoma. J Cutan Pathol. 2012;39(3):324–30. doi: 10.1111/j.1600-0560.2011.01858.x. Epub 2012/02/18. [DOI] [PubMed] [Google Scholar]
- 12.Ladstein RG, Bachmann IM, Straume O, Akslen LA. Prognostic importance of the mitotic marker phosphohistone H3 in cutaneous nodular melanoma. J Invest Dermatol. 2012;132(4):1247–52. doi: 10.1038/jid.2011.464. Epub 2012/02/03. doi:jid2011464 [pii] 10.1038/jid.2011.464. [DOI] [PubMed] [Google Scholar]


