Abstract
Parkinson's disease (PD) is a neurodegenerative disease causing both motor and non-motor symptoms. Drooling, an excessive pooling and spillover of saliva out of the oral cavity, is one of the non-motor symptoms in PD patients that produces various negative physical and psychosocial consequences for patients and their caregivers. At present, the pathophysiology of drooling in PD is not completely certain; however, impaired intra-oral salivary clearance is likely the major contributor. There are neither standard diagnostic criteria nor standard severity assessment tools for evaluating drooling in PD. In accordance with the possible pathophysiology, dopaminergic agents have been used to improve salivary clearance; however, these agents are not completely effective in controlling drooling. Various pharmacological and nonpharmacological treatment options have been studied. Local injection with botulinum toxin serotypes A and B into major salivary glands is most effective to reduce drooling. Future research to explore the exact pathophysiology and develop standard diagnostic criteria and standard severity assessment tools are needed to formulate specific treatment options and improve patient care.
Keywords: Drooling, Sialorrhea, Parkinson's disease, Botulinum toxin
1. Introduction
Drooling may occur in many neurological disorders including neuromuscular diseases such as myasthenia gravis, amyotrophic lateral sclerosis (ALS) and oculopharyngeal muscular dystrophy, neurodegenerative diseases such as Parkinson's disease (PD), multiple system atrophy (MSA), progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB) and corticobasal degeneration (CBD), and cerebrovascular diseases. Drooling is generally defined as excessive pooling and poor control of saliva in the oral cavity that might be caused by impaired salivary clearance whereas sialorrhea refers to overflow or overproduction of saliva [1]. Regrettably, both terms are sometimes used interchangeably. If patients have drooling, they might subsequently spill saliva from their oral cavity, or might aspirate the saliva causing aspiration pneumonia. Other possible negative consequences are poor oral hygiene and social embarrassment. In PD, drooling is considered a non-motor symptom. This article focuses on the prevalence, associative factors, negative impacts of drooling, normal physiology of salivation and swallowing, pathophysiology of drooling, assessment tools, and treatment options for drooling in PD.
2. Methods
References for this review were identified through searches of PubMed using the search terms “Drooling and Parkinson's disease”, “Sialorrhea and Parkinson's disease” and “Treatment of drooling in Parkinson's disease”. We mainly selected papers that were published between January 1973 to August 2014. Only reports published in English were included. We cited references reflecting personal selection of the review authors.
3. Prevalence, Associated Factors and Negative Impacts of Drooling in PD
Due to the lack of a standard definition and criteria for diagnosing drooling in PD patients, estimates of prevalence vary. Previous studies showed that prevalence ranged from 10 to 84% (Table 1) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Various tools such as the Unified Parkinson's Disease Rating Scale (UPDRS) part II [12,13,14,15]; Scales for Outcomes in PD for Autonomic Symptoms (SCOPA-AUT) [7,16]; PD non-motor symptoms questionnaire (PDNMSQuest) [8,10]; and different types of screening questionnaires [2,3,4,5,6,7,10,11] were used to screen drooling. The factors associated with drooling have been reported. However, results vary among studies and the conclusion remains unclear. Factors possibly associated with drooling were severity of PD [2,14], male gender [3,10], aging [6], hallucinations [11], duration of PD [13], the sum of the scores of UPDRS part II and III greater than 28 points, dysarthria, dysphagia, orthostatic hypotension, and a history of using antidepressants [12]. Drooling during PD can have negative impact for both patients and caregivers. Many negative physical sequelae were reported to follow the course of drooling such as perioral dermatitis, poor oral hygiene, bad breath, increased amount of intra-oral occult bacteria, eating and speaking difficulty, and an increased rate of respiratory tract infection from silent aspiration of saliva [11,17,18,19,20,21]. Psychosocially, drooling PD patients showed poor quality of life (QoL), i.e., social embarrassment and increasing emotional distress [6,11]. In addition, drooling patients affected their caregivers by increasing their burden, depression and anxiety, and reducing their QoL [16].
Table 1.
Prevalence of drooling in Parkinson's disease
| Year | Reference | Screening tools | Number surveyed | Prevalence (%) |
|---|---|---|---|---|
| 2012 | Damian et al. [16] | SCOPA-AUT | 62 | 81 |
| 2012 | Ozdilek et al. [15] | UPDRS part II: salivation subscore | 50 | 84 |
| 2012 | Rana et al. [14] | UPDRS part II: salivation subscore | 307 | 40 |
| 2012 | Perez-Lloret et al. [13] | UPDRS part II: salivation subscore | 419 | 37 |
| 2011 | Müller et al. [12] | UPDRS part II: salivation subscore | 207 | 42 |
| 2010 | Leibner et al. [11] | Questionnaire: 7-item drooling survey questionnaire | 58 | 59 |
| 2008 | Cheon et al. [10] | PD-NMSQuest | 74 | 32 |
| 2008 | Nicaretta et al. [9] | UPDRS part II: salivation subscore | 134 | 10 |
| 2007 | Martinez-Martin et al. [8] | PD-NMSQuest | 525 | 42 |
| 2007 | Verbaan et al. [7] | SCOPA-AUT | 420 | 73 |
| 2007 | Kalf et al. [6] | Questionnaire: “Do you suffer from involuntary loss of saliva (drooling)?” | 216 | 49 |
| 2002 | Siddiqui et al. [5] | Questionnaire: rating 0-4 point for detecting severity of symptoms 0 = normal 1 = rare (one per month) 2 = occasional (one per week) 3 = frequent (one per day) 4 = constant. |
44 | 52 |
| 2002 | Volonté et al. [4] | Questionnaire: Present or absent nocturnal sialorrhea | 65 | 15 |
| 2000 | Scott et al. [3] | Questionnaire: present or absent drooling | 943 | 40 |
| 1991 | Edwards et al. [2] | Questionnaire: rating 0-4 point for detecting severity of symptoms 0 = normal 1 = rare (one per month) 2 = occasional (one per week) 3 = frequent (one per day) 4 = constant. |
96 | 70 |
UPDRS: Unified Parkinson's Disease Rating Scale; SCOPA-AUT: Scales for Outcome in Parkinson's disease; autonomic; PD-NMSQuest: Parkinson's disease non-motor symptoms questionnaire
4. Normal Physiology of Salivation and Swallowing
The processes of salivation are controlled by both sympathetic and parasympathetic nervous system. However, facilitation of ingestion and swallowing are mainly contributed by parasympathetic nervous system. The parasympathetic afferent pathways receive unconditioned reflex stimulation from the pharynx and esophagus. Then, signals are conducted via the vagus and spinal splanchnic nerves to the salivary center located in the medulla. The parasympathetic outputs are conducted via two different pathways including the glossopharyngeal nerve, which then innervates the otic ganglion, and, subsequently, to the parotid glands via the auriculotemporal nerve and the facial nerve through the chorda tympani nerve to the submandibular ganglia and then innervates the submandibular and sublingual glands via the lingual nerve [22].
The normal physiology of human swallowing is composed of three phases: oral, pharyngeal, and esophageal. The oral phase is voluntary whereas pharyngeal and esophageal phases are involuntary. When swallowing begins, the oropharyngeal phase uses more than 30 different muscles to coordinate and precisely time moving the food bolus to the esophagus. The upper esophageal sphincter (UES) subsequently opens and the bolus passes through the esophagus by peristalsis into the stomach [23]. The central motor control areas include the premotor cortex, primary motor cortex, basal ganglia, pedunculopontine nuclei, and cerebellum; they project descending motor outputs to the medullary swallowing center which includes a swallowing central pattern generator and its interneurons such as the nucleus of the solitary tract. After that, the medullary swallowing center provides the outputs to the structures involved in the swallowing process such as the tongue, larynx, pharynx, and upper esophagus. Lingual muscles are controlled by the motor output of the hypoglossal nucleus while laryngeal, pharyngeal and upper esophageal muscles are controlled by motor output of the nucleus ambiguus [24]. The oropharyngeal phase is most affected in PD patients.
5. Pathophysiology of Drooling in PD
Drooling is more prominent during the “off” period. Two major domains possibly influencing the pathophysiology of drooling in PD have been proposed: one is an abnormality of salivary production and the other is insufficient salivary clearance. Overproduction of saliva might cause drooling. However, many studies showed that drooling PD patients produced less saliva compared to normal controls [25,26,27]. The exact mechanisms causing decreased salivary production are not understood [26]. A possible explanation is dopamine deficiency. Previous studies in both invertebrate and vertebrate animal models showed that dopamine modulates salivary secretion [28,29]. Experimental studies in rats demonstrated that activation of central and peripheral dopamine receptors produced salivary secretion [29]. Supportive evidence consists of lesions at the striatum, globus pallidus, or its output pathway, which is the lateral mesencephalic reticular formation, could significantly decrease salivary secretion [30]. A pathological study showed Lewy bodies in the superior cervical ganglion, cervical sympathetic trunk, peripheral vagus nerve, and submandibular glands [31]. Another study used Tc-99m scintigraphy to measure the activity of salivary production and speed of salivary excretion of the parotid glands in drooling PD patients compared to healthy controls. The result showed that salivary production in drooling PD patients and healthy controls was the same. However, the speed of salivary excretion to a discrete stimulus in drooling PD patients was significantly higher compared to healthy controls [32]. According to the above-cited evidence, increasing salivary production should not be a main contributor to the pathophysiology of drooling in PD. However, increasing speed of salivary excretion might partially contribute to its pathophysiology.
Swallowing dysfunction in PD patients, in which the oropharyngeal phase is a major component, is the other domain that might contribute to drooling. Oropharyngeal dysphagia in PD patients can result from bradykinesia. A previous animal study showed that 6-hydroxydopamine (6-OHDA) injected rat models exhibited slow tongue protrusion speed and that average tongue press time was significantly longer compared to normal controls [33]. Another study showed that the maximum tongue pressure in advanced PD patients was lower compared to early or moderate PD patients, and that oropharyngeal transit time was negatively correlated with tongue movement speed [34]. Both studies reflect the fact that PD patients have bradykinesia and poor muscle control of the tongue. Therefore, dysfunction of the motor control of the tongue contributes to the pathophysiology of dysphagia and, therefore, also possibly drooling. A videofluorographic study of 6-OHDA rat models showed that the parkinsonian rat models had higher rates of aberrant food bolus movement compared to normal controls [35]. Another study using barium swallow with videofluoroscopy in drooling PD patients demonstrated a direct correlation between the severity of dysphagia and the severity of drooling [36]. Therefore, oropharyngeal dysphagia might be a major contributor to the pathophysiology of drooling in PD. In addition, upper esophageal dysmotility might also affect dysphagia and drooling. The data from previous manometric studies demonstrated evidence of impaired UES relaxation in PD patients compared to normal controls. However, this factor cannot be the sole cause of dysphagia if patients have sufficient pharyngeal propulsive forces and clearance mechanisms [37,38].
In addition, a recent study showed that severe hypomimia, unintentional mouth opening and stooped posture with dropped head, could cause drooling in PD patients by losing the ability to maintain saliva within the oral cavity [39]. In contrast, there is no obvious evidence that medication-induced dyskinesia can produce drooling. The possible domains contributing to the pathophysiology of drooling in PD are summarized in Figure 1.
Figure 1.
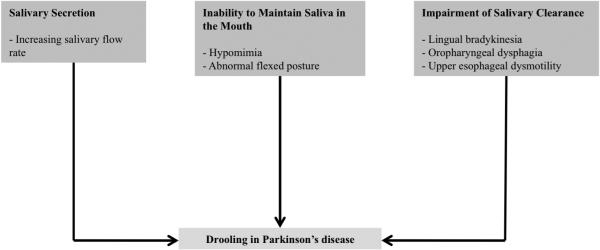
Possible pathophysiology of drooling in Parkinson's disease
6. Assessment Tools for Drooling in PD
The assessment tools to evaluate drooling in PD include both objective and subjective measures. Objective tools were developed to measure the volume of saliva and salivary flow. The limitations of these tools are that they are time-consuming and cannot evaluate the psychosocial impairment. Therefore, subjective tools were developed. The subjective measures in many previous studies were the UPDRS part II salivary subscores to evaluate drooling treatment responses and visual analog scales (VAS) to assess the frequency, familial (VAS-FD) and social distress (VAS-SD); however, not all scales are validated. Three drooling-specific rating scales including the Drooling Severity and Frequency Scale (DSFS), Drooling Rating Scale (DRS) and Sialorrhea Clinical Scale for PD (SCS-PD) have been used to evaluate drooling in PD. The DSFS, a semi-quantitative scale, was used in studies to evaluate drooling in PD and cerebral palsy (CP). The scale is composed of two domains: (a) the severity of drooling rated on a five-point scale and (b) frequency of drooling rated on a four-point scale. Since the DSFS is easy to administer it is widely used. However, the limitations of this scale are no assessment of the psychosocial impact, no validation and no evidence of correlation between this scale and the objective measures of salivary secretion.
With the DRS, patients are rated for severity of drooling by 0 to 3 points. The DRS is scored for the preceding week while sitting, standing, staying in bed, talking, and eating or drinking. The advantages of this scale are ease of use and evaluation of drooling in various situations, but the limitation is the lack of psychosocial evaluation. The SCS-PD was developed to cover social and functional impairment with respect to the severity and frequency of drooling. Patients rate a score from 0 to 3 points per question for seven questions covering the severity, frequency and feeling of discomfort during day-time, night-time, eating, speaking and social participation within the preceding week. The two advantages of this scale are coverage of the social and functional impairment and also validation using saliva volume measurements in PD patients and healthy volunteers. This scale was originally made and validated in Spanish and then translated into English. Therefore, the language translation might be an important factor contributing to measurement bias.
Recommendations from the Movement Disorders Society (MDS) do not specify which rating scale should be the standard subjective tool. However, they suggest that all three rating scales can be used to evaluate drooling in PD patients [40].
Another consideration for assessing drooling in PD is assessment of swallowing function especially in the oropharyngeal phase. Earlier Nilsson et al. [41] used the ROSS test to measure the peak suction pressure, suction time, bolus volume, and oral-pharyngeal transit time; however, this test has some limitations such as complexity and inability to visualize the process. At present, videofluoroscopic examination is the most common method for evaluation of swallowing disorders, and many studies [42,43,44] have used this tool to assess swallowing function. The advantage of this tool are real-time visualization and more details in terms of onset and offset of oral transit time and pharyngeal transit time, number of tongue pumps while the bolus is in the oral cavity, and rating the penetration-aspiration scale.
7. Treatment Options for Drooling in PD
First, treatment should begin by withdrawing medications that aggravate drooling such as cholinesterase inhibitors, clozapine or quetiapine. Next, the target might be to improve motor symptoms by using dopaminergic medications or by performing deep brain stimulation if the motor symptoms otherwise justify these approaches. However, the response of drooling is usually only partial and there is clearly a need for a specific adjunctive treatment for this problem. Specific treatment options for drooling in PD are both pharmacological and nonpharmacological.
7.1. Pharmacological treatments
The groups of medications that have been studied are anticholinergics, adrenergic receptor antagonists, and botulinum neurotoxin (BoNT), both serotypes A (BoNT-A) and B (BoNT-B). Paragraphs below and Table 2 summarize the evidence and current recommendations of pharmacological treatment options for drooling in PD.
Table 2.
Potential medications commonly used for treating drooling in Parkinson's disease
| Medication | Mechanism of action | Dose | Route of administration |
|---|---|---|---|
| Glycopyrrolate [47] | Anticholinergic: Blocks muscarinic acetylcholine receptor; unable to cross blood-brain barrier | 1–2 mg twice or three-times daily | Oral |
| Ipratropium bromide [46] | Anticholinergic: Muscarinic cholinergic receptor antagonist without specificity for subtypes; unable to cross blood-brain barrier | 21 μg four-times daily | Sublingual spray |
| Atropine [45] | Anticholinergic: Competitive inhibitor of muscarinic acetylcholine receptors; crossing blood-brain barrier | 0.5 mg twice daily | Sublingual drop |
| Clonidine [52] | α-2 adrenergic receptor agonist | 0.15 mg daily | Oral |
| Modafinil [52] | α-1 adrenergic receptor agonist | 100 mg daily | Oral |
| OnabotulinumtoxinA [53,54,55,56,57,58,59] | Reducing presynaptic acetylcholine release | 5-50 units per each parotid gland 5 units per each submandibular gland |
Local injection |
| AbobotulinumtoxinA [60,61,62] | Reducing presynaptic acetylcholine release | 75-146.2 units per each parotid gland 78.7 units per each submandibular gland |
Local injection |
| RimabotulinumtoxinB [63,64,65,66,67] | Reducing presynaptic acetylcholine release | 500-2000 units per each parotid gland 250 units per each submandibular gland |
Local injection |
7.1.1. Anticholinergics
Blocking cholinergic receptors, especially subtype M3, can minimize salivary secretion. Therefore, anticholinergics can be used to reduce drooling. However, because available agents are not selective for M3 receptors, they might produce undesirable adverse effects such as confusion, hallucinations, constipation, urinary retention, and drowsiness. Sublingual atropine, sublingual ipratropium bromide spray, oral glycopyrrolate and intra-oral tropical tropicamide were studied in drooling patients with PD whereas oral trihexyphenidyl, benztropine and transdermal scopolamine have not been. In an open-labeled pilot study using sublingual atropine in 6 drooling PD and 1 drooling PSP patients, results showed that 1 drop of 1% atropine solution twice daily for a 1-week period demonstrated a statistically significant decline in salivary production both objectively using the changing weight of dental rolls after placing intra-orally for 5-minutes before and after receiving treatment, and subjectively using self-reported drooling severity, rating score from 1 (normal) to 5 points (severe). Adverse events occurred in 3 patients: 1 with delirium and 2 with hallucinations [45].
A study of administering sublingual ipratropium bromide was conducted in a 5-week, randomized double-blind, placebo-controlled, cross-over study to assess efficacy and safety in 17 PD patients with bothersome drooling. The primary outcome was the changing weight of cotton rolls before and after receiving treatment. Secondary outcomes were subjective ratings of the severity and frequency of drooling using home diaries, UPDRS part II salivation subscores, parkinsonian disability using UPDRS, and adverse events. The results showed no significant difference in objective measurement at the end of 2 weeks of treatment with ipratropium bromide compared to placebo. However, there was a mild effect on the subjective measurement. In addition, there were no significant differences in the number of adverse events between the ipratropium bromide and placebo groups [46].
A 4-week, randomized, double-blind, placebo-controlled cross-over trial with 1 mg of oral glycopyrrolate administered three times daily in 23 drooling PD patients was conducted. Change in sialorrhea scoring scale (SSS) scores in terms of a greater than 30% improvement was assessed. The difference in the means of SSS scores between the placebo and glycopyrrolate groups was a secondary outcome. The results were statistically significant in both primary and secondary outcomes (p=0.021 and p=0.011, respectively). There were no statistically significant differences in adverse events between the treatment and placebo groups [47]. The efficacy and safety of intra-oral tropical tropicamide was studied in 12 drooling PD patients. Results showed no significant improvement of VAS between placebo and treatment groups for each dose without any adverse events [48].
In conclusion, according to the current recommendations of MDS for treating drooling in PD with anticholinergics, glycopyrrolate is efficacious, but there is lack of evidence for treating longer than 1 week. There are insufficient data regarding its safety. There is not enough information about the efficacy and safety of ipratropium bromide spray to treat drooling [49].
7.1.2. Adrenergic receptor agonists
The effect of α-2 adrenergic receptors might partially contribute to drooling. Clozapine and yohimbine, α-2 adrenergic receptor antagonists, were reportedly associated with drooling as an adverse effect [50,51]. Therefore, activation of α-2 adrenergic receptors might reduce drooling. Clonidine improved drooling in a small randomized, double-blinded, placebo-control study in 32 drooling PD patients. Seventeen subjects were treated with clonidine and 15 received placebo. The assessment tool measured how many times each subject had to clear their saliva in a 5-minute period. Evaluation was performed at baseline, 1 and 3 months after randomization. Results showed that clonidine significantly improved the number of times of clearing saliva at both time periods [52]. Oral modafinil 100 mg daily was reported to be beneficial for drooling in patients with PD. However, modafinil is an α-1 receptor agonist; therefore, the reduced drooling might be related to the improvement of dysphagia rather than hypersalivation [52]. The efficacy of modafinil needs further investigation.
In conclusion, there are no current recommendations for using adrenergic receptor agonists to treat drooling in PD. However, clonidine and modafinil might be considered according to the results of previous small studies.
7.1.3. Botulinum toxin injection
The mechanism of action of BoNT is inhibition of acetylcholine release. Two serotypes, BoNT-A and BoNT-B, were studied in drooling PD patients. Results after local injection of BoNT into the salivary glands are inhibition of cholinergic parasympathetic and postganglionic sympathetic activity causing reduction of salivary secretion. Studies of both BoNT-A and BoNTB are summarized in Table 3.
Table 3.
Studies of botulinum neurotoxin A and B for treating drooling patients with Parkinson's disease
| Study | Type of study |
Type of BoNT |
Number of cases |
Dose (Units per each side) |
USG | Outcome measurements |
Results | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| Jost et al. 1999 [53] | Case series | OnabotulinumtoxinA | 5 | 5 units per each parotid gland | No | Rating by the patient and his or her spouse | 2 with good (normal salivation), 2 with moderate (decreased salivation), 1 with no change | No |
| Pal et al. 2000 [54] | Open-label | OnabotulinumtoxinA | 9 | 7.5 units then, 8 weeks later 15 units per each parotid gland | No | DSFS and weight of dental rolls placed in the mouth for 5 min | 8 patients had significant reduction of objective saliva production. Approximately 35% reduction in mean value of salivary production at the end of study | Dryness of mouth |
| Su et al. 2006 [55] | Open-label | OnabotulinumtoxinA | 15 | 15 units for each parotid gland and 5 unit per each submandibular gland | No | DSFS and weight of dental rolls placed in the mouth for 10 min | Significant reduction in objective saliva production at 4 weeks (p < 0.01) and improvement of DSFS score. | Dryness of mouth |
| Santamato et al. 2008 [56] | Open-label | OnabotulinumtoxinA | 18 | 15 units for each parotid gland | Yes | DSFS | Significant improvement of DSFS at 4 weeks | No |
| Friedman et al. 2001 [57] | Open label, case-control | OnabotulinumtoxinA | 11 | 5 units per each parotid gland | No | Weight of dental rolls placed in the mouth for 2 min | Significant reduction in saliva production at 1 week (p < 0.0001 vs baseline) | No |
| Dogu et al. 2004 [58] | Randomized placebo-control | OnabotulinumtoxinA | 15 | 30 units for each parotid gland; 7 with and 8 without ultrasound guidance | Yes | VAS and weight of dental rolls placed in the mouth for 5 min | Significant reductions in saliva production at 1, 4 and 12 weeks (p = 0.001 vs. baseline) in ultrasound guidance group and significant reductions from baseline in VAS scores. | Dryness of mouth |
| Lagalla et al. 2006 [59] | Randomized double-blind, placebo-control | OnabotulinumtoxinA | 16 with treatment and 32 with placebo | 50 units per each parotid gland | No | VAS for drooling frequency, VAS-FD, VAS-SD and weight of dental rolls placed in the mouth for 5 min | Significant reduction in objective saliva production, VAS for drooling frequency, VAS-FD and VAS-FS at 4 weeks | Transient swallowing difficulty |
| Nobrega et al. 2006 [60] | Case series | AbobotulinumtoxinA | 21 | 125 units per each parotid gland | Yes | DSFS | Significant Improvement of DSFS at 4 weeks | Dryness of mouth |
| Lipp et al. 2003 [61] | Randomized double-blind, placebo-control | AbobotulinumtoxinA | 32 (20 with PD, 12 with ALS); 7 with placebo, 8 with 18.7 units, 9 with 37.5 units and 8 with 75 units group | 18.7, 37.5, or 75 units per each parotid gland | No | 6-item questionnaire, weight of dental rolls placed in the mouth for 5 min and mechanical counter once a week for a 12-hour | Saliva reduction of 50% and significant improvement of counter measurement in group treated with 75 units | No reported |
| Mancini et al. 2003 [62] | Randomized double-blind, placebo-control | AbobotulinumtoxinA | 20 (14 with PD, 6 with MSA); 10 with placebo and 10 with treatment group | 146.2 units per each parotid gland and 78.7 units per each submandibular gland | Yes | DSFS | Significant reduction in DSFS at 1 week (p = 0.005 vs placebo) | No |
| Racette et al. 2003 [63] | Open-label | RimabotulinumtoxinB | 9 | 1000 units per each parotid gland | No | VAS and weight of dental rolls placed in the mouth for 5 min | Significant improvement of VAS score (P < 0.001) | Transient dryness of mouth |
| Contarino et al. 2007 [64] | Open-label | RimabotulinumtoxinB | 9 | 1000 units per each parotid gland and 250 units per each submandibular gland | Yes | DSFS, VAS and weight of dental rolls placed in the mouth for 5 min | Significant reduction of objective saliva production at 1 week and significant improvement of DSFS and VAS score at 1 week. | Dryness of mouth |
| Ondo et al. 2004 [65] | Randomized double-blind, placebo-control | RimabotulinumtoxinB | 16; 8 with placebo and 8 with treatment group | 1000 units per each parotid gland and 250 units per each submandibular gland | No | DSFS, VAS and DRS | Significant improvement of DSFS (p < 0.001), VAS (p < 0.001) and DRS (p < 0.05) | Dryness of mouth, worsening gait difficulty, neck pain and diarrhea |
| Lagalla et al. 2009 [66] | Randomized double-blind, placebo-control | RimabotulinumtoxinB | 36; 18 with placebo and 18 with treatment group | Total dose 4000 units per bilateral parotid glands | No | DSFS, VAS-FD, VAS-SD and weight of dental rolls placed in the mouth for 5 min | Significant reduction of objective salivary production at 4 weeks (p < 0.0001) and significant improvement Of DSFS, VAS-FD and VAS-SD | Transient dysphagia and transient weakness of chewing |
| Chinnapongse et al. 2012 [67] | Randomized double-blind, placebo-control | RimabotulinumtoxinB | 54; 15 with placebo, 14 with 1500 units, 12 with 2500 units and 13 with 3500 units group | Placebo, 500, 1000, 1500 units per each parotid gland and placebo and fixed dose 250 units per each submandibular gland in treatment group | No |
Investigator: DSFS, CGI, UPDRS part II; salivation and swallowing subscore Subject: DSFS, PGI, UPDRS part II; salivation and drooling impact scale |
All subjective evaluation by both investigator and subject significantly improved at 4 weeks comparing to baseline | Dryness of mouth and viscous saliva |
| Guidubaldi et al. 2011 [68] | Randomized double-blind, cross-over | AbobotulinumtoxinA and RimabotulinumtoxinB | 27 (12 with PD and 15 with ALS); 13with BoNT-A and 14with BoNT-B group |
AbobotulinumtoxinA: 100 units per each parotid gland and 25 units per each submandibular gland RimabotulinumtoxinB: 1000 units per each parotid gland and 250 units per each submandibular gland |
Yes | DSFS, VAS, DRS and weight of dental rolls placed in the mouth for 5 min |
Latency: Significantly shorter after BoNT-B (3.2 ± 3.7 days) than that after BoNT-A (6.6 ± 4.1 days; P = 0.002) 1 week: BoNT-B treatments reduced the cotton roll weights more than that of BoNT-A (P = 0.024) and slightly better subjective scales than BoNT-A 1month: BoNT-B slightly better subjective scales than BoNT-A 2months: No significant differences between BoNT-A and B in both objectively and subjectively measurements |
Dryness of mouth and viscous saliva |
Two types of BoNT-A, onabotulinumtoxinA and abobotulinumtoxinA, have been used to treat drooling in PD. Seven studies including 1 case series [53], 3 open-label studies [54,55,56], 1 open-labelled case-control study [57], 1 randomized placebo-control study [58] and 1 randomized, double-blinded, placebo-control study [59] used onabotulinumtoxinA for treating drooling patients with PD. OnabotulinumtoxinA was injected into the parotid glands for all studies. One study included MSA and DLB patients whose submandibular glands were injected [55]. No studies compared injection of the parotid glands with the submandibular glands. Five studies used a blind injection technique [53,54,55,57,59] whereas 2 studies used ultrasound guidance [56,58]. Santamato et al. conducted an open-label study using ultrasound-guided toxin injection in 18 drooling PD patients while Dogu et al. conducted a randomized control study comparing toxin injection in 15 drooling PD patients divided into arms using (n=8) and not-using (n=7) ultrasound guidance. In terms of pre- and post-treatment evaluation, 2 studies only used subjective assessment [53,56], 1 only used objective assessment [57], and 4 used both subjective and objective assessment [54,55,58,59]. The subjective assessment tools included reporting from patients and their spouses, DSFS and VAS for drooling severity, frequency, VASFS and VAS-SD. The objective assessment was the percent change of weight of dental roll after placement in the mouth for 2, 5 or 10 minutes. Duration of evaluation after start of treatment ranged from 1 to 16 weeks. All studies agreed that onabotulinumtoxinA injection, dosage ranging from 5 to 50 units and 5 units per parotid and submandibular gland, respectively, significantly reduced drooling in PD, MSA and DLB patients and improved subjective or objective assessments for approximately 4 months. In addition, injecting the toxin under ultrasound guidance might have provided more accuracy and more reduction in salivary production compared to the blind injection technique.
AbobotulinumtoxinA was also studied in drooling PD patients. Three studies including 1 case series [60] and 2 randomized double-blind, placebo-control studies [61,62] were published. AbobotulinumtoxinA was injected into the parotid glands for all studies. The study conducted by Lipp et al. included ALS, MSA and CBD patients [61]. The study conducted by Mancini et al. included MSA and injected submandibular glands [62]. Only one study used a blind injection technique [61] whereas 2 studies used ultrasound guidance [60,62]. Nobrega et al. reported a case series of abobotulinumtoxinA injection under ultrasound guidance in 21 drooling PD patients while Mancini et al. conducted a randomized, double-blinded, placebo-control study using ultrasound-guided toxin injection in 20 drooling patients (14 with PD and 6 with MSA) divided into 2 groups of 10 patients, treatment or placebo. These studies conducted by Nobrega et al. and Mancini et al. used DSFS as a subjective assessment while a study conducted by Lipp et al. used percent change of weight of dental rolls after placing in the mouth for 5-minutes as an objective assessment and a mechanical counter for spitting in a 12-hour period as a semi-objective assessment. Duration of evaluation from start of treatment ranged from 1 to 4 weeks. All studies agreed that abobotulinumtoxinA injection, with doses ranging from 75 to 146.2 units and 78.7 units per parotid and submandibular gland, respectively, significantly reduced drooling in PD, ALS, MSA and CBD patients in terms of either improved subjective or objective assessments. This effect lasted for 1 to 4 months. In addition, a previous study conducted by Kalf et al. showed no statistically significant difference between parotid and submandibular gland injection with abobotulinumtoxinA.
RimabotulinumtoxinB, the only available BoNT-B, has also been studied in drooling PD patients. To date, 5 studies using rimabotulinumtoxinB to treat drooling PD patients including 2 open-label studies [63,64] and 3 randomized double-blind, placebo-control studies [65,66,67] were published. RimabotulinumtoxinB was injected into the parotid glands for all studies. The study conducted by Contarino et al. included ALS patients. Three studies also injected submandibular glands [64,65,67]. Four studies used a blind injection technique [63,65,66,67] whereas 2 studies used ultrasound guidance [61]. The subjective assessment tools used in the studies included DSFS, VAS for drooling severity, VAS-FS, VAS-SD and DRS while the objective assessment was percent change of weight of dental rolls after placing in the mouth for 5-minutes. Duration of evaluation from start of treatment ranged from 1 to 4 weeks. All studies agreed that rimobotulinumtoxinB injection in doses ranging from 500 to 2000 units and 250 units per parotid and submandibular gland, respectively, significantly reduced drooling in PD and ALS patients in terms of improved subjective or objective assessments. This effect lasted up to 4.8 months.
Guidubaldi et al. conducted a randomized, double-blinded, placebo-controlled crossover study comparing between BoNT-A and B injection in 27 drooling patients (15 with ALS and 12 with PD) under ultrasound guidance. Parotid gland was injected with either 100 units of abobotulinumtoxinA or 1000 units of rimabotulinumtoxinB while the submandibular gland was injected with either 25 units of abobotulinumtoxinA or 250 units of rimabotulinumtoxinB. All patients were evaluated by DSFS, VAS, DRS and by change of weight of dental roll after placing in the mouth for 5-minutes at baseline, 1 and 4 weeks, and every 4 weeks until no benefit was observed. At 1 month, BoNT-B showed improvement in DSFS and DRS more than BoNT-A; however, there were no significant differences between both groups at 2 months [68].
In conclusion, as confirmed in the current recommendations of the MDS, both BoNT-A and BoNT-B are efficacious for symptomatically controlling drooling in PD [49]. Onset of effect of both BoNT-A and B starts at 1 week, and lasts for approximately 3 to 5 months after injection. Injecting BoNT-A or B under ultrasound guidance might provide more benefit; no obvious evidence showed a significant difference in term of efficacy between BoNT-A and B. The common adverse effect after injecting BoNT is dryness of mouth which is generally mild. The anatomical landmarks for injecting the parotid and submandibular glands are in Figure 2.
Figure 2. Landmark for injecting parotid and submandibular gland.
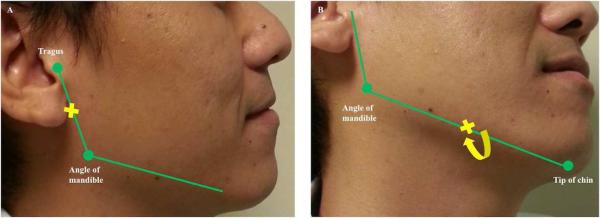
(A) Parotid gland: Drawing the imaginary line starts from the tragus to angle of the mandible; then the mid-point of this line is the landmark for injecting botulinum neurotoxin into the gland. (B) Submandibular gland: Drawing the imaginary line, along with the length of body of the mandible, starts from angle of the mandible to tip of the chin; then one finger breadth medial to the mid-point of this line is the landmark for injecting botulinum neurotoxin into the glands.
7.2. Non-pharmacological treatments
Many non-pharmacological approaches such as chewing gum, behavioral modification, radiotherapy (RT) and surgical treatment were reported. However, only 2 studies mainly involving PD patients were published [69,70]. Mark et al. conducted a randomized placebo-control study involving 6 PD patients to evaluate the effect of behavioral modification. Patients were instructed to consciously swallow their saliva each time when they heard the sound. Results showed a significant reduction of DRS; however, the magnitude of effect decreased at 3 months compared to 1 month. The authors concluded that self-motivation was important in increasing the benefit with this intervention [69]. Postma et al. reported a case series of 28 drooling patients (22 with PD, 1 with vascular parkinsonism, 3 with MSA and 2 with PSP) who received a bilateral 12 Gy of RT to the parotid and superior parts of the submandibular glands to reduce drooling. The authors used UPDRS part II salivation subscore and shortened Parkinson's Disease Questionnaire-8 for evaluating efficacy of treatment and QoL, respectively, at pre-RT, 1 and 6 months post-RT. Drooling improved significantly at 1 month post-RT and this effect lasted for 1 year. Common adverse events were loss of taste and dry mouth; however, 75% of these adverse events were transient. QoL improved significantly in the long term [70]. To date, there is no study that particularly investigated the effect of deep brain stimulation (DBS) on drooling in PD patients. To the extent that drooling is caused by a swallowing problem, if DBS affected swallowing, there could be an influence on drooling. A systematic review showed no effect of DBS on swallowing [71], but a recent result showed a deleterious effect with unilateral subthalamic nucleus DBS [72]. It seems unlikely that DBS will help drooling.
In conclusion, there are no current recommendations for using non-pharmacological treatments to treat drooling in PD. However, behavioral modification and, in refractory cases, RT might be considered as an adjunctive therapy.
8. Conclusion
Drooling produces important negative consequences for both PD patients and their caregivers. While the main problem seems to be failure of swallowing, most of the treatments are directed to reducing salivary secretion. At present, local injection with BoNT into major salivary glands is the most effective therapeutic option. There are some areas of uncertainty that need further research including addressing the pathophysiology and standardizing diagnostic criteria and severity assessment tools. Developing more specific therapeutic options would be valuable to improve patients’ quality of life.
Highlights.
Drooling is the one of the common non-motor symptoms in PD patients.
Drooling produces various negative consequences for patients and their caregivers.
The standard diagnostic criteria and severity assessment tools are still lacking.
At present, the pathophysiology of drooling in PD is not completely certain.
Local injection with BoNT into salivary glands is the most effective treatment.
Acknowledgement
Devera Schoenberg, M.Sc., contributed to this review article as the editor of this manuscript.
The Faculty of Medicine, Siriraj Hospital, Mahidol University has awarded a fellowship to Dr.Prachaya Srivanitchapoom for doing research in Parkinson's disease and Movement Disorders at Human Motor Control Section (HMCS), National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, USA. The Indo-US Science Technology Forum (IUSTTF) has awarded a fellowship to Dr.Sanjay Pandey to do research in Parkinson's disease and Movement Disorders at HMCS, NINDS, NIH, Bethesda, USA.
Roles of the Authors
Dr.Prachaya Srivanitchapoom and Dr.Sanjay Pandey contributed in manuscript preparation by writing the first draft, review and critique.
Dr.Mark Hallett has contributed in the manuscript preparation by reviewing, critiquing, revising and editing it.
Financial disclosures of all authors (related to research covered in this article and for the preceding 12 months):
Dr. Prachaya Srivanitchapoom: None
Dr. Sanjay Pandey: None
Dr. Mark Hallett: Dr. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Editorial Board of 20 journals, and received royalties from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, and Elsevier. He has received honoraria for lecturing from Columbia University. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds came from the Kinetics Foundation, for studies of instrumental methods to monitor Parkinson's disease, and BCN Peptides, S.A., for treatment studies of blepharospasm, Merz, for a study of hand dystonia, and Allergan via Mt. Sinai, for a study of the use of ultrasound to treat dystonia and spasticity.
Funding sources for study: NINDS Intramural Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dand P, Sakel M. The management of drooling in motor neuron disease. Int J Palliat Nurs. 2010;16:560–64. doi: 10.12968/ijpn.2010.16.11.80024. [DOI] [PubMed] [Google Scholar]
- 2.Edwards LL, Pfeiffer RF, Quigley EMM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord. 1991;6:151–6. doi: 10.1002/mds.870060211. [DOI] [PubMed] [Google Scholar]
- 3.Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson's disease symptom profile. Acta Neurol Scand. 2000;102:37–43. doi: 10.1034/j.1600-0404.2000.102001037.x. [DOI] [PubMed] [Google Scholar]
- 4.Volonté MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson's disease. Neurol Sci. 2002;23:S121–22. doi: 10.1007/s100720200099. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui MF, Rast S, Lynn MJ, Auchus AP. Pfeiffer RF.Autonomic dysfunction in Parkinson's disease: a comprehensive symptom survay. Parkinsonism Relat Disord. 2002;8:277–84. doi: 10.1016/s1353-8020(01)00052-9. [DOI] [PubMed] [Google Scholar]
- 6.Kalf JG, Smit AM, Bloem BR, Zwarts MJ, Munneke M. Impact of drooling in Parkinson's disease. J Neurol. 2007;254:1227–32. doi: 10.1007/s00415-007-0508-9. [DOI] [PubMed] [Google Scholar]
- 7.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333–41. doi: 10.1212/01.wnl.0000266593.50534.e8. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Martin P, Schapira A, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–29. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 9.Denise Hack Nicaretta DH, de Rosso ALZ, Maliska C, Costa MMB. Scintigraphic analysis of the parotid glands in patients with sialorrhea and Parkinson's disease. Parkinsonism Relat Disord. 2008;14:338–41. doi: 10.1016/j.parkreldis.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Cheon SM, Ha MS, Park MJ, Kim JW. Nonmotor symptoms of Parkinson's disease: prevalence and awareness of patients and families. Parkinsonism Relat Disord. 2008;14:286–90. doi: 10.1016/j.parkreldis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Leibner J, Ramjit A, Sedig L, Dai YF, Wu SS, Jacobson IV C, et al. The impact of and the factors associated with drooling in Parkinson's disease. Parkinsonism Relat Disord. 2010;16:475–77. doi: 10.1016/j.parkreldis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Muller B, Larsen JP, Wentzel-Larsen T, Skeie GO, Tysnes OB. Autonomic and sensory symptoms and signs in incident, untreated Parkinson's Disease: frequent but mild. Mov Disord. 2011;26:65–72. doi: 10.1002/mds.23387. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Lloret S, Ne`gre-Page`s L, Ojero-Senard A, Damier P, Deste'e A, Tison F, et al. Oro buccal symptoms (dysphagia, dysarthria, and sialorrhea) in patients with Parkinson's disease: preliminary analysis from the French COPARK cohort. Eur J Neurol. 2012;19:28–37. doi: 10.1111/j.1468-1331.2011.03402.x. [DOI] [PubMed] [Google Scholar]
- 14.Rana AQ, Yousuf MS, Awan N, Fattah A. Impact of progression of Parkinson's disease on drooling in various ethnic groups. Eur Neurol. 2012;67:312–14. doi: 10.1159/000336054. [DOI] [PubMed] [Google Scholar]
- 15.Ozdilek B, Gunal DI. Motor and non-motor symptoms in Turkish patients with Parkinson's disease affecting family caregiver burden and quality of life. J Neuropsychiatry Clin Neurosci. 2012;24:478–83. doi: 10.1176/appi.neuropsych.11100315. [DOI] [PubMed] [Google Scholar]
- 16.Damian A, Adlerb CH, Hentz JG, Shill HA, Caviness JN, Sabbagh MN, et al. Autonomic function, as self-reported on the SCOPA-autonomic questionnaire, is normal in essential tremor but not in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:1089–93. doi: 10.1016/j.parkreldis.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloem BR, Kalf JG, van de Kerkhof PCM, Zwarts MJ. Debilitating consequences of drooling. J Neurol. 2009;256:1382–83. doi: 10.1007/s00415-009-5144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einarsdóttir ER, Gunnsteinsdóttir H, Hallsdóttir MH, Sveinsson Sigurjón, Jónsdóttir SR, O' lafsson VG, et al. Dental health of patients with Parkinson's disease in Iceland. Spec Care Dentist. 2009;29:123–7. doi: 10.1111/j.1754-4505.2009.00075.x. [DOI] [PubMed] [Google Scholar]
- 19.Nóbrega AC, Rodrigues B, Melo A. Silent aspiration in Parkinson's disease patients with diurnal sialorrhea. Clin Neurol Neurosurg. 2008;110:117–9. doi: 10.1016/j.clineuro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues B, Nóbrega AC, Sampaio M, Argolo N, Melo A. Silent saliva aspiration in Parkinson's disease. Mov Disord. 2011;26:138–41. doi: 10.1002/mds.23301. [DOI] [PubMed] [Google Scholar]
- 21.Nóbrega AC, Rodrigues B, Melo A. Is silent aspiration a risk factor for respiratory infection in Parkinson's disease patients? Parkinsonism Relat Disord. 2008;14:646–8. doi: 10.1016/j.parkreldis.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Boyce W, Bakheet MR. A review of a vexing, often unrecognized sign of oropharyngeal and esophageal disease. J Clin Gastroenterol. 2005;39:89–97. [PubMed] [Google Scholar]
- 23.Merello M. Sialorrhoea and drooling in patients with Parkinson's disease: epidemiology and management. Drugs Aging. 2008;25:1007–19. doi: 10.2165/0002512-200825120-00003. [DOI] [PubMed] [Google Scholar]
- 24.Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23:1065–75. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- 25.Bateson M, Gibberd FB, Wilson RSE. Saliva symptoms in Parkinson disease. Arch Neurol. 1973;29:274–5. doi: 10.1001/archneur.1973.00490280086013. [DOI] [PubMed] [Google Scholar]
- 26.Bagheri H, Damase-Michel C, Lapeyre-Mestre M, Cismondo S, O'Connell D, Senard JM, et al. A study of salivary secretion in Parkinson's disease. Clin Neuropharmacol. 1999;22:213–5. [PubMed] [Google Scholar]
- 27.Proulx M, de Courval FP, Wiseman MA, Panisset M. Salivary production in Parkinson's disease. Mov Disord. 2005;20:204–7. doi: 10.1002/mds.20189. [DOI] [PubMed] [Google Scholar]
- 28.Marg S, Walz B, Blenau W. The effects of dopamine receptor agonists and antagonists on the secretory rate of cockroach (Periplaneta americana) salivary glands. J Insect Physiol. 2004;50:821–30. doi: 10.1016/j.jinsphys.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Koga T, Kobashi M, Mizutani M, Tsukamoto G, Matsuo R. Area postrema mediates gastric motor response induced by apomorphine in rats. Brain Res. 2003;960:122–31. doi: 10.1016/s0006-8993(02)03801-5. [DOI] [PubMed] [Google Scholar]
- 30.Pazo JH, Belforte JE. Basal ganglia and functions of the autonomic nervous system. Cell Mol Neurobiol. 2002;22:645–54. doi: 10.1023/a:1021844605250. [DOI] [PubMed] [Google Scholar]
- 31.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol. 2010;119:703–13. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 32.Nicaretta DH, de Rosso AL, Maliska C, Costa MM. Scintigraphic analysis of the parotid glands in patients with sialorrhea and Parkinson's disease. Parkinsonism Relat Disord. 2008;14:338–41. doi: 10.1016/j.parkreldis.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Ciucci MR, Russell JA, Schaser AJ, Doll EJ, Vinney LM, Connor NP. Tongue force and timing deficits in a rat model of Parkinson disease. Behav Brain Res. 2011;222:315–20. doi: 10.1016/j.bbr.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T. Impaired food transportation in Parkinson's disease related to lingual bradykinesia. Dysphagia. 2011;26:250–5. doi: 10.1007/s00455-010-9296-y. [DOI] [PubMed] [Google Scholar]
- 35.Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2013;28:95–104. doi: 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nóbrega AC, Rodrigues B, Torres AC, Scarpel RD, Neves CA, Melo A. Is drooling secondary to a swallowing disorder in patients with Parkinson's disease? Parkinsonism Relat Disord. 2008;14:243–5. doi: 10.1016/j.parkreldis.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson's disease. Gastroenterology. 1996;110:383–92. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 38.Sung HY, Kim JS, Lee KS, Kim YI, Song IU, Chung SW, et al. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson's disease. Mov Disord. 2010;25:2361–8. doi: 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- 39.Kalf JG, Munneke M, van den Engel-Hoek L, de Swart BJ, Borm GF, Bloem BR, et al. Pathophysiology of diurnal drooling in Parkinson's disease. Mov Disord. 2011;26:1670–6. doi: 10.1002/mds.23720. [DOI] [PubMed] [Google Scholar]
- 40.Evatt ML, Chaudhuri KR, Chou KL, Cubo E, Hinson V, Kompoliti K, et al. Dysautonomia rating scales in Parkinson's disease: sialorrhea, dysphagia, and constipation--critique and recommendations by movement disorders task force on rating scales for Parkinson's disease. Mov Disord. 2009;24:635–46. doi: 10.1002/mds.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative assessment of oral and pharyngeal function in Parkinson's disease. Dysphagia. 1996;11:144–50. doi: 10.1007/BF00417905. [DOI] [PubMed] [Google Scholar]
- 42.Johnston BT, Li Q, Castell JA, Castell DO. Swallowing and esophageal function in Parkinson's disease. Am J Gastroenterol. 1995;90:1741–6. [PubMed] [Google Scholar]
- 43.Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson's disease. Dysphagia. 1998;13:95–100. doi: 10.1007/PL00009562. [DOI] [PubMed] [Google Scholar]
- 44.Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson's disease. Dysphagia. 2008;23:26–32. doi: 10.1007/s00455-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 45.Hyson HC, Johnson AM, Jog MS. Sublingual atropine for sialorrhea secondary to parkinsonism: a pilot study. Mov Disord. 2002;17:1318–20. doi: 10.1002/mds.10276. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen TR, Galpern WR, Asante A, Arenovich T, Fox SH. Ipratropium bromide spray as treatment for sialorrhea in Parkinson's disease. Mov Disord. 2007;22:2268–73. doi: 10.1002/mds.21730. [DOI] [PubMed] [Google Scholar]
- 47.Arbouw ME, Movig KL, Koopmann M, Poels PJ, Guchelaar HJ, Egberts TC, et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology. 2010;74:1203–7. doi: 10.1212/WNL.0b013e3181d8c1b7. [DOI] [PubMed] [Google Scholar]
- 48.Lloret SP, Nano G, Carrosella A, Gamzu E, Merello M. A double-blind, placebo-controlled, randomized, crossover pilot study of the safety and efficacy of multiple doses of intra-oral tropicamide films for the short-term relief of sialorrhea symptoms in Parkinson's disease patients. J Neurol Sci. 2011;310:248–50. doi: 10.1016/j.jns.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26:S42–80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davydov L, Botts SR. Clozapine-induced hypersalivation. Ann Pharmacother. 2000;34:662–5. doi: 10.1345/aph.19259. [DOI] [PubMed] [Google Scholar]
- 51.Chatelut E, Rispail Y, Berlan M, Montastruc JL. Yohimbine increases human salivary secretion. Br J Clin Pharmacol. 1989;28:366–8. doi: 10.1111/j.1365-2125.1989.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou KL, Evatt M, Hinson V, Kompoliti K. Sialorrhea in Parkinson's disease: a review. Mov Disord. 2007;22:2306–13. doi: 10.1002/mds.21646. [DOI] [PubMed] [Google Scholar]
- 53.Jost WH. Treatment of drooling in Parkinson's disease with botulinum toxin. Mov Disord. 1999;14:1057–9. doi: 10.1002/1531-8257(199911)14:6<1057::aid-mds1033>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Pal PK, Calne DB, Calne S, Tsui JK. Botulinum toxin A as treatment for drooling saliva in Parkinson's disease. Neurology. 2000;54:244–7. doi: 10.1212/wnl.54.1.244. [DOI] [PubMed] [Google Scholar]
- 55.Su CS, Lan MY, Liu JS, Chang CC, Lai SL, Wu HS, et al. Botulinum toxin type A treatment for parkinsonian patients with moderate to severe sialorrhea. Acta Neurol Taiwan. 2006;15:170–6. [PubMed] [Google Scholar]
- 56.Santamato A, Ianieri G, Ranieri M, Megna M, Panza F, Fiore P, et al. Botulinum toxin type A in the treatment of sialorrhea in Parkinson's disease. J Am Geriatr Soc. 2008;56:765–7. doi: 10.1111/j.1532-5415.2008.01612.x. [DOI] [PubMed] [Google Scholar]
- 57.Friedman A, Potulska A. Quantitative assessment of parkinsonian sialorrhea and results of treatment with botulinum toxin. Parkinsonism Relat Disord. 2001;7:329–32. doi: 10.1016/s1353-8020(00)00073-0. [DOI] [PubMed] [Google Scholar]
- 58.Dogu O, Apaydin D, Sevim S, Talas DU, Aral M. Ultrasound-guided versus ‘blind’ intraparotid injections of botulinum toxin-A for the treatment of sialorrhoea in patients with Parkinson's disease. Clin Neurol Neurosurg. 2004;106:93–6. doi: 10.1016/j.clineuro.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Lagalla G, Millevolte M, Capecci M, Provinciali L, Ceravolo MG. Botulinum toxin type A for drooling in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord. 2006;21:704–7. doi: 10.1002/mds.20793. [DOI] [PubMed] [Google Scholar]
- 60.Nóbrega AC, Rodrigues B, Torres AC, Enzo A, Melo A. Does botulinum toxin decrease frequency and severity of sialorrhea in Parkinson's disease?. J Neurol Sci. 2007;253:85–7. doi: 10.1016/j.jns.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 61.Lipp A, Trottenberg T, Schink T, Kupsch A, Arnold G. A randomized trial of botulinum toxin A for the treatment of drooling. Neurology. 2003;61:1279–81. doi: 10.1212/wnl.61.9.1279. [DOI] [PubMed] [Google Scholar]
- 62.Mancini F, Zangaglia R, Cristina S, Sommaruga MG, Martignoni E, Nappi G, et al. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type A in the treatment of drooling in parkinsonism. Mov Disord. 2003;18:685–8. doi: 10.1002/mds.10420. [DOI] [PubMed] [Google Scholar]
- 63.Racette BA, Good L, Sagitto S, Perlmutter JS. Botulinum toxin B reduces sialorrhea in parkinsonism. Mov Disord. 2003;18:1059–61. doi: 10.1002/mds.10484. [DOI] [PubMed] [Google Scholar]
- 64.Contarino MF, Pompili M, Tittoto P, Vanacore N, Sabatelli M, Cedrone A, et al. Botulinum toxin B ultrasound-guided injections for sialorrhea in amyotrophic lateral sclerosis and Parkinson's disease. Parkinsonism Relat Disord. 2007;13:299–303. doi: 10.1016/j.parkreldis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Ondo WG, Hunter C, Moore W. A double-blind placebo-controlled trial of botulinum toxin B for sialorrhea in Parkinson's disease. Neurology. 2004;62:37–40. doi: 10.1212/01.wnl.0000101713.81253.4c. [DOI] [PubMed] [Google Scholar]
- 66.Lagalla G, Millevolte M, Capecci M, Provinciali L, Ceravolo MG. Long-lasting benefits of botulinum toxin type B in Parkinson's disease-related drooling. J Neurol. 2009;256:563–7. doi: 10.1007/s00415-009-0085-1. [DOI] [PubMed] [Google Scholar]
- 67.Chinnapongse R, Gullo K, Nemeth P, Zhang Y, Griggs L. Safety and efficacy of botulinum toxin type B for treatment of sialorrhea in Parkinson's disease: a prospective double-blind trial. Mov Disord. 2012;27:219–26. doi: 10.1002/mds.23929. [DOI] [PubMed] [Google Scholar]
- 68.Guidubaldi A, Fasano A, Ialongo T, Piano C, Pompili M, Mascianà R, et al. Botulinum toxin A versus B in sialorrhea: a prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson's disease. Mov Disord. 2011;26:313–9. doi: 10.1002/mds.23473. [DOI] [PubMed] [Google Scholar]
- 69.Marks L, Turner K, O'Sullivan J, Deighton B, Lees A. Drooling in Parkinson's disease: a novel speech and language therapy intervention. Int J Lang Commun Disord. 2001;36:282–7. doi: 10.3109/13682820109177898. [DOI] [PubMed] [Google Scholar]
- 70.Postma AG, Heesters M, van Laar T. Radiotherapy to the salivary glands as treatment of sialorrhea in patients with parkinsonism. Mov Disord. 2007;22:2430–5. doi: 10.1002/mds.21752. [DOI] [PubMed] [Google Scholar]
- 71.Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson's disease: a systematic review. Parkinsonism Relat Disord. 2013;19:783–8. doi: 10.1016/j.parkreldis.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troche MS, Brandimore AE, Foote KD, Morishita T, Chen D, Hegland KW, et al. Swallowing Outcomes Following Unilateral STN vs. GPi Surgery: A Retrospective Analysis. Dysphagia. 2014;29:425–31. doi: 10.1007/s00455-014-9522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


