Abstract
Previous electrophysiological investigations have evaluated movement-related beta (14–28 Hz) oscillatory activity in healthy participants. These studies have described an abrupt decrease in beta activity that starts before movement onset, and a sharp increase in beta power that peaks after movement termination. These neural responses have been respectively termed the event-related beta desynchronization or pre-movement beta ERD, and the post-movement beta rebound (PMBR). Previous studies have shown that a variety of movement parameters and demographic factors (e.g., age) modulate the amplitude of these oscillatory responses, and in the current study we evaluated whether the amplitudes follow a biological temporal rhythm (e.g., circadian), as it is known that spontaneous beta levels increase from morning to afternoon in some brain areas. To this end, we used magnetoencephalography (MEG) to evaluate oscillatory activity during a right hand finger-tapping task in four participants who were recorded at three different times (09:00, 12:00, 16:00) on three consecutive days (i.e., 36 total MEG sessions). All MEG data were corrected for head motion and examined in the time-frequency domain using beamforming methods. We found a significant linear increase in beta ERD amplitude from 09:00 to 16:00 hours in the left precentral gyrus, left premotor cortices, left supplementary motor area (SMA), and the right precentral and postcentral gyri. In contrast, the amplitude of the PMBR was very steady across the day in all brain regions except the left SMA, which exhibited a linear increase from morning to afternoon. Finally, beta levels during the baseline period also increased from 09:00 to 16:00 in most regions of the cortical sensorimotor network. These data show that both the pre-movement beta ERD and spontaneous beta levels strongly increase from morning to afternoon in the motor cortices, which may indicate that the amplitude of the beta ERD response is determined by the spontaneous beta level during the motor planning period.
Keywords: movement, circadian, desynchronization, magnetoencephalography, motor planning
Introduction
Over the past decade, numerous magnetoencephalography (MEG) and electroencephalography (EEG) studies have examined movement-related oscillatory responses in healthy participants (Cheyne et al., 2008; Gaetz et al., 2010, 2011; Hall et al., 2011; Jurkiewicz et al., 2006; Muthukumaraswamy, 2010; Tzagarakis et al., 2010; Wilson et al., 2010, 2011). These electrophysiological methods have excellent spatiotemporal resolution, which has allowed neural activity serving individual movements to be decomposed into planning, execution, and termination stages, and these three stages of movement have been tentatively linked to three distinct oscillatory responses (Cheyne et al., 2008; Gaetz et al., 2010, 2011; Hall et al., 2011; Jurkiewicz et al., 2006; Muthukumaraswamy, 2010; Tzagarakis et al., 2010; Wilson et al. 2010, 2011). Briefly, there is an event-related desynchronization (ERD) in the beta-frequency range (14–28 Hz) that peaks before movement onset and continues slightly after movement execution, which is termed the pre-movement beta ERD response. There is an event-related synchronization (ERS) in the gamma-frequency range that coincides with the onset of movement and is relatively brief (i.e., ~200 ms), and finally there is a post-movement beta ERS that reaches maximum amplitude slightly after the termination of movement (Cheyne et al., 2008; Gaetz et al., 2010, 2011; Hall et al., 2011; Heinrichs-Graham et al., in press; Jurkiewicz et al., 2006; Tzagarakis et al., 2010; Wilson et al., 2010, 2011). This latter response is generally termed the post-movement beta rebound (PMBR). For clarity, the precise frequency range (e.g., 14–28 Hz) and time window varies slightly between studies, but this variance likely reflects demographic factors and/or small differences in the MEG data analysis approach or task design (e.g., see Cheyne et al., 2008).
In addition to characterizing the oscillatory dynamics, many studies to date have evaluated how specific task parameters, diseases, therapies, and/or demographic variables (e.g., age) modulate the amplitude of beta responses. For example, a recent MEG study demonstrated that the amplitude of the beta ERD linearly scaled with the directional uncertainty of the movement (Tzagarakis et al., 2010), which is in agreement with a previous study showing that the beta ERD was more strongly lateralized when the upcoming movement was cued to one side with certainty versus ambiguous cueing (Doyle et al., 2005). There are also data showing that diazepam, a GABA-A modulator, accentuates the pre-movement beta ERD, but not the PMBR or the movement-related gamma response (Hall et al., 2011). Studies in patient populations have shown that the amplitude of the beta ERD is reduced in Parkinson’s disease (Heinrichs-Graham et al., in press), and that all three oscillatory responses are abnormal in adolescents with psychosis (Wilson et al., 2011). MEG studies of stroke patients have reported that effective motor-related therapies are associated with a decrease in PMBR amplitude following treatment (Wilson et al., 2011b). Other studies have shown that the amplitude of the PMBR increases as a function of age, with little-to-no synchronization occurring in younger children (Gaetz et al., 2011). Age also modulates the beta ERD, but this effect varies by motor region (Wilson et al., 2010). Finally, limb-dependent differences in the location and peak frequency of the response have been shown with the beta ERD and PMBR (Kurz et al., in press; Wilson et al., 2010).
While these motor-related oscillatory responses have been widely studied and appear to be quite robust, little is known about their test-retest reliability or whether factors like muscle fatigue, mental fatigue, participant satiety, sleepiness/arousal level, or circadian clock modulate the responses. There is reasonable evidence that resting-state power in the beta frequency band is weakest in the morning hours and that it increases during the late afternoon in healthy adults (Cacot et al., 1995; Gundel et al., 1983; Toth et al., 2007). There is also abundant evidence that motor performance is influenced by the biological clock (Carrier and Monk, 2000; Drust et al., 2005; Gueugneau and Papaxathis, 2010; Jasper et al., 2009), and a recent fMRI study of finger-tapping showed that time-of-day effects could be discerned in multiple motor regions (Peres et al., 2011). Unfortunately, the relationship between fMRI activation and neural oscillatory activity in electrophysiology is only partially understood, which prevents any strong conclusions regarding circadian effects on motor-related oscillations. In the current study, we used MEG to evaluate neural oscillatory activity during a finger-tapping task in four male participants who were recorded at three different times, 09:00, 12:00, and 16:00, on three consecutive days (i.e., nine MEG sessions per participant). As a control condition, we also examined resting-state beta activity levels in these participants at the same time points. Our primary goal was to determine whether the amplitude of motor-related beta oscillatory activity varied depending on the circadian clock, and if so to identify the critical brain regions that exhibit a chronologically-dependent response. Based on previous findings of increased resting-state beta during the later afternoon, we hypothesized that the amplitude of the beta ERD and PMBR responses would be stronger at 16:00 relative to 09:00, and that day-to-day variability would be minimal indicating a strongly reliable response.
Materials and methods
Participant selection
Four healthy males who were right handed participated in the study. The mean age was 31.5 years-old (range: 24–39) and all participants had at least 18 years of formal education. Exclusionary criteria included any pre-existing major psychiatric or neurological disorder, active brain infection, presence of brain neoplasm or space-occupying lesion, history of head trauma, current or history of substance abuse, and the MEG Laboratory’s standard exclusion criteria (e.g., orthodontic braces, extensive dental work, ferromagnetic implants, pacemakers, etc.). Written informed consent was obtained following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, who reviewed and approved the study protocol.
Experimental Paradigm
Participants were instructed to abstain from alcohol and to sleep normally throughout the study and for several days prior to study initiation. Normal sleep was defined as obtaining one’s personal average amount of sleep each night (not more or less), and we expect compliance was high as all participants were laboratory personnel. Participants underwent three MEG recordings per day (09:00, 12:00, and 16:00) for three consecutive days, and each of the nine sessions included the same resting-state and finger tapping experiments. Throughout these experiments, participants were seated in a custom chair within the magnetically-shielded room with their head positioned in the helmet-shaped MEG sensor array. During the movement task, participants were instructed to fixate on a centrally-presented cross hair and to perform a single flexion-extension of the second metacarpus phalangeal (i.e., index finger) of the right hand each time a dot reached the 12 o’clock position. This dot completed one full rotation around a clock-like circle without tick marks or numbers every 6 s, and was meant to serve as a pacing device (Heinrichs-Graham et al., in press; Wilson et al., 2013). The precise timing of movement onset was determined by a fiber optic switch, whereby a signal was emitted from one side of a groove that functioned as the finger rest area. Initiation of movement allowed the signal beam to contact a sensor on the opposite side of the groove and this event generated a trigger pulse that was recorded with the MEG data. Each participant performed approximately 120 trials. Participants also completed a six minute block of eyes-open rest (i.e., fixation on a crosshair) during each MEG session. Total MEG recording time was ~17 minutes per session (including both tasks).
Structural Magnetic Resonance Imaging (sMRI)
High-resolution neuroanatomic images were acquired using a Philips Achieva 3T X-series scanner. The T1-weighted sagittal images were obtained with an eight channel head coil using a 3D fast field echo sequence with the following parameters: TR = 8.1 ms; TE = 3.7 ms; field of view: 24 cm; matrix: 256 × 256; slice thickness: 1 mm with no gap; in-plane resolution: 0.9375 × 0.9375 mm; sense factor: 1.5. The structural volumes were used for MEG coregistration and spatial normalization.
MEG Data Acquisition & MRI Coregistration
All recordings were conducted in a one-layer magnetically-shielded room (MSR) with active shielding engaged. With an acquisition bandwidth of 0.1–330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta Neuromag system with 306 magnetic sensors, including 204 planar gradiometers and 102 magnetometers (Elekta, Helsinki, Finland). Using MaxFilter (v2.1.15; Elekta), MEG data from each participant were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (tSSS; Taulu et al., 2005; Taulu and Simola, 2006).
Prior to MEG measurement, four coils were attached to the participant’s head and the locations of these coils, together with the three fiducial points and scalp surface, were determined with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, including the scalp surface points, each participant’s MEG data were coregistered with their T1-weighted MRI data prior to source space analyses (i.e., beamforming). MRI data were aligned parallel to the anterior and posterior commissures and were transformed into standard space using BESA MRI (Version 2.0; BESA GmbH, Gräfelfing, Germany).
MEG Preprocessing, Time-Frequency Transformation, & Statistics
Cardio-artifacts were removed from the data using signal-space projection (SSP) and the projection operator was accounted for during source reconstruction (Uusitalo and Imoniemi, 1997). Artifact rejection was based on a fixed threshold method, supplemented with visual inspection. In the motor task, epochs were of 5.0 s duration (−2.0 to 3.0 s), with 0.0 s defined as movement onset and the baseline being the −2.0 to −1.2 s time window. For each participant and session, at least 100 artifact-free epochs remained for further analysis, and this number did not statistically differ between the nine MEG sessions (p > 0.8). These artifact-free epochs were transformed into the time-frequency domain using complex demodulation (bandwidth: 4–50 Hz; resolution: 2.0 Hz, 25 ms; Hoechstetter et al., 2004; Papp and Ktonas, 1977), and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These data were normalized by dividing the power value of each predetermined time-frequency bin by the respective bin’s baseline power, which was calculated as the mean power during the −2.0 to −1.2 s time period.
The specific time-frequency windows used for imaging were determined by statistical analysis of the spectrograms corresponding to each of the 204 gradiometer-type sensors across all participants and MEG sessions. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants and sessions. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants and sessions were derived and subjected to the beamforming analysis; the same frequency bands were also used for the resting-state analyses. The two time-frequency bins of interest (see Results) were each significant in a cluster of neighboring gradiometers. We defined the precise time-frequency parameters using the single sensor with the highest t-value, but the results would have been identical had we used any of the gradiometers surrounding the peak sensor.
MEG Source Imaging & Statistics
Using the time-frequency windows determined by the analysis described above, cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001; Van Veen et al., 1997), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images are derived from the cross spectral densities of all combinations of MEG sensors averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged pre-stimulus noise period of equal duration and bandwidth (van Veen et al., 1997). In principle, the beamformer operator generates a spatial filter for each grid point, which passes signals without attenuation from the given neural region while minimizing interference from activity in all other brain areas (see Hillebrand et al., 2005, for a review). MEG preprocessing and imaging used the Brain Electrical Source Analysis software (BESA version 6.0; BESA GmbH, Gräfelfing, Germany).
Normalized source power was computed for the selected frequency bands over the entire brain volume per participant and session at 4.0 × 4.0 × 4.0 mm resolution. Prior to statistical analysis, each participant’s functional images, which were co-registered to their anatomical images prior to beamforming, were transformed into standardized space using the transform previously applied to the structural MRI volume and spatially resampled. The resulting 3D maps of functional brain activity were statistically evaluated as follows using a mass univariate approach based on the general linear model. Essentially, we first examined the effect of time (i.e., 09:00, 12:00, and 16:00) on each of the two significant time-frequency responses across all participants and days, and the resulting statistical parametric maps were thresholded at (p < 0.01) and adjusted for multiple comparisons using a cluster criterion of (k = 300). Importantly, these pairwise comparisons were only to identify regions-of-interest, and subsequently we extracted the peak-voxel value from each of these regions in each participant. These amplitude values were subjected to repeated-measures ANOVA, with day (3 levels) and time (3 levels) as within-subjects factors, to evaluate the effect of time and day on neuronal activity in each brain region. In addition, we derived a virtual sensor time series from each of the peak voxels to quantify the amplitude of beta activity during the pre-movement baseline period, and these data were also evaluated using repeated-measures ANOVA for circadian clock effects.
Finally, analysis of the resting-state MEG recordings focused on the regions-of-interest identified in the motor task. Neural activity estimates were extracted from these regions using an inverse spatial filtering approach (Franzen et al., 2013; Wilson et al., 2013b; see also Scherg et al., 2002) and these data were transformed into the frequency domain using Fourier analysis. We focused on the amplitude of oscillatory activity in the same beta band that was identified in the motor task through permutation testing. Estimates of resting-state beta activity in the regions-of-interest were evaluated using the same ANOVA model described above for the motor task.
Results
MEG Sensor-Based Time-Frequency Analyses
Sensor-level spectrograms were statistically examined using nonparametric permutation testing to derive the precise time-frequency bins for follow up beamforming analyses. The results showed significant (p < 0.05; corrected) beta event-related desynchronization in a subset of gradiometers near the left sensorimotor cortex in each group, which stretched from 14–28 Hz during the −0.4 to 0.2 s time window (0.0 s = movement onset). In addition, significant beta event-related synchronization activity in the same 14–28 Hz band was detected during the 0.8 to 1.4 s time window in roughly the same set of gradiometers near the left sensorimotor cortices (p < 0.05; corrected). These two neural responses correspond closely to the oscillatory motor activity identified in many previous studies, and are typically termed the pre-movement beta ERD and the post-movement beta rebound (PMBR) responses. These two time-frequency bands, and a window of equal bandwidth and duration from the baseline period, were imaged using a beamformer to derive the spatial location of these significant movement-related neural oscillatory responses.
MEG Beamforming Analyses
Pre-movement beta ERD
Source analysis of the beta ERD response across participants revealed strong desynchronization centered on the left motor hand knob region (Yousry et al., 1997) of the precentral gyrus in each of the three sessions (i.e., 09:00, 12:00, and 16:00). Group mean beamformer images for the beta ERD in each session are shown in Figure 1. Pairwise comparisons were performed to identify regions-of-interest for the effect of time on beta ERD amplitude. These comparisons showed that the amplitude of the beta ERD did not significantly change in any brain area between 09:00 and 12:00 (p > 0.01, cluster-corrected), but significantly increased from 09:00 to 16:00 and from 12:00 to 16:00 in the left precentral gyrus, left premotor cortices, left SMA, and the right precentral and postcentral gyri (p < 0.01, cluster-corrected; Figure 2). We extracted the peak voxel value from each of these brain regions and evaluated the effects of day and time using repeated-measures ANOVA. To avoid potential biases from partial volume effects (i.e., differences in the sensitivity of the imaging device to neural activity in different brain areas), an ANOVA was conducted for each brain region, which is consistent with the mass univariate approach that is typical in neuroimaging. For the left precentral gyrus, our results indicated a main effect of time F(2,6) = 11.41 (p < 0.01) without an effect of day or a day-by-time interaction effect, and the within-subject contrasts indicated a significant linear increase in beta ERD amplitude across time F(1,3) = 12.94 (p < 0.05; Figure 3). Similarly, a main effect of time F(2,6) = 17.68 (p < 0.01) without a main effect of day or the day-by-time interaction was detected in the left premotor cortices, and the within-subjects contrasts indicated a significant linear increase component F(1,3) = 26.08 (p < 0.01; Figure 3). Neuronal activity in the left SMA also varied as a function of time F(2,6) = 13.57 (p < 0.01), but not day or their interaction; within-subject contrasts again revealed a linear change F(1,3) = 45.17 (p < 0.01). Finally, main effects of time without day effects or day-by-time interactions were detected in the right precentral gyrus F(2,6) = 17.18 (p < 0.01) and right postcentral gyrus F(2,6) = 6.63 (p = 0.03); in both cases, within-subject contrasts indicated a significant linear component (right precentral gyrus: F(1,3) = 23.73 (p < 0.01); right postcentral gyrus F(1,3) = 9.75 (p = 0.05; Figure 3)).
Figure 1.
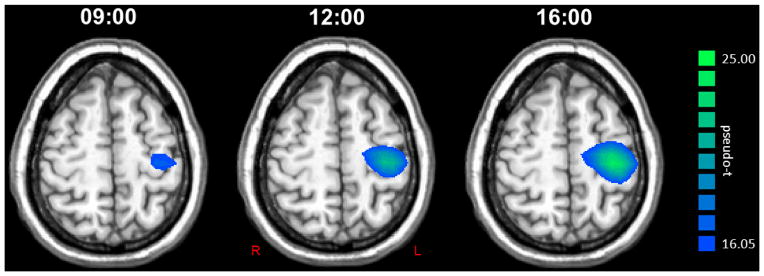
Group mean beamformer images (pseudo-t) of the pre-movement beta ERD response at each time point. The group average for the 09:00 session is shown to the left, the 12:00 session in the middle, and the 16:00 session is shown on the right next to color scale bar. Each image is the average of 12 images, as all four participants completed a MEG session at each time point for three consecutive days. As can be easily discerned, the amplitude of the beta ERD increased in the left precentral gyrus from 09:00 to 12:00 and from 12:00 to 16:00. All images are shown in radiological convention.
Figure 2.
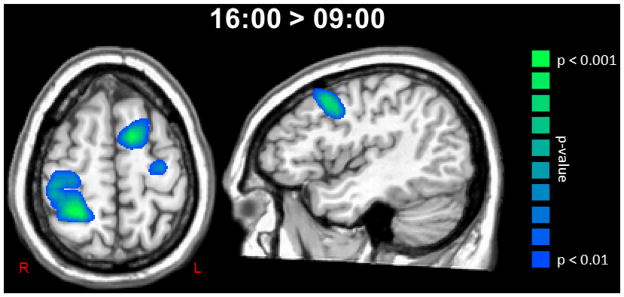
Significant increases in beta ERD amplitude were observed in a widespread network of sensorimotor regions. To the left, an axial image shows areas of the left precentral gyrus, left supplementary motor area, and the right precentral and postcentral gyri where the amplitude of the pre-movement beta ERD increased from 09:00 to 16:00 hours (p < 0.01, cluster-corrected). Significant increases in beta ERD amplitude from 09:00 to 16:00 were also observed in the left premotor cortices (sagittal image). Both images are shown at the same threshold (p < 0.01, cluster-corrected) and in radiological convention. Color scale bar is shown on the far right.
Figure 3.
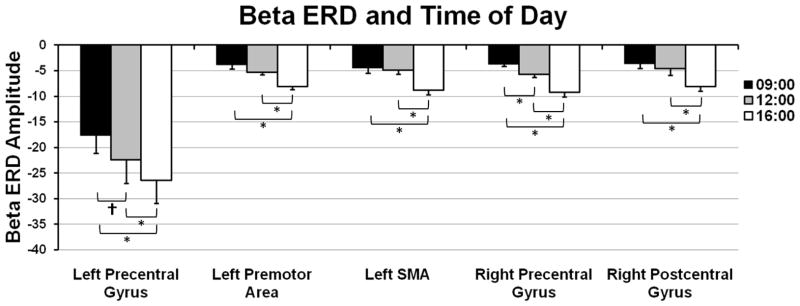
The amplitude of the pre-movement beta ERD increased from 09:00 to 16:00 and from 12:00 to 16:00 in five sensorimotor brain regions. Data are shown in pseudo-t units (y-axis) and correspond to the group mean value of the maximum difference voxel (16:00 > 09:00), which was computed using the beta ERD amplitude for this voxel taken from each participant per MEG session. A significant linear increase in beta ERD amplitude was observed across time in each brain region (p < 0.05). Error bars indicate one standard error of the mean and significant differences based on pairwise comparisons have been marked (* = p < 0.05; † = p < 0.10).
Post-movement beta rebound (PMBR)
Source analysis of the PMBR across participants revealed strong synchronization of beta neural responses near the left precentral gyrus in each of the three sessions (i.e., 09:00, 12:00, and 16:00). Group mean beamformer images of the PMBR in each session are shown in Figure 4. As with the beta ERD analysis, pairwise comparisons were performed to identify regions-of-interest for the effect of time on PMBR amplitude. These comparisons showed that neural activity did not significantly differ in any brain region between 09:00 and 12:00 (p > 0.01, cluster-corrected). In contrast, the PMBR significantly increased between 09:00 and 16:00 and from 12:00 to 16:00 in the left SMA (p < 0.01, cluster-corrected; Figure 5). We extracted the peak voxel value from the left SMA of each participant and subjected these data to repeated-measures ANOVA, with day and time as within-subject factors. Our results indicated a significant main effect of time F(2,6) = 9.18 (p < 0.01) without an effect of day or a day-by-time interaction effect, and the within-subject contrasts indicated a significant linear increase in PMBR amplitude across time F(1,3) = 9.76 (p = 0.05; Figure 5).
Figure 4.
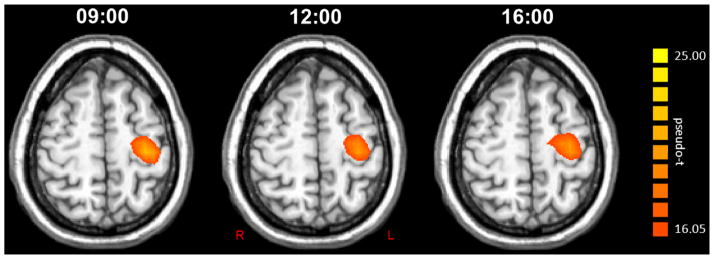
Group mean beamformer images (pseudo-t) of the post-movement beta rebound (PMBR) at each time point. The group average for the 09:00 session is shown to the left, the 12:00 session in the middle, and the 16:00 session on the right next to the color scale bar. Each image is the average of 12 images, as all four participants completed a MEG session at each time point for three consecutive days. As can be discerned, the amplitude of the PMBR was very stable across all time points in the left precentral gyrus. Images are shown in radiological convention.
Figure 5.
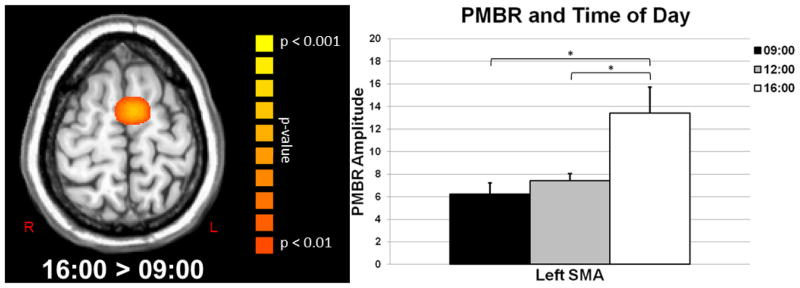
Significant increases in the amplitude of the post-movement beta rebound (PMBR) were observed in the left supplementary motor area (SMA). (Left) Axial image showing neural areas where significant increases were observed in the PMBR from 09:00 to 16:00 (p < 0.01, cluster-corrected); color scale bar appears to the right. (Right) Bar graph showing the significant linear increase in PMBR amplitude within the left SMA. Data are shown in pseudo-t units and correspond to the group mean amplitude value of the maximum difference voxel (16:00 > 09:00), which was computed using the PMBR amplitude for this voxel taken from each participant per MEG session. Error bars indicate one standard error of the mean and significant differences based on pairwise comparisons have been marked (* = p < 0.05).
Baseline Activity (14–28 Hz)
Although the beta ERD and PMBR are both metrics of change in oscillatory power from the baseline to the target period, understanding whether beta activity during the baseline period (i.e., pre-movement) fluctuates from morning to afternoon is critical to interpreting our key findings. Thus, we evaluated the amplitude of beta activity during the baseline by extracting virtual sensors from the peak voxels used in the beta ERD statistics. In the left precentral gyrus, these analyses showed a significant main effect of time F(2,6) = 4.98 (p < 0.05) without an effect of day or a day-by-time interaction effect, and the within-subject contrasts indicated a significant linear increase in beta across time F(1,3) = 7.77 (p < 0.05; Figure 6). Beta activity in the left SMA also varied as a function of time F(2,6) = 6.17 (p < 0.05), but not day or their interaction; within-subject contrasts again revealed a linear effect F(1,3) = 13.92 (p < 0.05; Figure 6). Neither main effects nor the day-by-time interaction was significant in the left premotor cortices, but the within-subjects contrasts showed a marginal linear increase across time F(1,3) = 8.96 (p = 0.058; Figure 6). Finally, main effects of time without day effects or day-by-time interactions were detected in the right precentral gyrus F(2,6) = 11.57 (p < 0.01) and right postcentral gyrus F(2,6) = 8.29 (p < 0.01); in both regions, within-subject contrasts revealed a significant linear change (right precentral gyrus: F(1,3) = 26.11 (p < 0.01); right postcentral gyrus F(1,3) = 8.78 (p < 0.05; Figure 6)).
Figure 6.
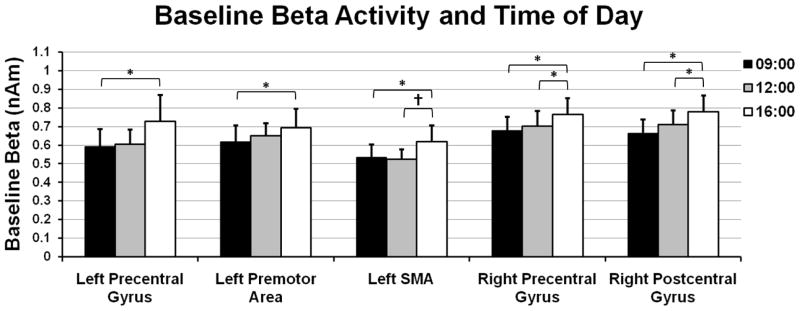
Beta activity levels during the baseline period (i.e., before movement) also increased from 09:00 to 16:00 in sensorimotor cortices. To evaluate time-related changes in baseline beta activity, we extracted virtual sensors from the peak voxels of the beta ERD response and computed the mean beta amplitude during the baseline period. The results indicated a significant linear increase in the amplitude of baseline beta activity across time (p < 0.05) in the left precentral gyrus, left SMA, left premotor cortices, and the right precentral and postcentral gyri. Error bars indicate one standard error of the mean. Significant differences based on pairwise comparisons have been marked (* = p < 0.05; † = p < 0.10).
MEG Resting-State Analyses
To evaluate whether these changes in beta activity were tied to the performance of a movement task and/or were specific to motor cortices, we also probed resting-state beta levels at each time point. For this analysis, we focused on beta activity in the precentral gyri bilaterally, the left SMA, left premotor, right postcentral gyrus, and the right supramarginal gyrus. These regions encompass our most important findings for the beta ERD and PMBR responses, plus a non-sensorimotor region to assess the presence of any global changes. These analyses showed a significant increase in beta amplitude across sensorimotor areas. Specifically, for the left precentral gyrus, the results indicated a significant main effect of time F(2,6) = 4.89 (p < 0.05) without an effect of day or a day-by-time interaction effect, and the within-subject contrasts indicated a significant linear increase in beta amplitude across time F(1,3) = 30.5 (p < 0.01; Figure 7). Neuronal activity in the left SMA also varied as a function of time F(2,6) = 13.91 (p < 0.01), but not day or their interaction; within-subject contrasts again revealed a linear change F(1,3) = 25.31 (p < 0.01; Figure 7). Neither main effects nor the day-by-time interaction was significant in the left premotor cortices, but the within-subjects contrasts indicated a marginal linear increase F(1,3) = 7.08 (p = 0.08; Figure 7). In the right precentral gyrus, a main effect of time F(2,6) = 5.74 (p < 0.05) without a main effect of day or day-by-time interaction was detected, and the within-subjects contrasts showed a significant linear increase component F(1,3) = 16.13 (p < 0.05; Figure 7). Similar to the left premotor cortices, neither main effects nor the day-by-time interaction was significant in the right postcentral gyrus, but a marginal linear increase was detected in the within-subjects contrasts F(1,3) = 7.63 (p = 0.07; Figure 7). Finally, we evaluated resting beta levels in the right supramarginal gyrus to assess whether beta increases were a global effect or more specific to the sensorimotor cortices. This analysis showed no main effects (both p’s > 0.5), nor a time-by-day interaction (p = 0.51), nor any type of linear or quadratic increase/decrease from 09:00 to 16:00.
Figure 7.
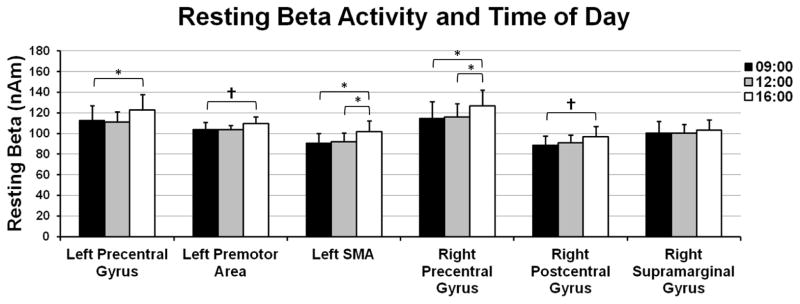
The amplitude of spontaneous beta activity during the resting-state also increased from 09:00 to 16:00 and from 12:00 to 16:00 in some sensorimotor brain regions. The amplitude of resting-state beta was extracted from brain areas where significant increases were observed in the PMBR or beta ERD responses. To assess for global effects, we also evaluated the amplitude of a non-motor brain region (i.e., the right supramarginal gyrus). A significant linear increase in the amplitude of spontaneous beta activity across time (p < 0.05) was observed in the left precentral gyrus, left supplementary motor area, and the right precentral gyrus. Linear trends were also found in the left premotor cortices and the right postcentral gyrus (p < 0.10), but not in the right supramarginal gyrus (p < 0.50). Error bars indicate a standard error of the mean and significant differences based on pairwise comparisons have been marked (* = p < 0.05; † = p < 0.10).
Discussion
We examined whether the amplitude of motor-related beta oscillatory responses varied with the circadian clock by examining neural activity during finger-tapping in four participants at three different times (09:00, 12:00, and 16:00) on three consecutive days. Our primary hypotheses were that the amplitude of the beta ERD and the PMBR would be stronger at 16:00 relative to 09:00, and that day-to-day variability would be minimal indicating a strongly reliable response. Our results support both of these conclusions, although more so for the beta ERD response, and we would add that baseline and resting beta levels also increased in motor-related brain regions. Our most important finding was the linear increase in beta ERD amplitude from the morning (09:00) to the afternoon (16:00) in a widespread network of motor regions that included the left precentral gyrus, left SMA, and left premotor cortices, as well as ipsilateral areas in the right precentral and postcentral gyri. We also observed a linear increase in the amplitude of the PMBR from 09:00 to 16:00, but this effect was restricted to the left SMA and much more concentrated in the 12:00 to 16:00 time window. Finally, spontaneous beta activity during the baseline period (motor task) and the resting-state also increased from 09:00 to 16:00 in a widespread network of cortical sensorimotor regions, but again this linear increase was mostly concentrated in the 12:00 to 16:00 time window. Below, we discuss the implications of these findings for understanding the underlying circuitry and basic neurophysiology of motor-related beta activity, and for good practice in MEG and EEG studies of motor-related oscillatory responses.
All participants generated a strong beta ERD response slightly before movement onset in all MEG sessions and this response was strongest in the left primary motor cortex, but could also be seen in other motor areas including the left premotor and SMA, as well as ipsilaterally in the right precentral and postcentral gyri. These results are consistent with many prior studies of beta activity in healthy adults and children (Gaetz et al., 2010; Hall et al., 2011; Heinrichs-Graham et al., in press; Jurkiewicz et al., 2006; Tzagarakis et al., 2010; Wilson et al., 2010, 2011, 2013), which have characterized this response as a marker of movement planning (Doyle et al., 2005; Kaiser et al., 2001). Interestingly, we found that the amplitude of the beta ERD significantly increased from morning to afternoon in recognized regions of the motor network, which has not been previously reported. Before discussing the mechanisms or functional significance of this increase, it is important to clarify that the beta ERD metric quantifies the relative decrease in beta amplitude associated with an event (e.g., movement onset). Thus, a stronger beta ERD response reflects a greater decrease in beta amplitude from the baseline to the target period, irrespective of whether the baseline period contained more or less beta activity. Taken in the current context, this chronological increase in the amplitude of the beta ERD may reveal a critical feature of motor cortex physiology. Essentially, the amplitude of beta activity during the pre-movement baseline period and resting-state increased from morning to afternoon in the motor cortices, and concomitantly the magnitude of the beta ERD also increased, which may suggest that the amplitude of the beta ERD is determined by the spontaneous beta level. Potentially, the number of cortical motor cells that are spontaneously synchronized at the beta rhythm may be modulated by the circadian clock, and if so this could underlie the increased resting-state and baseline beta during the afternoon. Likewise, input from the SMA and other frontal regions during motor planning would disrupt this synchronization and the greater number of involved cells would produce a stronger beta ERD compared to that observed earlier in the circadian cycle. An alternative view is that the number of involved cells remains constant, but that the degree of beta synchrony amongst these cells increases throughout the day, perhaps due to increased thalamocortical drive later in the circadian cycle. Both of these interpretations would be consistent with the current data. Importantly, several prior studies have connected the beta ERD amplitude to the directional certainty of an upcoming movement, with the most predictable movements being associated with the strongest beta ERD (Doyle et al., 2005; Kaiser et al., 2001; Tzagarakis et al., 2010), and we do not propose that the current findings are in any way contradictory. Such studies are generally conducted with the conditions presented together in a randomized fashion, and given our findings of a slow linear change across the day it is safe to assume that the baseline beta level was relatively stable throughout the experiments. We propose that these earlier findings reflect a different mechanism, in that when the upcoming movement is known the key neuronal population can be fully programmed based on top-down input from the SMA and other frontal areas. This may involve dissipation of local beta oscillatory activity and increased activity in other bands (e.g., gamma; Cheyne et al., 2008; Wilson et al., 2010), as these cells become engaged in movement execution. In contrast, when the parameters of the upcoming movement are not fully known, this key neural population cannot be identified and consequently many cortical motor cells continue to oscillate at the beta rhythm. Of course, other interpretations are also possible and our views remain speculative until further data becomes available.
A strong PMBR was also detected after movement termination in all participants and MEG sessions and this response was strongest in the left precentral gyrus (i.e., primary motor cortex), which is in agreement with many previous studies (Gaetz et al., 2010, 2011; Hall et al., 2011; Jurkiewicz et al., 2006; Tzagarakis et al., 2010; Wilson et al., 2010, 2011). Unlike our beta ERD findings, the amplitude of the PMBR did not significantly change in the primary motor cortices from 09:00 to 16:00, and in fact was remarkably similar across all MEG sessions. However, a significant circadian effect was detected in the left SMA and this effect took the form of a linear increase in the PMBR amplitude from 09:00 to 16:00, which is much like that observed for the beta ERD response in this same brain area. This specificity for the left SMA was surprising, especially since we observed progressively stronger beta ERD responses and spontaneous beta activity in a widespread network of motor regions. There are two competing hypotheses for the purpose of the PMBR, both of which are relevant to the current findings. The first hypothesis, the idling hypothesis, posits that the PMBR functions to return the motor cortex to its baseline, as the motor cortices is highly synchronous when at rest (Pfurtscheller, 1992; Pfurtscheller et al., 1996; Pfurtscheller and Lopes da Silva, 1999). The second, newer hypothesis speculates that the PMBR is an active cortical motor inhibitory mechanism (Cassim et al., 2001; Gaetz et al., 2010; Pfurtscheller et al., 1997; Salmelin et al., 1995), and/or an index of afferent sensory neuronal input to the motor cortices (i.e., proprioceptive and exteroceptive; Cassim et al., 2001; Houdayer et al., 2006; Parkes et al., 2006). In the current study, the idling hypothesis would predict a significantly stronger PMBR in the afternoon due to the increased beta ERD amplitude at this time-of-day, as such would be needed to return the motor cortices to their baseline level of beta activity. While our findings in the left SMA are consistent with this, our lack of findings in other motor regions is clearly contradictory. In particular, we observed no hint of a circadian clock effect in the left precentral gyrus, despite a very robust effect for the beta ERD in this brain region. Furthermore, even in the left SMA, the maximum PMBR response was about one centimeter posterior, more medial, and slightly superior to the maximum beta ERD response; the idling hypothesis would predict more spatial correspondence between these maxima. In regard to the second “inhibitory and/or afferent input” hypothesis of PMBR function, the SMA is known to have abundant, direct connections with the primary motor and somatosensory cortices, as well as the cerebellum, and the stronger PMBR response in the left SMA could reflect greater afferent input later in the day. Although speculative, stronger multi-sensory feedback (e.g., proprioceptive, visual, somatosensory) later in the day could serve a compensatory role, as motor performance is known to fluctuate throughout the day (Carrier and Monk, 2000; Drust et al., 2005) and there is some evidence that kinematic awareness may be highest during the afternoon and evening hours (Gueugneau and Papaxathis, 2010). Finally, we think it is worth noting that the previously described “overshoot” in the PMBR response (relative to baseline beta levels; Pfurtscheller et al., 1996, 1997) became smaller from 09:00 to 16:00 in all sensorimotor regions except the left SMA. In these other regions, the amplitude of the PMBR remained stable across time despite increases in the beta ERD amplitude and baseline beta levels. Such differences in response profile across time has not been previously reported and highlights a distinction between the beta ERD and PMBR, which likely has implications for understanding the functional significance of these motor-related oscillatory responses. Future studies should more closely evaluate the relationship between PMBR amplitude and spontaneous beta levels.
In summary, we examined whether motor-related beta oscillatory activity was modulated by the circadian clock. Our primary findings were that the amplitude of the beta ERD linearly increased from 09:00 to 16:00 in a widespread network of sensorimotor regions that included the left precentral gyrus, left premotor cortices, left SMA, and the right precentral and postcentral gyri. This increase in beta ERD amplitude was concomitant with an increase in spontaneous beta activity (i.e., during the baseline period and the resting-state), and we propose that these findings indicate that the observed beta ERD amplitude is determined by the baseline beta level during movement planning. Unlike the beta ERD, we found that the PMBR linearly increased from 09:00 to 16:00 in only one brain area (i.e., left SMA), with negligible changes in the region with the strongest PMBR response in all sessions (i.e., left precentral gyrus). We propose that these findings indicate that the amplitude of the PMBR is not tightly coupled to spontaneous beta levels or the amplitude of the beta ERD in most sensorimotor regions, which long-term is a critical insight for gaining a more complete understanding of the functional significance of the PMBR in motor performance. These data may also be informative to biophysical models of the genesis and modulation of sensorimotor cortical rhythms (e.g., Jones et al., 2009), and may suggest circadian influences on thalamocortical drive. Finally, our results are especially relevant to the design of future studies of oscillatory motor activity. We observed robust increases in beta ERD amplitude in the afternoon relative to the morning, and future studies of the beta ERD could harness this finding by simply scheduling more participants for afternoon MEG/EEG sessions. Such practice should increase the signal-to-noise ratio and potentially the study’s sensitivity to the effects of interest.
Highlights.
Each participant performed a finger-tapping task during nine MEG recording sessions
MEG recordings occurred at 09:00, 12:00, and 16:00 on three consecutive days
MEG data was examined in the time-frequency domain and imaged using a beamformer
Significant linear increase across time in beta ERD amplitude in sensorimotor areas
Beta ERD amplitude may be determined by spontaneous beta levels during the baseline
Acknowledgments
This work was supported by NIH grant R01 MH103220 (TWW), a Kinman-Oldfield Award for Neurodegenerative Research from the University of Nebraska Medical Center (TWW), and a grant from the Nebraska Banker’s Association. The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cacot P, Tesolin B, Sebban C. Diurnal variations of EEG power in healthy adults. Electroenceph Clin Neurophysiol. 1995;94:305–312. doi: 10.1016/0013-4694(94)00298-y. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH. Circadian rhythms of performance: new trends. Chronobiol Int. 2000;17:719–732. doi: 10.1081/cbi-100102108. [DOI] [PubMed] [Google Scholar]
- Cassim F, Monaca C, Szurhaj W, Bourriez JL, Defebvre L, Derambure P, et al. Does post- movement beta synchronization reflect an idling motor cortex? Neuroreport. 2001;12:3859–3863. doi: 10.1097/00001756-200112040-00051. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42:332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Doyle LM, Yarrow K, Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol. 2005;116:1879–1888. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Drust B, Waterhouse J, Atkinson G, Edwards B, Reilly T. Circadian rhythms in sports performance, an update. Chronobiol Int. 2005;22:21–44. doi: 10.1081/cbi-200041039. [DOI] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: A basis for exact inference. Stat Sci. 2004;19:676–685. [Google Scholar]
- Franzen JD, Heinrichs-Graham E, White ML, Wetzel MW, Knott NL, Wilson TW. Atypical coupling between posterior regions of the default mode network in attention-deficit/hyperactivity disorder: a pharmaco-magnetoencephalography study. J Psychiatry Neurosci. 2013;38(5):333–340. doi: 10.1503/jpn.120054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98(2):694–9. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueugneau N, Papaxanthis C. Time-of-day effects on the internal simulation of motor actions: psychophysical evidence from pointing movements with the dominant and non-dominant arm. Chronobiol Int. 2010;27:620–639. doi: 10.3109/07420521003664205. [DOI] [PubMed] [Google Scholar]
- Gundel A, Hilbig A. Circadian acrophases of powers and frequencies in the waking EEG. Int J Neurosci 1983. 1983;22:125–33. doi: 10.3109/00207459308987391. [DOI] [PubMed] [Google Scholar]
- Hall SD, Stanford IM, Yamawaki N, McAllister CJ, Rönnqvist KC, Woodhall GL, Furlong PL. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56(3):1506–10. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter- Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb Cortex. 2013 May 3; doi: 10.1093/cercor/bht121. In press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Labyt E, Cassim F, Bourriez JL, Derambure P. Relationship between event-related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations. Clin Neurophysiol. 2006;117:628–636. doi: 10.1016/j.clinph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Jasper I, Häußler A, Baur B, Marquardt C, Hermsdörfer J. Circadian variations in the kinematics of handwriting and grip strength. Chronobiol Int. 2009;26:576–594. doi: 10.1080/07420520902896590. [DOI] [PubMed] [Google Scholar]
- Jones SR, Pritchett DL, Sikora MA, Stufflebeam SM, Hämäläinen M, Moore CI. Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: rhythmogenesis and modulation of sensory-evoked responses. J Neurophysiol. 2009;102:3554–3572. doi: 10.1152/jn.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci Lett. 2001;312:149–152. doi: 10.1016/s0304-3940(01)02217-0. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–1885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Parkes LM, Bastiaansen MC, Norris DG. Combining EEG and fMRI to investigate the post-movement beta rebound. Neuroimage. 2006;29:685–696. doi: 10.1016/j.neuroimage.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Peres I, Vetter C, Blautzik J, Reiser M, Pöppel E, Meindl T, Roenneberg T, Gutyrchik E. Chronotype predicts activity patterns in the neural underpinnings of the motor system during the day. Chronobiol Int. 2011;28(10):883–889. doi: 10.3109/07420528.2011.619084. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. Int J Psychophysiol. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Jr, Neuper C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol. 1996;98:281–293. doi: 10.1016/0013-4694(95)00258-8. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hämäläinen M, Kajola M, Hari R. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage. 1995;2:237–243. doi: 10.1006/nimg.1995.1031. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. J Clin Neurophysiol. 2002;19(2):91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Trans Signal Process. 2005;53:3359–3372. [Google Scholar]
- Toth M, Kiss A, Kosztolanyi P, Kondakor I. Diurnal alterations of brain electrical activity in healthy adults: a LORETA study. Brain Topogr. 2007;20(2):63–76. doi: 10.1007/s10548-007-0032-3. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 2010;30:11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo M, Ilmoniemi R. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Fleischer A, Archer D, Hayasaka S, Sawaki L. Oscillatory MEG motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil Neural Repair. 2011b;25(2):188–193. doi: 10.1177/1545968310378511. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Human Brain Mapping. 2013b;34(3):566–574. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O’Neill J, Knott NL, Fox HS, Swindells S. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J Neuroimmune Pharmacol. 2013;8(4):965–74. doi: 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]


