Abstract
Neurosteroids are involved in sex-specific epilepsies. Allopregnanolone and related endogenous neurosteroids in the brain control excessive neuronal excitability and seizure susceptibility. Neurosteroids activate GABA-A receptors, especially extrasynaptic αβδ-GABA-A receptor subtypes that mediate tonic inhibition and thus dampen network excitability. Our studies over the past decade have shown that neurosteroids are broad-spectrum anticonvulsants and confer seizure protection in various animal models. Neurosteroids also exert antiepileptogenic effects. There is emerging evidence on a critical role for neurosteroids in the pathophysiology of the sex-specific forms of epilepsies such as catamenial epilepsy, a menstrual cycle-related seizure disorder in women. Catamenial epilepsy is a neuroendocrine condition in which seizures are clustered around specific points in the menstrual cycle, most often around the perimenstrual or periovulatory period. Apart from ovarian hormones, fluctuations in neurosteroid levels could play a critical role in this gender-specific epilepsy. Neurosteroids also regulate the plasticity of synaptic and extrasynaptic GABA-A receptors in the hippocampus and other regions involved in epilepsy pathology. Based on these studies, we proposed a neurosteroid replacement therapy for catamenial epilepsy. Thus, neurosteroids are novel drug targets for pharmacotherapy of epilepsy.
Keywords: Allopregnanolone, neurosteroid, progesterone, catamenial epilepsy, GABA-A receptor
Introduction
Neurosteroids are steroids synthesized within the brain with unconventional, rapid effects on neuronal excitability. Neurosteroids and their precursor steroid hormones play an important role in the neuronal excitability, seizure susceptibility, and pathophysiology of epilepsy. The term neurosteroid refers to steroids that are synthesized de novo in the nervous system from cholesterol, independent of the peripheral steroidogenic endocrine glands (Baulieu, 1981). It has been known since the 1940s, from the pioneering work of Hans Selye, that naturally occurring steroids such as the ovarian steroid progesterone and the adrenal steroid deoxycorticosterone can exert anesthetic and anticonvulsant actions (Selye, 1941; 1942; Clarke et al., 1973). In the early 1980s, the synthetic steroid alphaxolone was found to enhance synaptic inhibition via an action on GABA-A receptors in the brain (Harrison and Simmonds, 1984). A major advance occurred when 5α-reduced metabolites of progesterone and deoxycorticosterone were also found to enhance GABA-A receptor function (Majewska et al., 1986). Consequently, it became evident that the anticonvulsant properties of progesterone and deoxycorticosterone are predominantly due to their conversion in the brain to neurosteroids allopregnanolone (3α-hydroxy-5α-pregnane-20-one, AP) and allotetrahydro-deoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one; THDOC), respectively (Reddy 2003; 2004; 2011; Carver and Reddy, 2013) (Fig. 1). This article describes the neurobiological aspects of neurosteroids with a special emphasis on catamenial epilepsy, a menstrual cycle-related seizure disorder in women. It focuses on the role of GABA-A receptor-modulating neurosteroids in regulating seizure susceptibility and pathophysiology of sex-specific epilepsies.
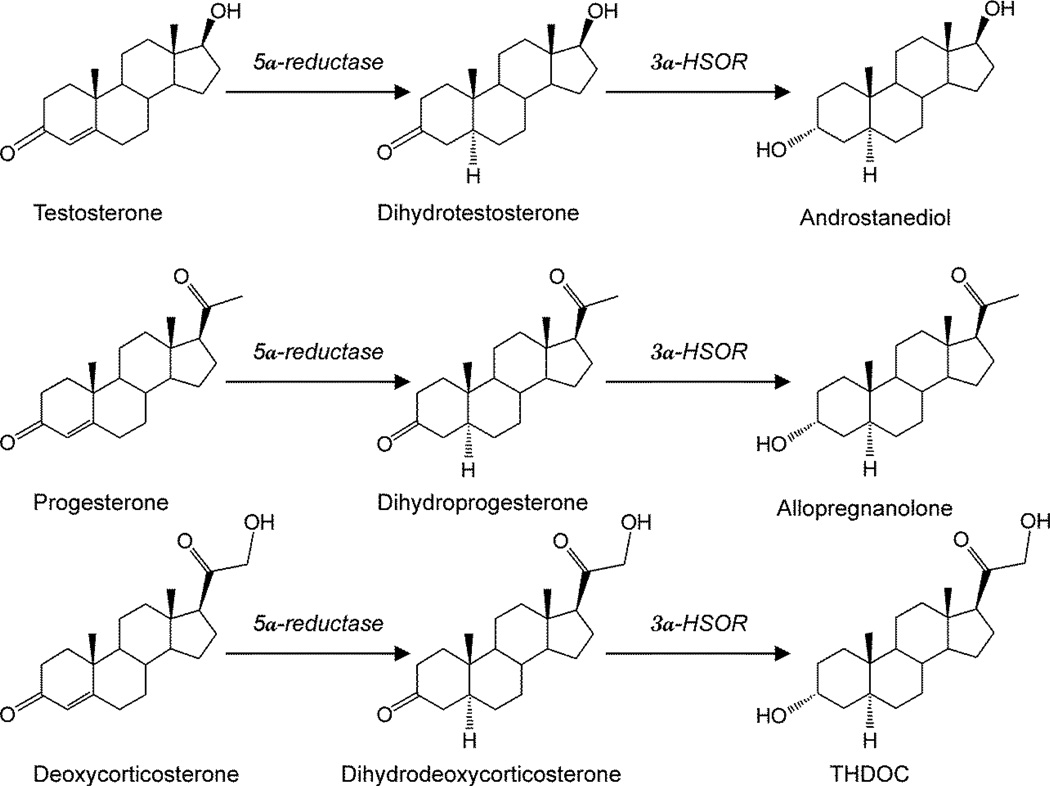
Figure 1. Biosynthesis of neurosteroids in the brain.
Enzymatic pathways for the production of three prototype neurosteroids allopregnanolone, allotetrahydro-deoxycorticosterone (THDOC) and androstanediol is illustrated from steroid precursors. Testosterone, progesterone, and deoxycorticosterone undergo two sequential A-ring reduction steps catalyzed by 5α-reductase and 3α-hydroxysteroid oxidoreductase (3α-HSOR) to form the 5α, 3α-reduced neurosteroids. The conversion of progesterone or deoxycorticosterone into neurosteroids occurs in several regions within the brain. The 5α-reductase, 3α-HSOR and other enzymes are present in the brain.
Neurosteroid Biosynthesis in the Brain
A variety of neurosteroids are synthesized in the brain (Baulieu, 1981; Kulkarni and Reddy, 1995). The most widely studied are allopregnanolone, THDOC, and androstanediol (Fig.1).There is now compelling evidence that all of the enzymes required for the biosynthesis of the neurosteroids from cholesterol are present in the brain (Stoffel-Wagner et al., 2000; 2003). Allopregnanolone and related neurosteroids are produced via sequential A-ring reduction of the steroid hormones by 5α-reductase and 3α-hydroxysteroid-oxidoreductase isoenzymes (Reddy, 2009a). The androgenic neurosteroid androstanediol (5α-androstan-3α,17β-diol) is synthesized from testosterone (Reddy, 2004a,b). In the periphery, the steroid precursors are mainly synthesized in the gonads, adrenal gland, and feto-placental unit, but synthesis of these neurosteroids likely occurs in the brain from cholesterol or from peripherally derived intermediates. Since neurosteroids are highly lipophilic and can readily cross the blood-brain barrier, neurosteroids synthesized in peripheral tissues can reach targets in the brain.
Precursor steroids may enter the brain from the blood circulation and can be converted to neurosteroids (Agís-Balboa et al., 2006). Recent evidence indicates that neurosteroids are present mainly in principal neurons in many brain regions that are relevant to focal epilepsies, including the hippocampus and neocortex (Agis-Balboa et al., 2006; Saalmann et al., 2007; Do Rego et al., 2009). This observation is consistent with the notion that neurosteroids function in an autocrine fashion in which they reach their targets by lateral membrane diffusion (Chisari et al., 2010). Neurosteroids are present in the neocortex. It is not clear if there are specific neocortical areas related to focal epilepsy such as the motor cortex that show increased presence of neurosteroids compared to cortical areas not normally associated with a high seizure potential. However, the rates of production and their specific control in different regions remain unclear. The biosynthesis of neurosteroids is controlled by the translocator protein (TSPO), formerly called peripheral or mitochondrial benzodiazepine receptor (Rupprecht et al., 2009). Activation of TSPO by endogenous signals and ligands facilitates the intramitochondrial flux of cholesterol and thereby promotes neurosteroid synthesis. It is suggested that TSPO ligands might be an alternative approach for neurosteroid therapeutics (Nothdurfter et al., 2011). Overall, all of the necessary enzymes required for neurosteroid synthesis are region-specific, cell-specific, and available within the brain, both in neurons and glia (Compagnone and Mellon, 2000).
Neurosteroid Activation of GABA-A Receptors
Neurosteroids rapidly alter neuronal excitability through direct interaction with GABA-A receptors (Harrison et al., 1984; 1987; Majewska et al., 1986; Gee et al., 1988; Hosie et al., 2007; 2009; Carver and Reddy, 2013). Activation of the GABA-A receptor by various ligands leads to an influx of chloride ions and to a hyperpolarization of the membrane that dampens the excitability. Allopregnanolone, THDOC and other structurally-related neurosteroids act as positive allosteric modulators and direct activators of GABA-A receptors (Fig. 2). At low concentrations, neurosteroids potentiate GABA-A receptor currents, whereas at higher concentrations, they directly activate the receptor (Harrison et al., 1987; Reddy and Rogawski, 2002). Like barbiturates, neurosteroid enhancement of GABA-A receptors occurs through increases in both the channel open frequency and channel open duration (Twyman and Macdonald, 1992; Lambert et al., 2009; Ramakrishnan and Hess, 2010).
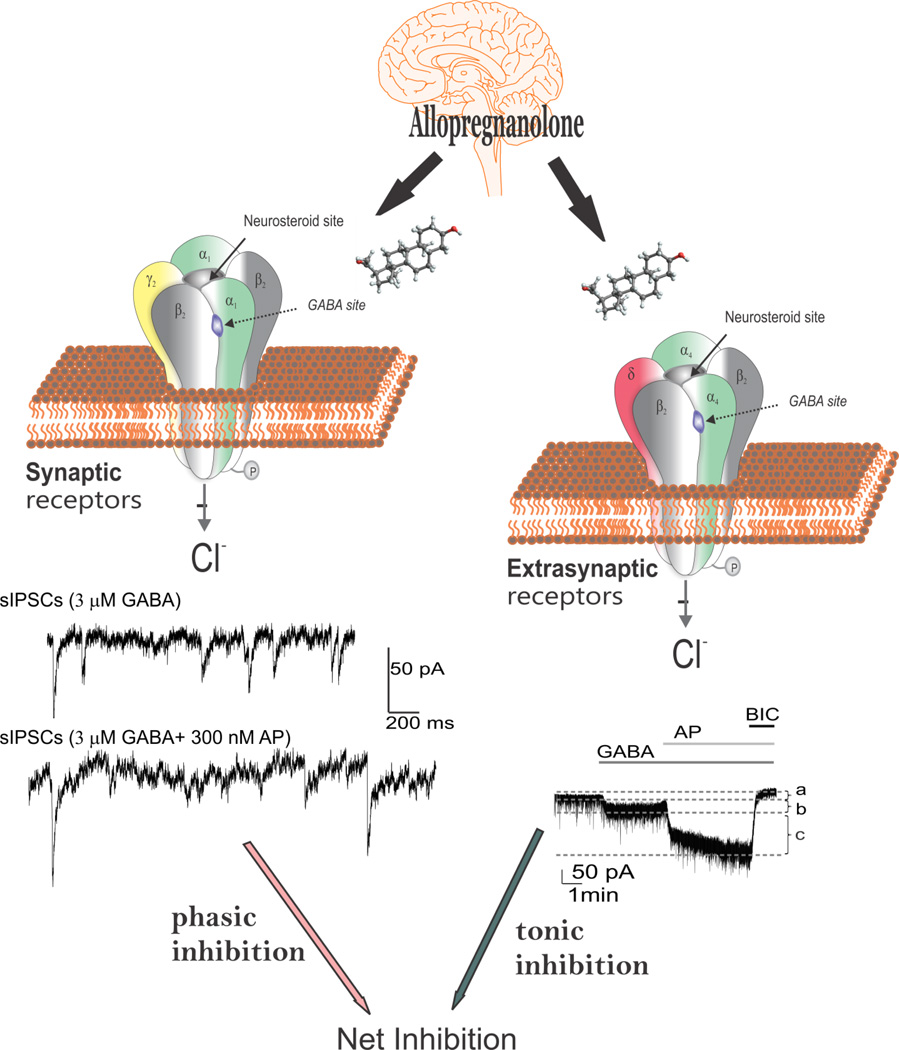
Figure 2. Neurosteroid modulation of synaptic and extrasynaptic GABA-A receptors.
Allopregnanolone and related neurosteroids binds and potentiate the GABA-A receptor function leading to enhanced inhibitory transmission. GABA-A receptors are pentamers with five protein subunits that form the chloride ion channel pore. Neurosteroids are thought to bind at the “neurosteroid binding site”, which is distinct from sites for GABA, benzodiazepines and barbiturates. Synaptic GABA-A receptors (2α2β1γ pentamers) mediate the phasic portion of GABAergic inhibition, while extrasynaptic GABA-A receptors (2α2βδ pentamers) primarily contribute to tonic inhibition in the hippocampus. Neurosteroids activate both synaptic and extrasynaptic receptors and enhance the phasic and tonic inhibition and thereby promote maximal net inhibition. AP, allopregnanolone; BIC, bicuculline
The GABA-A receptor is a pentamer consisting of five subunits that form a chloride channel. Sixteen subunits (α1-6, β1-3, γ1-3, δ,ε,θ, and π subunits) have been identified so far. The GABA site is located at the interface between α and β subunits. Benzodiazepines bind at the interface between α and γ subunits and interact with subunit combinations α1,2,3,5β2γ2. Neurosteroid‘s effect on GABA-A receptors occurs by binding to discrete sites on the receptor-channel complex that are located within the transmembrane domains of the α-and β-subunits (Hosie et al., 2006; 2007), which they access by lateral membrane diffusion (Chisari et al., 2009; 2010). The binding sites for neurosteroids are distinct from the recognition sites for GABA, benzodiazepines, and barbiturates (Hosie et al., 2009). Androgenic neurosteroids such as androstanediol may interact with these sites, and a recent study indicates that this agent is a positive allosteric modulator of GABA-A receptors (Reddy and Jian, 2010).
The molecular nature of neurosteroid binding sites is under intense scrutiny. The effects of neurosteroids on GABA-A receptors occur by binding to discrete sites on the receptor-channel complex that are located within the transmembrane domains of the α and β subunits (Hosie et al., 2007) (Fig.2). The specific binding sites of neurosteroids on the GABA-A receptor are quite different from sites for drug such as benzodiazepines and barbiturates. Having the ability to potentiate GABAergic current at 10 – 500 nM concentrations and to autonomously, directly induce receptor channel opening at larger concentrations (> 500 nM) (Belelli et al., 2002), neurosteroids appear to operate on a wider array of receptor isoforms and are thus less specific in binding as compared with benzodiazepines, which have very high affinity (Kd in sub-nanomolar range). In fact, the neurosteroid enhancement of binding is thought to be due to allosteric interaction with an altogether different site on the receptor. It has been proposed that neurosteroids may bind receptors from intracellular access or at a site within the neuronal plasma membrane (Akk et al., 2009). Studies of recombinant GABA-A receptor isoforms indicate that neurosteroids act on most subunit configurations (Puia et al., 1993; Carver and Reddy, 2013). This distinguishes neurosteroids from benzodiazepines, which only act on GABA-A receptors that contain γ2 subunits and do not contain α4 or α6 subunits. In general, the specific α subunit type may influence neurosteroid efficacy, whereas the γ subunit type may affect both the efficacy and potency of neurosteroid modulation (Lambert et al., 2009). The δ-containing receptors possess a significantly higher affinity for GABA than other receptor subtypes. Neurosteroids therefore can markedly enhance the current generated by δ-subunit-containing GABA-A receptors even in the presence of saturating GABA concentrations (Carver and Reddy, 2013).
The GABA-A receptor mediates two types of GABAergic inhibition, now stratified into synaptic (phasic) or extrasynaptic (tonic) inhibition (Fig. 2). Phasic inhibition is attributed to the inhibitory postsynaptic current (IPSC) resulting from membrane GABA-A receptors opening in response to rapid release of GABA across the synapse (Farrant and Nusser, 2005). Vesicular release of GABA into the synapse generates a local peak concentration of GABA that may reach millimolar levels. Tonic inhibition is persistent inhibitory current mediated by perisynaptic or extrasynaptic receptors in response to ambient or extracellular GABA at low micromolar levels (Glykys and Mody, 2007). Tonic current enables shunting inhibition to control gain of neuronal excitability (Mitchell and Silver, 2003). Although GABA activates synaptic (γ2-containing) GABA-A receptors with high efficacy, GABA activation of the extrasynaptic (δ-containing) GABA-A receptors are limited to low-efficacy activity characterized by minimal desensitization and brief openings. Physiological tonic currents of GABA receptors are dependent on the pentamer subunit composition and fairly independent of physiological levels of ambient exogenous GABA.
Neurosteroids act on all GABA-A-receptor isoforms. However, they have large effects on extrasynaptic δ-subunit containing GABA-A receptors that mediate tonic currents (Wohlfarth et al., 2002; Belelli et al., 2002). The potentiation of δ-subunit-containing receptors by THDOC and other neurosteroids is selective for channels with low-efficacy gating characteristics marked by brief bursts and channel openings in conditions of both low and high GABA concentrations, and thereby neurosteroids can preferentially increase the efficacy of these receptors based on pharmacokinetics which are not yet fully understood (Bianchi and Macdonald, 2003). Neurosteroids therefore markedly enhance the current generated by δ-subunit-containing receptors even in the presence of saturating GABA concentrations. Consequently, GABA-A receptors that contain the δ-subunit are highly sensitive to neurosteroid potentiation and mice lacking δ-subunits show drastically reduced sensitivity to neurosteroids (Mihalek et al., 1999; Spigelman et al., 2002). Tonic current causes a steady inhibition of neurons and reduces their excitability. Neurosteroids therefore could play a role in setting the level of excitability by potentiation of tonic inhibition during seizures that elevate ambient GABA levels.
Anticonvulsant and Antiepileptogenic Activity of Neurosteroids
Allopregnanolone and related neurosteroids are powerful anticonvulsants. Neurosteroids exhibit broadspectrum anticonvulsant effects in diverse rodent seizure models (Reddy, 2010; 2011). Neurosteroids protect against seizures induced by GABA-A receptor antagonists, including pentylenetetrazol and bicuculline, and are effective against pilocarpine-induced limbic seizures and seizures in kindled animals (Kokate et al., 1994; Belelli et al., 1989; Frye, 1995; Wieland et al., 1995; Reddy et al., 2004). The potencies of neurosteroids in models where they confer seizure protection vary largely in accordance with their activities as positive allosteric modulators of GABA-A receptors (Reddy, 2004a; 2004b; Kaminiski et al., 2005). Neurosteroids are highly active in the 6-Hz model, an electrical paradigm in which limbic-like seizures are induced by electrical stimulation of lower frequency and longer duration than in the maximal electroshock test (Kaminiski et al., 2004). However, neurosteroids are inactive or only weakly active against seizures elicited by maximal electroshock (Reddy, 2010). Neurosteroid‘s ability to suppress seizures is stereoselective. Androstanediol, but not its 3β-epimer, produces a dose-dependent suppression of behavioral and electrographic seizures in the mouse hippocampus kindling (Reddy and Jian, 2010).
Neurosteroids are highly effective in suppressing seizures due to withdrawal of GABA-A receptor modulators including neurosteroids and benzodiazepines, as well as other types of agents such as ethanol and cocaine (Reddy and Rogawski, 2001; Tsuda et al., 1997; Devaud et al., 1996). In contrast to benzodiazepines, where utility in the chronic treatment of epilepsy is limited by tolerance, anticonvulsant tolerance is not evident with neurosteroids (Kokate et al., 1998; Reddy and Rogawski, 2000a). Thus, neurosteroids have the potential to be used in the chronic treatment of epilepsy. Unlike benzodiazepines, neurosteroids are able to modulate all isoforms of GABA-A receptors, including those that contain benzodiazepine-insensitive α4 and α6 subunits or lack the obligatory γ2 subunit required for benzodiazepine-sensitivity.
Recent studies suggest that neurosteroids play a role in epileptogenesis (Edwards et al., 2001; Biagini et al., 2006; 2009a; 2010; Reddy et al., 2010; Reddy, 2013a). Using the kindling model, we demonstrated that the development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors (Reddy and Mohan, 2011). To explore mechanisms underlying the observed seizure resistance, we investigated the role of neurosteroids using finasteride, a 5α-reductase inhibitor that blocks the synthesis of progesterone-derived neurosteroids. Progesterone produced a significant delay in the rate of kindling and pretreatment with finasteride blocked progesterone‘s inhibition of kindling epileptogenesis (Reddy and Ramanathan, 2012). These findings are consistent with a contributory role of neurosteroids in limbic epileptogenesis. Thus, it is possible that inhibition of neurosteroid synthesis could incite mechanisms that may promote epileptogenesis.
The P450scc is a critical enzyme for the biosynthesis of neurosteroids in the brain. Following pilocarpine-induced status epilepticus in the rat, the P450scc is upregulated for several weeks, suggesting that it may be associated with promotion of neurosteroidogenesis (Biagini et al., 2009). Ordinarily, rats develop spontaneous recurrent seizures following a latent period of similar duration to the period during which P450scc is elevated. The role of neurosteroids in delaying seizure onset in the pilocarpine model is confirmed using finasteride, which can exacerbate seizures by inhibition of neurosteroid synthesis. Inhibiting neurosteroid synthesis with finasteride accelerated the onset of spontaneous recurrent seizures (Biagini et al., 2006), suggesting that endogenous neurosteroids play a role in restraining epileptogenesis, or at least act to inhibit the expression of seizures.
The development of epilepsy is linked to complex alterations in neuroplastic mechanisms. Dysregulation of neurosteroid synthesis may also play a role. This premise is being tested in various epileptogenic models (Reddy, 2013). We investigated the role of the prototype endogenous neurosteroid allopregnanolone in controlling limbic epileptogenesis. Treatment with finasteride, a neurosteroid synthesis inhibitor, resulted in a significant increase in epileptogenesis in the hippocampus kindling model (Ramanathan and Reddy, 2011). Exogenous administration of allopregnanolone, at doses that produce levels similar to gonadotropins, markedly inhibited epileptogenesis. In female epilepsy rats, finasteride treatment exacerbates seizure frequency (Lawrence et al., 2010). The exact mechanisms are unclear. Neurosteroid-mediated increase in tonic inhibition in the hippocampus could inhibit the spread of the seizure discharge from the hippocampal focus and thereby suppress the rate of development of behavioral kindled seizure activity without affecting the focal electrographic discharges. Increased tonic inhibition by allopregnanolone is shown to impair the N-methyl-D-aspartate (NMDA) receptor-mediated excitability in the hippocampus (Shen et al., 2010). It is likely that such a mechanism may underlie the neurosteroid‘s disease-modifying effects in epileptogenic models.
Role of Neurosteroids in Sex-Specific Forms of Epilepsy
Sex difference in seizure susceptibility is one of the long-standing issues of epilepsy. Clinical evidence shows gender-and age-related expression in many seizure syndromes. The incidence of epilepsy is generally higher in males than in females (Hauser, 1997; Christensen et al., 2005). More women than men are diagnosed with idiopathic generalized epilepsy, but localization-related symptomatic epilepsies are more frequent in men, and cryptogenic localization-related epilepsies are more frequent in women (Hauser, 1997; Christensen et al., 2005). Overall, there is considerable evidence indicating that men exhibit greater seizure susceptibility than females, while many females exhibit greater fluctuations in susceptibility to seizures, including menstrual cycle-related changes in seizure activity (Hauser, 1997; Christensen et al., 2005). Sexbased differences in seizure sensitivity may arise from variations between men and women in factors such as body weight, steroid hormones, cytochrome P450 activity and biologic differences in neuronal networks in the brain. Changes in seizure sensitivity are also evident at puberty, which is associated with rigorous changes in reproductive hormones and behavioural patterns (Reddy, 2009). The relationship between menstrual cycle and seizure sensitivity in females is well known and is greatly influenced by hormonal fluctuations associated with menstrual cycle phases.
Neurosteroids may be involved in the physiological regulation of seizure susceptibility in individuals with epilepsy. Endogenous neurosteroids may affect seizure situations in catamenial epilepsy, stress, temporal lobe epilepsy, and alcohol withdrawal (Reddy, 2009a; Kim et al., 2010). However, it is noteworthy that there is no evidence that alterations in neurosteroid levels in the absence of preexisting epilepsy can induce epileptogenesis. There is emerging evidence that neurosteroids may play a role in limbic epileptogenesis (Reddy et al, 2013). Neurosteroids that enhance the GABAergic inhibition within the brain are potent anticonvulsants and they regulate neural excitability networks by enhancing the phasic and tonic inhibition in the hippocampus, a critical region involved in the limbic epilepsy. Therefore, neurosteroids may represent a rational treatment strategy for modification of acquired epileptogenesis and retarding secondary epileptogenesis in chronic epilepsy. It is possible that alterations in neurosteroidogenesis may play a role as inciting factors in the development and persistence of limbic epilepsy (Reddy and Mohan, 2011). Our recent work provides important new evidence that the availability of neurosteroids does indeed critically influence the propensity for seizures (Reddy and Zeng, 2007). We used epileptic female rats that had experienced status epilepticus, and monitored spontaneous seizure activity for up to 5 months. The epileptic animals exhibited about 2 seizures per day, each lasting approximately a minute. Gonadotropin-induced increase in neurosteroids was associated with reduced seizure intensity. However, when neurosteroids were withdrawn by using the neurosteroid synthesis inhibitor finasteride, a significant (two-fold) increase in seizure frequency was observed (Reddy, 2009a). These findings are confirmed in a recent study that utilized ovariectomized epileptic animals (Lawrence et al., 2010).
Neurosteroids may play a key role in chronic epilepsy. Neurosteroid modulation of tonic activation of extrasynaptic GABA-A receptors can regulate excitability during epileptogencity. Given the complex plasticity of GABA-A receptors in epilepsy, it is difficult to predict the functional outcome of altered subunit compositions. A consistent finding from studies that have used various models of chronic epilepsy is that tonic conductances are largely preserved in epileptic brain around the time when synaptic inhibition is reduced (Sun et al., 2007; Zhang et al., 2007). Studies in a status epilepticus model of temporal lobe epilepsy (TLE) have shown a striking reduction in δ-subunit containing GABA-A receptors in the dentate gyrus (Peng et al., 2004; Zhang et al., 2007), suggesting that neurosteroid effects on nonsynaptic GABA-A receptors may be reduced. There was a compensatory increase in γ2-subunit, so that tonic inhibition is preserved, though the efficacy of THDOC in modulating tonic current is decreased. In addition, neurosteroid modulation of synaptic currents is diminished in dentate gyrus granule cells and α4 subunit-containing receptors are expressed at synaptic sites (Sun et al., 2007). All of these changes may exacerbate seizures in epileptic animals but do not affect the efficacy of neurosteroids because they act on all GABA-A receptor isoforms.
Progesterone has antiseizure properties and plays an important role in epilepsy. Women with epilepsy are prone to seizures in response to decreased levels of progesterone during perimenstrual periods (Herzog et al., 1997; Reddy, 2009). However, progesterone‘s molecular mechanism of action in seizure activity is not fully understood. Progesterone‘s cellular actions are mediated by the progesterone receptor (PR), a member of the nuclear receptor superfamily of transcription factors (Li and O‘Malley, 2003). PRs are expressed in the brain with high levels in the hypothalamus and moderate levels in the limbic areas (Parsons et al.,1982; Auger and De Vries,2002). PRs are widely distributed in the hippocampus (Kato et al., 1994; Alves et al., 2000; Brinton et al., 2008), but their physiological significance remains unclear. Progesterone‘s seizure protection is undiminished in PR knockout (PRKO) mice and occurs mainly by its conversion to allopregnanolone, a neurosteroid modulator of GABA-A receptors (Reddy et al., 2004). Recently, we investigated the role of PRs in limbic epileptogenesis using multiple approaches for the intervention of the PR pathway (Reddy and Mohan, 2011). The PRKO mouse exhibited an increased resistance to epileptogenesis in kindling models. Lack of PRs markedly impaired the persistence of seizure expression at four weeks after kindling development. Selective inhibition of PRs in the brain by antisense oligos also resulted in a significant decrease in epileptogenesis in wild-type mice (Reddy and Mohan, 2011). Overall, these results indicate that the PR pathway plays an important role in promoting epileptogenesis, long-term stability of this epileptic-like state, and modulating progesterone‘s ability to suppress seizures. However, the mechanisms underlying PR-mediated epileptogenesis remain unclear. PRs, as transcription factors, are most likely to regulate GABA-A receptor subunit plasticity in the hippocampus. PRs may promote epileptogenesis by influencing synaptic plasticity and tonic inhibition in the hippocampus.
Catamenial Epilepsy
Epilepsy is characterized by the unpredictable occurrence of seizures. However, seizures do not occur randomly in many women with epilepsy. Seizure clusters occur with a temporal periodicity following circadian or lunar cycles. In women with epilepsy, seizure periodicity may conform to the menstrual cycle according to a “menstrual clock” provided by a common phase marker of the onset of menses (Gowers, 1881). Catamenial epilepsy, derived from the Greek word kataminios, meaning “monthly”, is characterized by seizures that cluster around specific points in the menstrual cycle (Fig.3). Catamenial epilepsy is a neuroendocrine condition in which seizures are most often clustered around perimenstrual or periovulatory period. Epilepsy affects an estimated 1.3 million women in the United States (Kaplan et al., 2007; Pennell, 2008). Catamenial epilepsy affects from 10 – 70% of women with epilepsy (Herzog et al., 2004; Bazan et al., 2005; Gilad et al., 2008; Reddy, 2009; 2013b). The large variation in prevalence of catamenial epilepsy is partly because of methodological differences such as the criteria used for defining seizure exacerbation in relation to menstrual cycle, patients‘ self-reporting, diaries, and other records of seizures relating to menses. Overall, these studies support the prevailing notion that at least 1 in every 3 women with epilepsy show catamenial seizure exacerbation. Catamenial epilepsy is a form of intractable epilepsy because catamenial seizures are often quite resistant to available drug treatments.
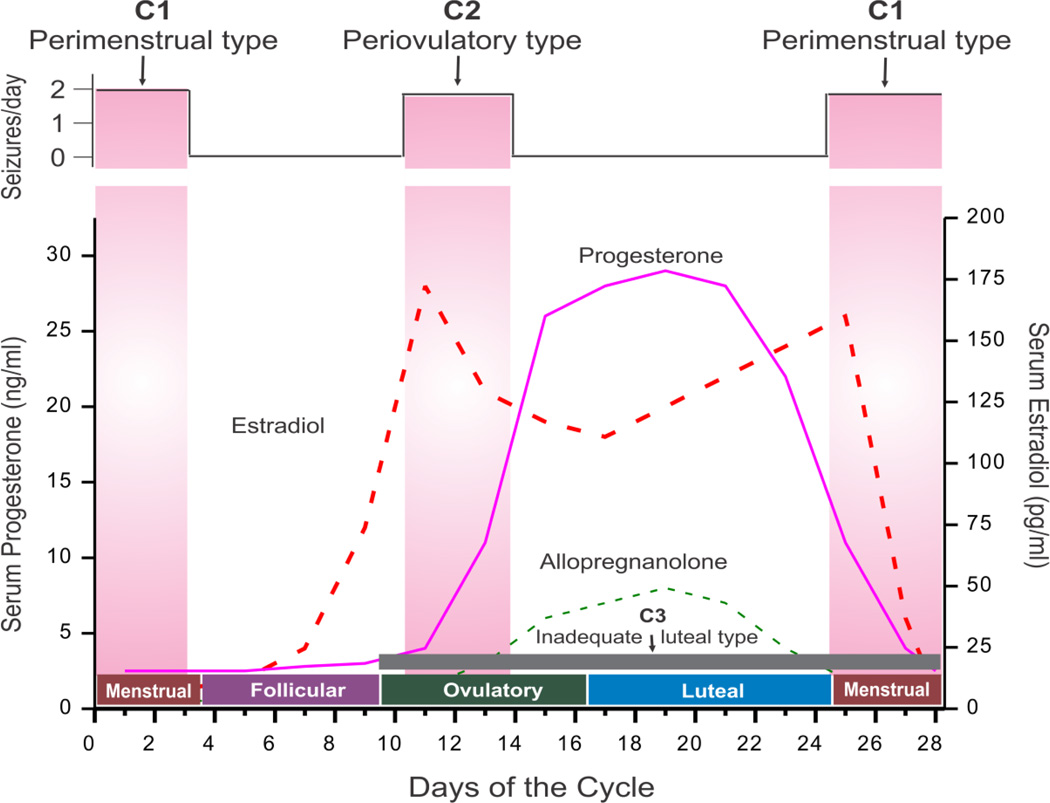
Figure 3. Schematic outline of neuroendocrine aspects of catamenial epilepsy.
The upper panel illustrates the relationship between seizure frequency and estradiol/progesterone (neurosteroid) levels. The lower panel illustrates the three types of catamenial epilepsy. The vertical bars (left and right) represent the likely period for the perimenstrual (C1) type, while the vertical bar (middle) represents the likely period for the periovulatory (C2) type. The horizontal dark gray bar (bottom) represents the inadequate luteal (C3) type that likely occur starting early ovulatory to menstrual phases. In general, the female reproductive cycle is estimated to last 29 days. Day 1 is the onset of menstruation, and ovulation occurs 14 days before the onset of menstruation. The menstrual cycle is divided into four phases: (i) menstrual phase, days −3 to +3; (ii) follicular phase, days +4 to +9; (iii) ovulatory phase, days +10 to +16; and (iv) luteal phase, days +17 to −4. The early follicular phase is associated with low levels of estrogens and progesterone. Estradiol is secreted in the second half of the follicular phase and increases to a peak at midcycle, while progesterone is elevated during the luteal phase and declines before menstruation begins. The neurosteroid allopregnanolone is increased in parallel to its precursor progesterone.
Three types of catamenial seizures, perimenstrual (C1), periovulatory (C2), and inadequate lutealphase (C3), have been identified (Herzog et al., 1997) (Fig. 3). The perimenstrual is the most common clinical type. The specific pattern of catamenial epilepsy can be identified simply by charting menses and seizures and obtaining a mid-luteal phase serum progesterone level to distinguish between normal and inadequate luteal phase cycles (Herzog et al., 2008; Quigg et al., 2009). The diagnosis of catamenial epilepsy is mainly based on the assessment of menstruation and seizure records. The simple approach of evaluation of catamenial epilepsy, that is, whether the patient's seizures tend to worsen at certain points of the menstrual cycle, is to record seizure diary in relation to menstrual cycle. Using the first day of menstrual bleeding as the first day of the cycle, the menstrual cycle is divided into four phases: (a) menstrual phase, days −3 to +3; (b) follicular phase, days +4 to +9; (c) ovulatory phase, days +10 to +16; and (d) luteal phase, days +17 to −4. The number of seizures in each phase is counted for at least 2 cycles and a two-fold or greater increase in frequency during a particular phase of the menstrual cycle can be used as diagnostic criteria of catamenial epilepsy.
In perimenstrual catamenial epilepsy (C1), women with epilepsy experience an increase in seizure activity before, during, or after the onset of menstruation (Reddy, 2009). Catamenial epilepsy is observed in women with both ovulatory and anovulatory cycles. Women with ovulatory cycles can experience either the perimenstrual or periovulatory catamenial types or even both within a single cycle (Bauer et al., 1998; Bauer, 2001). The diagnosis of ovulatory or anovulatory cycles is often made by estimating the midluteal phase progesterone levels. Progesterone levels lower than 5 ng/ml during days 20 through 22 of the cycle would certainly indicate inadequate luteal phase. Patients can be examined by pelvicabdominal ultrasound to measure size of mature graffian follicle as a sign of ovulation. In one study (Herzog et al., 2004), about 16.5% of subjects were found to have anovulatory cycles. These women showed a third type of catamenial epilepsy, referred to as inadequate luteal phase or anovulatory luteal seizures.
Changes in seizure activity in women can occur during changes in reproductive status (i.e. entering puberty, during pregnancy or after menopause). Although there is no overall consensus, puberty can affect the course of epilepsy. A significant increase in the incidence of generalized tonic-clonic seizures is observed in adolescents with epilepsy during puberty as compared with before puberty (Nijima and Wallace, 1989; Rosciszewka, 1987). Catamenial seizures can originate in women at puberty or in adolescent females with regular menstrual cycles. During puberty, the level of steroid hormones increases and menstrual period begins. Because steroid hormones influence seizure susceptibility, seizure types may change as females with epilepsy go through puberty. There is little information on the relationship between epilepsy and menopause. Natural reductions in steroid hormones around perimenopause and menopause are associated with alterations in frequency or severity of seizures in women with epilepsy (Abbasi et al., 1999; Harden et al., 1999; 2006).
Neuroendocrine Mechanisms of Catamenial Epilepsy
Catamenial epilepsy is a multifaceted condition attributed to numerous causes. Epilepsy typically develops due to certain genetic defect or often after a presumed precipitating injury. Catamenial epilepsy, in many cases, is assumed to be an acquired disorder and currently there is no clear evidence of genetic components. There is some evidence, however, to suggest that certain intrinsic properties of the brain such as the laterality and focality of the epileptic focus may play a role in the susceptibility of women with epilepsy to catamenial epilepsy (Herzog, 2007; Quigg et al., 2009). A variety of mechanisms such as fluctuations in antiepileptic drug (AED) levels, changes in water and electrolyte balance, and physiological variation in ovarian hormone secretion have been proposed as causes for catamenial epilepsy (see Reddy, 2009; 2013). Overall, cyclical changes in the circulating levels of estrogens and progesterone are now widely accepted to play a central role in the development of this condition (Fig.3).
Estrogens as proconvulsant hormones
Estradiol has been known to play a role in the exacerbation of seizures in women with epilepsy (Logothetis et al., 1959; Backstrom, 1976; Jacono and Robinson, 1987). Plasma estradiol levels are found to increase during both the follicular and luteal phase of the normal menstrual cycle (Fig.3). Backstrom (1976) was the first investigator to characterize the relationship between seizures and steroid hormones. In women with epilepsy, a positive correlation between seizure susceptibility and the estrogen-to-progesterone ratio was observed, peaking in the premenstrual and preovulatory periods and declining during the midluteal phase. Logothetis and colleagues (1959) have demonstrated that intravenous infusions of estrogen were associated with rapid interictal epileptiform activity in women with epilepsy and seizures were exacerbated when estrogen was given premenstrually. Therefore, it is thought that estrogens may facilitate some forms of catamenial seizures observed during these phases. The periovulatory catamenial exacerbation has been attributed to the midcycle surge of estrogen that is relatively unopposed by progesterone until early luteal phase (Logothesis et al., 1959). An increase in the ratio of estrogen-to-progesterone levels during perimenstrual period (described below) might at least partly contribute to the development of perimenstrual seizure exacerbation (Bonuccelli et al., 1989; Herzog et al., 1997). Nevertheless, the exact relationship between circulating estrogens and the perimenstrual or anovulatory catamenial seizures remains unclear.
The neuronal excitability mechanisms of estrogens are complex. Physiological receptors for estrogens include multiple membrane-associated and cell nuclear receptors (ERα and ERβ). Moreover, the nuclear receptors can also localize to the plasma membrane, where they can activate numerous signaling pathways. Estradiol can also activate a G-protein coupled membrane estrogen receptor with actions on many downstream signal transduction cascades (Scharfaman and MacLusky, 2006). Apart from classical estrogen receptor-mediated effects, estradiol affects neuronal excitability due to its organizational effects on synaptic structure and function. This mechanism may be apparent in estradiol‘s ability to enhance glutamate receptor-mediated excitatory neurotransmission (Smith et al., 1988; Wong and Moss, 1994) and decrease GABAergic inhibition (Murphy et al., 1998). Estradiol acts on neurons within the limbic system, cerebral cortex and other regions important for seizure susceptibility. Both direct effects on glutamate receptor subtypes and indirect effects through an increase in dendritic spine density of hippocampal NMDA receptor have been shown to be involved in estradiol modulation of the NMDA receptor function (Woolley and McEwen, 1994; Woolley et al., 1997; Rudick and Woolley, 2001). Chronic exposure of rats to estradiol increases the number and density of dendritic spines and excitatory synapses on hippocampal neurons that could increase the synchronization of synaptically driven neuronal firing in the hippocampus. This mechanism could be relevant to estradiol‘s proconvulsant effects in animal models. Estrogens appear to increase excitability by other mechanisms, including modulation of neuropeptides. There is evidence that estradiol increase the levels of brain-derived neurotrophic factor (BDNF) in hippocampus, which has been shown to have both protective actions and increase hippocampal excitability (Scharfman and Maclusky, 2006). Estradiol and BDNF, both influences neuropeptide Y, which has many effects that could influence seizures and epilepsy (Veliskova and Velisek, 2007). Therefore, the net effect of estradiol is difficult to predict due to multiple underlying mechanisms.
Progesterone as an anticonvulsant hormone
Progesterone plays a key role in catamenial epilepsy. Progesterone has consistent anticonvulsant and antiepileptic properties in animals and humans. Progesterone has long been known to have antiseizure activity in a variety of animal models of epilepsy (Craig, 1966; Landgren et al., 1978). In recent years, numerous studies have confirmed the powerful anticonvulsant activity of progesterone in diverse animal seizure models (see Reddy, 2009). Recent studies in our lab confirm the antiepileptogenic effects of progesterone in the kindling model of epileptogenesis (Reddy et al., 2010; Reddy and Mohan, 2011). There are two mechanisms by which progesterone affects reproduction and seizure susceptibility: binding to progesterone receptors (PRs) and being metabolized to the neurosteroid allopregnanolone. In progesterone-responsive target cells, progesterone binds to cytoplasmic PRs and the hormone-nuclear receptor complexes translocate to the cell nucleus where they activate or silence the transcription of downstream gene networks, thus affecting the physiological response of the target cell. There is strong evidence that the antiseizure effects of progesterone are not related to interactions with classical PRs, because antiseizure activity of progesterone was undiminished in PR knockout mice (Reddy et al., 2004). Further studies established that 5α-reduced neurosteroids are responsible for the seizure protection conferred by progesterone (Reddy et al., 2004).
In women with epilepsy, natural cyclic variations in progesterone during the menstrual cycle could influence catamenial seizure susceptibility (Fig.3). Seizures decrease in the mid-luteal phase when serum progesterone levels are high and increase premenstrually when progesterone levels fall and there is a decrease in the serum progesterone-to-estrogen ratio (Backstrom, 1976; Bonucelli et al., 1989; Herzog et al., 2001). Changes in progesterone levels have been directly correlated with catamenial seizures (Tuveri et al., 2008; El-Khayat et al., 2008). Despite some limitations, these findings provide evidence that disruption in ovarian cycle-related fluctuations in progesterone can be correlated to catamenial seizure exacerbation. The emerging evidence clearly indicates that perimenstrual catamenial seizures are associated with a rapid decline in progesterone around menstruation.
In clinical studies progesterone has been found to reduce seizures (Backstrom et al., 1984; Herzog, 2009). Previous open-label studies suggest that the cyclic administration of adjunctive natural progesterone supplement may lessen seizure frequency by over 50% in the majority of women with catamenially-exacerbated, intractable seizures (Herzog, 2009). Oral synthetic progestins, in contrast, have not shown significant efficacy. In a recently completed, NIH-sponsored Phase 3 trial, progesterone‘s efficacy was evaluated in women with epilepsy (Herzog et al., 2013). In this randomized, placebo-controlled, double-blind, multicenter clinical trial, Herzog and colleagues assessed the short term efficacy and safety of adjunctive cyclic progesterone therapy in the treatment of intractable seizures in 462 women with partial epilepsy. There was no significant difference in the proportions of responders for all seizures combined between progesterone and placebo in women with catamenial and noncatamenial epilepsy. However, the prespecified exploratory findings suggest that the level of perimenstrual seizure exacerbation is a significant predictor of the responder rate with progesterone therapy. Therefore, progesterone therapy may provide a clinically significant benefit for many women with perimenstrual catamenial epilepsy.
Neurosteroids as endogenous anticonvulsants
Neurosteroids play a critical role in catamenial seizure susceptibility in women with epilepsy. When neurosteroid levels fluctuate, loss of seizure control can occur. Neurosteroids allopregnanolone and THDOC have been implicated in perimenstrual seizure exacerbations in women with normal menstrual cycle. It is hypothesized that withdrawal of progesterone -derived neurosteroids leads to enhanced excitability predisposing to seizures (Reddy et al., 2001; 2012; Reddy, 2013b). In addition, plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in perimenstrual catamenial epilepsy. Animal studies have shown that prolonged exposure to allopregnanolone followed by withdrawal such as that occurs during menstruation causes a marked increase in expression of α4-subunit, a key subunit linked to enhanced neuronal excitability, seizure susceptibility and benzodiazepine resistance (Smith et al., 2007; Gangisetty and Reddy, 2010). These neuroendocrine changes can result in reduced inhibition resulting in enhanced excitability, which, among other effects, predisposes to seizures.
Neurosteroid withdrawal
However, the signaling mechanisms underlying the neurosteroid-withdrawal upregulation of α4-subunit expression remain unclear. The role of PRs and the transcription factor early growth response factor-3 (Egr3) in regulation of the GABA-A receptor α4-subunit expression in the hippocampus was investigated in a mouse neurosteroid withdrawal paradigm (Gangisetty and Reddy, 2010). Neurosteroid withdrawal induced a threefold increase in α4-subunit expression in wild-type (WT) mice, but this upregulation was undiminished in PR knockout mice. The expression of the transcription factor Egr3, which controls α4-subunit transcription, was increased significantly following neurosteroid withdrawal in WT and PR knockout mice. Neurosteroid withdrawal-induced α4-subunit upregulation was completely suppressed by antisense Egr3 inhibition. These results support that neurosteroid withdrawal-induced upregulation of GABA-A receptor α4-subunit expression is mediated by the Egr3 via a PR-independent signaling pathway.
It is thought that catamenial seizures may occur due to multiple mechanisms. Perimenstrual type (C1) occurs in women with ovulatory cycle possibly due to a sharp decline (“withdrawal”) in the serum level of progesterone and, consequently, of the level of progesterone-derived anticonvulsant neurosteroids in the brain around the perimenstrual period. The estradiol/neurosteroid ratio is highest during menstruation. Because neurosteroids potentiate GABA-A receptor-mediated inhibition, the rapid loss of neurosteroid-mediated inhibition, such as that which occurs before, during or after the onset of menses, could exacerbate seizures in many women with catamenial epilepsy. Periovulatory type (C2) occurs in women with ovulatory cycles, possibly due to estradiol surge just before ovulation, and low neurosteroid levels do not offset the estradiol-induced excitation because the rise of anticonvulsant neurosteroid levels would not occur until after ovulation. The relatively low neurosteroid inhibition and marked estradiol excitation could lead to periovulatory seizures. Inadequate luteal-type (C3) occurs in women with anovulatory cycles possibly due to a loss of neurosteroid-mediated inhibition during luteal phase for a prolonged time and also due to elevated estrogen levels. Progesterone secretion that occurs normally during the luteal phase is markedly decreased during anovulatory cycles, resulting in abnormally low levels of neurosteroid in the brain.
Preclinical models
Based on neuroendocrinology of neurosteroids, a variety of models have been described in animals that partially resemble catamenial seizure patterns. In the first category of models, attempts are made to mimic the luteal phase by inducing extended high levels of progesterone and estrogens followed by rapid decline to simulate the menstruation in normal rodents. These include pseudopregnancy, chronic progesterone, and progesterone (neurosteroid) withdrawal models (Smith et al., 1998; Moran and Smith, 1998; Reddy et al., 2001). The second category of models is based on the naturally occurring estrous cycle or administration of exogenous hormones that simulate the specific stages of estrous cycle in ovariectomized rats (Frye et al., 1998; Frye and Bayon, 1998). These physiological models better mimic the normal ovarian cycle. In the third category of models, epilepsy animals are exposed to steroid hormones and neurosteroid withdrawal conditions, and the frequency and severity of spontaneous or evoked seizures are utilized as indices of catamenial-like seizure exacerbation (Reddy and Zeng, 2007; Gangisetty and Reddy, 2010; Reddy et al., 2012).
Neurosteroid replacement therapy
Based on neurosteroid physiology, we developed a rat model of perimenstrual catamenial epilepsy (Reddy et al., 2001; Reddy and Rogawski, 2001). Rodents have a 4 to 5 day estrous cycle and studies of fluctuations in seizure susceptibility in cycling female rodents have not led to results that are relevant to the human menstrual cycle. In order to provide a model that more closely mimics the human situation, a condition of elevated progesterone was created in rats by gonadotropin treatment. This resulted in prolonged high circulating levels of estrogen and progesterone similar to those that occur in the luteal phase of the menstrual cycle. Then, to simulate the withdrawal of allopregnanolone that occurs at the time of menstruation, the animals were treated with finasteride (a 5α-reductase and neurosteroid synthesis inhibitor) 11 days after the initiation of gonadotropin treatment. Withdrawal of neurosteroids has led to decreased seizure threshold and increased seizure activity (Reddy et al., 2001), suggesting that endogenous neurosteroids do modulate seizure susceptibility. We made further advances in this model by utilizing epilepsy rats (Reddy and Zeng, 2007) and mouse kindling model (Reddy et al., 2012). In epilepsy rats, we demonstrated that prolonged exposure followed by withdrawal of the neurosteroid allopregnanolone is associated with a significant (two-fold) increase in seizure frequency. These findings were confirmed in an independent study (Lawrence et al., 2010).
The neurosteroid withdrawal model of catamenial epilepsy was used to investigate therapies for perimenstrual catamenial epilepsy (Reddy and Rogawski, 2000b; 2001; Reddy et al., 2012). A key result is that conventional AEDs, including benzodiazepines and valproate, are less potent in protecting against seizures during the period of enhanced seizure susceptibility following neurosteroid withdrawal. This pharmacoresistance appears to mimic the situation in women with catamenial epilepsy, where breakthrough seizures occur despite treatment with antiepileptic drugs. In contrast to the results with conventional antiepileptic drugs, neurosteroids, including allopregnanolone, THDOC and their 5α-isomers, were found to have enhanced activity in the catamenial epilepsy model (Reddy and Rogawski, 2001; Reddy et al., 2012). Therefore, we proposed a “neurosteroid replacement” approach to treat catamenial seizure exacerbations (Reddy and Rogawski, 2009). A neurosteroid could be administered in a “pulse” prior to menstruation and then withdrawn, or continuously administered throughout the month. The neurosteroid would be administered at low doses to avoid sedative side effects. Such low doses are expected to contribute little anticonvulsant activity during most of the menstrual cycle, but may prevent the occurrence of perimenstrual catamenial seizures.
GABA-A receptor subunit plasticity
Neurosteroids exhibit enhanced anticonvulsant activity in perimenstrual catamenial epilepsy, a neuroendocrine condition associated with neurosteroid withdrawal (NSW) (Reddy et al., 2012). However, the molecular mechanisms underlying such enhanced neurosteroid sensitivity remain unclear. Recently, we report a novel plasticity of extrasynaptic δ-containing GABAA receptors in the dentate gyrus in a mouse perimenstrual model of NSW (Carver et al., 2014). A significant increase occurred in δ-subunit, but not α1, α2, β2 and γ2 subunits, in the dentate gyrus of mice subjected to NSW paradigm. Electrophysiological studies confirmed enhanced sensitivity to allopregnanolone in NSW animals. Allopregnanolone produced a greater potentiation of tonic currents in granule cells of NSW animals. Moreover, such enhanced allopregnanolone sensitivity was not evident in δ-subunit knockout mice subjected to similar withdrawal paradigm. Overall, perimenstrual NSW is associated with striking upregulation of extrasynaptic δ-containing GABA-A receptors that mediate tonic inhibition and neurosteroid sensitivity in the dentate gyrus. These findings may represent a molecular rationale for neurosteroid therapy of catamenial epilepsy.
The ovarian cycle profoundly affects susceptibility to seizures and epileptogenesis. The molecular mechanisms underlying these changes are poorly understood. The estrous cycle models have been used to demonstrate that the structure of extrasynaptic GABA-A receptor undergoes drastic alterations due to changing levels of progesterone during the ovarian cycle (Maguire et al., 2005; Wu et al., 2013). During the late diestrous phase (associated with high progesterone levels), expression of the δ-containing GABA-A receptors was elevated, which was associated with an increase in tonic inhibition and diminished seizure susceptibility in mice. During the estrous phase (associated with low progesterone levels), tonic inhibition was reduced by 50% with corresponding increases in both seizure susceptibility and epileptogenesis in female mice (Wu et al., 2013). These cyclic alterations in the δ-subunit are also observed following exogenous progesterone treatment in ovariectomized female mice (Maguire and Mody, 2007). Unlike the phasic inhibition mediated by the γ-containing GABA-A receptors, the δ-containing GABA-A receptors are highly sensitive to neurosteroids (Mihalek et al., 1999; Stell et al., 2003).
Recently, we reported a novel role of extrasynaptic, δ-containing GABAA receptors as crucial mediators of the estrous cycle-related changes in neuronal excitability in mice, with hippocampus subfield specificity (Wu et al., 2013). The δ-subunit expression was undiminished by age, ovariectomy, and in mice lacking progesterone receptors, but was significantly reduced by finasteride, a neurosteroid synthesis inhibitor. In diestrus, there is greater potentiation of GABA currents by allopregnanolone in granule cells than in CA1 pyramidal cells. The baseline conductance and allopregnanolone potentiation of tonic currents in dentate granule cells are higher than in CA1 pyramidal cells. Susceptibility to epileptogenesis is lower at diestrous that estrous stage (Wu et al., 2013). These findings are consistent with the possibility that the abundance of extrasynaptic δ-containing GABA-A receptors is increased during diestrous, likely due to elevated neurosteroids, and thereby contributes to allopregnanolonesensitive GABAergic currents in the hippocampus, a key region for the pathophysiology of epilepsy (Wu et al., 2013). It is suggested that deficiencies in regulatory mechanisms controlling normal cycling of the δ-subunit-containing GABA-A receptors in the hippocampus could be a potential molecular mechanism for catamenial seizures.
Menopause and catamenial seizures
There is little information on the relationship between neurosteroids and menopause. Natural reductions in steroid hormones around perimenopause and menopause are associated with alterations in frequency or severity of seizures in women with epilepsy (Abassi et al., 1999; Harden et al., 1999, 2006). There is emerging clinical evidence suggesting that menopause is associated with the increase in seizures in about 30% of women with epilepsy, but there is no consensus on these findings. Some women going through menopause have fewer seizures and many experience no change at all. Hormone replacement therapy is significantly associated with an increase in seizure frequency during menopause, and this is more likely in women with a history of catamenial epilepsy (Harden et al., 2006; Harden, 2008). It has been suggested that seizures may improve after menopause, especially in the women with catamenial epilepsy (Roste et al., 2008). Nevertheless, neurosteroid mechanisms may have implications in menopause associated with cessation of menstrual cycle. Perimenstrual catamenial epilepsy occurs in women with normal menstrual cycle possibly due to a sharp decline (“withdrawal”) in the serum level of progesterone and, consequently, of the level of progesterone-derived anticonvulsant neurosteroids in the brain around perimenstrual period. Such seizure exacerbation may be absent or reduced in menopause. Other catamenial types such as periovulatory type related to estrogen excess may also be reduced in menopause. However, the levels of neurosteroids in perimenopause and menopause are not characterized completely. It is likely that menopause may be associated with low levels of anticonvulsant neurosteroids in the brain that may enhance susceptibility to seizures.
Conclusions and Perspectives
Neurosteroids that enhance the GABAergic neurotransmission are potent anticonvulsants and may regulate various neuronal excitability networks. Neurosteroids are believed to play a role in the regulation of seizure susceptibility in the setting of preexisting epilepsy. Menstrual and stress related fluctuations in seizures may be related to alterations in brain neurosteroid levels. Catamenial epilepsy is a gender-specific form of epilepsy that impacts a substantial proportion (~70%) of women with epilepsy. Although ovarian hormones play a central role, the exact cause of catamenial epilepsy is unknown. Currently experimental studies have indicated a clear role of estrogen, progesterone, and endogenous neurosteroids in the pathophysiology of catamenial epilepsy. Although there are several forms of catamenial epilepsy, neurosteroids have been implicated only in the seizure exacerbation that occurs around the perimenstrual period. It is hypothesized that withdrawal of progesterone-derived neurosteroids leads to enhanced brain excitability, and predisposition to seizures. In addition to neurosteroid fluctuations, there is emerging evidence that plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in catamenial epilepsy. Animal studies have shown that prolonged exposure to allopregnanolone, followed by withdrawal such as that occurs during menstruation, causes a marked increase in expression of the extrasynaptic α4 and δ-subunits, which are linked to enhanced neuronal excitability, seizure susceptibility and benzodiazepine resistance. Overall, these neuroendocrine changes can result in reduced inhibition, resulting in enhanced excitability, which, among other effects, predisposes to catamenial seizures.
Neurosteroids are novel drug targets for epilepsy. Neurosteroids may represent a rational treatment strategy for perimenstrual catamenial epilepsy. There are two modes for therapeutic application of neurosteroid mechanisms: (i) manipulation of the endogenous system; and (ii) an exogenous systemic administration. Cyclical or regular exogenous administration appears more amenable for titration. However, natural neurosteroids such as allopregnanolone have severe limitations because they have a short half-life, are orally-inactive, and may produce hormonal effects due to their metabolism to hormonally-active compounds. Synthetic analogs of neurosteroids may overcome these obstacles and the side effects associated with natural neurosteroid therapy. Although neurosteroids seems to be the most direct approach to the treatment of catamenial epilepsy, there is only limited anecdotal data available to support their use. Ganaxolone, the synthetic 3β-methyl derivative of allopregnanolone, is the only neurosteroid that has been evaluated for the treatment of epilepsy in humans (Monaghan et al., 1999; Reddy and Woodward, 2004). Ganaxolone has similar pharmacological properties to the natural neurosteroids such as allopregnanolone. An alternative strategy is to administer specific agents (e.g. TSPO agonists) that stimulate endogenous production of neurosteroids in the brain. They may also be useful for treatment of epilepsy. Both strategies can be helpful in the fight against epilepsy. Nonetheless, in the future synthetic neurosteroids and neurosteroid synthesis modulators may find utility in the treatment of some forms of epilepsy.
Highlights.
-
▶
This article describes the role of neurosteroids in sex-specific epilepsies.
-
▶
Neurosteroids are anticonvulsants and also exert antiepileptogenic effects.
-
▶
Neurosteroids such as allopregnanolone play a key role in catamenial epilepsy.
-
▶
Alterations of GABA receptor plasticity & function are evident in epilepsy models.
-
▶
Neurosteroid replacement therapy is useful for sex-specific forms of epilepsies.
Acknowledgments
The original research described in this article was supported in part by the NIH/NINDS grants NS051398, NS052158, NS071597 and U01NS083460 (to D.S.R.) and the seed grant of TAMHSC Women’s Health in Neuroscience (WHIN) program. The content is solely the responsibility of the author and do not necessarily represent the official views of the NIH.
Abbreviations
- AED
antiepileptic drug
- AP
allopregnanolone (3α-hydroxy-5α-pregnane-20-one)
- BDNF
brain-derived neurotrophic factor
- GABA
γ-aminobutyric acid
- EGR3
early growth response factor-3
- NSW
neurosteroid withdrawal
- THDOC
allotetrahydro-deoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one)
- TSPO
translocator protein
- IPSC
inhibitory postsynaptic current
- NMDA
N-methyl-D-aspartate
- TLE
temporal lobe epilepsy
- PR
progesterone receptor
- PRKO
progesterone receptor knockout mice
- WT
wild-type mice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abassi F, Krumholz A, Kittner SJ, Langenberg P. Effects of menopause on seizures in women with epilepsy. Epilepsia. 1999;42:205–210. doi: 10.1111/j.1528-1157.1999.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Akk G, Covery DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. The influence of the membrane on neurosteroid actions at GABAA receptors. Psychoneuroendocrinology. 2009;34S:S59–S66. doi: 10.1016/j.psyneuen.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER-alpha) gene-disrupted mice. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Auger CJ, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol. Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Zetterlund B, Blom S, Romano M. Effect of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol. Scand. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Bauer J. Interactions between hormones and epilepsy in female patients. Epilepsia. 2001;42(Suppl 3):20–22. doi: 10.1046/j.1528-1157.2001.042suppl.3020.x. [DOI] [PubMed] [Google Scholar]
- Bauer J, Burr W, Elger CE. Seizure occurrence during ovulatory and anovulatory cycles in patients with temporal lobe epilepsy: a prospective study. Eur. J. Neurol. 1998;5:83–88. doi: 10.1046/j.1468-1331.1998.510083.x. [DOI] [PubMed] [Google Scholar]
- Baulieu E-E. Steroid hormones in the brain: several mechanisms? In: Fuxe F, Gustafsson JA, Wetterberg L, editors. Steroid Hormone Regulation of the Brain. Oxford: Pergamon Press; 1981. pp. 3–14. [Google Scholar]
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arg Neuro-Psiquiatria. 2005;63(3B):751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neurosteroids and epileptogenesis in the pilocarpine model: Evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Panuccio G, Avoli M. Neurosteroids and epilepsy. Curr Opin Neurol. 2010;23:170–176. doi: 10.1097/WCO.0b013e32833735cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA-A receptor channels from low-to high-efficacy gating patterns. J Neurosci. 2003;23(34):10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors:form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230(2):151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS. Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABA-A receptors mediated tonic inhibition and neurosteroid sensitivity. J Neurosci. 2014 doi: 10.1523/JNEUROSCI.0596-14.2014. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABAA receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Anderson H, Friis ML, Sidenius P. Gender differences in epilepsy. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Clarke RS, Dundee JW, Carson IW. Proceedings: A new steroid anaesthetic-althesin. Proc R Soc Med. 1973;66:1027–1030. doi: 10.1177/003591577306601023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Craig CR. Anticonvulsant activity of steroids: separability of anticonvulsant from hormonal effects. J. Pharmacol. Exp. Ther. 1966;153:337–343. [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Mo V, Burnham WM, MacLusky NJ. Gonadectomy unmasks an inhibitory effect of progesterone on amygdala kindling in male rats. Brain Res. 2001;889:260–263. doi: 10.1016/s0006-8993(00)03147-4. [DOI] [PubMed] [Google Scholar]
- El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla R. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–1626. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory them: phasic and tonic activation of GABAA receptors. Nat Rev. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neuroactive steroid 3α,5α-THP has anti-seizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ, Bayon LE. Finasteride blocks the reduction in ictal activity produced by exogenous estrous cyclicity. J. Neuroendocrinol. 1998;10:291–296. doi: 10.1046/j.1365-2826.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Gilad R, Sadeh M, Rapoport A, Dabby R, Lampl Y. Lamotrigine and catamenial epilepsy. Seizure. 2008;17:531–534. doi: 10.1016/j.seizure.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gowers WR. Their causes, symptoms, and treatment. London: J & A Churchill; 1881. Epilepsy and other chronic convulsive diseases; p. 197. [Google Scholar]
- Harden CL, Leppik I. Optimizing therapy of seizures in women who use oral contraceptives. Neurology. 2006;67(Suppl 4):S56–S58. doi: 10.1212/wnl.67.12_suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- Harden CL. Hormone replacement therapy: will it affect seizure control and AED levels? Seizure. 2008;17(2):176–180. doi: 10.1016/j.seizure.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Herzog AG, Nikolov BG, Koppel BS, Christos PJ, Fowler K, Labar DR, Hauser WA. Hormone replacement therapy in women with epilepsy: a randomized, double-blind placebo-controlled study. Epilepsia. 2006;47:1447–1451. doi: 10.1111/j.1528-1167.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pulver MC, Ravdin L, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interactions with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Hauser WA. Incidence and prevalence. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 47–57. [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Hormonal therapies: progesterone. Neurotherapeutics. 2009;6:383–391. doi: 10.1016/j.nurt.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG. Is there a lateralized asymmetry in the sensitivity of the brain to hormones in epilepsy? Epilepsy and Behavior 2007. 2007;11:157–159. doi: 10.1016/j.yebeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM The NIH Progesterone Trial Study Group. Sensitivity and specificity of the association between catamenial seizure patterns and ovulation. Neurology. 2008;70:486–487. doi: 10.1212/01.wnl.0000278102.55701.d0. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Friedman MN, Freund S, Pascual-Leone A. Transcranial magnetic stimulation evidence of a potential role for progesterone in the modulation of premenstrual corticocortical inhibition in a woman with catamenial seizure exacerbation. Epilepsy Behav. 2001;2:367–369. doi: 10.1006/ebeh.2001.0232. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM Smithson SD and the Progesterone Trial Study Group. Progesterone versus placebo therapy for women with epilepsy: A randomized clinical trial. Neurology. 2013 doi: 10.1212/WNL.0b013e318259e1f9. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AD, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAA receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Jacono JJ, Robinson J. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle in epileptic women. Epilepsia. 1987;28:571–577. doi: 10.1111/j.1528-1157.1987.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Gasior M, Carter RB, Witkin JM. Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur J Pharmacol. 2003;474:217–222. doi: 10.1016/s0014-2999(03)02086-7. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–877. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan PW, Norwitz ER, Ben Menachem E, Pennell PB, Druzin M, Robinson JN, Gordon JC. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 2007;11:283–291. doi: 10.1016/j.yebeh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav. 1994;28:454–463. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- Kim GH, Lee HW, Park H, Lee SK, Lee SA, Kim YI, Song HK, Shin DJ, Hong SB. Seizure exacerbation and hormonal cycles in women with epilepsy. Epilepsy Res. 2010;90(3):214–220. doi: 10.1016/j.eplepsyres.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neuroactive steroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpineand kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35(8):1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Res. 1999;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Reddy DS. Neurosteroids: a new class of neuromodulators. Drugs of Today. 1995;31:433–455. [Google Scholar]
- Kuruba R, Reddy DS. Neuroprotective Effects of GABAergic Agents in the Rat Model of refractory status epilepticus. Soc Neurosci Abstr. 2011 #338.08 (C45). [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABA-A receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Landgren S, Bäckström T, Kalistratov G. The effect of progesterone on the spontaneous interictal spike evoked by the application of penicillin to the cat's cerebral cortex. J. Neurol. Sci. 1978;36:119–133. doi: 10.1016/0022-510x(78)90166-1. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous Neurosteroid Synthesis Modulates Seizure Frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, O‘Malley BW. Unfolding the actions of progesterone receptors. J Biol Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- Logothetis J, Harner R, Morrel F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. 1959;9:352–360. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA-A receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27(9):2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABA-A receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Molec Pharm. 2007;71:539–548. doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Gentet LJ, Dempster J, Belelli D. GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol. 2007;583:1021–1040. doi: 10.1113/jphysiol.2007.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrock DW, Lee DA. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1999;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith S. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J. Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima S, Wallace SJ. Effects of puberty on seizure frequency. Dev. Med. Child. Neurol. 1989;31:174–180. doi: 10.1111/j.1469-8749.1989.tb03976.x. [DOI] [PubMed] [Google Scholar]
- Nothdurfter C, Rammes G, Baghai TC, Schüle C, Schumacher M, Papadopoulos V, Rupprecht R. TSPO (18 kDa) as a target for novel anxiolytics with a favourable side-effect profile. J Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02166.x. (in press). [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell PB. Antiepileptic drugs during pregnancy: What is known and which AEDs seem to be safest? Epilepsia. 2008;49(suppl.9):43–55. doi: 10.1111/j.1528-1167.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG the Progesterone Trial Study Group. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology. 2009;73:223–227. doi: 10.1212/WNL.0b013e3181ae7adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG. NIH Progesterone Trial Study Group: Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology. 2009;73(3):223–227. doi: 10.1212/WNL.0b013e3181ae7adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L, Hess GP. Mechanism of potentiation of a dysfunctional epilepsy-linked mutated GABA-A receptor by a neurosteroid (3alpha, 21-dihydroxy-5alpha-pregnan-20-one): transient kinetic investigations. Biochemistry. 2010;49(36):7892–7901. doi: 10.1021/bi901241g. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Wu Y, Richerson GB. Postdepolarization potentiation of GABA-A receptors: A novel mechanism regulating tonic conductance in hippocampal neurons. J Neurosci. 2010;30(22):7672–7684. doi: 10.1523/JNEUROSCI.0290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol. Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. Neuroreport. 2004a;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17β-estradiol. Neuroscience. 2004b;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009a;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310(1):230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334:1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000a;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000b;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:303–310. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neuroactive steroids modulates GABAA receptor function and seizure susceptibility. J Neurosci. 2002;42:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010b;89:254–260. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Woodward R. Ganaxolone: a prospective overview. Drugs Future. 2004;29:227–242. [Google Scholar]
- Reddy DS, Zeng YC. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 2007;21(6):A1179–A11179. [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Frontiers in Endocrinology. 2011;2(38):1–11. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Hormones & Behavior. 2013a;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of hormones and neurosteroids in epileptogenesis. Frontiers in Cellular Neuroscience. 2013a;7:1–20. doi: 10.3389/fncel.2013.00115. (article 115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Ramanathan G. Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy & Behavior. 2012;25:92–97. doi: 10.1016/j.yebeh.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59(7–8):573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. The Journal of Pharmacology and Experimental Therapeutics. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31(2):650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol. 2011;2:38. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosciszewska D. In: Epilepsy and menstruation. Hopkins A, Epilepsy, editors. London: Chapman & Hall; 1987. pp. 373–381. [Google Scholar]
- Roste LS, Tauboll E, Svalheim S, Gjerstad L. Does menopause affect the epilepsy? Seizure. 2008;17:172–175. doi: 10.1016/j.seizure.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J. Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325(5939):490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABA-A receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. Anesthetics of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. [Google Scholar]
- Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (metrazol) J. Lab. Clin. Med. 1942;27:1051–1053. [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABA-A receptors in shaping learning deficits at puberty in mice. Science. 2010;327(5972):1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA-A receptors: focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABA-A receptor α4-subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamateevoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–282. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Beyenburg S, Watzka MS, Blumcke I, Bauer J, Schramm J, Bidlingmaier F, Elger CE. Expression of 5α-reductase and 3α-hydroxysteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 2000;41:140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Ludwig M, Clusmann H, Bidlingmaier F, Casarosa E, Luisi S, Elger CE, Beyenburg S. Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 2003;54:11–19. doi: 10.1016/s0920-1211(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Suzuki T, Misawa M. Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam-withdrawn mice by the neuroactive steroid 5α-pregnan-3α,21-diol-20-one (alloTHDOC) Addiction Biol. 1997;2:455–460. doi: 10.1080/13556219772516. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neuroactive steroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísková J, Velísek L. Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide-Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Belluzzi JD, Stein L, Lan NC. Comparative behavioral characterization of the neuroactive steroids 3α-OH,5α-pregnan-20-one and 3α-OH,5β-pregnan-20-one in rodents. Psychopharmacology. 1995;118:65–71. doi: 10.1007/BF02245251. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ-subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. Patch-clamp analysis of direct steroidal modulation of glutamate receptorchannels. J. Neuroendocrinol. 1994;6:347–355. doi: 10.1111/j.1365-2826.1994.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-Daspartate receptor-dependent mechanism. J. Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]