Abstract
Purpose
To report additional ocular outcomes of intensive treatment of hyperglycemia, elevated blood pressure, and dyslipidemia in the ACCORD Study.
Design
Double 2 × 2 factorial multicenter randomized clinical trials in men and women with type 2 diabetes who had established cardiovascular disease and/or cardiovascular risk factors. In the glycemia trial targets of intensive and standard treatment were HbA1c <6.0% and 7.0-7.9%, respectively, and in the blood pressure trial systolic blood pressure of <120 mm Hg and <140 mm Hg, respectively. The dyslipidemia trial compared fenofibrate plus simvastatin vs. placebo plus simvastatin.
Participants
Of the 5273 ACCORD-Eye participants, 3,472 were enrolled and 2856 had 4-year data (85% of survivors).
Methods
Eye examinations and fundus photographs were taken at baseline and year 4. Photographs were graded centrally for retinopathy severity and features of macular edema using the Early Treatment Diabetic Retinopathy Study (ETDRS) methods.
Primary Outcome Measure
3 or more steps progression on the ETDRS person scale or treatment of retinopathy with photocoagulation or vitrectomy.
Results
As previously reported, there were significant reductions in the primary outcome in the glycemia and dyslipidemia trials, but no significant effect in the blood pressure trial. Results were similar for retinopathy progression by 1, 2, and 4 or more steps on the person scale and for ≥2 steps on the eye scale. In the subgroup of patients with mild retinopathy at baseline, effect estimates were large (Odds Ratios ∼0.30, P<0.001), but did not reach nominal significance for participants with no retinopathy or for those with moderate to severe retinopathy at baseline.
Conclusions
Slowing of progression of retinopathy by intensive treatment of glycemia was observed in ACCORD participants, whose average age and diabetes duration were 62 and 10 years, respectively, and who had established cardiovascular disease and/or cardiovascular risk factors. The effect appeared stronger in patients with mild retinopathy. Similar slowing of progression was observed in patients treated with fenofibrate, while no effect was observed with intensive BP treatment. This is the second study to confirm the benefits of fenofibrate in reducing diabetic retinopathy progression and fenofibrate should be considered for treatment of diabetic retinopathy.
Introduction
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial included three randomized comparisons that evaluated the effects of intensive blood glucose and blood pressure control, and the combination of fenofibrate and statin vs. statin monotherapy therapy for dyslipidemia on the occurrence of cardiovascular events in patients with type 2 diabetes who also had established cardiovascular disease and/or additional cardiovascular risk factors1. As previously reported, none of the three intensive treatments was demonstrated to have a beneficial effect on the primary cardiovascular outcome2-5. After 3.5 years of follow-up the glycemia trial was stopped because of increased mortality in the intensive treatment group2,5. The ACCORD Eye Study was designed to evaluate the effects of the three interventions on the development and progression of diabetic retinopathy in a subset of ACCORD Study participants6. As previously reported, the main results of the ACCORD Eye Study were reductions in retinopathy progression in both the intensive glycemia and fenofibrate plus statin treatment groups, but not in the intensive BP-treatment group7. Here we examine components of the primary eye outcome, present additional pre-specified and exploratory analyses of primary and secondary outcome measurements and compare results between trials and in subgroups.
Methods
The ACCORD Study
The designs of the ACCORD study and the ACCORD Eye Study are described elsewhere1,6. The ACCORD study was approved by the institutional review board of each clinical center. Briefly, the ACCORD study was a multi-center study with a total of 10,251 participants randomly assigned in equal numbers to two glycemia management treatment arms. The intensive treatment arm aimed to achieve and maintain HbA1c level <6.0%. The standard treatment arm targeted an HbA1c range of 7.0-7.9% with an expected median value of approximately 7.5%. Of these participants, 5,518 with moderate dyslipidemia were randomly assigned in a double masked fashion to placebo or fenofibrate, 160 mg daily, in addition to statin, aiming to lower triglyceride levels and to raise high-density lipoprotein cholesterol (HDL) levels. Participants in both the placebo and the fenofibrate groups also took simvastatin to lower low-density lipoprotein (LDL) cholesterol levels. The other 4,733 participants, who had systolic blood pressures of 130-180 mm Hg, were simultaneously randomized to one of two hypertension management protocols. The intensive treatment arm targeted systolic blood pressure <120 mmHg and the standard treatment arm <140 mmHg. The primary outcome of the ACCORD Trial was the composite endpoint of the time until the first occurrence of non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death.
The ACCORD Eye Study
The primary aim of the ACCORD Eye study was to examine the effect of each of the 3 interventions on the development and progression of diabetic retinopathy. Participants who had proliferative diabetic retinopathy (PDR) previously treated with laser and or/vitrectomy were excluded from the ACCORD Eye Study, while all other ACCORD subjects at participating sites were eligible. The eye study protocol was reviewed by the institutional review board of each clinical center and signed informed consent was obtained from each participant. The ACCORD Eye Study consisted of standardized eye examinations conducted by a study ophthalmologist or optometrist and color fundus photography of 7 standard stereoscopic fields, scheduled for baseline and year 4 of follow-up. The eye examinations included visual acuity measurement and dilated examination of the anterior segment and fundus. The fundus photographs were graded centrally by trained personnel who had no knowledge of the medical status or the treatment assignment of the participants. Baseline and year 4 photographs were graded independently of each other. For assessment of retinopathy progression, the Early Treatment Diabetic Retinopathy Study (ETDRS) diabetic retinopathy severity scale, which combines the severity levels from both eyes for each person, was used,8 with minor modifications7, providing 17 steps from no diabetic retinopathy in either eye to high risk PDR in both eyes. The ETDRS diabetic macular edema (DME) severity scale was used to assess development of and change in DME on stereoscopic fundus photographs of the macular9. This scale classifies individual eyes by combining extent of retinal thickening within 1 disc diameter (DD) of the macular center with degree of thickening at the center. The scale has 10 steps, beginning with absence of retinal thickening and ending with ≥3 disc areas (DA, 1 DA=2.54 mm2) of thickening within 1 disc diameter (DD, 1 DD = 1.8 mm) of center and thickening at center ≥2 times that of “reference thickness,” defined as the maximum thickness of normal retina 0.5-1.0 DD from center. Change in DME between baseline and year 4 was assessed in one eye of each patient, choosing the eye in the higher step on the scale at baseline or, if both eyes were in the same step at baseline, the eye in the higher step at year 4. An extension of the ETDRS grading system was used to assess change in estimated areas of hard exudates and retinal thickening within 2 DD of the macular center10. Estimates from right and left eyes were summed. The hard exudates scale has 10 steps extending from 0 to ≥0.5 DA and the retinal thickening scale has 12 steps extending from 0 to ≥10 DA. In addition, the entire ACCORD cohort had visual acuity measurements with ETDRS logarithmic visual acuity charts at the medical clinics at baseline and every 2 years. Questionnaires regarding ocular surgery such as cataract surgery, vitrectomy and laser photocoagulation were also administered at each annual study visit.
ACCORD Eye Study Measures of Outcome
The primary outcome of the ACCORD Eye study was a composite of 3 or more steps of progression along the ETDRS diabetic retinopathy severity scale for persons or treatment of diabetic retinopathy with photocoagulation or vitrectomy in either eye. Secondary and exploratory outcomes included alternative definitions of progression, development of the primary outcome in retinopathy severity subgroups, development of retinopathy in participants free of it at baseline, change in photographic measures of macular edema and change in visual acuity.
Statistical Methods
Descriptive statistics and proportions are presented. The comparison of proportions of participants reaching an outcome at 4 years between groups was made using logistic regression models with likelihood ratio tests. Covariates included clinical network and whether the participant had a previous cardiovascular event. Separate models were used for the glycemia, lipid, and blood pressure comparisons. For the glycemia comparison, we also included indicator variables for fenofibrate, intensive blood pressure treatment, and trial (blood pressure vs. lipid). For the lipid and blood pressure comparisons, we also included an indicator variable for intensive glycemia treatment. Tests for interactions were also made using likelihood ratio tests by adding the interactions to the appropriate model. No adjustment for multiplicity has been made in this paper.
Results
From January 2001 to October 2005, 10,251 participants were recruited in the main ACCORD trial. From October 2003 to February 2006, 3472 eligible participants were enrolled in the ACCORD Eye Study. Of these 2856 (85% of survivors) returned for the second eye examination and fundus photographs.
Baseline Demographic and Systemic Characteristics
Baseline characteristics of participants in the ACCORD Eye Study have been presented previously by treatment group, as have levels of glycated hemoglobin, blood pressure, triglycerides and high-density lipoprotein cholesterol during the trial7. In summary (Table 1), at baseline mean age was 62 years, diabetes duration 10 years, HbA1c 8.2%, blood pressure 135/75 mm Hg, and BMI 32 kg/m2. Approximately 38% of participants were female and 32% non-white, 31% had previous cardiovascular disease, 14% were current smokers and 32% were taking insulin. During the trial there were clinically and statistically significant separations between intensive and standard treatment groups for each of the three interventions (HbA1c 6.4% vs. 7.5%. triglycerides 120 vs. 148 mg/dl, systolic blood pressure 118 vs. 134 mm Hg)7.
Table 1.
Baseline characteristics of ACCORD-Eye study participants (N=2856).
| Characteristic | mean (SD) or n (%) |
|---|---|
| Age (years) | 61.6 (6.4) |
| <55 | 170 (6.0%) |
| 55 to <65 | 1865 (65.3%) |
| ≥65 years | 821 (28.7%) |
| Female | 1090 (38.2%) |
| Race: | |
| White | 1935 (67.7%) |
| African American | 437 (15.3%) |
| Hispanic | 185 (6.5%) |
| Others | 299 (10.5%) |
| Educational Level | |
| Less than high school | 338 (11.8%) |
| High school graduation | 673 (23.6%) |
| Some college | 1017 (35.6%) |
| College and post-graduate | 826 (28.9%) |
| Prior Cardiovascular Disease | 895 (31.3%) |
| Smoking Status | |
| Never | 1188 (41.6%) |
| Former | 1280 (44.8%) |
| Current | 387 (13.6%) |
| Duration of Diabetes (years) | 10.0 (7.1) |
| <5 | 652 (23.0%) |
| 5 to <15 | 1542 (54.4%) |
| ≥15 | 640 (22.6%) |
| Hemoglobin A1C % | 8.2 (1.0) |
| <7.5% | 608 (21.3%) |
| 7.5 to <9% | 1652 (57.9%) |
| 9 to 12% | 585 (20.5%) |
| >12% | 8 (0.3%) |
| Blood pressure (mm Hg) | |
| Systolic | 134.5 (17.0) |
| Diastolic | 74.9 (10.5) |
| Cholesterol (mg/dl) | |
| Total | 179.7 (41.1) |
| HDL | 41.9 (11.3) |
| LDL | 100.7 (32.7) |
| Triglyceride | 195.1 (162.6) |
| Body Mass Index (BMI) | 32.4 (5.5) |
| Medications | |
| Oral hypoglycemic agents | 2288 (80.1%) |
| Insulin | 892 (32.2%) |
| Aspirin | 1573 (55.1%) |
| NSAIDs | 66 (2.3%) |
Cardiovascular Outcomes
In the main ACCORD Study, none of the three treatment strategies resulted in a significant decrease in the combined rates of heart attack, stroke, or cardiovascular death, compared with standard treatments. However, over about 3.5 years of follow-up, participants assigned to intensive glycemia treatment had a 22% higher risk of death (5.0% vs. 4.0%) and a three times higher risk of hypoglycemic episodes requiring medical intervention (10.5% vs. 3.5%), compared with participants assigned to standard glycemia treatment2. The Data and Safety Monitoring Committee recommended stopping the glycemia trial prior to its scheduled completion, and the Steering Committee stopped the trial on February 20082.
Baseline Ocular Characteristics
Of the 2856 participants with follow-up, at baseline 48% had no retinopathy, 21% had microaneurysms only, 20% had mild nonproliferative diabetic retinopathy (NPDR) and the remainder had moderate to severe NPDR or mild PDR (Table 2). Whether assessed by the ETDRS DME scale or by estimated areas of retinal thickening and hard exudates within 2DD of center, less than 10% of participants had photographically documented macular edema. By all of these measures, edema, when present, was nearly always mild or very mild. The distributions of visual acuity and history of prior cataract surgery at baseline for ACCORD Eye Study participants differed only slightly from those for all ACCORD participants (Table 3).
Table 2. Baseline Ocular Characteristics of ACCORD Eye Participants.
| Characteristics | N (%) |
|---|---|
| Baseline Severity of Diabetic Retinopathy | |
| None (level 10, step 1) | 1370 (48.0%) |
| Microaneurysms only (levels 20≤20, steps 2-3) | 598 (21.0%) |
| Mild NPDR (levels 35≤35, steps 4-5) | 569 (19.9%) |
| Moderate NPDR (levels 43≤43, steps 6-7) | 177 (6.2% |
| Moderately severe NPDR (levels 47≤47, steps 8-9) | 101 (3.5%) |
| Severe NPDR or PDR (level 53<53 or greater, steps 10-17 | 39 (1.4%) |
| Macular Edema (worse eye) | |
| None (ETDRS DME Severity 1A) | 2579 (92.1%) |
| Mild (1B-1C) | 159 (5.7%) |
| Moderate (2-3B) | 44 (1.6%) |
| Severe (4-5C) | 15 (0.5%) |
| Hard exudates area, total per person | |
| None (0 DA) | 2590 (90.7%) |
| Very mild (>0 and <0.016 DA) | 117 (4.1%) |
| Mild (≥0.016 and <0.125 DA) | 128 (4.5%) |
| Moderate (≥0.125 DA) | 20 (0.7%) |
| Retinal thickening, total per person | |
| None (0 DA) | 2621 (91.9%) |
| Very mild (>0 and <0.49 DA) | 95 (3.3%) |
| Mild (≥0.49 and <1.69 DA) | 75 (2.6%) |
| Moderate (≥1.69 and <6.25 DA) | 49 (1.7%) |
| Severe (≥6.25 DA) | 12 (0.4%) |
Table 3.
Baseline Visual Acuity and History of Cataract Surgery of the ACCORD Eye and ACCORD cohorts (N (%)).
| Characteristic | ACCORD Eye | ACCORD |
|---|---|---|
| Cataract surgery | ||
| None | 2639 (92.6%) | 8085 (89.1%) |
| Unilateral | 73 (2.6%) | 374 (4.1%) |
| Bilateral | 139 (4.9%) | 618 (6.8%) |
| Visual Acuity (better eye) | ||
| 20/20 or better (VA score: 84 or more) | 548 (19.8%) | 1367 (15.6%) |
| <20/20 to 20/40 (≥69 to <84) | 1738 (62.8%) | 5354 (61.1%) |
| <20/;40 to 20/160 (≥39 to <69) | 450 (16.3%) | 1854 (21.1%) |
| Worse than 20/160 (<39) | 32 (1.2%) | 194 (2.2%) |
Ocular Outcomes
Components of the Primary Outcome
The primary outcome for all participants (intensive and standard treatment groups combined) is presented in Table 4 (available at http://aaojournal.org), by baseline retinopathy severity step on the ETDRS scale, and by components of the primary outcome definition. The 10 cases in the last column of the table 4 (available at http://aaojournal.org), (“Vitrectomy only”), initially counted as progressing on the basis of vitrectomy alone, are now considered not to have progressed, because review of photographs and eye examination forms suggested that in these cases vitrectomy was more likely to have been done for epiretinal membrane than for diabetic retinopathy.
Revised primary outcome, overall and in subgroups
The first 2 rows of Table 5, for all severity levels combined, present, respectively, the primary results as previously published and the revised results reported here. As expected, results were essentially the same: statistically significant benefit for intensive glycemia and dyslipidemia treatments and no benefit for intensive blood pressure control. The remaining rows of the table present the revised primary outcome in baseline retinopathy subgroups based on Table 4 (available at http://aaojournal.org). In none of the trials was there evidence of treatment benefit in the small subgroup of patients in Steps 10-17, who had PDR or severe NPDR and in whom most outcomes were based on photocoagulation without ≥3-step progression. In the glycemia trial progression rates were lower in patients assigned to intensive treatment in each of the other subgroups. The difference was large and statistically significant only for Steps 2-4, patients with microaneurysms only in one or both eyes or with mild NPDR in only one eye (OR 0.30, 95% CI 0.15, 0.59; P=0.0002). For the other 3 subgroups odds ratios (ORs) ranged from 0.69 to 0.78. Results for the lipid trial were similar to those in the glycemia trial: little or no evidence of benefit for patients in Step 1, almost identical benefit for Steps 2-4 (OR 0.27, 95% CI 0.12, 0.63; P=0.0009) and somewhat larger effect estimates of benefit for Steps 5-6 (OR 0.41, P=0.09) and Steps 7-9 (OR 0.44, P=0.21). Among patients in Step 1 progression occurred in 22/375 (5.9%) of those assigned to placebo vs. 25/401 (6.2%) of those assigned to fenofibrate, while among those in all higher steps combined corresponding proportions were 55/412 (13.3%) and 24/405 (5.9%); P for interaction was 0.0092.
Table 5.
Four-year rates DR severity progression, overall and in subgroups.
| Outcome | Glycemia Trial | Lipid Trial | Blood Pressure Trial | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive | Standard | OR (95% CI) P | Fenofibrate | Placebo | OR (95% CI) P | Intensive | Standard | OR (95% CI) P | |
| Original Total with Progression (≥ 3-step, PC or vitrectomy) | 0.073 (104/1429) | 0.104 (149/1427) | 0.67 (0.51,0.87) P=0.0025 | 0.065 (52/806) | 0.102 (80/787) | 0.60 (0.42,0.86) P=0.0056 | 0.104 (67/647) | 0.088 (64/616) | 1.23 (0.84,1.79) P=0.29 |
| Revised Total with Progression (≥ 3-step or PC | 0.068 (97/1429) | 0.102 (145/1427) | 0.64 (0.49,0.84) P=0.0010 | 0.061 (49/806) | 0.098 (77/787) | 0.59 (0.40,0.86) P=0.0049 | 0.099 (64/647) | 0.084 (52/616) | 1.21 (0.82,1.78) P=0.33 |
| Baseline step* 1 no DR | 0.057 (39/683) | 0.071 (49/687) | 0.78 (0.50,1.21) P=0.27 | 0.062 (25/401) | 0.059 (22/375) | 1.12 (0.61,2.03) P=0.72 | 0.067 (21/314) | 0.071 (20/280) | 1.00 (0.53,1.92) P=0.99 |
| Baseline steps 2-4 Ma or mild DR one eye, no DR or Ma only other | 0.027 (12/439) | 0.084 (38/453) | 0.30 (0.15,0.59) P=0.0002 | 0.030 (8/264) | 0.101 (26/258) | 0.27 (0.12,0.63) P=0.0009 | 0.046 (8/173) | 0.041 (8/197) | 1.22 (0.44,3.40) P=0.71 |
| Baseline steps 5-6 mild - moderate NPDR | 0.090 (19/210) | 0.119 (21/176) | 0.69 (0.35,1.36) P=0.28 | 0.068 (6/88) | 0.135 (14/104) | 0.41 (0.14,1.18) P=0.09 | 0.081 (8/99) | 0.126 (12/95) | 0.61 (0.23,1.62) P=0.32 |
| Baseline steps 7-9 moderate- moderately severe NPDR | 0.198 (16/81) | 0.256 (22/86) | 0.74 (0.33,1.62) P=0.45 | 0.128 (6/47) | 0.250 (10/40) | 0.44 (0.12,1.62) P=0.21 | 0.310 (13/42) | 0.237 (9/38) | 2.23 (0.64,7.74) P=0.20 |
| Baseline steps 10-17 Severe NPDR or PDR | 0.667 (10/15) | 0.583 (14/24) | ** | 0.667 (4/6) | 0.5 (5/10) | ** | 0.706 (12/17) | 0.5 (3/6) | ** |
Model did not converge as some cells had too few people.
Comparison of intensive vs. standard glycemia and lipid treatment effects within the dyslipidemia trial suggests that these effects may be additive (Table 6). Progression of ≥3-steps or photocoagulation was observed in 19 of 400 patients (4.8%) assigned to both intensive treatments vs. 50/381 (13.1%) of those assigned to neither. When either treatment was intensive and the other standard the 4-year progression rates were intermediate and similar (6.7% and 7.4%).
Table 6.
Four-year rates of ETDRS ≥3 Step Progression or photocoagulation by randomization group.
| Lipid Treatment Trial | BP Treatment Trial | ||||
|---|---|---|---|---|---|
|
| |||||
| Glycemia | Fenofibrate | Placebo | Intensive | Standard | TOTALS |
|
| |||||
| Intensive | 0.048 (19/400) | 0.067 (27/406) | 0.086 (27/315) | 0.078 (24/308) | 0.068 (97/1429) |
| Standard | 0.074 (30/406) | 0.131 (50/381) | 0.111 (37/332) | 0.091 (28/308) | 0.102 (145/1427) |
| TOTALS | 0.061 (49/806) | 0.098 (77/787) | 0.099 (64/647) | 0.084 (52/616) | 0.085 (242/2856) |
Alternative outcome definitions using the ETDRS retinopathy severity scale
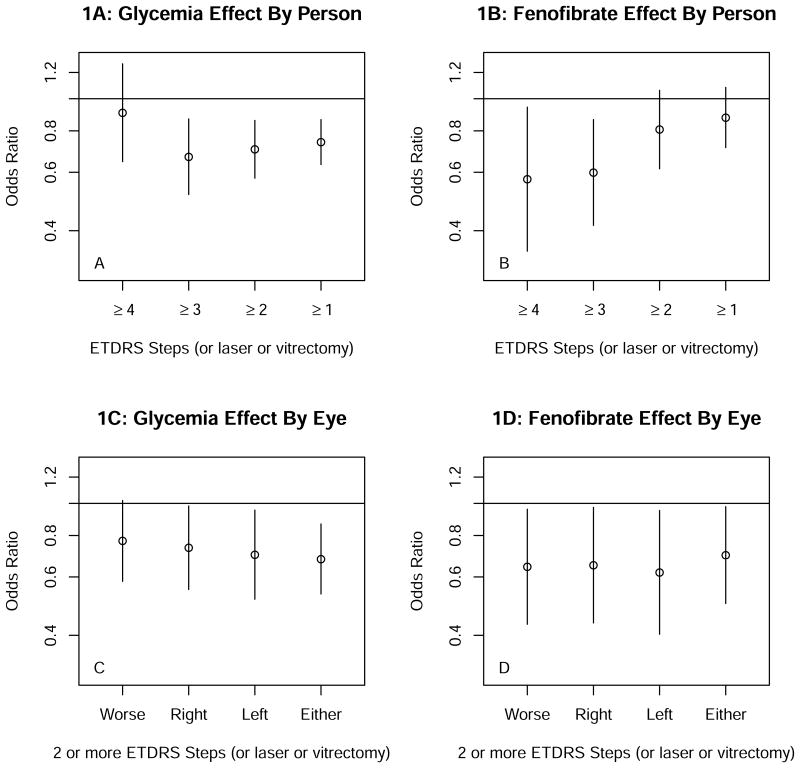
Alternatives to the outcome of 3 or more step progression on the ETDRS person scale or photocoagulation treatment for DR are shown in Figure 1. For the glycemia trial progression by 1 or more, 2 or more and 3 or more steps on the person scale or photocoagulation (Figure 1A) gave similar results, with statistically significant OR of 0.63 to 0.73 and upper limits of the 95% CIs ≤0.85, but for progression by 4 or more steps the OR was 0.85 and non-significant. This difference appeared to be explained, at least in part, by the smaller number of ≥ 4-step events (132 vs. 243 ≥3-step events) and the greater proportion of ≥4- step events represented by events attributed to photocoagulation alone. For the lipid trial (Figure 1B) only the 3 or more and 4 or more step outcomes gave statistically significant OR (0.60 and 0.57, respectively). Within each trial results for ≥2 steps on the eye scale or photocoagulation (Figures 1C and 1D) were similar for right, left, worse, or either eye; OR ranged from 0.61 to 0.73 but 95% CI tended to be wider than those for the person scale.
Figure 1.
A displays results of the glycemic trial with the diabetic retinopathy progression by 1 or more, 2 or more, and 3 or more steps on the ETDRS person scale or photocoagulation which were all statistically significant. Progression by 4 or more steps 2 was not statistically significant.
B shows the results of the lipid trial with the only the 3 or more and the 4 or more step progression of diabetic retinopathy along the person scale were statistically significant.
C shows the progression of diabetic retinopathy by 2 or more steps by the worse, right, left or either eye were similar in the glycemia trial.
D shows the progression of diabetic retinopathy by 2 or more steps by the worse, right, left or either eye were similar in the lipid trial.
Development of any retinopathy
For the 1370 patients with no diabetic retinopathy at baseline development of “any retinopathy”, defined alternatively as ≥1, 2, 3, or 4 steps of progression on the ETDRS scale or photocoagulation for diabetic retinopathy is shown in Table 7 (available at http://aaojournal.org). There was no suggestion of treatment benefit in any trial (P≥0.2). Similarly, for participants with diabetic retinopathy at baseline, analyses were conducted for regression or improvement in diabetic retinopathy along the ETDRS scale (Table 7A and B, available at http://aaojournal.org).
Other morphologic outcomes
None of the measures of change in macular edema between baseline and year 4 (worsening on the ETDRS DME severity scale or change in areas of hard exudates or retinal thickening) suggested any treatment benefit in any of the three trials (Table 8, available at http://aaojournal.org photocoagulation. In the glycemia trial they were seen with equal frequency in the intensive and standard treatment groups.
Change in Visual Acuity and Occurrence of Cataract Surgery (Supplementary Table 9, available at http://aaojournal.org)
In the entire ACCORD cohort, annual rates of change in ETDRS visual acuity score by ≥15 letters were approximately 3.6% for worsening and 7.7% for improvement. Annual rates of worsening to 20/50 or worse and to 20/200 or worse were approximately 5.1% and 0.5%, respectively. There were no clinically or statistically significant differences by treatment group. Annual rates of cataract surgery were approximately 3.0%. In the glycemia trial there was a small difference of little clinical significance: 2.88% in the intensive treatment group vs. 3.25% in the standard group (hazard ratio 0.884 (95% CI 0.788, 0.990, P=0.036).
Discussion
The previously reported findings of reductions in diabetic retinopathy progression resulting from the intensive treatment of hyperglycemia and dyslipidemia studied in ACCORD7 are confirmed by the analyses reported here and extended by analyses of subgroups and of alternative outcome measures. In both trials 4-year rates of the primary outcome, a composite of 3 or more steps of progression along the ETDRS diabetic retinopathy severity scale for persons or treatment of diabetic retinopathy with photocoagulation or vitrectomy in either eye, were reduced by similar amounts, from 10.2% with standard to 6.8% with intensive glycemia treatment (P=0.0010) and from 9.8% with placebo to 6.1% with fenofibrate (P=0.0049). Two recent smaller trials that enrolled patients similar to those enrolled in ACCORD and used similar photographic assessments of progression reported similar small (2-5%) absolute risk reductions with intensive glycemia treatment that were not statistically significant11,12. In the UKPDS, in patients with newly diagnosed type 2 diabetes, 6-year rates of ≥2-step progression were 27.8% and 23.0% in the standard and intensive treatment groups, respectively (P=0.017), yielding an absolute risk difference of 4.8% 13, similar to that observed for ACCORD 4-year rates of ≥2-step progression (18.6% and 13.6%, respectively, P=0.0002). In earlier long-term trials, published two decades ago, in which risk differences tended to be larger, follow-up was longer and levels of baseline HbA1c and/or HbA1c differences between intensive and standard treatment groups tended to be greater14-16.
Our finding of an apparently stronger treatment effect in patients with mild DR than in those with none was surprising. Our ACCORD cohort was similar in age and duration to the WESDR cohort with onset of diabetes at age >30 years and both cohorts had about 50% with no retinopathy at baseline of follow-up17. However, about 30% of this WESDR group progressed 2 or more steps on the ETDRS scale in 4 years,18 whereas only 12% of our ACCORD group did. This lesser rate of progression limited our power somewhat to determine an effect of intensive treatment and it also may reflect a change in medical care decades after these WESDR analyses, that result in lower event rates. Indeed, the most recent WESDR cohort had markedly decreased annualized progression rates among patients with similar glycemic and blood pressure control and similar duration of diabetes19. In the Diabetes Control and Complications Trial of type 1 diabetes a substantial reduction in ≥3-step progression was already present at 4 years in the large group of patients with no DR at baseline (14.1% and 3.5% in standard and intensive treatment groups, respectively), but in smaller subgroups with very mild to moderate NPDR similar results were evident only after 6 to 9 years14.
In the lipid trial our finding of little or no effect of fenofibrate in patients with no retinopathy at baseline but a strong effect in those with mild NPDR is similar to findings in the FIELD substudy of 1012 patients using photographic methods similar to those of ACCORD20. In the FIELD substudy the primary outcome was progression by ≥2 steps on the ETDRS eye scale (if the eyes of a patient differed at baseline choosing the worse eye, otherwise the right eye) a degree of progression similar to that represented by 3 steps on the person scale used in ACCORD. Among patients with no diabetic retinopathy at baseline, progression occurred in 43 of 368 (11.7%) patients assigned to placebo vs. 43 of 377 (11.4%) assigned to fenofibrate plus statin (P=0.87), while among patients with retinopathy (mostly minimal or mild) the corresponding proportions were 14/96 (14.6%) and 3/98 (3.1%, P=0.004); P for interaction was 0.01920. In the main FIELD study, as judged by incidence of photocoagulation during follow-up, the beneficial effect of fenofibrate was found in patients without, as well as in those with, a history of diabetic retinopathy at baseline, with somewhat stronger evidence in the former20,21. The authors point out that as yet undiagnosed retinopathy may have been present in some of these patients, as neither photographs nor fundus examinations were required at entry.
In the UKPDS both progression of retinopathy (to about 40% at 9 years in those with no retinopathy at the time of diagnosis) in photographs and incidence of photocoagulation documented clinically demonstrated the beneficial effect of intensive glycemia treatment22. However, with the latter assessment the effect was evident only after 9 years of follow-up, as compared to 6 years by photographic assessment. In the main ACCORD glycemia trial there was no difference by treatment group in the incidence of photocoagulation assessed by patient report at each annual visit23.
The lack of treatment effect for macular edema was disappointing, but perhaps not surprising when assessed only at the 4 year visit in patients with generally no or only mild edema at baseline and very few events.
Limitations of the ACCORD Eye study included photographic documentation of retinopathy status at only the baseline and 4-year visits and missing 4-year photographs and eye examinations in 15% of participants. Strengths include the generally high quality of the fundus photographs and the use of established methods for their assessment, as well as the opportunities provided by the large number of patients enrolled for subgroup analyses and for exploratory comparisons of glycemia effects within the lipid, and blood pressure trials.
Glycemia control has been the cornerstone in the management of diabetic patients and the results of ACCORD Eye demonstrate the significant ocular beneficial effect of improved glucose control. The beneficial effect of fenofibrate with a statin vs statin alone appears to slow the progression of diabetic retinopathy when mild or moderate retinopathy is present. This is supported by data from the FIELD Study. Recommendations of treatment with fenofibrate and statin for persons with evidence of mild to moderate diabetic retinopathy would be reasonable for people who have no contraindications to the therapy and especially those who cannot achieve good glycemic control. For those patients without evidence of diabetic retinopathy, further studies might be needed in the future either with larger sample size or a surrogate measure of progression to evaluate the role of fenofibrate in primary prevention of diabetic retinopathy. However, it is also important to note that very mild diabetic retinopathy with the occasional microaneurysm may be easily missed on clinical examination by the diabetologist, internist, the general ophthalmologist or even the retinal specialist. Such patients may benefit from fenofibrate therapy.
In summary, our principal conclusion is unchanged from that of the initial ACCORD-Eye study report: the slowing of retinopathy progression by intensive treatment of glycemia, previously established for type 1 diabetes and for some patients with type 2 diabetes, also extends to patients like those participating in ACCORD, whose average age and diabetes duration were 62 and 10 years, respectively, and who had established cardiovascular disease and/or cardiovascular risk factors. In patients with dyslipidemia, retinopathy progression was slowed by fenofibrate to a similar degree to that observed for intensive treatment in the glycemia trial and to that reported in the FIELD photographic substudy21. In both trials, treatment effect tended to be greater in eyes with very mild NPDR at baseline. In our original paper7 we saw a non-significant interaction between fenofibrate and gender (P=0.11, unadjusted for multiplicity) with a greater benefit for men than women. We found no effect on DME or visual acuity in any of the three trials. Clinically, these findings must be considered in the context of those from the main ACCORD trial: increased mortality in the intensive glycemia treatment group (in which the target HbA1c level was <6%) and no benefit on CVD outcomes in any of the trials. However, this information adds to our knowledge of these treatments and may be helpful in the design of future studies of patients like those enrolled in ACCORD.
Supplementary Material
Acknowledgments
Dr. Faramarz Ismail-Beigi receives research grants from Novo-Nordisk.
Dr. Leiter is a consultant to AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Roche, Sanofi, Servier, and Takeda. Dr. Leiter receives research grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Roche, and Sanofi. He also serves on the speakers bureaus for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Takeda
Dr. Danis is on the board of EyeKor LLC. He is a consultant for Allergan, Galaxo-Smith-Kline, Thrombogenics (Data and safety monitoring board), Oraya, and Eli Lilly.
Supported by contracts (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute and the National Institutes of Health, with additional support from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Eye Institute, the National Institute on Aging, and the Centers for Disease Control and Prevention. General Clinical Research Centers provided support at many sites. The following companies donated study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca Pharmaceuticals, Bayer HealthCare, Closer Healthcare, GlaxoSmith-Kline Pharmaceuticals, King Pharmaceuticals, Merck, Novartis Pharmaceuticals, Novo Nordisk, Omron Healthcare, Sanofi-Aventis U.S., and Takeda Pharmaceuticals.
Footnotes
There are no conflicts of interest from the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: design and methods. Am J Cardiol. 2007;99(Suppl):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 2.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The ACCORD Study Group. Effect of intensive blood pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ACCORD Study Group. Long term Effects of Intensive Glucose Lowering on Cardiovascular Outcomes. N Engl J Med. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew EY, Ambrosius WT, Howard LT, et al. Rationale, design, and methods of the Action to Control Cardiovascular Risk in Diabetes Eye Study (ACCORD-EYE) Am J Cardiol. 2007;99(12A):103i–11i. doi: 10.1016/j.amjcard.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 7.ACCORD Study Group and ACCORD Eye Study Group. Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. N Engl J Med. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–83. [PubMed] [Google Scholar]
- 9.Gangnon R, Davis M, Hubbard L, et al. A Severity Scale for Diabetic Macular Edema (DME) Developed from ETDRS Data. Invest Ophthalmol Vis Sci. 2008;49:5041–7. doi: 10.1167/iovs.08-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner T, Sander B, Larsen M, et al. An Extension of the Early Treatment Diabetic Retinopathy Study (ETDRS) System for Grading of Diabetic Macular Edema in the Astemizole Retinopathy Trial. Current Eye Research. 2006;31:535–547. doi: 10.1080/02713680600746112. [DOI] [PubMed] [Google Scholar]
- 11.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 12.Beulens JW, Patel A, Vingerling JR, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–2036. doi: 10.1007/s00125-009-1457-x. [DOI] [PubMed] [Google Scholar]
- 13.United Kingdom Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 14.The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin dependent diabetes mellitus: the Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 15.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 16.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Reitnopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. doi: 10.1001/archopht.1989.01070010250031. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmol. 2008;115(11):1859–68. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomized controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 21.FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 22.Kohner EM, Stratton IM, Aldington SJ, Turner RC, Matthews DR. Microaneurysms in the development of diabetic retinopathy (UPKDS 42). UK Prospective Diabetes Study Group. Diabetologica. 1999;42(9):1107–12. doi: 10.1007/s001250051278. [DOI] [PubMed] [Google Scholar]
- 23.Ismail-Beigi F, Craven T, Banerji M, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.