Abstract
Dietary therapy has been used to treat many individuals with epilepsy that are refractory to anti-epileptic drugs. The mechanisms for how dietary therapy confers seizure protection are currently not well understood. We evaluated the acute effects of glucose and β-hydroxybutyrate (the major circulating ketone body) in conferring seizure protection to the EL mouse, a model of multifactorial idiopathic generalized epilepsy. EL mice were fed either a standard diet unrestricted or a calorie-restricted standard diet to achieve a body weight reduction of 20–23%. D-glucose, 2-deoxy-D-glucose, and β-hydroxybutyrate were supplemented in the drinking water of calorie-restricted mice for 2.5 hours prior to seizure testing to simulate the effect of increased glucose availability, decreased glucose utilization, and increased ketone availability, respectively. Seizure susceptibility, body weight, plasma glucose and β-hydroxybutyrate were measured over a nine-week treatment period. Additionally, excitatory and inhibitory amino acids were measured in the brains of mice using 1H NMR. Glutamate decarboxylase activity was also measured to evaluate the connection between dietary therapy and brain metabolism. We found that lowering of glucose utilization is necessary to confer seizure protection with long-term (>4 weeks) calorie restriction, whereas increased ketone availability did not affect seizure susceptibility. In the absence of long-term calorie restriction, however, reduced glucose utilization and increased ketone availability did not affect seizure susceptibility. Brain excitatory and inhibitory amino acid content did not change with treatment, and glutamate decarboxylase activity was not associated with seizure susceptibility. We demonstrate that reduced glucose utilization is necessary to confer seizure protection under long-term calorie restriction in EL mice, while acute ketone supplementation did not confer seizure protection. Further studies are needed to uncover the mechanisms by which glucose utilization influences seizure susceptibility.
Keywords: calorie restriction, ketone bodies, epilepsy, 2-deoxy-D-glucose (2-DG), ketogenic diet
1. Introduction
Dietary therapy, specifically the ketogenic diet, has been used to successfully manage seizures in individuals that are refractory to anti-epileptic drug (AED) therapy [1]. Despite the success of the ketogenic diet for individuals who are refractory to AED therapy, many individuals on the ketogenic diet do not have their epilepsy fully controlled. Consequently, alternative dietary therapies are suggested to control seizures, including calorie restriction [2–4]. Evidence from animal models demonstrates that calorie restriction is anticonvulsant and antiepileptic [5–7].
Calorie restriction and ketogenic diets result in similar physiological changes, namely an increase in circulating ketone bodies. Calorie restriction results in lowered circulating glucose, while the ketogenic diet may lower circulating glucose, depending on the amount of calories consumed [5, 8, 9]. While calorie restriction and the ketogenic diet are hypothesized to have similar mechanisms in conferring seizure protection, preclinical evidence suggests that these mechanisms are not the same [10]. Additionally, while changes in circulating glucose and ketone bodies are associated with changes in seizure susceptibility, the influence of circulating glucose and ketone bodies on seizure susceptibility and brain metabolism are not well understood [11, 12]. Understanding the role that glucose and ketone bodies have in conferring seizure protection can aid in designing and tailoring effective dietary therapies for individual patients or lead to pharmacological therapy that mimics dietary therapy.
Our goal was to evaluate the acute roles of glucose and β-hydroxybutyrate (the major circulating ketone body) in conferring seizure protection to the EL mouse, a model of multifactorial idiopathic generalized epilepsy [13–15]. Seizures in EL mice can be managed through AED and dietary therapy, which makes the EL mouse an ideal model to study the mechanisms through how seizure protection is conferred [5, 6, 16, 17]. We previously found that EL mice given a calorie-restricted ketogenic diet had reduced seizure susceptibility, but that subsequent acute supplementation of glucose resulted in significantly increased seizure susceptibility [18]. We confirmed these results using a calorie-restricted standard mouse chow diet, and additionally analyzed the acute effect of lowering glucose utilization or increasing β-hydroxybutyrate availability on seizure susceptibility. Since calorie restriction chronically lowers glucose utilization, we simulated acute increased glucose utilization by supplementing glucose in the drinking water of calorie-restricted mice immediately before seizure testing. We simulated acute low glucose utilization conditions by using acute supplementation of the glycolytic inhibitor 2-deoxy-D-glucose (2-DG), a proconvulsant and anticonvulsant compound [19]. Additionally, we supplemented β-hydroxybutyrate in the drinking water of the mice to simulate increased ketone body availability. We found that in conjunction with long-term calorie restriction (>4 weeks), seizure protection in the EL mouse was linked to reduction of glucose utilization. Acute supplementation of β-hydroxybutyrate, in conjunction with long-term calorie restriction, did not increase seizure protection in the EL mouse. In the absence of long-term calorie restriction, neither acutely lowering glucose utilization nor supplementing β-hydroxybutyrate conferred seizure protection.
2. Materials and Methods
2.1 Mice
The inbred, epilepsy-prone EL mice were originally obtained from J. Suzuki (Tokyo Institute of Psychiatry) and maintained in the Boston College Animal Care Facility. The mice were group housed (2–4 mice per cage) and kept on a 12-hour light/dark cycle with lights on at 6 am and lights off at 6 pm. All mice were tested at 10 am and fed at 11 am. Mice were assigned cage mates by matching body weights during the pre-trial period to minimize calorie intake differences. Mice had free access to weighed food, measured water, and their cage mates throughout the study. Females were used for these studies, as adult males die sporadically with age from acute uremia poisoning due to urinary blockage and retention [20]. Seizures in EL mice commence with sexual maturity, at approximately 60–75 days of age [6, 13]. The procedures for animal use were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Boston College Institutional Animal Care and Use Committee.
2.2 Seizure Susceptibility, Seizure Testing, and Seizure Phenotype
The seizure susceptibility paradigm for testing EL mice, along with the seizure phenotypes, has been previously described [5, 13]. Briefly, mice were tested for seizures once per week through a tail suspension paradigm. Mice were characterized as seizure-susceptible if they experienced a generalized seizure. A generalized seizure in the EL mouse, which has been electroencephalographically validated, is characterized by loss of consciousness, loss of postural equilibrium, and head and limb clonus, which may also be accompanied by vocalization and incontinence [6, 13, 14]. An erect-forward arching Straub tail, which indicates spinal cord activation, is also observed in EL mice having generalized seizures. EL mice that displayed vocalization and twitching without full progression to a generalized seizure were designated as not seizure susceptible [13]. Mice experiencing a generalized seizure during the weekly testing paradigm were assigned a seizure susceptibility score of 1, whereas mice not experiencing a generalized seizure were assigned a seizure susceptibility score of 0. The seizure susceptibility score for each mouse was averaged over multiple tests and the mean seizure susceptibility score for each group was determined.
2.3 Diet
All mice were fed with standard rodent chow ad libitum during the pre-trial period (Prolab RMH 3000; PMI LabDiet, Richmond, IN, USA). This is the standard mouse pellet diet, which contains 4.2 kcal/g of total energy. According to manufacturer specifications, this diet is comprised of 520 g carbohydrates, 120 g fat, 225 g protein, and 45 g fiber per 1 kg of food. During the study phase, SD-UR mice received rodent chow ad libitum, whereas all SD-R groups were calorie restricted on rodent chow to reduce mouse body weights by 20–23%. Food was weighed and administered daily. Water was provided ad libitum to all mice throughout the study. Food and water intake was tracked for each cage and averaged to calculate individual intake. During the study phase, 2.5 hours prior to seizure testing, water bottles in each cage were changed to contain a measured amount of water and either water alone, water plus 25 mM D-glucose (Sigma Aldrich, St. Louis, MO), water plus 25 mM D-glucose and 8 mM 2-deoxy-D-glucose (2-DG) (Sigma Aldrich), or water plus 25 mM D-glucose and 50 mM β-hydroxybutyrate (bOHB) (Sigma Aldrich). Immediately before seizure testing, all water bottles were changed back to water alone.
2.4 Pre-Trial Period
The pre-trial period began when the mice were 40 ± 1 days of age and lasted for seven weeks. During this time, all mice were group-housed and fed ad libitum. They were tested for seizure susceptibility once per week. Only mice that had demonstrated at least one generalized seizure during the pre-trial period entered the study. The mean number of seizures that mice experienced before beginning the study was 2.57 ± 0.13 (Standard Error of the Mean).
2.5 Treatment Period
After the 7-week pre-trial period, the mice were placed into five groups (n = 13–15 mice/group). All mice were then fasted for 16 hours to establish a similar metabolic state at the start of the experiment. The mice in each group were assigned to one of five dietary conditions:
-
1)
Standard diet (SD) fed ad libitum with water alone prior to seizure testing (SD-UR),
-
2)
SD restricted to achieve a 20–23% body weight reduction with water alone prior to seizure testing (SD-R),
-
3)
SD restricted to achieve a 20–23% body weight reduction with water plus 25 mM glucose prior to seizure testing (SD-R[Glu]),
-
4)
SD restricted to achieve a 20–23% body weight reduction with water plus 25 mM glucose and 8 mM 2-DG prior to seizure testing (SD-R[Glu][2-DG]),
-
5)
SD restricted to achieve a 20–23% body weight reduction with water plus 25 mM glucose and 50 mM bOHB prior to seizure testing (SDR[Glu][bOHB]).
The treatment period lasted for a total of 10 weeks, and included weekly seizure testing through 9 weeks.
2.6 Collection of Plasma and Brain
Blood was collected at the end of the pre-trial phase (1 hr after seizure testing) and on week 9 (1 hr after seizure testing) through submandibular bleeding into heparinized tubes. Plasma was collected by centrifuging blood at 3,000 × g for 10 minutes at 4°C and stored at −80°C until analysis. Whole brain was collected one week after the end of seizure testing (week 10) through snap freezing in liquid nitrogen. The whole brain collection procedure occurred immediately after a 2.5 hr administration of water from each respective group to mimic the metabolic state of the mouse during the seizure-testing paradigm.
2.7 Measurement of plasma glucose and β-hydroxybutyrate
Glucose was measured spectrophotometrically using the Trinder Assay (StanBio, Boerne, TX). β-hydroxybutyrate was measured enzymatically using a modification of the Williamson et al. procedure [21].
2.8 Measurement of brain hexokinase activity
Hexokinase activity was measured from the cerebrum using a hexokinase colorimetric assay kit (Sigma Aldrich). Hexokinase activity was standardized to protein levels using the Bio-Rad protein assay (Hercules, CA).
2.9 Metabolite Extraction and 1H-NMR Analysis
Whole brain samples were extracted with ice-cold 2:1 methanol:chloroform. Samples were centrifuged at 8,000 × g for 20 minutes at 4°C and the supernatant was collected. Extraction was performed one more time on the pellets and the supernatants were pooled. Samples were dried down under a stream of nitrogen and lyophilized. The samples were rehydrated in deuterium oxide (D2O) for 1H NMR analysis.
1H NMR spectra of the extracts were obtained on a Varian VNMRS 600 spectrometer at 599.688 MHz at 25°C using a 9,615.4 Hz sweep width, 32,768 data points collected, and a 1.0 s relaxation delay. 64 transients were collected and the resulting free induction decay was processed with a line broadening of 0.5 Hz. Metabolite ratios for lactate, N-acetylasparate (NAA), γ-aminobutyric acid (GABA), glutamate, aspartate, and taurine were calculated by integrating the selected reference peaks (Figure 1) [22]. The area under the curve for each reference peak was standardized to the area under the curve for phosphocreatine plus creatine.
Figure 1.
Representative 1H NMR spectra from an EL mouse brain obtained at 600 MHz. Reference peaks correspond to the following metabolites: 1, lactate; 2, N-acetylaspartate (NAA); 3, γ-aminobutyric acid (GABA); 4, glutamate; 5, aspartate; 6, phosphocreatine plus creatine; 7, taurine [22].
2.10 Measurement of Glutamate Decarboxylase Activity
Glutamate decarboxylase (GAD) activity was measured fluorescently with an NADPH-coupled assay, according to the methods of Wolf and Klemisch [23]. All reagents for this assay were obtained from Sigma Aldrich. Briefly, frozen whole brain tissue was homogenized in buffer (0.1 M sodium phosphate buffer with 1 mM 2-aminoethylisothironium bromide, 0.1% Triton X-100, and 20 µM pyridoxal 5’-phosphate; pH 7.0) and centrifuged at 5,000 × g for 30 minutes at 4°C. The supernatant was collected and mixed with buffer containing glutamate (0.1 M sodium phosphate buffer with 50 mM L-glutamate, 250 µM pyridoxal 5’-phosphate, and 0.4% beta-mercaptoethanol; pH 7.0) to drive the GAD reaction for 15 minutes at 37°C. The reaction was stopped with the addition of 0.25 N HCl. Samples were placed in a dry bath at 100°C for 5 minutes to destroy endogenous NADPH. Samples were mixed in a buffer with GABase (0.3 M Tris buffer with 6 mM alpha-ketoglutarate, 0.1 M NADP+, 6 mM beta-mercaptoethanol, and 10 U GABase; pH 8.4) for 15 minutes at 37°C to drive the indicator reaction to completion. NADPH is formed in an equimolar ratio with GABA formation, and NADPH was measured fluorescently with an excitation wavelength of 340 nm and an emission wavelength of 460 nm.
2.11 Statistical Analysis
All statistical analyses were performed using SPSS Software (IBM SPSS Statistics, Version 21). One-way ANOVA was used to evaluate any significant differences in body weight, seizure susceptibility, plasma glucose, plasma β-hydroxybutyrate levels, brain metabolites, and GAD activity between treatment groups. Differences were considered significant at p < 0.05. All values are presented as mean ± standard error of the mean (SEM).
3. Results
3.1 Influence of Treatments on Body Weight
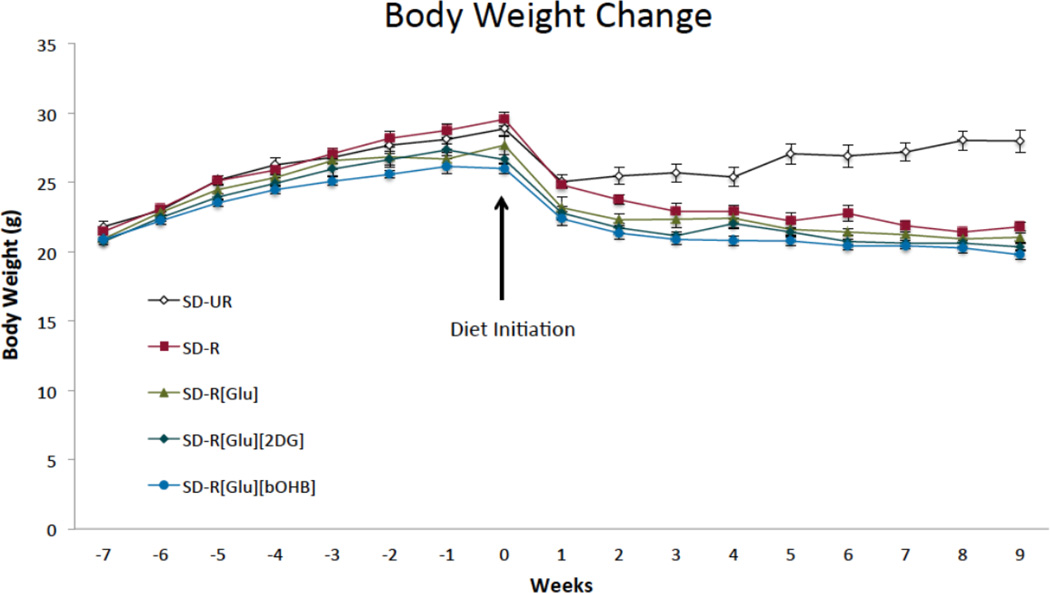
The average body weight at the start of the treatment period was 27.7 ± 0.3 g. The treatments, with the exception of SD-UR, lowered body weights by 20–23%, and did not lead to any statistical difference in body weights for the calorie restricted groups (Figure 2).
Figure 2.
Effect of dietary treatment on body weight. At week 0, dietary treatment was initiated. From weeks 2–9, body weights in the SD-UR group were significantly higher than all SD-R groups (p < 0.01). Values are displayed as mean ± SEM (n = 13–15 mice/group).
3.2 Water Intake and Drug Dosages
Water intake during the 2.5 hr treatment period was similar across all groups (1.59 ± 0.07 mL). The mean glucose intake in the groups receiving glucose was similar at 7.62 ± 0.52 mg. This was a dose of 375 mg/kg for glucose. Mean 2-DG intake was 2.01 ± 0.19 mg for the SD-R[Glu][2DG] group and mean bOHB intake was 9.52 ± 0.07 mg for the SD-R[Glu][bOHB] group. The doses for 2-DG and bOHB were 100 mg/kg and 475 mg/kg, respectively. The addition of 2-DG decreased brain hexokinase activity in the SD-R[Glu][2-DG] group (3.5 ± 1.2 nM/min/mg protein) compared to all other groups combined (12.5 ± 2.5 nM/min/mg protein).
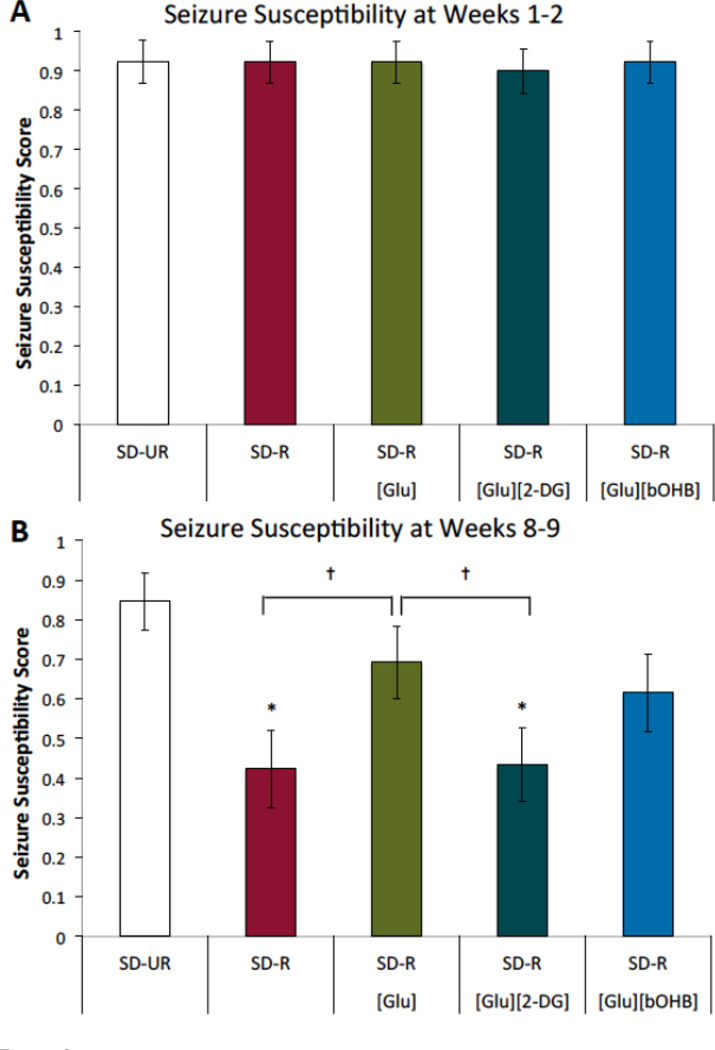
3.3 Influence of Treatments on Seizure Susceptibility
In the absence of long-term calorie restriction, the acute treatments of glucose, glucose plus 2-DG, and glucose plus bOHB did not affect seizure susceptibility (Figure 3A). With long-term calorie restriction, however, treatment differences were observed (Figure 3B). The SD-UR group displayed high seizure susceptibility, which was reduced through calorie restriction, as seen in the SD-R group. The addition of glucose to the drinking water of the mice for 2.5 hrs prior to seizure testing increased seizure susceptibility. The increase in seizure susceptibility was blocked by the addition of 2-DG to the drinking water. Adding 50 mM bOHB to the drinking water, which was the highest concentration of bOHB in water that EL mice could tolerate before restricting water intake, did not ameliorate the effects of 25 mM glucose on seizure susceptibility.
Figure 3.
Seizure susceptibility in EL mice with dietary treatment. (A) Seizure susceptibility for weeks 1–2. Seizure susceptibility scores were pooled for weeks 1–2 to assess the effect of acute glucose, glucose plus 2-DG, and glucose plus bOHB treatment in the absence of long-term calorie restriction. Seizure susceptibility was similar across all groups. (B) Seizure susceptibility for weeks 8–9. Seizure susceptibility scores were pooled for weeks 8–9, as the seizure susceptibilities were consistent within groups at the study end-point. Seizure susceptibility was reduced in the SD-R group, and the effect of calorie restriction was blocked by the addition of 25 mM D-glucose in the drinking water prior to testing (SD-R[Glu]). The addition of 8 mM 2-DG to the 25 mM D-glucose in the drinking water (SD-R[Glu][2-DG]) significantly lowered seizure susceptibility and returned seizure susceptibility levels to that of SD-R. 50mM bOHB did not ameliorate the effect of glucose on seizure susceptibility (SD-R[Glu][bOHB]). Values are displayed as mean ± SEM (n = 13–15 mice/group). *p < 0.01 compared to the SD-UR group; †p < 0.05.
3.4 Influence of Treatments on Plasma glucose and β-hydroxybutyrate levels
At the beginning of the study, all mice had similar plasma glucose (10.8 ± 0.1 mM) and bOHB levels (0.40 ± 0.02 mM). The plasma glucose levels after 9 weeks of treatment were significantly lower in all SD-R groups, compared to SD-UR (Figure 4A). Plasma bOHB levels were significantly higher in all SD-R groups, compared to SD-UR (Figure 4B). The addition of glucose, 2-DG, and bOHB to the drinking water did not alter plasma glucose and bOHB levels across SD-R groups.
Figure 4.
Influence of dietary treatment on plasma glucose and β-hydroxybutyrate levels in EL mice after 9 weeks of treatment. (A) Plasma glucose levels were significantly reduced in all of the SD-R groups. (B) Plasma β-hydroxybutyrate levels were significantly raised in all of the SD-R groups. Values are displayed as mean ± SEM (n = 13–15 mice/group). *p < 0.01.
3.5 Influence of Treatments on Brain Metabolites
The brain metabolites of lactate, NAA, GABA, glutamate, aspartate, and taurine were measured using 1H NMR spectroscopy. The excitatory amino acids, glutamate and aspartate, along with the inhibitory amino acids, GABA and taurine, were unchanged in the brain of all dietary groups (Table 1). NAA, a neuronal marker of pathology, was also unchanged in all groups. Lactate, a neuronal energy substrate, was additionally unchanged in all groups.
Table 1.
Effect of Diet and Treatment on Brain Metabolites
| Metabolite* | ||||||
|---|---|---|---|---|---|---|
| Treatment Group |
Lactate |
N- acetylaspartate |
GABA Glutamate |
Aspartate | Taurine | |
| SD-UR | 40.8 ±2.1 | 57.0 ±6.5 | 16.6 ±2.2 | 75.6 ±2.2 | 18.1 ±1.9 | 57.9 ±3.9 |
| SD-R | 36.6 ±1.4 | 56.3 ±3.9 | 12.7 ±0.7 | 73.1 ±2.9 | 15.3 ±0.8 | 56.5 ±3.0 |
| SD-R[Glu] | 39.1 ±1.9 | 55.8 ±1.9 | 10.9 ±0.7 | 70.9 ±4.1 | 13.4 ±1.3 | 53.1 ±2.8 |
| SD-R[Glu][2DG] | 40.5 ±2.6 | 57.0 ±2.9 | 10.2 ±1.4 | 74.8 ±1.8 | 13.3 ±1.9 | 53.2 ±2.5 |
| SD-R[Glu][bOHB] | 34.4 ±4.0 | 57.2 ±2.7 | 11.9 ±0.7 | 72.9 ±2.0 | 15.0 ±0.2 | 57.2 ±2.2 |
Values are ratios of 1H NMR peaks standardized to 1H NMR phosphocreatine + creatine peaks, represented as mean ± SEM. n = 4–6 mice per group.
3.6 Influence of Treatments on Brain GAD Activity
Brain GAD activity was significantly increased across all calorie-restricted groups compared to the SD-UR group (Figure 5). GAD activity was similar across all calorie-restricted groups, indicating that acute treatments of glucose, 2-DG, and bOHB did not affect GAD activity.
Figure 5.
Effect of dietary treatment on brain glutamate decarboxylase (GAD) activity. GAD activity is significantly increased in all calorie-restricted groups, compared to the SD-UR group. GAD activity is similar across all calorie-restricted groups. Values are displayed as mean ± SEM (n = 5–6 mice/group). *p < 0.05 compared to the SD-UR group.
4. Discussion
We confirmed from previous work that long-term calorie restriction significantly reduces seizure susceptibility in EL mice [5], and that acute addition of glucose abolishes the seizure-protective effects of calorie restriction [18]. We found that reduced glucose utilization is necessary for acutely managing seizures with dietary therapy in EL mice, whereas acute supplementation of bOHB does not have a seizure-protective role for EL mice, in the presence of increased glucose availability. Interestingly, in the absence of long-term calorie restriction, the acute reduction of glucose utilization did not decrease seizure susceptibility.
The induction of ketosis is a common motif among dietary therapies that are used to treat epilepsy. Our data indicate that acute supplementation of bOHB was unable to confer seizure protection in EL mice. The ketone body bOHB, which is the major circulating ketone body, has not demonstrated acute anticonvulsant activity in a variety of models, whereas the ketone body acetoacetate has been shown to have anticonvulsant activity [12]. We administered bOHB in the drinking water, as the EL mice did not restrict water intake. Additionally, β-hydroxybutyrate dehydrogenase in the liver readily interconverts bOHB and acetoacetate to maintain a circulating equilibrium, and the 2.5 hr treatment period would allow ample time for the equilibrium to be maintained [21, 24].
Our results therefore suggest that acute ketone body supplementation does not confer seizure protection in EL mice. This is consistent with our previous results demonstrating that chronic bOHB supplementation in the drinking water of calorie-restricted EL mice did not result in additional anticonvulsant activity compared to calorie-restricted controls [18]. We cannot completely rule out that supplementing higher levels of ketone bodies may be anticonvulsant in the EL mouse, however a solution of 50 mM bOHB was the highest concentration EL mice could tolerate before restricting fluid intake. Furthermore, plasma levels of bOHB were not increased with bOHB supplementation, which suggests rapid utilization or blood clearance of bOHB. While bOHB may not be readily utilized for energy in the initial stages of calorie restriction, supplemented bOHB should be readily metabolized with long-term calorie restriction as brain cells upregulate activity of enzymes important in ketone body metabolism, although this did not result in protection against seizures [5, 11, 24].
In contrast to ketone supplementation, acutely decreasing glucose utilization resulted in seizure protection for EL mice under long-term calorie restriction. Decreased glucose utilization was achieved with administration of 2-DG, a glycolytic inhibitor that produces the metabolite 2-deoxy-D-glucose-6-phosphate, which allosterically inhibits the phosphorylating action of hexokinase, a rate-limiting step of glycolysis [25, 26]. 2-DG is readily taken up into the brain of EL mice, and we confirmed that 2-DG administration reduced hexokinase activity in the brain of EL mice [27]. Acute supplementation of 2-DG did not affect circulating glucose levels. While decreasing glucose utilization conferred seizure protection in mice under long-term calorie restriction, it did not confer seizure protection with short-term calorie restriction. This suggests that the mechanisms for seizure control from a calorie-restricted diet are multifaceted.
The circulating levels of glucose and ketones were not associated with changes in seizure protection due to acute administration of glucose, 2-DG, or bOHB, as circulating glucose and ketones did not change with acute treatment under long-term calorie restriction. This is consistent with our previous work demonstrating that acute supplementation of glucose does not lead to an increase in circulating glucose levels [18]. This is also consistent with other work demonstrating that low circulating blood glucose leads to rapid blood glucose clearance in humans after a bolus of glucose [28], and ketone bodies are rapidly cleared from plasma in rats under calorie-restricted conditions [29]. Rapid blood glucose clearance is associated with increased cerebral glucose utilization and seizures [18, 30]. Rapid bOHB clearance does not appear to be associated with seizure incidence.
Seizure susceptibility can be acutely changed with the addition of glucose or 2-DG; we therefore examined the hypothesis that calorie restriction and ketosis leads to mitochondrial flux and changes the brain handling of amino acids, particularly the conversion of glutamate to GABA [31]. While we found no changes in brain amino acid levels, a previous report examining seizures in rat pups had reported no changes in brain GABA levels related to seizure activity, but had reported changes in GAD levels related to seizure activity [32]. This suggested that the metabolic flux of glutamate decarboxylation to GABA is more representative of changes in seizure-related brain metabolism than steady-state levels of brain amino acids. We found that GAD activity was increased across all calorie-restricted groups compared to the SD-UR group, but was similar across all calorie-restricted groups, regardless of seizure susceptibility. This demonstrated that increased GAD activity was not associated with acute seizure protection. Increased GAD activity may be necessary under long-term calorie restriction to confer seizure protection, but other processes must also be responsible for conferring acute seizure protection. Our present data also does not rule out that increased GAD activity may be a side effect of calorie restriction that is unrelated to seizure protection. This result underscores the utility of the present testing paradigm in parsing the effects of calorie restriction from seizure protection, and therefore revealing effects of calorie restriction that are dissociated from seizure protection, such as increased GAD activity.
A possible mechanism that would confer both chronic and acute seizure protection in EL mice that remains consistent with our present data is the hypothesis that metabolism of ketones raises the free energy of ATP hydrolysis, which leads to an increase in the resting membrane potential of neurons and thereby inhibits aberrant synchronous neuronal discharges [24, 33–35]. Efficient brain ketone metabolism requires upregulating monocarboxylic acid transporters and relevant ketone body metabolizing enzymes, which occurs after prolonged fasting, calorie restriction or a ketogenic diet [36, 37]. Therefore, providing exogenous ketones or acutely decreasing glucose utilization would not confer seizure protection until ketones can be efficiently metabolized to increase the resting membrane potential of neurons. During prolonged fasting or calorie restriction, when glucose becomes available, neurons will utilize glucose through glycolysis, as glycolysis produces ATP faster than the oxidative steps of the tricarboxylic acid cycle and the electron transport chain [11, 38]. This acute glucose utilization would lead to a neuronal depolarization, which could increase aberrant synchronous neuronal discharges and allow breakthrough seizures [18, 39]. The present and previous results with dietary therapy in EL mice remain consistent with the hypothesis of ketone body metabolism leading to neuronal hyperpolarization [18].
5. Conclusions
We demonstrated that under long-term calorie restriction, decreasing glucose utilization is necessary for seizure protection. Ketone body supplementation did not provide acute seizure protection to EL mice with or without long-term calorie restriction. In the absence of long-term calorie restriction, decreased glucose utilization did not provide seizure protection to the EL mouse.
Highlights.
-
-
Acute 2-DG supplementation reduces glucose utilization and seizures in EL mice
-
-
Increasing ketone availability does not affect seizure susceptibility in EL mice
-
-
Short-term calorie restriction does not provide seizure protection to EL mice
-
-
Brain excitatory and inhibitory amino acid content was not associated with seizures
Acknowledgements
The authors wish to thank Thomas N. Seyfried for helpful comments regarding the design and analysis of the study. The authors also wish to thank Bhumi Patel, Jacqueline Fung, Sonia Iosim, and Matthew R. Linnehan for assistance with implementing the dietary therapy and seizure testing. This work was supported in part from NIH grant NS055195, the Jasse Walsh Scholarship (JJM), and the Boston College Research Expense Fund.
Abbreviations
- 2-DG
2-deoxy-D-glucose
- SD
standard mouse chow diet
- UR
unrestricted feeding (ad libitum)
- R
calorie restricted feeding
- Glu
D-glucose
- bOHB
beta-hydroxybutyrate
- NAA
N-acetylaspartate
- GAD
glutamate decarboxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 2.Hartman AL, Rubenstein JE, Kossoff EH. Intermittent fasting: a "new" historical strategy for controlling seizures? Epilepsy Res. 2013;104:275–279. doi: 10.1016/j.eplepsyres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuen AW, Sander JW. Rationale for using intermittent calorie restriction as a dietary treatment for drug resistant epilepsy. Epilepsy Behav. 2014;33C:110–114. doi: 10.1016/j.yebeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Kossoff EH, Rowley H, Sinha SR, Vining EP. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49:316–319. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 5.Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metabolism. 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–1378. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- 7.Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 8.Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metabolism. 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meidenbauer JJ, Ta N, Seyfried TN. Influence of a Ketogenic Diet, Fish-Oil, and Calorie Restriction on Plasma Metabolites and Lipids in C57BL/6J Mice. Nutr Metabolism. 2014;11:23. doi: 10.1186/1743-7075-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman AL, Zheng X, Bergbower E, Kennedy M, Hardwick JM. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–1402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–537. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 12.Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6:406–414. doi: 10.1016/j.nurt.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todorova MT, Burwell TJ, Seyfried TN. Environmental risk factors for multifactorial epilepsy in EL mice. Epilepsia. 1999;40:1697–1707. doi: 10.1111/j.1528-1157.1999.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki J. Paroxysmal discharges in the electroencephalogram of the El mouse. Experientia. 1976;32:336–338. doi: 10.1007/BF01940824. [DOI] [PubMed] [Google Scholar]
- 15.Meidenbauer JJ, Mantis JG, Seyfried TN. The EL mouse: a natural model of autism and epilepsy. Epilepsia. 2011;52:347–357. doi: 10.1111/j.1528-1167.2010.02898.x. [DOI] [PubMed] [Google Scholar]
- 16.Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–940. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagatomo I, Akasaki Y, Nagase F, Nomaguchi M, Takigawa M. Relationships between convulsive seizures and serum and brain concentrations of phenobarbital and zonisamide in mutant inbred strain EL mouse. Brain Res. 1996;731:190–198. doi: 10.1016/0006-8993(96)82386-9. [DOI] [PubMed] [Google Scholar]
- 18.Mantis JG, Meidenbauer JJ, Zimick NC, Centeno NA, Seyfried TN. Glucose reduces the anticonvulsant effects of the ketogenic diet in EL mice. Epilepsy Res. 2014;108:1137–1144. doi: 10.1016/j.eplepsyres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Gasior M, Yankura J, Hartman AL, French A, Rogawski MA. Anticonvulsant and proconvulsant actions of 2-deoxy-D-glucose. Epilepsia. 2010;51:1385–1394. doi: 10.1111/j.1528-1167.2010.02593.x. [DOI] [PubMed] [Google Scholar]
- 20.Todorova MT, Dangler CA, Drage MG, Sheppard BJ, Fox JG, Seyfried TN. Sexual dysfunction and sudden death in epileptic male EL mice: inheritance and prevention with the ketogenic diet. Epilepsia. 2003;44:25–31. doi: 10.1046/j.1528-1157.2003.11402.x. [DOI] [PubMed] [Google Scholar]
- 21.Williamson DH, Mellanby J, Krebs HA. Enzymatic determination of D(--)-b-hydroxybutyric acid and acetoacetic acid in blood. J Biochem. 1962;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Wolf R, Klemisch H. Adaptation of an enzymatic fluorescence assay for L-glutamic acid decarboxylase. Anal Biochem. 1991;192:78–81. doi: 10.1016/0003-2697(91)90187-x. [DOI] [PubMed] [Google Scholar]
- 24.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostag Leukotr Ess. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Gueron M. The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: characterization by 31P NMR and metabolic implications. Biochimie. 1992;74:867–873. doi: 10.1016/0300-9084(92)90070-u. [DOI] [PubMed] [Google Scholar]
- 26.Horton RW, Meldrum BS, Bachelard HS. Enzymic and cerebral metabolic effects of 2-deoxy-D-glucose. J Neurochem. 1973;21:507–520. doi: 10.1111/j.1471-4159.1973.tb05996.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakamoto Y, Nakayama S, Suzuki J. Cerebral uptake of [14C]deoxyglucose during the entire seizure and the recovery period in an El mouse. Epilepsy Res. 1990;5:43–48. doi: 10.1016/0920-1211(90)90064-3. [DOI] [PubMed] [Google Scholar]
- 28.Verdonk CA, Rizza RA, Gerich JE. Effects of plasma glucose concentration on glucose utilization and glucose clearance in normal man. Diabetes. 1981;30:535–537. doi: 10.2337/diab.30.6.535. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins RA, Mans AM, Davis DW. Regional ketone body utilization by rat brain in starvation and diabetes. Am J Physiol. 1986;250:E169–E178. doi: 10.1152/ajpendo.1986.250.2.E169. [DOI] [PubMed] [Google Scholar]
- 30.Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. Am J Physiol Heart Circ Physiol. 2000;279:H1346–H1354. doi: 10.1152/ajpheart.2000.279.3.H1346. [DOI] [PubMed] [Google Scholar]
- 31.Yudkoff M, Daikhin Y, Melo TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Ann Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arias C, Valero H, Tapia R. Inhibition of brain glutamate decarboxylase activity is related to febrile seizures in rat pups. J Neurochem. 1992;58:369–373. doi: 10.1111/j.1471-4159.1992.tb09320.x. [DOI] [PubMed] [Google Scholar]
- 33.Veech RL, Kashiwaya Y, Gates DN, King MT, Clarke K. The energetics of ion distribution: the origin of the resting electric potential of cells. IUBMB Life. 2002;54:241–252. doi: 10.1080/15216540215678. [DOI] [PubMed] [Google Scholar]
- 34.Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–247. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 36.Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- 37.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIlwain H, Tresize MA. The glucose, glycogen and aerobic glycolysis of isolated cerebral tissues. Biochem J. 1956;63:250–257. doi: 10.1042/bj0630250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huttenlocher PR. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]