Abstract
To feed or breathe, the oral opening must connect with the gut. The foregut and oral tissues converge at the primary mouth, forming the buccopharyngeal membrane (BPM), a bilayer epithelium. Failure to form the opening between gut and mouth has significant ramifications, and many craniofacial disorders have been associated with defects in this process. Oral perforation is characterized by dissolution of the BPM, but little is known about this process. In humans, failure to form a continuous mouth opening is associated with mutations in Hedgehog (Hh) pathway members; however, the role of Hh in primary mouth development is untested. Here, we show, using Xenopus, that Hh signaling is necessary and sufficient to initiate mouth formation, and that Hh activation is required in a dose-dependent fashion to determine the size of the mouth. This activity lies upstream of the previously demonstrated role for Wnt signal inhibition in oral perforation. We then turn to mouse mutants to establish that SHH and Gli3 are indeed necessary for mammalian mouth development. Our data suggest that Hh-mediated BPM persistence may underlie oral defects in human craniofacial syndromes.
Keywords: Hedgehog, Wnt, Primary mouth, Xenopus, Mouse, Buccopharyngeal membrane, Stomodeum, Fibronectin
Introduction
To feed or breathe the oral opening must connect with the gut. The primary mouth marks the location of this interface, and perforation is essential (Dickinson and Sive, 2006; Hardin and Armstrong, 1997; McClay et al., 1992; Poelmann et al., 1985; Soukup et al., 2013; Takahama et al., 1988; Watanabe et al., 1984). Despite the fundamental importance of the primary mouth, little is known about the molecular control of its development. In mammals, the buccopharyngeal membrane (BPM) is hidden internally, behind the expanding facial prominences and is surrounded by the brain and cardiac tissues, making mammalian primary mouth development a challenging process to investigate (Poelmann et al., 1985; Soukup et al., 2013; Theiler, 1969; Waterman, 1977). However, a series of elegant studies have shown that Xenopus laevis is a tractable model for understanding primary mouth development (Dickinson and Sive, 2007, 2006, 2009; Jacox et al., 2014; Kennedy and Dickinson, 2014).
In mammals and amphibians the mouth opening forms as a result of contact between invaginating primary mouth ectoderm and foregut endothelium (Fig. 1A) (Dickinson and Sive, 2006, 2009; Soukup et al., 2013; Waterman, 1977, 1985; Waterman and Schoenwolf, 1980). In Xenopus, invaginating ectoderm appears as a depression called the stomodeum (Dickinson and Sive, 2006), and this depression deepens as apoptosis and cell intermingling thin the epithelium (Dickinson and Sive, 2006, 2009; Poelmann et al., 1985). The basement membrane (BM) separating foregut endoderm and stomodeal ectoderm dissolves to permit intercalation of the epithelial bilayer and subsequent oral perforation (Dickinson and Sive, 2006, 2009; Soukup et al., 2013; Waterman, 1977, 1985; Waterman and Schoenwolf, 1980) (Fig. 1).
Fig. 1.
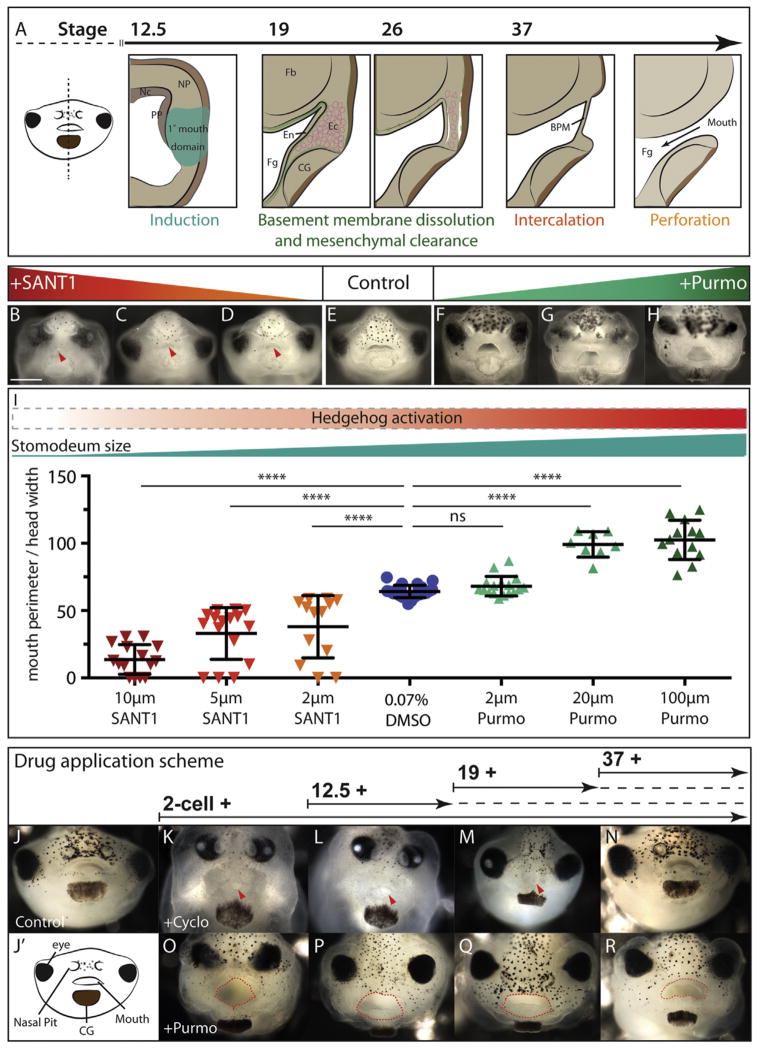
Hedgehog perturbation affects the size of the oral opening. (A) Schematic illustrating primary mouth development. Frontal view of Xenopus tadpole indicates sectional plane for schematics. Stage 12.5, primary mouth induction occurs anterior to the prechordal plate (PP), notochord (Nc) and neural plate (NP). Stage 19 foregut endoderm (Fg) abuts mouth ectoderm (Ec, pink), separated by fibronectin-rich basement membrane (BM, green), between forebrain (Fb) and cement gland (CG). BM dissolves and mesenchymal clearance thins stomodeum (green dashed line indicates BM). Stage 37, buccopharyngeal membrane (BPM) formation. Stage 40, BPM perforation. (B–D) Frontal view of stage 45 tadpoles incubated from 2-cell stage with 10 μM (B), 5 μM (C), or 2 μM SANT1 (D) (B, n=13/13, C, n=16/16, D, n=15/15). Primary mouth is indicated by red arrowhead. (E) Control tadpole, 0.07% DMSO (n=26/26). (F–H) Tadpoles incubated with 2 μM (F), 20 μM (G) or 100 μM purmorphamine (H). Increase in mouth size was observed with increasing concentrations of purmorphamine (F, n=70/70, G, n=43/43, H, n=154/154). (I) Quantification of mouth size for 10 μM, 5 μM, or 2 μM SANT1, 0.07% DMSO, 2 μM, 20 μM or 100 μM purmorphamine, where mouth perimeter is normalized to width of the head. ****P<0.001. Scheme indicating primary mouth size (green) in relationship to Hh activity (red bar). (J) Stage 45 control tadpole. (J′) Facial anatomy schematic. (K–N) Tadpoles incubated with 250 μM cyclopamine from 2-cell stage (K), between stages 12.5–19 (L), 19–37 (M), or from 37 (N). (O–R) Tadpoles treated with 100 μM purmorphamine from the 2-cell stage (O), between stages 12.5–19 (P), 19–37 (Q), or from stage 37 (R).
At present, only a single signaling system has been identified as a molecular regulator of primary mouth development. In Xenopus, Wnt signal inhibition is necessary for stomodeal specification and perforation (Dickinson and Sive, 2009). Wnt/β-catenin signaling is necessary to promote transcriptional activation of the basement membrane component fibronectin (FN) (Gradl et al., 1999), while Wnt inhibitors Crescent and Frzb-1 are required within the stomodeum for dissolution of the basement membrane separating foregut endoderm and oral ectoderm (Dickinson and Sive, 2009). Loss of either inhibitor results in a small, imperforate primary mouth. Concomitantly, facial Wnt-8 gain-of-function is sufficient to suppress stomodeum formation (Dickinson and Sive, 2009). However, the stomodeal ectoderm becomes unresponsive to Wnts long before perforation, suggesting that Wnts do not directly control mouth opening (Dickinson and Sive, 2009). Therefore, it is necessary to consider other signaling pathways during primary mouth morphogenesis.
In mammals, virtually nothing is known about the molecular control of primary mouth formation, but several craniofacial syndromes, including CHARGE, Down, Holzgreve–Wagner–Rehder, Greig cephalopolydactyly syndrome (GCPS) and synostotic syndromes, as well as cleft palate, have been associated with persistent BPM (Kliegman, 2011; DéMurger et al., 2014; Pillai et al., 1990; Verma and Geller, 2009). Notably, Holzgreve–Wagner–Rehder syndrome involves cleft palate and postaxial polydactyly, phenotypes associated with Hh perturbation (Legius et al., 1988). Furthermore, a recent publication reports that GCPS—characterized by mutations in the Hh effector Gli3—caused oral anomalies in all prenatal cases observed, and in one instance a complete absence of the oral opening (DéMurger et al., 2014). We therefore tested the requirements for Hedgehog signaling during primary mouth development. We present data suggesting that Hh signaling is required for BPM dissolution in both Xenopus and mouse. Moreover, we show that Hh signaling is required in a dose-dependent fashion to control basement membrane dissolution and endoderm–ectoderm intercalation. These data significantly advance our understanding of primary mouth development and pinpoint a novel role for Hh signaling during this process.
Results and discussion
Hedgehog is required for primary mouth perforation
In Xenopus, the primary mouth is externally accessible and offers a tractable system for studying oral perforation (Dickinson and Sive, 2006). Moreover, Xenopus embryos are easily treated with chemical modulators. Thus, early effects of Hh perturbation can be readily bypassed (Hollemann et al., 2007; Lewis and Krieg, 2014; Peyrot et al., 2011). We asked whether Hh loss of function, using the potent inhibitor cyclopamine or SANT1, could perturb primary mouth development (Chen et al., 2002; Peyrot et al., 2011; Williams et al., 2003). Indeed, incubation with either cyclopamine or SANT1 resulted in ablation of the stomodeum and the primary oral opening (Fig. 1B–D compared to E). Hh pathway activation is therefore essential for primary mouth development.
Hedgehog regulates primary mouth size
As loss of Hh activation resulted in a small or absent primary mouth, we asked whether increasing the levels of Hh could modulate mouth size. Purmorphamine is a well-established Hh agonist (Dessaud et al., 2007; Sinha and Chen, 2006; Stanton and Peng, 2010), and continuous incubation of embryos with purmorphamine caused a dramatic increase in primary mouth size (Fig. 1F–H compared to E, and I). Therefore, Hh activation is both necessary and sufficient to drive an increase in mouth size. This effect on oral size was specific, as both inhibition and activation of Hh signaling, from the 2-cell stage, resulted in broadly similar changes in head proportions (Supplemental Fig. S1D and E); despite this, cyclopamine and purmorphamine had opposing effects on the oral opening. Furthermore, the cement gland and nasal pits, two anterior structures that develop in close proximity to the mouth, were not dramatically altered upon either treatment (Fig. 1B–H and Supplemental Fig. S1A–C). These data suggest that Hh-mediated regulation of primary mouth development is specific to the mouth, and can be uncoupled from early morphogenetic defects elsewhere in the craniofacial region.
We then tested whether mouth size is sensitive to graded levels of Hedgehog signaling by applying a range of drug dosages (Fig. 1B–I). To quantify the change in mouth size we measured the perimeter of the stomodeum after incubation with 2, 5 or 10 μM SANT1, 0.7% DMSO (control), or 2, 20, 100 μM purmorphamine. As incubation with either Hh modulator significantly decreased head size (Fig. S1E–F) we chose to normalize mouth perimeter to the width of the head, measured as the distance between the outer edges of the eyes. We found that increasing levels of purmorphamine caused a dose dependent enlargement in primary mouth size (Fig. 1F–I). Conversely, cyclopamine or SANT1 treatments caused a dose dependent reduction or complete loss of the stomodeum (Fig. 1B–I). Together, these data suggest that an intermediate level of Hh activation is required to determine normal mouth size.
The oral opening is sensitive to Hedgehog throughout development
Previous studies have shown that primary mouth specification is susceptible to Wnt manipulation for a short time window, up to stage 24, prior to appearance of the stomodeum (Dickinson and Sive, 2009). Therefore, we asked whether Hh activation functions throughout primary mouth development or during a brief period. We tested sensitivity to Hh perturbation during induction, basement membrane dissolution, or perforation stages by treating with either drug from the 2-cell stage, between stages 12 and 19, stages 19 and 37, or from stage 37 (see Fig. 1A). At all stages up to perforation, we found that purmorphamine treatment was sufficient to increase mouth size (Fig. 1N–Q). In contrast, tadpoles were sensitive to Hh inhibition only until perforation stages (stage 37) (Fig. 1I–M). This suggests that initial specification of the mouth requires early Hh activation. However, perforation of the mouth may be separately controlled as a later increase in Hh signaling is sufficient to expand mouth size.
Hedgehog signaling regulates stomodeal basal lamina dissolution
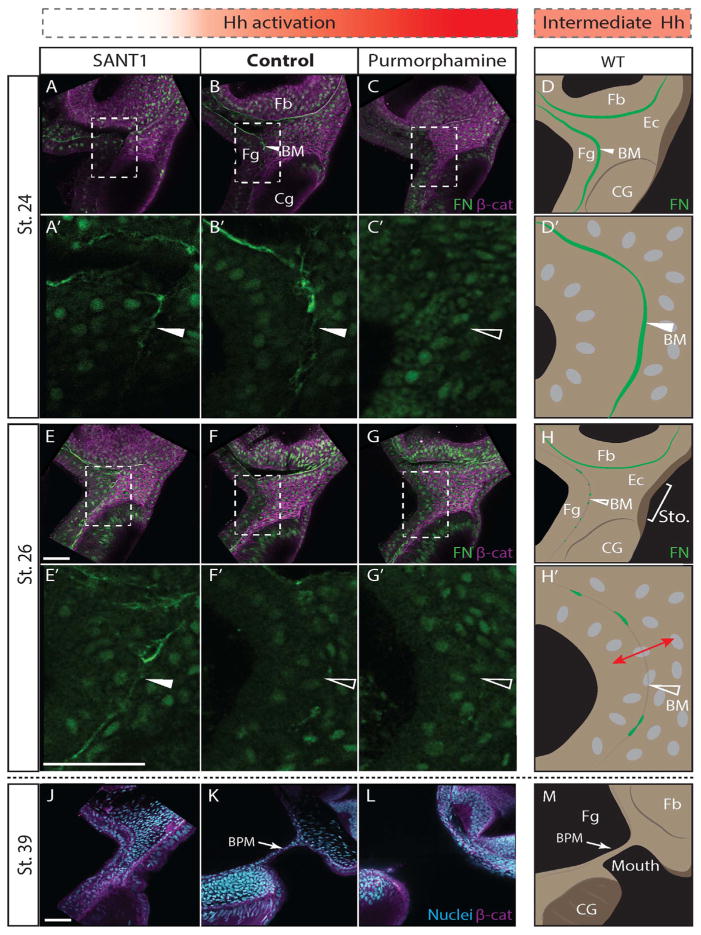
We next sought to understand the cell biological mechanism of Hh action during primary mouth development. Prior to intercalation stages, a basement membrane comprising fibronectin and laminin separates endoderm and ectoderm (Dickinson and Sive, 2006), and the buccopharyngeal membrane forms after contact between the foregut endoderm with stomodeal ectoderm. Endoderm–ectoderm contact initiates basal lamina dissolution, which permits cell intercalation prior to perforation (Dickinson and Sive, 2006). Therefore, we considered whether the basement membrane component fibronectin was maintained after cyclopamine or SANT1 treatment. Fibronectin immunoreactivity marks the stomodeal BM at stage 24 (Fig. 2B), which is lost by stage 26 (Fig. 2E). After cyclopamine treatment, basement membrane fibronectin was maintained at stage 26 (Fig. 2A and D), suggesting that Hh is necessary for basement membrane dissolution.
Fig. 2.
Hedgehog signaling is necessary and sufficient for basement membrane dissolution. (A–C, E–G′) Sagittal sections through primary mouth stained for β-catenin in magenta, fibronectin (FN) and nuclei in green. (Fg) foregut, (BM) basement membrane, (Fb) forebrain, (Cg) cement gland. (A–C) Stage 24. (A) Embryo treated with 10 μM SANT1 from the 2-cell stage. (A′) Magnified view of endoderm–ectoderm interface and basement membrane. FN is observed (white arrowhead) (n=7). (B) Control. (B′) FN immunofluorescence indicates the presence of BM between foregut and ectoderm (white arrowhead, n=7). (C) Embryo treated with 250 μM purmorphamine. (C′) No FN immuofluorescence is observed between foregut and ectoderm (open white arrowhead, n=6/7). (D–D′) Schematic indicating anatomy of sections represented in B–B′. Fibronectin-rich basement membrane separates foregut and ectoderm. (E–G′) Stage 26. (E) Embryo treated with 10 μM SANT1. (E′) shows persistent FN immunofluorescence (white arrowhead, n=8). (F) Stage 26 control. (F′) Almost no BM FN is observed in controls (open arrowhead, n=12). (G) Stage 26 embryo treated with 100 μM purmorphamine. (G′) No FN immuofluorescence is observed (open white arrowhead, n=10). (H–H′) Schematic indicating anatomy of WT sections represented in F–F′. Fibronectin-rich basement membrane is absent or broken, foregut and ectoderm cells mix (red arrow). (J–L) Sagittal sections of stage 39 tadpoles stained for β-catenin (magenta) and DAPI. (J) Tadpole treated with 10 μM SANT1 (n=5). (K) Control tadpole. BPM is observed as a single layer epithelium (n=6). (L) Tadpole treated with 100 μM purmorphamine. No BPM is present (n=4). Scale bars indicate 50 μm. (M) Schematic indicating anatomy of WT sections represented in (K). Buccopharyngeal membrane (BPM) separates foregut from external environment and indicates site of future mouth.
This raised the possibility that increasing Hh activation might ectopically promote basement membrane dissolution. Indeed, in contrast to control and cyclopamine treatments, fibronectin was significantly diminished or completely absent in stage 24 purmorphamine treated embryos (Fig. 2C). Therefore, Hh is necessary and sufficient to promote basement membrane dissolution. Precocious loss of the basement membrane could promote premature endoderm–ectoderm mixing and increase the duration or amount of intercalation. This mechanism is consistent with our observations that increased Hh activity causes a dose dependent increase in mouth size.
Previous studies suggested that basement membrane maintenance reduces mouth size by inhibiting endoderm–ectoderm intercalation and buccopharyngeal membrane formation (Dickinson and Sive, 2009). This prompted us to examine the BPM: in stage 39 control embryos, a one-cell thick BPM is observed indicating that endoderm and ectoderm cells have intercalated into a single cell layer (Fig. 2B). In contrast, after cyclopamine treatment, endoderm and ectoderm were morphologically distinct (Fig. 2A), and the intervening mesenchyme was still present. This phenotype is consistent with a lack of mesenchymal clearance and intercalation, and suggests that Hh is required for timely BM dissolution and BPM formation.
As BM maintenance can inhibit intercalation in the stomodeum, we hypothesized that premature BM dissolution could promote intercalation and premature perforation. We compared purmorphamine treated embryos to controls at stage 39, when the buccopharyngeal membrane is evident but has thinned. The BPM was absent in embryos treated with purmorphamine (compare Fig. 2L to K). Therefore, Hh activation is sufficient to promote premature perforation and primary mouth opening.
Stomodeal inhibition of Wnt is downstream of Hh signaling
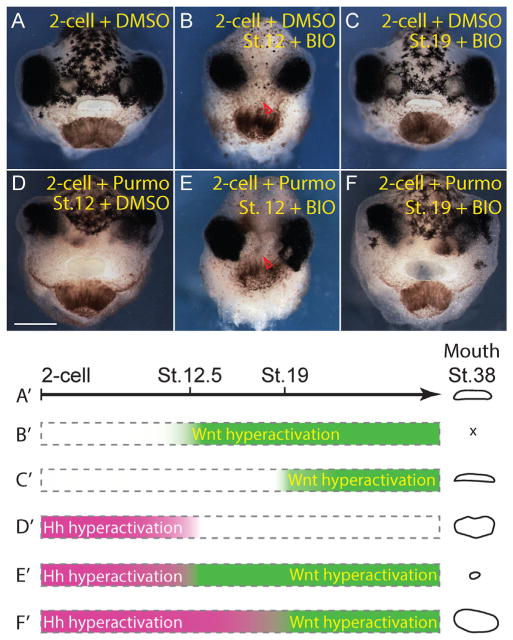
Basement membrane dissolution is expedited after either gain of Hh signaling (Fig. 2) or loss of the Wnt inhibitors Frzb-1 and Crescent (Dickinson and Sive, 2009). Our data suggest that Hh signals are required for stomodeal formation during an earlier developmental window than that reported for stomodeal Frzb-1 or Crescent. Therefore, we asked if Wnt signal inhibition functions downstream of Hh activation in determining primary mouth size (Fig. 3). In this case, activation of Wnt should be able to rescue mouth enlargement caused by Hh gain of function. To test this, we first treated embryos with DMSO or purmorphamine at the 2-cell stage. After washout at stage 12.5 or 19, embryos were treated with a glycogen synthase kinase-3 (GSK-3) inhibitor, BIO, which activates β-catenin dependent Wnt signaling (Meijer et al., 2003). Consistent with previous reports on Wnt inhibition (Dickinson and Sive, 2009), BIO treatment during neurulation (stage 12.5–19), after incubation with DMSO (2 cell-stage-12.5), caused a complete loss of the mouth (Fig. 3B), while BIO treatment from stage 19 did not affect mouth size (Fig. 3C). Embryos treated with BIO from stage 12.5, after incubation with purmorphamine, showed a reversal of purmorphamine induced stomodeal expansion, with a complete loss of the oral opening (compare Fig. 3E to D). Conversely, BIO treatment from stage 19, after incubation with purmorphamine, was unable to reverse the effect of increased Hh signaling (Fig. 3F). These data suggest that stomodeum is refractory to inhibition of Wnt signals after stage 19, consistent with previous reports showing that Frzb-1/Crescent needs to be down regulated after stage 19 (Dickinson and Sive, 2009).
Fig. 3.
Wnt inhibition acts downstream of Hh signaling during BPM dissolution. (A–F) Stage 38 tadpoles. (A) Control tadpole incubated with 0.7% DMSO continuously from 2-cell stage. (B) Tadpole incubated with 0.7% DMSO, then 15 μM BIO from stage 12.5 (n=34). (C) Tadpole incubated with 0.7% DMSO, then 15 μM BIO from stage 19 (n=34). (D) Tadpole incubated with 100 μM purmorphamine, then 0.07% DMSO from stage 12.5 (n=34). Primary mouth is enlarged. (E) Tadpole incubated with 100 μM purmorphamine, then 15 μM BIO from stage 12.5 (n=34). (F) Tadpole incubated with 100 μM purmorphamine, then 15 μM BIO from stage 19 (n=34). Scale bars indicate 100 μm. (G) Schematic indicating drug application scheme and outcome. Red arrowheads (B and E) indicate absence of primary mouth.
As Hh and Wnt signaling function antagonistically in many contexts (Akiyoshi, 2006; Cain et al., 2009; He et al., 2006; Lee et al., 2000; Varnat et al., 2010; Wheway et al., 2013), we propose that SHH signals, expressed in the prechordal plate (Ekker et al., 1995), activates facial Wnt inhibitor expression, such as Crescent and Frzb-1, which in turn mediates basal lamina dissolution (Dickinson and Sive, 2009). As prolonged Hh signaling causes continued enlargement of the mouth, but Wnt sensitivity is short-lived, Hh is likely to act independently of Wnt during later oral opening (Fig. 4D).
Fig. 4.
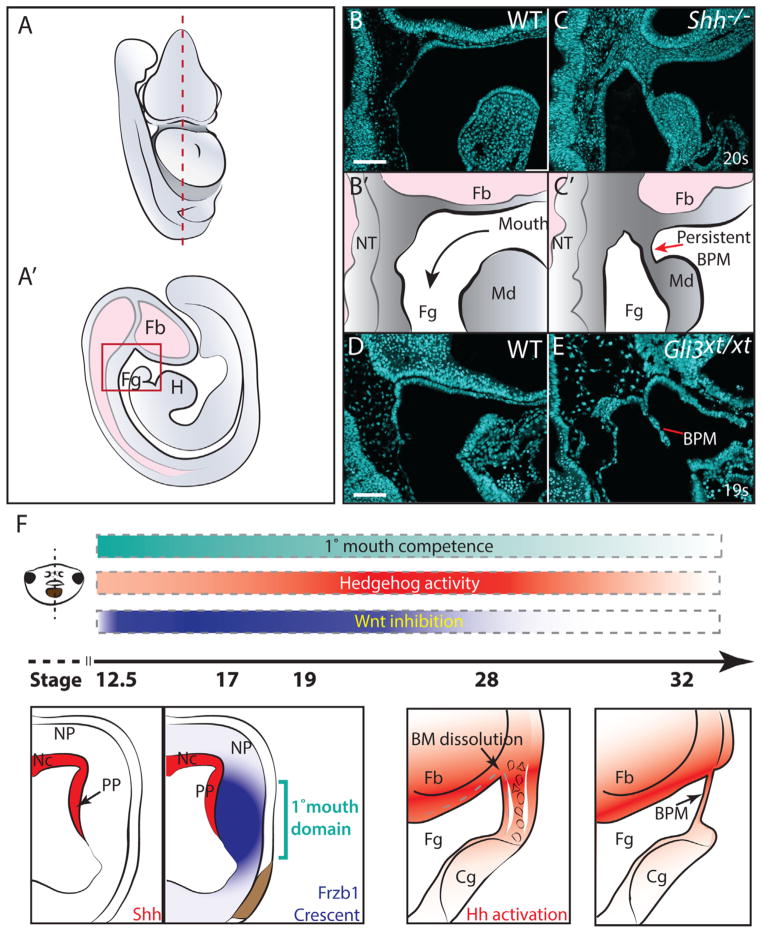
Sonic hedgehog and Gli3 are required for perforation of the mammalian BPM. (A) Schematic frontal view of E9.0 mouse embryo. Dashed line indicates section plane. (B) Schematic of section. Red box indicates region represented in panels (C) and (D′). (Fb) forebrain, (Fg) pharyngeal foregut, (H) Heart. (C–D) Nuclei are stained with DAPI (cyan) (B) Sagittal section of E9.0 (20 somite) Shh+/+ embryo (n=3). (B′) Schematic illustrating anatomy in (B). (C) Sagittal section through E9.0 Shh−/− embryo (n=3). (C′) Schematic illustrating anatomy depicted in (C). (D–E) Sagittal sections through E9.0 (19 somite) Gli3+/+ and Gli3xt/xt embryos, respectively. Remnant buccopharyngeal membrane (BPM) is observed in Shh−/− and Gli3xt/xt embryos. Nuclei are stained with DAPI (cyan). Scale bars indicate 100 μm. B–C are littermates, as are D–E. (F) Proposed model of primary mouth formation in Xenopus. Frontal schematic indicates sectional plane of diagrams. Cyan bar indicates stages of primary mouth competence. Red bar indicates level of Hh activation while blue indicates Wnt inhibition, where white is none, and bright color is high. Wnt inhibitors Crescent and Frzb-1 are expressed in primary mouth ectoderm (blue). Primary mouth ectoderm is induced adjacent to Hh signal where Wnt inhibition is highest (cyan bracket). By stage 28, BM is indicated by white outlines, and mesenchymal cells are illustrated in black. Hh signal activation (red) is highest ventral to the forebrain (Fb). (Cg) and (Fg) indicate cement gland and foregut, respectively. Wnt inhibitors are no longer expressed, and stomodeum is refractive to Wnt activation. By stage 39, endoderm–ectoderm intercalation forms monolayer buccopharyngeal membrane (BPM). Hh signal activation is required through intercalation stages.
Hh regulates mammalian BPM perforation
Our data demonstrate a key role for Hh signaling in Xenopus mouth development; genetic evidence suggests that this is also the case in humans (DéMurger et al., 2014; Legius et al., 1988). To ask if Hh loss of function might perturb mammalian BPM perforation, we examined mice mutant for Sonic hedgehog (Shh). In humans, the BPM disappears by 15 days gestation, while in mice perforation of the BPM occurs by the 17-somite stage at E9 (Poelmann et al., 1985; Theiler, 1969; Standring, 2009). Strikingly, cross sections of E9 embryos revealed that the BPM was inappropriately retained in Shh mutants (Fig. 4C).
In humans, Greig cephalopolydactyly (GCPS) and Pallister–Hall syndromes are caused by mutation of the Hedgehog effector Gli3 (DéMurger et al., 2014; Legius et al., 1988). Both syndromes are associated with an absence of oral perforation (DéMurger et al., 2014). We examined BPM perforation using mice carrying the Gli3xt-J allele which models GCPS (Hui and Joyner, 1993). We found that loss of Gli3 in this mutant was sufficient to retard dissolution of the BPM (Fig. 4D and E). This outcome is of interest because it provides insights into the mechanism of Gli3 action. Biochemically, Gli3 is a transcription factor; it is believed that in the absence of Hh, Gli3 represses Hh target genes, while Hh activation converts Gli3 into a transcriptional activator (Blaess et al., 2008; Wang et al., 2007). Our data are notable because in the context of human Gli3 mutations, it has been unclear whether the oral phenotypes reflect a requirement for Gli3 as a transcriptional activator or as a repressor (DéMurger et al., 2014). Because BPM persistence in Gli3xt-J/xt-J mice phenocopies Shh mutant mice, our data support the argument that Gli3 acts as a transcriptional activator during oral perforation.
Anatomical characterization in diverse systems including sea urchins, urodeles, Xenopus, and mouse have suggested that interactions between foregut and stomodeal ectoderm are important for oral perforation (Dickinson and Sive, 2006; Hardin and Armstrong, 1997; McClay et al., 1992; Poelmann et al., 1985; Soukup et al., 2013; Takahama et al., 1988; Theiler, 1969; Watanabe et al., 1984; Waterman, 1977). However, molecular regulation of primary mouth opening is largely untested, especially as perforation occurs early in development and perturbation of major signaling cascades results in broad cranial defects. Moreover, because the mammalian primary mouth develops internally, defects in its development are obscured by secondary oral phenotypes. Indeed, the evidence of human BPM anomalies is primarily anecdotal and persistent BPM is frequently unreported due to the severity of associated phenotypes (Verma and Geller, 2009).
Taken together, we provide the first demonstration of a role for HH signaling in primary mouth development, and moreover we provide the first data directly revealing a molecular mechanism in mammalian BPM development. Our data suggest that HH and Gli3 are required to drive basement membrane dissolution, endoderm–ectoderm intercalation and perforation of the primary mouth. These experiments also illustrate the feasibility of using Xenopus to provide testable hypotheses in mammals. Combined, these data provide novel evolutionary insights into the genetic regulation governing oral opening, and may be relevant to poorly studied human anomalies, such as persistent BPM and atresia.
Materials and methods
Animals
X. laevis embryos were cultured using standard methods (Sive et al., 2000). Staging was according to Nieuwkoop and Faber (1994). Shh mutants were initially generated by crossing floxed mutants to Sox2-Cre (Lewis et al., 2001). Gli3xt-J is a spontaneously occurring intergenic deletion of Gli3 (Hui and Joyner, 1993). Mice were a kind gift from Dr. Steven Vokes.
Chemical inhibitors
Xenopus embryos were incubated in 12-well plates, 10 embryos per well. For Hh perturbation 250 μM, 50 μM, or 5 μM cyclopamine (Cayman Chemicals), 20 μM, 5 μM, or 2 μM SANT1 (Sigma), or 2 μM, 20 μM, or 100 μM purmorphamine (inSolution, Sigma) were added to media. Control embryos were incubated in 0.7% DMSO in media. For single dose experiments 250 μM cyclopamine, 20 μM SANT1 and 100 μM purmorphamine were used.
Immunohistochemistry
Immunohistochemistry was performed according to standard protocols using a polyclonal anti-β-catenin antibody (Santa Cruz 7199) or monoclonal 4H2 anti-fibronectin antibody (1:200) (Danker et al., 1993) (kind gift from Douglas DeSimone), revealed by an Alexa Fluor-conjugated secondary antibody (1:200). DNA was visualized with 0.1% DAPI. Embryos were cleared in benzyl benzoate:benzyl alcohol and staining visualized using a Zeiss 700 microscope.
Supplementary Material
Acknowledgments
Funding
This work was funded by grants from Biotechnology and Biological Sciences Research Council (BBSRC) (BB/E013872/1 and BB/I021922/1) and Wellcome Trust (081880/Z/06/Z) to K.J.L., as well as a King’s College London Dental studentship to K.J.L. and T.B. The National Institute of Dental and Craniofacial Research/National Institutes of Health (NIDCR/NIH) (F32DE023272) funded J.T. Work in the J.B.W. lab is supported by grants from the National Institute of General Medical Sciences (GM074104)/National Heart, Lung and Blood Institute (HL117164); J.B.W. is an Early Career Scientist of the Howard Hughes Medical Institute.
We are grateful to Roman Khonsari, Jordan Lewandowski and Steven Vokes for valuable discussion and the Vokes lab for kind gift of mice. We thank Marc Dionne for critical reading of the manuscript.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2014.09.029.
Footnotes
Author contributions
T.B. J.T. and K.J.L. conceived project. J.T. and T.B. performed experiments. J.T., T.B., J.B.W., and K.J.L. analyzed and interpreted the data. J.T. designed all figures and all authors contributed to the manuscript.
References
- Akiyoshi T. Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving Wnt signalling activation. Gut. 2006;55:991–999. doi: 10.1136/gut.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess S, Stephen D, Joyner AL. Gli3 coordinates three-dimensional patterning and growth of the tectum and cerebellum by integrating Shh and Fgf8 signaling. Development. 2008;135:2093–2103. doi: 10.1242/dev.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain JE, Islam E, Haxho F, Chen L, Bridgewater D, Nieuwenhuis E, Hui CC, Rosenblum ND. GLI3 repressor controls nephron number via regulation of Wnt11 and Ret in ureteric tip cells. PLoS One. 2009;4:e7313. doi: 10.1371/journal.pone.0007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker K, Hacke H, Ramos J, DeSimone D, Wedlich D. V(+)-fibronectin expression and localization prior to gastrulation in Xenopus laevis embryos. Mech Dev. 1993;44:155–165. doi: 10.1016/0925-4773(93)90064-5. [DOI] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Sive H. Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary. Semin Cell Dev Biol. 2007;18:525–533. doi: 10.1016/j.semcdb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dickinson AJG, Sive H. Development of the primary mouth in Xenopus laevis. Dev Biol. 2006;295:700–713. doi: 10.1016/j.ydbio.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Dickinson AJG, Sive HL. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–1081. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DéMurger FDE, Ichkou A, Mougou-Zerelli S, Le Merrer M, Goudefroye GER, Delezoide A-L, Quélin C, Manouvrier S, Baujat G, Fradin M, et al. New insights into genotype–phenotype correlation for GLI3 mutations. Eur J Hum Genet. 2014:1–11. doi: 10.1038/ejhg.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC, McGrew LL, Lai CJ, Lee JJ, von Kessler DP, Moon RT, Beachy PA. Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis. Development. 1995;121:2337–2347. doi: 10.1242/dev.121.8.2337. [DOI] [PubMed] [Google Scholar]
- Gradl D, Kühl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J, Armstrong N. Short-range cell–cell signals control ectodermal patterning in the oral region of the sea urchin embryo. Dev Biol. 1997;182:134–149. doi: 10.1006/dbio.1996.8436. [DOI] [PubMed] [Google Scholar]
- He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, Luxon BA, Xie J. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J Biol Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- Hollemann T, Tadjuidje E, Koebernick K, Pieler T. Manipulation of hedgehog signaling in Xenopus by means of embryo microinjection and application of chemical inhibitors. Methods Mol Biol. 2007;397:35–45. doi: 10.1007/978-1-59745-516-9_3. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Jacox LA, Dickinson AJ, Sive H. Facial transplants in Xenopus laevis embryos. J Vis Exp. 2014 Mar 26;(85) doi: 10.3791/50697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AE, Dickinson AJ. Quantitative analysis of orofacial development and median clefts in Xenopus laevis. Anat Rec. 2014;297:834–855. doi: 10.1002/ar.22864. [DOI] [PubMed] [Google Scholar]
- Kliegman R. Nelson Textbook of Pediatrics. W B Saunders Company; Philadelphia, PA: Elsevier/Saunders; 2011. [Google Scholar]
- Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127:109–118. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- Legius E, Moerman P, Fryns JP, Vandenberghe K, Eggermont E. Holzgreve–Wagner–Rehder syndrome: Potter sequence associated with persistent bucco-pharyngeal membrane. A second observation. Am J Med Genet. 1988;31:269–272. doi: 10.1002/ajmg.1320310203. [DOI] [PubMed] [Google Scholar]
- Lewis C, Krieg PA. Reagents for developmental regulation of hedgehog signaling. Methods. 2014;66:390–397. doi: 10.1016/j.ymeth.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- McClay DR, Armstrong NA, Hardin J. Pattern formation during gastrulation in the sea urchin embryo. Development 1992. 1992;(Supplement):33–41. [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus Laevis (Daudin) Garland Science; New York: 1994. [Google Scholar]
- Peyrot SM, Wallingford JB, Harland RM. A revised model of Xenopus dorsal midline development: differential and separable requirements for Notch and Shh signaling. Dev Biol. 2011;352:254–266. doi: 10.1016/j.ydbio.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai KG, Kamath VV, Kumar GS, Nagamani N. Persistent buccopharyngeal membrane with cleft palate. A case report. Oral Surg Oral Med Oral Pathol. 1990;69:164–166. doi: 10.1016/0030-4220(90)90319-n. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Dubois SV, Hermsen C, Smitsvan Prooije AE, Vermeij-Keers C. Cell degeneration and mitosis in the buccopharyngeal and branchial membranes in the mouse embryo. Anat Embryol. 1985;171:187–192. doi: 10.1007/BF00341413. [DOI] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: a laboratory manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Soukup V, Horácek I, Cerny R. Development and evolution of the vertebrate primary mouth. J Anat. 2013;222:79–99. doi: 10.1111/j.1469-7580.2012.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring S. Gray’s Anatomy, the anatomical basis of clinical practice. 40. Churchill Livingstone, Elsevier; London: 2009. [Google Scholar]
- Stanton BZ, Peng LF. Small-molecule modulators of the sonic hedgehog signaling pathway. Mol Biosyst. 2010;6:44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- Takahama H, Sasaki F, Watanabe K. Morphological changes in the oral (buccopharyngeal) membrane in urodelan embryos: development of the mouth opening. J Morphol. 1988;195:59–69. doi: 10.1002/jmor.1051950106. [DOI] [PubMed] [Google Scholar]
- Theiler K. On the anlage of the fore-gut in the house mouse and the formation of furrows on the surgace of the egg-cylinder. Z Anat Entwick-lungsgesch. 1969;128:40–46. [PubMed] [Google Scholar]
- Varnat F, Zacchetti G, Altaba ARI. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev. 2010;127:73–81. doi: 10.1016/j.mod.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Verma SP, Geller K. Persistent buccopharyngeal membrane: report of a case and review of the literature. Int J Pediatr Otorhinolaryngol. 2009;73:877–880. doi: 10.1016/j.ijporl.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Wang C, Rüther U, Wang B. The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol. 2007;305:460–469. doi: 10.1016/j.ydbio.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Sasaki F, Takahama H. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the anuran embryo. Anat Rec. 1984;210:513–524. doi: 10.1002/ar.1092100312. [DOI] [PubMed] [Google Scholar]
- Waterman RE. Ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the hamster embryo. Dev Biol. 1977;58:219–229. doi: 10.1016/0012-1606(77)90088-4. [DOI] [PubMed] [Google Scholar]
- Waterman RE. Formation and perforation of closing plates in the chick embryo. Anat Rec. 1985;211:450–457. doi: 10.1002/ar.1092110412. [DOI] [PubMed] [Google Scholar]
- Waterman RE, Schoenwolf GC. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the chick embryo. Anat Rec. 1980;197:441–470. doi: 10.1002/ar.1091970408. [DOI] [PubMed] [Google Scholar]
- Wheway G, Abdelhamed Z, Natarajan S, Toomes C, Inglehearn C, Johnson CA. Aberrant Wnt signalling and cellular over-proliferation in a novel mouse model of Meckel=Gruber syndrome. 2013;377:55–66. doi: 10.1016/j.ydbio.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Williams JA, Guicherit OM, Zaharian BI, Xu Y, Chai L, Wichterle H, Kon C, Gatchalian C, Porter JA, Rubin LL, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.