Abstract
Background and aims
Cannabis-dependent participants with depressive disorder are less likely to achieve abstinence with venlafaxine-XR (VEN-XR) treatment. Individuals on VEN-XR reported more severe withdrawal, despite not reducing their smoking behavior. We hypothesized that withdrawal-like symptoms, likely medication side effects, led to continued marijuana smoking in this group.
Methods
We conducted a secondary analysis using Marijuana Withdrawal Checklist (MWC) scores and urine THC to test whether severity of withdrawal-like symptoms mediates the relationship between VEN-XR treatment and continued marijuana smoking. We included 103 participants (VEN-XR = 51, Placebo = 52). Marijuana use was dichotomized into smoking (THC > 100 ng/ml) and non-smoking (THC ≤ 100 ng/ml) weeks. MWC scores were obtained weekly. We used three models in a regression based mediation analysis.
Results
The estimated risk of smoking marijuana was greater for individuals on VEN-XR in weeks 7–9, even when controlling for MWC scores (week 7 Risk Difference (RD) = 0.11, p = 0.034; week 8 RD = 0.20, p = 0.014), and higher scores mediated this effect. In weeks 10 and 11, the estimated effect was stronger (week 10 RD = 0.03, p = 0.380; week 11 RD = 0.07, p = 0.504), and worse withdrawal-like symptoms more fully accounted for continued marijuana smoking in the VEN-XR group, according to the models.
Conclusions
Individuals treated with VEN-XR had more severe withdrawal-like symptoms, which mediated their continued marijuana smoking. Noradrenergic agents, such as VEN-XR, may negatively impact treatment outcomes in cannabis-dependent patients attempting to reduce or stop their use.
Keywords: Cannabis, Marijuana, Treatment, Venlafaxine, Cannabis Withdrawal, Marijuana Withdrawal
1. Introduction
Cannabis is the most widely used illicit drug in the world, and prevalence rates of cannabis use disorders are relatively higher worldwide than for other drugs of abuse (UNODC, 2011). Cannabis withdrawal is common among regular users trying to quit or reduce their use (Cornelius et al., 2008; Hasin et al., 2008) and withdrawal can be a powerful motivator to continue using marijuana, contributing to early relapse (Allsop et al., 2012; Budney et al., 2008). Conversely, reduction in withdrawal symptoms is associated with positive clinical outcomes in randomized-controlled trials: individuals receiving gabapentin had attenuated withdrawal and reduced marijuana use (Mason et al., 2012), and individuals treated with dronabinol had decreased withdrawal and increased study retention (Levin et al., 2011).
We previously reported on a 12-week randomized controlled trial of venlafaxine-XR (VEN-XR) for comorbid cannabis dependence and depression, and found that participants receiving VEN-XR were significantly less likely to achieve abstinence than individuals receiving placebo, despite their depression improving (Levin et al., 2013). The findings of more marijuana smoking in the VEN-XR group were unexpected, and prompted us to consider the role of withdrawal symptoms. Because individuals receiving VEN-XR did not significantly reduce their smoking behavior, they would not be expected to experience more severe cannabis withdrawal. However, we speculated that the overlap in the symptom profiles of cannabis withdrawal and VEN-XR side effects contributed to a higher burden of withdrawal-like symptoms in the VEN-XR group. This finding would be clinically important, especially if it interferes with the individual’s ability to reduce or stop smoking marijuana.
VEN-XR is a serotonin and norepinephrine reuptake inhibitor that increases norepinephrine activity at higher doses. Evidence from preclinical and human laboratory studies suggests that noradrenergic hyperactivity may be an important feature of cannabis withdrawal. Precipitated withdrawal in cannabis-dependent mice has been alleviated by the alpha-2 agonist clonidine, which decreases noradrenergic release (Lichtman et al., 2001), and by Prostaglandin E2, an end-product of the arachidonic acid cascade which also inhibits norepinephrine release (Anggadiredja et al., 2003). Human laboratory studies have shown that bupropion SR, a dopamine and norepinephrine reuptake inhibitor, worsened withdrawal symptoms in dependent marijuana smokers (Haney et al., 2001), while the alpha-2 agonist lofexidine, which acts similarly to clonidine and decreases noradrenergic activity, decreased cannabis withdrawal and reduced self-administration (Haney et al., 2008). Thus, side effects of VEN-XR include symptoms associated with increased noradrenergic activity and may mimic withdrawal symptoms to experienced marijuana users who are medication-naïve.
Here, we examine the relationship between VEN-XR treatment, withdrawal symptom scores and marijuana use in a secondary analysis. We hypothesized that worse symptom scores on the Marijuana Withdrawal Checklist (MWC) contributed to continued marijuana smoking in the VEN-XR group, accounting for their higher urine THC levels relative to the placebo group in the later weeks of the study.
2. Methods
2.1. Participants
Individuals were men and non-pregnant females between the ages of 18–60, cannabis-dependent with active use, had major depressive disorder or dysthymia, and at least 3 months duration of depressive symptoms. We excluded participants with a history of mania, schizophrenia, or psychotic disorder; dependence on other substances requiring medical intervention; risk for suicide; seizure disorder or an unstable medical condition. We also excluded participants currently taking psychotropic medications and those with a prior trial of treatment with venlafaxine.
2.2. Study design
We have conducted a secondary analysis of the data from a randomized, placebo-controlled, double-blind, 12-week clinical trial of VEN-XR for cannabis dependence and depression (Levin et al., 2013). The study began with a placebo lead-in week followed by randomization. Participants (n = 22) who had a clinically significant improvement in depressive symptoms during the lead-in were not randomized. All other consented individuals were randomized to placebo or VEN-XR, titrated up to 225 mg over 3 weeks post-randomization. In week 4, if individuals did not score “very much improved” on the Clinical Global Impression scale, they were titrated up to 375 mg of placebo or VEN-XR. Medication doses were reduced if the dose increases were poorly tolerated due to side effects. All individuals received weekly cognitive behavioral therapy/relapse prevention therapy (CBT/RPT), and visited the clinic twice weekly for assessments.
2.3. Measures
Urine THC concentration (creatinine-corrected) was examined as a longitudinal variable. The Marijuana Withdrawal Checklist (MWC), a 29-item instrument in which participants are asked to rate the severity of each symptom on a scale of 0–3 (0 = none, 1 = mild, 2 = moderate, 3 = severe), was given weekly to assess the severity of cannabis withdrawal over the past 24 h. There is significant overlap between withdrawal symptoms on the MWC and VEN-XR side effects, which are likely also captured in the MWC symptom scores. Urine specimens were collected and withdrawal symptoms were recorded twice weekly at each clinic visit.
2.4. Data analysis
In the current study, we used marijuana withdrawal scores on the MWC as a measure of withdrawal-like symptoms and we used urine THC concentration as a measure of marijuana use to test our hypothesis that withdrawal score severity mediates the relationship between VEN-XR and increased marijuana smoking. We used a regression-based mediation approach (Baron and Kenny, 1986), generalized to accommodate dichotomous outcomes where risk differences are used to compute mediation effects (Imai et al., 2010). In our analysis the primary marijuana outcome was dichotomized into either a smoking week (THC > 100 ng/ml) or a non-smoking week (THC ≤ 100 ng/ml). The cutoff of 100 ng/mL was used as the point between positive and negative to decrease the probability of false positives (Budney et al., 2000, 2006; Carpenter et al., 2009).
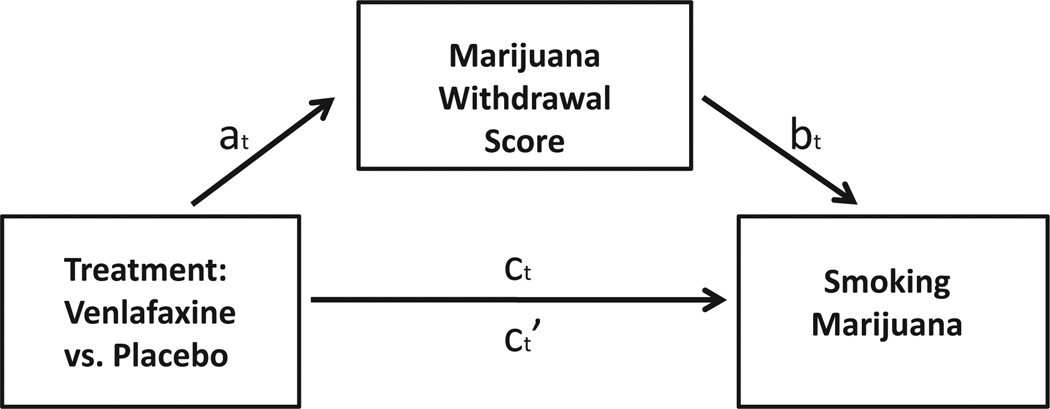
Three different models were evaluated to determine the relationships between treatment group, cannabis withdrawal scores, and marijuana smoking (see Fig. 1). All models used techniques of longitudinal generalized linear mixed modeling with appropriate distribution and link function, random intercept, and autoregressive correlation structure to account for the within-subject correlations of the repeated measures.
Fig. 1.
Mediation analysis. at = The estimated effect of treatment with VEN-XR on marijuana withdrawal. bt = The estimated effect of marijuana withdrawal on marijuana smoking when treatment with VEN-XR is included in the model. ct= The total estimated effect of treatment with VEN-XR on marijuana smoking when marijuana withdrawal is not included in the model. ct′= The direct estimated effect of treatment with VEN-XR on marijuana smoking when marijuana withdrawal is included in the model.
Model 1 used a log-linear model with time, treatment, and time by treatment interactions as predictors to test the relationship of withdrawal scores and treatment group and show that treatment was associated with withdrawal scores (see Fig. 1, relationship at). Without this relationship, withdrawal scores cannot be evaluated as a potential mediator. Significantly higher withdrawal scores were found in weeks 7–12, which allowed us to evaluate in Model 2 and Model 3 the potential mediation effect of withdrawal scores on increased marijuana smoking in the VEN-XR group for those weeks. Model 2 estimated the magnitude of the effect of VEN-XR treatment on marijuana smoking without controlling for withdrawal scores (Fig. 1, relationship ct), using a logistic model with time, treatment, and time by treatment interaction as predictors. Model 3 estimated the magnitude of the effect of VEN-XR on marijuana smoking with controlling for withdrawal scores (Fig. 1, relationship ct′), using a logistic model with time, treatment, withdrawal score, time by treatment, and time by withdrawal score interactions. In Model 3 we also tested the significance of the association between withdrawal scores and marijuana smoking (Fig. 1, relationship bt). The effect of VEN-XR treatment on marijuana smoking for each of the weeks of interest was expressed as a risk difference (RD). The percent change in risk differences between Model 2 and Model 3 was calculated and provides the estimated proportion of the effect that is mediated by withdrawal scores. The difference in risk differences between Model 2 and Model 3 was calculated and provides the estimated amount of mediation.
In the 3 models discussed above, no additional covariates were adjusted for. Urine data was only collected during the study, with THC urine level from the first visit included in the outcome for week 1. Thus, a baseline THC urine was not used as a covariate. There were no differences in demographic characteristics between treatment arms (Levin et al., 2013) and thus no demographic characteristics were adjusted for.
For weeks 10 and 11, which showed the strongest estimated mediation effect of withdrawal scores on marijuana smoking, we also tested for significant differences between the treatment and placebo groups for each item on the MWC using the Mann–Whitney U test for a nonparametric distribution.
3. Results
3.1. Characteristics of the sample
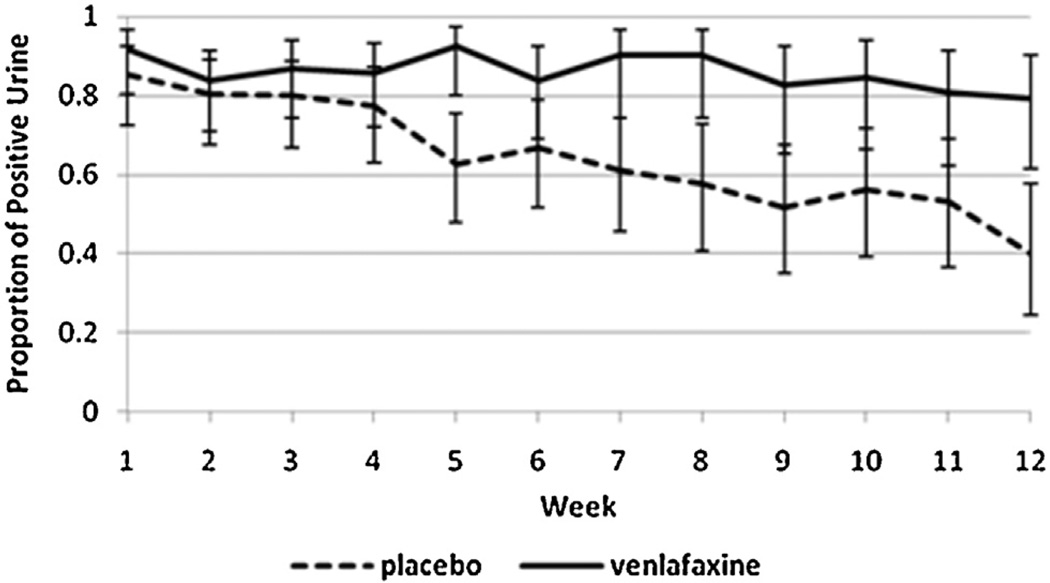
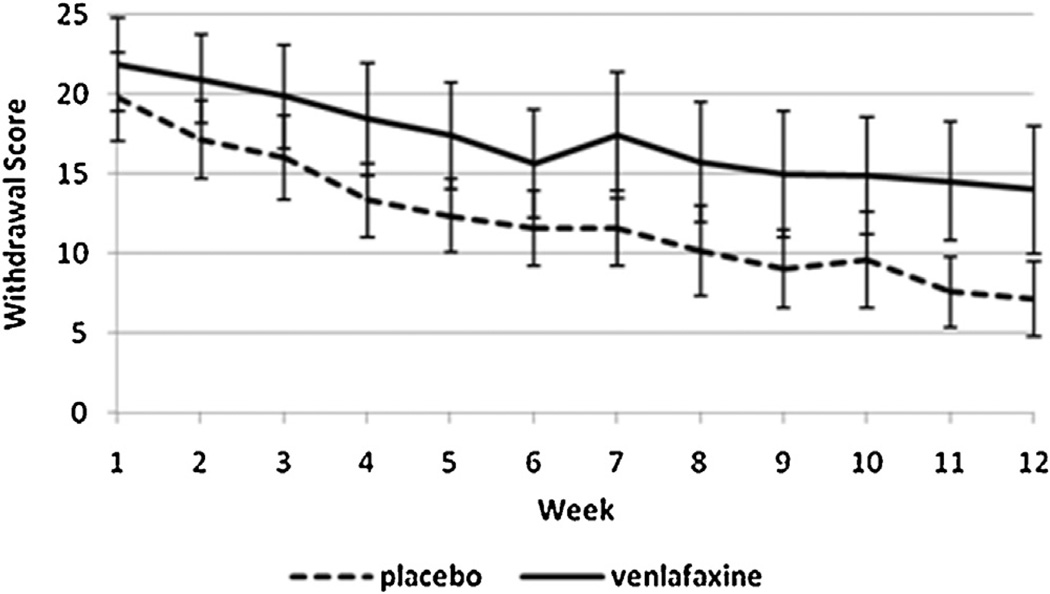
One hundred and three individuals were included in the original study and in this secondary analysis (VEN-XR = 51, PBO = 52). Participants did not significantly differ on baseline or clinical characteristics (age, gender, race, education, employment status, married status, marijuana use, depression scores). The sample was a heavy-using population, with the mean usage at 27.4 days out of 30, and the baseline grams used per using day was 2.6 (SD = 2.8) (Levin et al., 2013). Side effects reported in the parent study showed significant group differences only for decreased libido in the VEN-XR group. We observed that the VEN-XR group had a greater proportion of positive urines than the placebo group, especially in the later weeks of the study (see Fig. 2). We also observed that the VEN-XR group had higher symptom scores on the MWC in the later weeks of the study, specifically weeks 7–12 (see Fig. 3).
Fig. 2.
Observed proportion of positive urine toxicologies over the weeks in the study by treatment arm.
Fig. 3.
Observed withdrawal symptoms averaged by treatment group (VEN-XR, n = 51; PBO, n = 52) and week. *Significant differences were found between treatment arms in the later weeks of the study (specifically, at weeks 7–12).
3.2. Model 1
In Model 1, significantly higher withdrawal scores were found in the VEN-XR group compared to the placebo group in weeks 7–12, but not in weeks 1–6 (see Table 1). The significant relationship between VEN-XR treatment and withdrawal scores in weeks 7–12 allowed us to evaluate the mediation effect of withdrawal scores on the relationship between VEN-XR treatment and marijuana smoking only in those weeks.
Table 1.
Mediation of VEN-XR on marijuana abstinence by week.
| Week | Model 1: Correlation analysis of VEN-XR and withdrawal scores |
Model 2: Estimated effect of VEN-XR on marijuana smoking |
Model 3: Estimated effect of VEN-XR on marijuana smoking controlling for standardized withdrawal scores |
Mediation effect: Amount of estimated mediation of withdrawal score on marijuan smoking in VEN-XR |
Mediation effect: Proportion of the effect of VEN-XR on marijuana smoking accounted for by marijuana withdrawal scores |
|
|---|---|---|---|---|---|---|
| Beta, p-value of effect at at time t |
Estimated risk difference for VEN-XR, p-value of effect c |
Estimated risk difference for VEN-XR, p-value of effect c’ |
Exponentiated beta, p-value of effect b |
Difference in risk differences = c’-c (model 2 compared to model 3) |
% Decrease in risk differences = 1-(c’/c) (model 2 compared to model 3) |
|
| 1 | 0.0201 | N/A | N/A | N/A | N/A | N/A |
| (0.8895) | ||||||
| 2 | 0.1548 | N/A | N/A | N/A | N/A | N/A |
| (0.2835) | ||||||
| 3 | 0.1147 | N/A | N/A | N/A | N/A | N/A |
| (0.4315) | ||||||
| 4 | 0.1265 | N/A | N/A | N/A | N/A | N/A |
| (0.3930) | ||||||
| 5 | 0.2795 | N/A | N/A | N/A | N/A | N/A |
| (0.0642) | ||||||
| 6 | 0.2956 | N/A | N/A | N/A | N/A | N/A |
| (0.0527) | ||||||
| 7 | 0.3199 | 0.2548 | 0.1123 | 7.2 239 | 0.1425 | 0.5593 |
| (0.0404) | (0.009) | (0.034) | (0.0013) | |||
| 8 | 0.4146 | 0.3719 | 0.1984 | 3.8259 | 0.1735 | 0.4665 |
| (0.0097) | (0.003) | (0.014) | (0.0034) | |||
| 9 | 0.4210 | 0.4013 | 0.2250 | 4.9567 | 0.1763 | 0.4393 |
| (0.0107) | (0.008) | (0.028) | (0.0017) | |||
| 10 | 0.4972 | 0.2925 | 0.0275 | 26.0531 | 0.2650 | 0.9060 |
| (0.0029) | (0.034) | (0.380) | (0.0003) | |||
| 11 | 0.6238 | 0.2912 | 0.0707 | 7.5427 | 0.2205 | 0.7572 |
| (0.0002) | (0.086) | (0.504) | (0.0007) | |||
| 12 | 0.5412 | 0.5519 | 0.3907 | 5.4794 | 0.1612 | 0.2921 |
| (0.00140) | (0.002) | (0.006) | (0.0030) | |||
3.3. Model 2
In weeks 7–10, the estimated risk of smoking marijuana was significantly greater for individuals in the VEN-XR group (see Table 1). In week 11, the estimated risk of smoking marijuana was still higher for the VEN-XR group, but only at a trend level (RD = 0.29, p = 0.086). Overall, VEN-XR had a significant negative effect on abstinence in the later weeks of the study.
3.4. Model 3
In weeks 7–9, the estimated risk of smoking marijuana for individuals in the VEN-XR group remained significantly higher than PBO, but with decreased magnitude compared to Model 2 (see Table 1, effect ct′). In weeks 10 and 11, the estimated risk difference of smoking marijuana between the VEN-XR group and PBO group was diminished when withdrawal scores were controlled for, and did not remain significant (week 10 RD = 0.03, p = 0.380; week 11 RD = 0.07, p = 0.504). In all of the weeks tested (weeks 7–12), withdrawal scores were significantly associated with marijuana smoking (see Table 1, effect bt).
3.5. Mediation effect
In each of the investigated weeks (weeks 7–12), the mediation effect of more severe withdrawal scores weakened the effect of VEN-XR treatment on increased marijuana smoking. The proportion of this decrease that can be attributed to the estimated mediation effect of withdrawal scores is shown by week in Table 1 (see Table 1, estimated mediation effect).
In weeks 7–9, the estimated mediation effect of withdrawal scores on marijuana smoking is only partial because the effect of VEN-XR treatment on marijuana smoking, though decreased, remained significant (Table 1, column 4).
For example, in week 7, we see that participants in the VEN-XR group are expected to have 38% higher withdrawal scores than participants in the placebo group, according to Model 1. Model 2 estimates that participants in the VEN-XR group have 25% greater risk of smoking marijuana compared to participants in the placebo group. Model 3 estimates that participants treated with VEN-XR have 11% higher risk of smoking marijuana when withdrawal scores are controlled for compared to participants treated with placebo, and one standard deviation increase in the withdrawal score would be expected to increase the odds of smoking marijuana by 7.2 times. The amount of mediation is 14%, which corresponds to a proportion of 56%. This means that in week 7, the models estimate that higher withdrawal scores account for 56% of the relationship between VEN-XR treatment and marijuana smoking.
In weeks 10 and 11, more severe withdrawal scores have a greater estimated mediation effect on the positive relationship between VEN-XR treatment and marijuana smoking. In week 10, for example, Model 2 estimates that the risk of smoking marijuana is 29% higher for those treated with VEN-XR relative to placebo. However, this increased risk loses significance when withdrawal scores are controlled for in Model 3 (estimated risk difference for marijuana smoking in VEN-XR group relative to placebo in week 10 = 2.75%, p = 0.380). For both weeks 10 and 11, the models estimate that higher withdrawal scores accounted for greater than 75% of the effect of VEN-XR treatment on marijuana smoking (% decrease in estimated risk difference week 10 = 0.906, week 11 = 0.757). This suggests that withdrawal scores more fully mediate the effect of VEN-XR treatment on marijuana smoking in those weeks, according to the model estimates.
In week 12, higher withdrawal scores are estimated to account for about 30% of the effect of VEN-XR treatment on marijuana smoking (% decrease in estimated risk difference = 0.2921), which means that the estimated mediation effect of withdrawal scores on marijuana smoking is smaller than in weeks 10 and 11.
3.6. Withdrawal symptom scores
For weeks 10 and 11, in which a stronger mediation effect of withdrawal scores was observed, we investigated the differences between VEN-XR treatment and placebo for each item on the 29-item MWC questionnaire. Significantly higher scores were reported for 9 items by individuals on VEN-XR, including shakiness (U = 1177.5, p = 0.010), sleep difficulty (U = 1261.5, p = 0.001), sweating (U = 1248.5, p = 0.001), nervousness (U = 1173.5, p = 0.023), increased appetite (U = 1167.5, p = 0.020), strange dreams (U = 1162.5, p = 0.024), dizziness (U = 1165.5, p = 0.0153), nausea (U = 1171.0, p = 0.0087) and yawning (U = 1148.0, p = 0.033).
4. Discussion
When we examined the relationship between VEN-XR, marijuana smoking, and symptoms scores on the Marijuana Withdrawal Checklist using a mediation analysis, we found that severity of symptoms mediated the increased marijuana smoking in patients on VEN-XR. Individuals treated with VEN-XR experienced more severe withdrawal-like symptoms in weeks 7–12, and according to the model estimates, the increased marijuana smoking we observed in the VEN-XR group during weeks 7–9 was attributable to more severe withdrawal symptom scores. In weeks 10 and 11, the estimated effect of withdrawal scores was greater, and increased marijuana smoking was more fully attributable to the severity of these withdrawal-like symptoms.
Many of the specific withdrawal scale items that were scored higher in the VEN-XR group were consistent with a state of noradrenergic hyperactivation, such as shakiness, sweating, nervousness, and sleep difficulties and were likely side effects from VEN-XR. We propose that these symptoms were experienced similarly to marijuana withdrawal, and thus may have hindered attempts to stop or reduce marijuana smoking.
Across the study weeks, withdrawal scores were decreasing in both groups and trending toward an increasing divergence between groups (see Fig. 3). This trend is consistent with the idea that withdrawal-like side effects were persisting in the VEN-XR group while cannabis withdrawal symptoms were resolving in the placebo group. Additionally, medication doses continued to be increased up to week 4 and beyond for those individuals with continuing depressive symptoms, increasing the burden of noradrenergic side effects as the study weeks progressed. Thus, it is possible that individuals receiving VEN-XR may have been attempting to temper these side effects by increasing their marijuana smoking, accounting for their higher urine THC in the later weeks of the study.
Our proposed mechanism is supported by existing evidence of noradrenergic hyperactivation in marijuana withdrawal (Anggadiredja et al., 2003; Budney et al., 2008; Haney et al., 2013; Lichtman et al., 2001) and by the pharmacology of VEN-XR, which inhibits norepinephrine reuptake at higher doses resulting in adverse effects consistent with noradrenergic potentiation (Harvey et al., 2000). Further support comes from clinical studies suggesting monoamine reuptake inhibitors worsen marijuana withdrawal (Carpenter et al., 2009; Haney et al., 2001), or are poorly tolerated (Tirado et al., 2008) in this population. In contrast, the alpha agonist lofexidine, which decreases noradrenergic activity, has shown to be beneficial in cannabis withdrawal (Haney et al., 2008).
There are several limitations to this study. First, this is a secondary, post hoc analysis from a medication efficacy trial, and findings must be interpreted in this context. Second, it is most likely that symptoms measured as marijuana withdrawal were primarily VEN-XR side effects. Nonetheless our finding that symptoms with a similar profile to cannabis withdrawal were significantly worse in the VEN-XR group and contributed to the overall higher withdrawal scores that mediated increased marijuana smoking is highly relevant. A final limitation is that this study was conducted in depressed individuals and the findings cannot be generalized directly to a non-depressed population.
Despite these limitations, our findings add significantly to our thinking about the pathophysiology and clinical management of marijuana withdrawal. We have replicated findings of worse outcomes for cannabis-dependent individuals treated with medications that increase noradrenergic tone, and we have provided a potential mechanism. Thus, noradrenergic agents may negatively impact cannabis-dependent patients who are attempting to stop or reduce their use, and further studies are needed to more directly test this theory.
Acknowledgments
Role of funding source
This research was supported by the National Institute on Drug Abuse grants R01DA15451, KO2 000465, K24 DA029647 and K24 DA022412.
Dr. Levin is a past consultant for Eli Lily and Company, Shire Pharmaceuticals Group, AstraZeneca and OrthoMcNeil Pharmaceutical Inc. She has also received research support from Eli Lily and Company, UCB PhamaInc., Shire Pharmaceuticals Group, AstraZeneca and OrthoMcNeil Pharmaceutical Inc. She currently receives medication from World Med for an ongoing study sponsored by the National Institutes on Drug Abuse and served as a consultant to GW Pharmaceuticals in 2011. Dr. Nunes served on an advisory board for Eli Lily and Company and was paid and honorarium and received reimbursement for travel expenses to an advisory board meeting in January 2012. Drs. Nunes, Bisaga, and Sullivan receive medication from Alkermes/Cephalon, Inc., for ongoing studies that are sponsored by the National Institute on Drug Abuse. Dr. Nunes also receives medication and/or placebo for research studies from Reckitt-Benckiser and Duramed Pharmaceuticals, and he has received or will receive from Brainsway devices under investigation and reimbursement for travel expenses for investigators meetings in connection with a Brainsway research study. Dr. Nunes also received a web-based behavioral intervention for a research study in data analysis from HealthSim, LLC.
Footnotes
Contributors
Dr. Levin, Dr. Mariani, Dr. Nunes, Dr. Bisaga and Dr. Sullivan designed the study and Dr. Levin wrote the protocol. Dr. Kelly wrote the first draft and conducted the literature search for this paper. Dr. Pavlicova and Mr. Glass undertook the statistical analysis. All authors contributed to and have approved the final manuscript.
Conflict of interest
Drs. Kelly, Pavlicova, and Mariani and Mr. Glass: none.
References
- Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, Budney AJ. Quantifying the clinical significance of cannabis withdrawal. PLoS ONE. 2012;7:e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggadiredja K, Yamaguchi T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Prostaglandin E2 attenuates SR141716A-precipitated withdrawal in tetrahydrocannabinol-dependent mice. Brain Res. 2003;966:47–53. doi: 10.1016/s0006-8993(02)04169-0. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J. Consult. Clin. Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J. Consult. Clin. Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J. Subst. Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am. J. Addict. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict. Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2013;38:1557–1565. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Harvey AT, Rudolph RL, Preskorn SH. Evidence of the dual mechanisms of action of venlafaxine. Arch. Gen. Psychiatry. 2000;57:503–509. doi: 10.1001/archpsyc.57.5.503. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J. Clin. Psychiatry. 2008;69:1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol. Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani J, Brooks DJ, Pavlicova M, Cheng C, Nunes E. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani J, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, Bisaga A, Sullivan MA, Carpenter KM. A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction. 2013;108:1084–1094. doi: 10.1111/add.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol. Biochem. Behav. 2001;69:181–188. doi: 10.1016/s0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Buffkins K, Kyle M, Adusumalli M, Begovic A, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado CF, Goldman M, Lynch K, Kampman KM, Obrien CP. Atomoxetine for treatment of marijuana dependence: a report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend. 2008;94:254–257. doi: 10.1016/j.drugalcdep.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World Drug Report. 2011 http://www.unodc.org/documents/data-and-analysis/WDR2011/World_Drug_Report_2011_ebook.pdf.