Abstract
Objective
The objective of this article is to review the current literature on Wnt5a and its signaling mechanism, along with its role in atherosclerosis. In addition, the significance of Wnt5a as a diagnostic marker and a potential therapeutic target is reviewed. Wnt5a, a secreted glycoprotein, belongs to a family of highly conserved proteins that regulate important processes such as cell fate specification, embryonic development, cell proliferation, migration, and differentiation in a variety of organisms. The complexity of Wnt5a signaling lies in the fact that Wnt5a can bind to different classes of frizzled receptors, receptor tyrosine kinase-like orphan receptor 2, as well as co-receptors such as low density lipoprotein receptor-related protein 5/6. Wnt5a signals primarily through the non-canonical pathway, where it mediates cell proliferation, adhesion, and movement. However, the role of Wnt5a in canonical signaling is still unresolved. Depending on the receptor availability, Wnt5a can serve to activate or inhibit the canonical Wnt signaling pathway. Due to the promiscuous nature of Wnt5a, it has been extremely difficult to fully understand its signaling mechanism. Wnt5a has recently emerged as a macrophage effector molecule that triggers inflammation. Perturbations in Wnt5a signaling have been reported in several inflammatory diseases, particularly in sepsis, rheumatoid arthritis, and atherosclerosis.
Conclusion
Both existing and emerging evidence suggests that the expression of Wnt5a is always up-regulated in these, and possibly other inflammatory disorders. This knowledge can be useful for targeting Wnt5a and/or its receptor and downstream signaling molecules for therapeutic intervention in inflammatory disorders.
Keywords: Wnt5a, macrophages, inflammation, atherosclerosis, inflammatory disorders
1. Introduction
Wingless (WNT) genes code for a family of secreted, lipid-modified glycoproteins that play a critical role in normal cellular processes (i.e. cell proliferation, migration, and differentiation). Wnt proteins are also known to affect diverse developmental processes like embryonic patterning, cell fate specification and generation of cell polarity in a variety of organisms [1, 2].
Wnt proteins can associate with the extracellular matrix and different cell types; many of which can bind to multiple Frizzled (Fz) receptors, belonging to a family of G-protein-coupled transmembrane proteins [2, 3]. Some, but not all Wnt proteins require the co-receptors low-density lipoprotein receptor-related protein (LRP) 5 and LRP 6, receptor tyrosine kinase-like orphan receptor 2 (Ror2) and Ryk receptor like tyrosine kinase to mediate diverse intracellular signaling pathways [3, 4]. Wnt5a, a member of the Wnt family of proteins, was first identified in the early 1990’s [2, 5, 6], and has been shown to play distinct roles during embryogenesis, organ homeostasis, and in adult cellular processes.
In the last decade numerous studies have pointed to an important role of Wnt5a in a variety of inflammatory disorders such as rheumatoid arthritis, tuberculosis, atherosclerosis and sepsis [2, 7-11]. The exact role of Wnt5a in disease pathogenesis remains unknown; however, several studies suggest that perturbations in Wnt5a signaling could result in abnormal Wtn5a expression resulting in disruption of its normal regulatory activity. This review will discuss Wnt5a signaling and its role in various inflammatory disorders, and how it could be used as potential therapeutic target. Further, this review will also discuss how the expression pattern of Wnt5a in these disease conditions can be exploited as a diagnostic marker.
2. Wnt proteins and signaling pathways
WNT genes are highly conserved throughout the metazoans and encode 19 Wnt proteins [3]. Wnts exhibit distinct roles, as they are known to be differentially expressed during embryogenesis and among various tissues. Several studies have led to a consensus that Wnt proteins can be exclusively divided into two different groups, “canonical” and “non-canonical” Wnts, which can activate the “canonical” and “non-canonical” signaling pathways, respectively [3]. This complex signaling capability could be due to the fact that several Wnt proteins are known to bind to different Fz receptors and co-receptors such as LRP5/6, as well as receptor tyrosine kinase Ror2/Ryk [3]. Depending on the ligand-receptor complex formed, Wnt signaling can lead to transduction of either canonical or non-canonical pathways. Additionally, the Wnt ligands can also act in an autocrine and/paracrine manner in cells expressing the appropriate receptors [12].
However, both of these pathways initially involve binding of Wnt to its receptor and recruitment of disheveled (DSH) proteins, followed by activation of different downstream effector molecules, and thus affecting different cellular processes [1, 2]. Regulation of Wnt signaling by secreted antagonists such as the Wnt inhibitory Factor-1 (WIF-1) and secreted frizzled related protein (sFRP) that can directly bind to the Wnt proteins, further increases the complexity of the Wnt signaling pathway [12, 13].
2.1 Canonical Wnt signaling
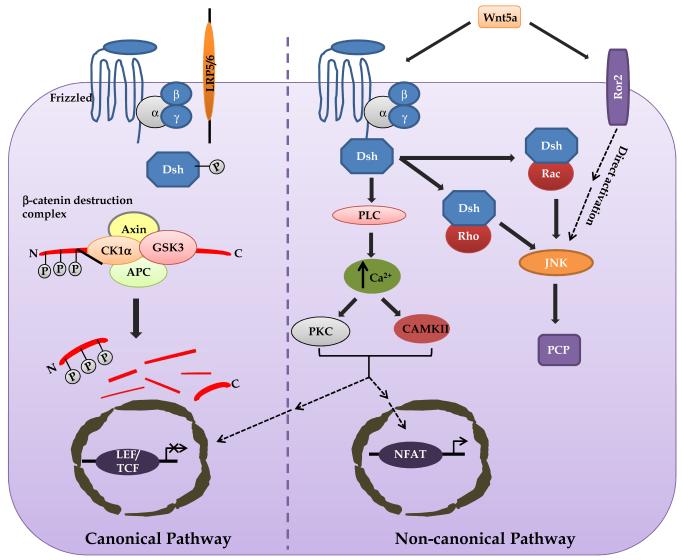
Canonical Wnt signaling induces cytosolic β-catenin stabilization and entry into the nucleus where it interacts with transcription factors to affect the transcription of various target genes. Wnt signaling through the canonical pathway, that involves β-catenin, has been extensively studied and is well understood (Fig. 1). In the absence of Wnt, cytoplasmic β-catenin is phosphorylated and ubiquitinated for proteasomal degradation by a “destruction complex” that consists of the proteins AXIN, glycogen synthase kinase 3 (GSK3), adenomatous polyposis coli (APC) and casein kinase 1α (CK1α). Interaction of canonical Wnts with Fz receptors and possibly LRP 5/6 at the cell surface leads to recruitment and activation of the DSH protein. Active DSH inhibits the “destruction complex” proteins by recruiting AXIN to the cell plasma membrane, which in turn bind to the cytoplasmic side of LRP 5/6. These events lead to decreased phosphorylation, and thus decreased proteosomal degradation of β-catenin. Increased levels of nuclear β-catenin can lead to transcriptional activation of target genes (e.g. cyclin D, cmyc) via interactions with DNA-bound transcription factors like T-cell factor/lymphoid enhancer factor [1, 3]. The canonical Wnt signaling pathway primarily modulates cell proliferation and cell fate, although it is also known to perform diverse functions throughout embryogenesis [1, 14]. In addition to its role in normal development, abnormal Wnt signaling has been linked with cancer, where it is thought to promote cancer cell proliferation and migration [15, 16].
Figure 1. Wnt signaling pathways.
In the absence of canonical Wnt, cytoplasmic β-catenin is phosphorylated by glycogen synthase kinase 3 (GSK3) and casein kinase 1α (CK1α). These form a recognition complex that recruits other components of the “destruction complex” – AXIN and adenomatous polyposis coli (APC), to ubiquitinate β-catenin for proteasomal degradation. Interaction of canonical Wnts with Frizzled receptors and LRP 5/6 at the cell surface leads to recruitment and activation of the disheveled (DSH) protein. Active DSH inhibits the “destruction complex” proteins by recruiting AXIN to the cell plasma membrane, which binds to the cytoplasmic side of receptor-related protein 5/6 (LRP 5/6) co-receptors. This inhibits the degradation complex, promotes the accumulation of β-catenin and its translocation to the nucleus. Increased levels of nuclear β-catenin leads to transcriptional activation of target genes via interaction with DNA-bound transcriptional factors like T-cell factor/lymphoid enhancer factor (TCF/LEF). The β-catenin-independent pathways are activated by non-canonical Wnt and specific combinations of Frizzled receptors. One branch of the non-canonical pathway involves the activation of small GTPases – Rho and Rac; the DSH–Rho and DSH-Rac GTPase complexes activate JUN N-terminal kinase (JNK) to regulate the cell polarity pathway (PCP). Another branch, when activated, leads to phospholipase C (PLC)-mediated increases in intracellular Ca2+ levels that further activates Ca2+/calmodulin-dependent protein kinase (CAMKII) and protein kinase C (PKC). Both of these kinases can activate the nuclear factor of activated T cells (NFAT) transcription factor to regulate gene expression. This signaling branch has been shown to mediate cell proliferation, migration and movement. Also, both CAMKII and PKC can block the canonical Wnt–β-catenin pathway by inhibiting β-catenin-dependent transcription of target genes.
2.2 Non-canonical Wnt signaling
The non-canonical Wnt signaling cascade does not involve β-catenin and the signaling is less well defined, since at least three different β-catenin-independent pathways have been shown to exist (Fig. 1). Wnt5a can activate the Wnt-Ca2+ signaling pathway to regulate convergent extension movements in Xenopus and zebrafish [17, 18]. In this pathway, non-canonical Wnts (e.g. Wnt5a) and Fz receptors (e.g. Fz-5) interact to activate DSH, which in turn stimulates phospholipase C (PLC) mediated release of intracellular Ca2+. These increased levels of Ca2+ lead to activation of either calmodulin dependent protein kinase II (CAMKII) or protein kinase C (PKC). Downstream activation events involving dephosphorylation of nuclear factor of activated T cell (NFAT) and its translocation to the nucleus leads to transcriptional modulation of target genes [3, 14, 19]. Ectopic expression of Wnt5a can stimulate intracellular Ca2+ release, increase CAMKII activity or result in translocation of PKC to the plasma membrane, depending on which receptors are present. Interestingly, the Wnt-Ca2+ pathway has also been shown to antagonize the β-catenin pathway. PKC, activated by the Wnt-Ca2+ signaling pathway blocks the canonical pathway upstream of β-catenin by phosphorylating DSH (Fig. 1). The Wnt-Ca2+ signaling pathway regulates cell movement, proliferation and migration, along with modulation of cell behavior and structural change in a variety of organisms [3, 12] [20].
The non-canonical planar cell polarity (PCP) pathway, originally identified in Drosophila, refers to a conserved pathway for uniform orientation of a common cell population along a specific axis within the epithelia. It involves activation of DSH via Wnt-receptor binding, which further activates small GTPase – Rho and Rac (Fig. 1). Active DSH can sometimes lead to the activation of Jun N-terminal kinase (JNK), instead of small GTPases (e.g. when Wnt5a binds to the receptor Ror2) [3]. Downstream interactions with other proteins lead to uniform polarization of a field of cells along the apical-basal axis. Several recent studies in vertebrates point to the importance of the PCP pathway and demonstrate its involvement in some important processes such as gastrulation and maintenance of cell orientation [3, 21].
2.3 Wnt5a – a non-canonical Wnt protein
Wnt5a, a member of the non-canonical Wnt family of proteins, has been shown to play diverse roles during normal regulatory processes. It has been reported that the WNT5a gene encodes two isoforms using alternative exons 1A and 1B and that transcription is based on multiple mechanisms such as NF-κB, TGFβ, NOTCH and Hedgehog signaling [22]. The Wnt5a protein has been shown to bind to several different receptors to elicit distinct cellular responses via non-canonical pathways. Wnt5a, like other Wnts, requires two important post-translational modifications to be fully functional. Palmitoylation and glycosylation of the Wnt5a protein are necessary for its binding to a specific receptor and for its secretion, respectively [2, 23-25]. Secreted Wnt5a protein is always lipid-modified, and therefore hydrophobic in nature. Due to this unique post-translational modification, it has become increasingly difficult to solubilize and biochemically characterize endogenous Wnt proteins in vitro.
Wnt5a is mostly associated with non-canonical Wnt signaling. For example, Wnt5a can bind to Ror2 to induce intracellular signaling and activation of the JNK pathway, simultaneously inhibiting the canonical β-catenin pathway [3, 26]. Wnt5a has also been shown to activate or block the β-catenin pathway [3, 27]. This dual signaling activity of Wnt5a could be partly due to its ability to bind to, and activate distinct receptors. Wnt5a binding to receptor Ror2 leads to inhibition of the β-catenin pathway, whereas Wnt5a binding to Fz-4 activates the β-catenin pathway [27]. Thus, Wnt5a can modulate diverse activities by signaling via different receptors.
In summary, Wnt5a mainly activates the non-canonical downstream signaling pathways that includes Wnt-Ca2+ and Wnt-PCP pathways, thereby inhibiting the canonical pathway. However, depending on the receptor availability, it can also activate the canonical pathway. Wnt-Ca2+ signaling activates CAMKII and PKC to regulate cell proliferation, migration and adhesion; whereas the PCP pathway acts via small GTPases or sometimes via JNK to regulate cell orientation. Activation of the canonical β-catenin pathway via Wnt5a is thought to regulate embryogenesis and cell fate specificity.
3. Wnt5a in atherosclerosis
Atherosclerosis, a chronic inflammatory disease, is the major risk factor in the development of cardiovascular disease, particularly coronary artery disease and stroke [28]. The pathogenesis of atherosclerosis is complex and involves interplay between a variety of cell types present in the arteries (endothelial cells and smooth muscle cells) as well as cells that migrate into the wall during the process (monocytes, lymphocytes, fibroblasts, dendritic cells) [29, 30]. The most critical step in atherogenesis is the interaction between cholesterol [specifically oxidized low density lipoproteins (ox-LDL)[31], dysfunctional endothelial cells and monocyte-derived macrophages [32]. These events result in the accumulation of macrophages/foam cells in the intima, and plaque formation which reduces the arterial diameter and finally triggers severe vascular or thrombotic events through multiple mechanisms [29, 30, 33].
The expression of Wnt5a has been previously demonstrated in endothelial cells and smooth muscle cells [34, 35]. Blumenthal et al. reported that microbial stimulation of monocyte-derived macrophages led to an increased expression of Wnt5a. Interestingly, the same group also reported that Wnt5a expression was not induced in activated lymphocytes, suggesting that activation induced expression of Wnt5a is restricted to certain cell types [8]. In order to establish more concrete evidence about the involvement of Wnt5a and its significant source, our lab is working on a signaling pathway that may play a critical role in the pathogenesis of atherosclerosis. Our group reported the expression of Wnt5a in macrophage-rich regions of both murine and human atherosclerotic lesions [9]. Wnt5a was found to be highly expressed in macrophage-rich regions of the plaques of ApoE−/− mice on a high fat diet compared to those from wild type mice on a regular diet. Similarly, Wnt5a and CD68 (a macrophage marker) were coincidentally expressed in the human atherosclerotic plaque regions. This, together with the finding that Wnt5a is upregulated in murine macrophages stimulated with lipopolysaccharide (LPS), indicates that Wnt5a is indeed a macrophage effector molecule [9].
More importantly, we found that ox-LDL a key trigger for atherogenesis can significantly induce the mRNA expression of Wnt5a in human monocyte-derived macrophages as well as in differentiated THP-1 cells [11]. In addition, we reported a correlation between the expression of Wnt5a and the histopathological severity of human atherosclerotic plaques, i.e. Wnt5a was found to be significantly higher in more advanced regions of the plaque compared to the less advanced regions. Using laser capture microdissection combined with quantitative real-time PCR, we demonstrated elevated transcripts of Wnt5a in the intima of the artery closer to the shoulder region of the plaque. Our lab was the first to show a positive correlation between Wnt5a expression and severity of atherosclerotic disease; Wnt5a expression increases as the atherosclerotic disease progresses to a much more advanced stage. Furthermore, using an ELISA method, we quantified the amount of Wnt5a in the serum of atherosclerotic and normal subjects. The results indicate that on average, the amount of Wnt5a protein present in the serum of atherosclerotic patients was significantly higher compared to that of healthy controls [36]. Recently, Wnt5a was shown to be involved in the regulation of cholesterol accumulation in mouse macrophages [37]. This finding is in line with results from our lab that show that Wnt5a modulates the expression of scavenger receptors in THP-1 cells, suggesting a role for Wnt5a during foam cell formation [38].
As mentioned previously, the expression of Wnt5a has been described in other cell types involved in the development of atherosclerosis, namely endothelial and smooth muscle cells [31]. Cheng et al. reported that Wnt5a, via non-canonical signaling, regulates endothelial cell proliferation [35]. Later, Kim et al. demonstrated that Wnt5a induces inflammation in cultured endothelial cells by up-regulation of cytokines like interleukin (IL)-8, IL-6, IL-1α etc. via the Wnt-Ca2+ signaling pathway. They also demonstrated that Wnt5a induces a robust increase in cyclooxygenase 2, an enzyme involved in pain and inflammation. Furthermore, Wnt5a enhanced endothelial cell permeability and invasiveness via extracellular matrix, clearly indicating its role in atherosclerosis [39]. Wnt5a RNA expression was first described in primary pulmonary artery smooth muscle cells (SMCs) [31] and recently, Wnt signaling has been reported to be involved in their proliferation, migration and survival via activation of both β- catenin-dependent and -independent pathways [40] [39]. Wnt5a is also involved in vascular calcification – an important event associated with the pathogenesis of atherosclerosis [41-43]. Xin H et al. [42] demonstrated that the levels of Wnt5a protein correlated with the extent of calcification in smooth muscle cell co-cultures, and that Wnt5a exerted this effect via the Wnt5a/Ror2 signaling mechanism. Moreover, vascular calcification can be inhibited by induction of Wnt5a antagonist sFRP2, suggesting that Wnt5a plays a major role in initiating arterial calcification [43].
4. Wnt5a expression in macrophages is regulated by inflammatory pathways
Inflammation is a complicated, dynamic and tightly regulated process that is part of the innate immune response. Inflammation can be induced by a variety of factors and is dependent upon interplay between different cell types in which monocyte/macrophages play a critical role [44, 45].
Depending on the types of cytokines and chemokines expressed during inflammation, macrophages show plasticity that leads to differentiation into a variety of macrophage phenotypes [46] [47] [48]. Although it is known that in a pro-inflammatory environment, macrophages differentiate into M1 or classic macrophages, while in an anti-inflammatory environment M2 or alternative phenotype macrophages predominate; the role of Wnt5a in this complicated scenario is still in debate [49]. In the last decade Wnt5a has been postulated to be a macrophage effector molecule or to act as a pro-inflammatory factor in macrophages, inducing inflammation. First, Wnt5a protein and mRNA has been described as being expressed in different inflammatory conditions such as rheumatoid arthritis, ATH, and tuberculosis. In all cases the expression of Wnt5a protein or mRNA has been associated with macrophages [8, 9, 50]. It has now become apparent that Wnt5a expression in macrophages is controlled at least by two different mechanisms. First, the up-regulation of Wnt5a in macrophages has been associated with activation of inflammatory cascades such as TLRs, IL-6, STAT3 or NF-κB pathways (Fig. 2). LPS or IFN-γ treated human or mouse macrophages show an increased expression of Wnt5a transcripts, along with other known inflammatory mediators like IL-6, IL-8, CCL2, and CCL4 [11] [9, 39, 51, 52]. In addition, LPS/IFN-γ also induced the expression of Fz-5 (an exclusive Wnt5a receptor) and CAMKII (a non-canonical downstream effector molecule) [53]. Blumenthal et al. [8] has reported that microbial stimulation of human macrophages induces the up-regulation of Wnt5a transcripts via Toll-like receptors (TLRs) and the NF-κB signaling pathway (Fig. 2). This, together with the fact that most of the TLR agonists can induce the expression of Wnt5a in human macrophages suggests that TLR signaling is an important activation mechanism for Wnt5a [8, 53]. Recently, our lab found elevated levels of TLR2 and TLR4 in advanced atherosclerotic arterial lesions, and this expression was coincident with that of Wnt5a [11, 36].
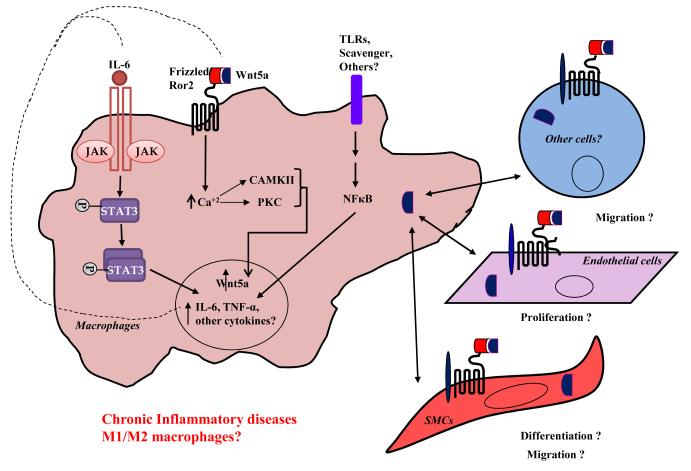
Figure 2. Working hypothesis of Wnt5a-mediated activation of macrophages that lead to inflammatory diseases.
Toll-like receptor (TLR)- or oxidized-LDL (ox-LDL)-induced activation of macrophages leads to transcription of Wnt5a via (a) NFκB signaling, (b) Ca2+/calmodulin-dependent protein kinase (CAMKII) signaling, or (c) protein kinase C (PKC)- dependent signaling pathways. Wnt5a, when secreted, can work in a paracrine manner to prime endothelial cells, smooth muscle cells, or other cell types (e.g. lymphocytes) by interacting with the frizzled or Ror2 receptors. Induced Wnt5a further mediates up-regulation of inflammatory mediators like IL-6, TNF-α through activated NFκB signaling pathway. All of these events together, result in sustained release of Wnt5a and other pro-inflammatory cytokines that cause cell migration, proliferation and differentiation, which can result in a severe inflammatory response.
Interestingly, Fujio et al. [54] demonstrated that stimulation of gp130, a subunit component of the IL-6 receptor, led to increased up-regulation of Wnt5a in cardiomyocytes. Conversely, Wnt5a expression was undetectable in cardiomyocytes overexpressing a dominant negative signal transducer and activator of transcription (STAT) 3, clearly indicating the requirement of active STAT3 for Wnt5a induction [54]. Later, several other groups reported that activated IL-6 signals via a janus kinase (JAK)-STAT3 signaling mechanism to induce the expression of Wnt5a (Fig. 2) [16, 22, 52]. Using Wnt5a integrative genomic analyses, it was shown that the NF-κB binding site is highly conserved within the Wnt5a promoter region, suggesting the involvement of the NF-κB signaling pathway in Wnt5a induction [22]. Since the JAK-STAT3/NF-κB/TLR signaling is almost always activated in inflammatory disorders (e.g. rheumatoid arthritis, atherosclerosis, etc.) it won’t be far-fetched to predict the up-regulation of Wnt5a in these, and possibly other disorders involving either of these signaling mechanisms.
Recently, it has been suggested that Wnt5a itself can trigger inflammation via signaling through macrophages. It has been reported that Wnt5a leads to JNK 1/2 phosphorylation in murine and human macrophages [55, 56]; also it was found that Wnt5a induces p38 and ERK MAPK pathways. It has also been reported that activation of THP-1 cells by Wnt5a via the non-canonical pathway involves JNK and NF-κB activation [57]. Recently, Yang L et al. [58] reported that the knockdown of Wnt5a in ApoE−/− mice could inhibit the NF-κB and MAPK signaling pathways, thereby mitigating the progression of atherosclerosis.
Moreover, other cells types like lymphocytes, endothelial cells, and smooth muscle cells that are known to express receptors for Wnt5a [8, 34], may mediate paracrine signaling upon Wnt5a-frizzled interactions to trigger an inflammatory response (Fig. 2). In this manner, the autocrine and paracrine signaling events can not only lead to increased production of Wnt5a and other inflammatory mediators, but also increased cell proliferation and migration – hallmarks of active inflammation thereby leading to severe diseased conditions. Thus, it is reasonable to conclude that Wnt5a triggers inflammation in atherosclerosis, and possibly other inflammatory diseases via signaling through macrophages.
5. Wnt5a in other inflammatory diseases
Sepsis is a systemic inflammatory disorder characterized by the presence of a known or suspected infection. Macrophages are the key players in eliciting a systemic inflammatory response that leads to more severe septic shock symptoms [10]. Using western blotting and immunohistochemistry, respectively, Periera et al. [53] demonstrated high levels of Wnt5a in the serum and bone marrow macrophages of septic patients. In an in vitro set of experiments that mimic the septic shock model, these investigators reported high expression of Wnt5a and Fz-5 in human macrophages. Additionally, Periera et al. [10, 53] established that Wnt5a secreted in this manner, can further stimulate the release of pro-inflammatory cytokines like IL-6, IL-8 and IL-1β. Wnt5a signaling via macrophages is thus critically involved in the pathogenesis of sepsis [53].
In rheumatoid arthritis (RA), a chronic inflammatory disorder that mainly targets the synovial joints, an inflammatory response of the synovium causes bone marrow-derived macrophages and synovial fibroblasts to transform and induce the expression of various inflammatory mediators [59, 60]. Sen et al [7] reported that synovial fibroblasts from synovial tissue of RA patients have increased levels of both Wnt5a and Fz-5. They also reported an increased production of IL-6 and IL-15 in normal synovial fibroblasts transfected with Wnt5a [50]. Most interestingly the inhibition of Wnt5a-Fz5 signaling by blocking antibodies or Wnt5a alone with RNA inhibition, down-regulate the expression of IL-6 and IL-15. This indicates the important role that Wnt5a signaling plays in the pathogenesis of RA [61].
In pulmonary tuberculosis – a granulomatous inflammatory disorder in humans, caused by Mycobacterium tuberculosis – macrophages, lymphocytes and fibroblasts initiate the inflammatory response that eventually leads to an infection, primarily in the lungs. It was reported an increased expression of Wnt5a and Fz-5 in the granulomatous lesions of pulmonary tuberculosis patients. Activated human macrophages, and not lymphocytes, show increased expression of both Wnt5 and Fz-5 [8]. This data confirms previous findings that Wnt5a expression is more pronounced in monocytes/macrophages, the primary inflammatory cell type.
Psoriasis vulgaris is one of the most common chronic inflammatory diseases of the skin that involves neutrophils, dendritic cells, subsets of T lymphocytes, epidermal keratinocytes and macrophages. Various cytokines (e.g. TNF-α, IL-1, IL-6) and chemokines (e.g. IL-8) have been implicated in the pathogenesis of psoriasis [62, 63]. Microarray analysis of several Wnt genes in psoriatic skin lesions show increased expression of Wnt5a. In fact, Wnt5a was the only protein to be selectively expressed in the psoriatic epidermis when compared to other Wnt genes [63]. Later, Romanowska et al. [64] reported significantly higher levels of Wnt5a in keratinocytes derived from psoriasis patients when compared to those derived from healthy controls. Additionally, both Wnt5a and Fz-5 were found to be highly up-regulated in human psoriatic skin lesions. Wnt5a expression was more pronounced around regions containing the inflamed neutrophil aggregates, activated dermal fibroblasts and endothelial cells. Moreover, this expression was highly compartmentalized in the basal epidermal layer of normal adult skin [64]. This marked difference in the expression pattern of Wnt5a and its receptor may be one of the mechanisms contributing to the chronic inflammatory state observed in psoriasis. However, further studies are required to clearly establish the role of Wnt5a in the pathogenesis of psoriasis.
Recently, the role of Wnt5a has also been studied in diabetes and obesity. The expression of Wnt5a has been reported in adipose tissue macrophages and in CD14 + monocytes from obese and type 2 diabetic subjects. Although the role of Wnt5a in low-grade inflammation remains under debate, in vitro studies show that Wnt5a plays a role in regulating adipogenesis [65].
Significant progress has been made in investigating the role of Wnt5a in various inflammatory disorders (e.g. RA, sepsis etc.) [8, 50, 53, 61]. The expression of Wnt5a has been reported in atherosclerotic lesions [9, 11, 36]; however, the mechanism of Wnt5a signaling, and its exact biological role in the pathogenesis of atherosclerosis is poorly understood. It would be of great value to understand and interpret the function(s) of Wnt5a in the inflammatory diseases, and apply them in context to atherosclerosis. Several studies related to inflammatory disorders have associated Wnt5a with macrophages, and based on these and our findings, it is fair to deduct that Wnt5a is involved in different stages of atherosclerosis [10, 11, 36]. In the early stages of atherosclerosis, inflammation of the endothelial wall can activate the endothelial cells to secret Wnt5a, and this Wnt5a could play an important role in the recruitment of monocytes to the site of injury [39]. Additionally, based on the recently reported role of Wnt5a in cholesterol accumulation, it is possible that Wnt5a may contribute in the formation of foam cells [37, 66]. During acute events or complications developed in vulnerable atherosclerotic plaques, Wnt5a may play a role as an inflammatory regulator by modulating the expression of biological mediators like IL-6, IL-1β, cyclooxygenase-2 etc. [39, 53]. Although further studies are clearly required to warrant these interpretations, it is plausible to state that Wnt5a is involved in almost all the aspects of the pathogenesis of atherosclerosis.
6. Future directions
Wnt5a can bind to different Fz and non-Fz receptors, depending on receptor availability. The ligand-receptor pair formed can affect various cellular processes by signaling primarily via the non-canonical pathway, although some reports point out that Wnt5a can also function to signal via the canonical pathway. Several intense studies need to be performed in order to more fully characterize Wnt5a signaling mechanisms.
Considerable data supports the fact that increased expression of Wnt5a and aberrant Wnt5a signaling may be a common theme in various inflammatory disorders [8-10, 61]. This unique expression pattern of Wnt5a could be exploited in the diagnostic identification of RA, atherosclerosis, sepsis etc. Due to its hydrophobic nature, it is difficult to conclusively detect Wnt5a in biological samples. Nevertheless, development of newer techniques has made it possible to isolate and detect this promiscuous protein in the serum of diseased patients. Recently, we developed a sandwich ELISA for the detection of recombinant Wnt5a [67] and used it to successfully detect Wnt5a in the serum of patients with atherosclerotic disease. We found that Wnt5a levels were significantly higher on patients with atherosclerotic disease than those from healthy controls [36]. Additionally, using immunoprecipitation, Periera et al. [53] demonstrated increased expression of Wnt5a in the serum of patients with sepsis. Based on these facts, it is not long before Wnt5a can indeed be used as a complementary prognostic marker for some, if not all inflammatory disorders.
Due to the varied role of Wnt5a in context to receptor binding, it is difficult to target for therapeutic intervention. But there have been some successful in vitro trials to block Wnt5a expression and/or Wnt5a signaling. Sen et al. [50]demonstrated increased expression of Wnt5a and pro-inflammatory cytokines in RA synovial fibroblast. Using siRNA directed against Wnt5a, they were successfully able to decrease the expression of Wnt5a and pro-inflammatory cytokines produced via Wnt5a signaling. Pereira et al. [53]used a similar approach to attenuate the increased expression of pro-inflammatory cytokines in an in vitro septic shock model. Importantly, Yang L et al. [58] recently reported that the knockdown of Wnt5a using the siRNA approach reduced the progression of atherosclerosis by inhibiting the NF-κB and MAPK signaling pathways. This study also demonstrated that Wnt5a silencing downregulated the expression of important inflammatory mediators in atherosclerotic ApoE−/− mice, thereby reducing the severity of the disease [58]. The use of siRNA as a therapeutic approach therefore looks promising, and more so, since the technology for siRNA delivery is continuously improving.
Since knowledge regarding Wnt5a downstream signaling mechanisms remains limited, another promising strategy could be the use of blocking antibodies targeted against transmembrane Fz receptors. Wnt5a is known to bind several receptors, some of which are Fz-3,-4,-5, Ror2 etc. The Fz proteins are typical G protein-coupled receptors, and can be easily manipulated pharmacologically. The added advantage of targeting extracellular Wnt5a receptors is that they are readily accessible for binding either designer antibodies or small molecule inhibitors. In the aforementioned study on Wnt5a signaling in RA, the investigators, using anti-Fz5 antibodies, were able to inhibit the levels of pro-inflammatory cytokines [50]. In a different approach, Pereira et al. used a soluble frizzled-related peptide 1, a soluble Wnt5a binding protein that specifically binds to and inhibits its interaction with the cell surface Fz receptor[10, 53]. However, the use of blocking antibodies or small molecule inhibitors in models of inflammatory disorders is limited due to incomplete knowledge regarding the specificities of Wnt5a-receptor pairing, and receptor availability. Although all these approaches seem to be technically feasible, several large-scale studies need to be performed to predict and minimize the potential adverse effects of targeted therapy.
Another issue to consider is the macrophage heterogeneity with respect to differential Wnt5a expression/signaling [68, 69]. Recently, the atherosclerotic plaque has been shown to contain two different phenotypes of macrophages – M1 and M2 [70]. Also, oxLDL has been shown to affect macrophage polarization towards the M1 or M2 phenotype [69, 71]. However, literature points towards a contradictory relationship between Wnt5a and these macrophage phenotypes. Some studies suggest that activated M1 macrophages are the highest source of Wnt5a, but others suggest that M2 macrophages, after activation can also secret Wnt5a. Our group is currently working on the expression of Wnt5a in association with macrophage polarization. Finally, considering that (a) Wnt5a can be secreted by different cell types (e.g. macrophages, endothelial cells, smooth muscle cells, etc.) [9] [35] [40] [39] , (b) Wnt5a works in an autocrine and paracrine manner, and (c) Wnts are tightly regulated and have complex signaling mechanisms, the analysis of the role of Wnt5a in the pathogenesis of atherosclerosis becomes more challenging.
In summary, Wnt5a signaling pathways are one of the most important and highly regulated signaling pathways in vertebrates. It involves interplay of distinct cell surface receptors and downstream effectors that lead to varied responses. Some of these responses regulate major developmental processes as well as cell fate specificity, patterning, proliferation and migration of various cell lineages. Dysfunctional regulation of Wnt5a signaling has been implicated in a wide range of diseases, including inflammatory disorders. While significant progress has been made in understanding Wnt5a signaling and its expression pattern in inflammatory diseases, more extensive studies are needed to decipher the exact role of Wnt5a in disease pathogenesis. These studies will help identify novel targets for therapeutic intervention along with predictive biomarkers for targeted therapy in inflammatory disorders.
Highlights.
We continued to explore the hypothesis that Wnt5a plays a role in atherosclerosis.
Perturbations in Wnt5a signaling have been reported in several inflammatory diseases.
We review current literature on Wnt5a signaling and its role in inflammation.
Wnt5a has emerged as a macrophage effector molecule that triggers inflammation.
We discuss the significance of Wnt5a as diagnostic marker and potential therapeutic target.
Acknowledgments
We are grateful to Dr. Kelly McCall for her critical comments and discussions on this manuscript. This work was supported by National Institute of Health (Grant 1R15HL092545 to R.M.).
Abbreviations
- Fz
Frizzled
- LRP
lipoprotein receptor-related protein
- Ror2
receptor tyrosine kinase-like orphan receptor
- DSH
disheveled
- CaMKII
calmodulin dependent protein kinase II
- PKC
protein kinase C
- NFAT
nuclear factor of activated T cell
- PCP
planar cell polarity
- JNK
Jun N-terminal kinase
- RA
rheumatoid arthritis
- IL
interleukin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF, et al. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavin BJ, McMahon JA, McMahon AP. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev. 1990;4:2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- 6.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 7.Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 9.Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 10.Pereira CP, Bachli EB, Schoedon G. The wnt pathway: a macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep. 2009;11:236–242. doi: 10.1007/s11883-009-0036-4. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt PMLC, House DL, Keller CM, Kohn LD, Silver MJ, McCall KD, et al. Increased Wnt5a mRNA Expression in Advanced Atherosclerotic Lesions, and Oxidized LDL Treated Human Monocyte-Derived Macrophages. The Open Circ and Vasc J. 2012;5:1–7. doi: 10.2174/1877382601205010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 14.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 15.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br. J Cancer. 2009;101:209–214. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCall KD, Harii N, Lewis CJ, Malgor R, Kim WB, Saji M, et al. High basal levels of functional toll-like receptor 3 (TLR3) and noncanonical Wnt5a are expressed in papillary thyroid cancer and are coordinately decreased by phenylmethimazole together with cell proliferation and migration. Endocrinology. 2007;148:4226–4237. doi: 10.1210/en.2007-0459. [DOI] [PubMed] [Google Scholar]
- 17.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Develop. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 18.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 19.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech Develop. 2001;106:61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 21.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nature Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 22.Katoh M, Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med. 2009;23:763–769. doi: 10.3892/ijmm_00000190. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol. 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 24.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 25.Smolich BD, McMahon JA, McMahon AP, Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell. 1993;4:1267–1275. doi: 10.1091/mbc.4.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 27.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:570–582. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 30.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 31.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Bioph Res Co. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Bioph Res Co. 2008;365:285–290. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- 36.Malgor R, Bhatt PM, Connolly BA, Jacoby DL, Feldmann KJ, Silver MJ, et al. Wnt5a, TLR2 and TLR4 are elevated in advanced human atherosclerotic lesions. Inflam Res. 2014;63:277–285. doi: 10.1007/s00011-013-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin L, Hu R, Zhu N, Yao HL, Lei XY, Li SX, et al. The Novel Role and Underlying Mechanism of Wnt5a in Regulating Cellular Cholesterol Accumulation. Clin Exp Pharmacol Physiol. 2014 doi: 10.1111/1440-1681.12258. [DOI] [PubMed] [Google Scholar]
- 38.Szymanski C, Noriko Kantake N, Kelly McCall K, Malgor R. Expression of Wnt5a and its effect on oxLDL uptake in THP-1 cells. FASEB J. 2014:LB534. [Google Scholar]
- 39.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 40.Mill C, George SJ. Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc Res. 2012;95:233–240. doi: 10.1093/cvr/cvs141. [DOI] [PubMed] [Google Scholar]
- 41.Doherty TM, Asotra K, Fitzpatrick LA, Qiao J-H, Wilkin DJ, Detrano RC, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci. 2003;100(20):11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin H, Xin F, Zhou S, Guan S. The Wnt5a/Ror2 pathway is associated with determination of the differentiation fate of bone marrow mesenchymal stem cells in vascular calcification. Int J Mol Med. 2013;31(3):583–588. doi: 10.3892/ijmm.2013.1242. [DOI] [PubMed] [Google Scholar]
- 43.Woldt E, Terrand J, Mlih M, Matz RL, Bruban V, et al. The nuclear hormone receptor PPARgamma counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012;3:1077. doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 45.Gui T, Shimokado A, Sun Y, Akasaka T, Muragaki Y. Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediators Inflamm. 2012;2012:693083. doi: 10.1155/2012/693083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 47.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon S. Alternative activation of macrophages. Nature Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 49.Bergenfelz C, Medrek C, Ekstrom E, Jirstrom K, Janols H, Wullt M, et al. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol. 2012;188:5448–5458. doi: 10.4049/jimmunol.1103378. [DOI] [PubMed] [Google Scholar]
- 50.Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh MC, Collins GD, Vandanmagsar B, Patel K, Brill M, Carter A, et al. Activation of Wnt5A signaling is required for CXC chemokine ligand 12-mediated T-cell migration. Blood. 2009;114:1366–1373. doi: 10.1182/blood-2008-08-175869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katoh M, Katoh M. STAT3-induced WNT5A signaling loop in embryonic stem cells, adult normal tissues, chronic persistent inflammation, rheumatoid arthritis and cancer. Int J Mol Med. 2007;19:273–278. [PubMed] [Google Scholar]
- 53.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28(3):504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 54.Fujio Y, Matsuda T, Oshima Y, Maeda M, Mohri T, Ito T, et al. Signals through gp130 upregulate Wnt5a and contribute to cell adhesion in cardiac myocytes. FEBS letters. 2004;573:202–206. doi: 10.1016/j.febslet.2004.07.082. [DOI] [PubMed] [Google Scholar]
- 55.Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol. 2011;90:553–559. doi: 10.1016/j.ejcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454–5459. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J, Chang W, Jung Y, Song K, Lee I. Wnt5a activates THP-1 monocytic cells via a beta-catenin-independent pathway involving JNK and NF-kappaB activation. Cytokine. 2012;60:242–248. doi: 10.1016/j.cyto.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Yang L, Chu Y, Wang Y, Zhao X, Xu W, Zhang P, et al. siRNA-mediated silencing of Wnt5a regulates inflammatory responses in atherosclerosis through the MAPK/NF-kappaB pathways. Int J Mol Med. 2014 Jul 22; doi: 10.3892/ijmm.2014.1860. doi:10.3892/ijmm.2014.1890. [DOI] [PubMed] [Google Scholar]
- 59.Karouzakis E, Neidhart M, Gay RE, Gay S. Molecular and cellular basis of rheumatoid joint destruction. Immunol letters. 2006;106:8–13. doi: 10.1016/j.imlet.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 60.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 61.Sen M, Carson DA. Wnt signaling in rheumatoid synoviocyte activation. Mod Rheumatol. 2002;12:5–9. doi: 10.3109/s101650200001. [DOI] [PubMed] [Google Scholar]
- 62.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 63.Reischl J, Schwenke S, Beekman JM, Mrowietz U, Sturzebecher S, Heubach JF. Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol. 2007;127:163–169. doi: 10.1038/sj.jid.5700488. [DOI] [PubMed] [Google Scholar]
- 64.Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, et al. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PloS one. 2009;4:e5354. doi: 10.1371/journal.pone.0005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bilkovski R, Schulte DM, Oberhauser F, Mauer J, Hampel B, Gutschow C, et al. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int J Obesity. 2011;35:1450–1454. doi: 10.1038/ijo.2011.6. [DOI] [PubMed] [Google Scholar]
- 66.Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, et al. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009;284(1):381–8. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kummitha CM, Mayle KM, Christman MA, 2nd, Deosarkar SP, Schwartz AL, McCall KD, et al. A sandwich ELISA for the detection of Wnt5a. J Immunol Methods. 2010;352:38–44. doi: 10.1016/j.jim.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis. 2011;214:345–349. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 71.Hirose K, Iwabuchi K, Shimada K, Kiyanagi T, Iwahara C, Nakayama H, et al. Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis. 2011;10:1. doi: 10.1186/1476-511X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]