Abstract
When all of the epilepsies are considered, sex differences are not always clear, despite the fact that many sex differences are known in the normal brain. Sex differences in epilepsy in laboratory animals are also unclear, although robust effects of sex on seizures have been reported, and numerous effects of gonadal steroids have been shown throughout the rodent brain. Here we discuss several reasons why sex differences in seizure susceptibility are unclear or are difficult to study. Examples of robust sex differences in laboratory rats, such as the relative resistance of adult female rats to the chemoconvulsant pilocarpine compared to males, are described. We also describe a novel method that has shed light on sex differences in neuropathology, which is a relatively new techniques that will potentially contribute to sex differences research in the future. The assay we highlight uses the neuronal nuclear antigen NeuN to probe sex differences in adult male and female rats and mice. In females, weak NeuN expression defines a sex difference that previous neuropathological studies have not described. We also show that in adult rats, social isolation stress can obscure the normal effects of 17β-estradiol to increase excitability in area CA3 of hippocampus. These data underscore the importance of controlling behavioral stress in studies of seizure susceptibility in rodents and suggest that behavioral stress may be one factor that has led to inconsistencies in outcomes of sex differences research. These and other issues have made it difficult to translate our increasing knowledge about the effects of gonadal hormones on the brain to improved treatment for men and women with epilepsy.
Keywords: estrogen, estradiol, progesterone, neurosteroid, androgen, menstrual cycle, estrous cycle, gender, seizure, epileptogenesis, temporal lobe epilepsy
I. INTRODUCTION
The fact that there are sex differences in some epilepsy syndromes is well documented (Christensen et al., 2005). For example, idiopathic generalized epilepsy (IGE) is more common in women (McHugh and Delanty, 2008). A type of reflex epilepsy called photosensitive epilepsy is also more common in women (Taylor et al., 2007). Focal cortical dysplasia is more common in males (Ortiz-Gonzalez et al., 2013) and a different type of malformation, perinodular heterotopia, is more common in males (Sisodiya et al., 1999).
However, sex differences in epilepsy are not always clear. In epidemiological studies, this may be due to pooling subjects with different epilepsy syndromes or neuropathology. If only some epilepsy syndromes and some brain areas exhibit strong sex differences, overrall there may be little evidence of differences. Indeed, in a recent comprehensive review, incidence of the epilepsies was lower in women than men but only by a small margin (McHugh and Delanty, 2008). Pooling subjects with different types of epilepsy and different causes of epilepsy is also likely to contribute to inconsistencies from one study to the next. If one study includes a large proportion of subjects with IGE, sex differences might be large, but if another study does not, reported differences could be less obvious. This issue could explain why the incidence of epilepsy was greater in men (57% men and 43% women) according to Kim et al. (2014) but not McHugh and Delanty (2008). Regarding prevalence, similar inconsistencies exist: the prevalence of epilepsy showed no differences between men and women in one study (Burneo et al., 2005), whereas a different cohort showed a higher prevalence of epilepsy in men (6.05/1000 vs. 5.18/1000; Sridharan and Murthy, 1999), which was also found in another group of subjects (Benamer and Grosset, 2009), but prevalence was higher in women than men in yet another group (Winkler et al., 2009). Incidence also varies: in a study from Italy there were no sex differences in the incidence of epilepsy (Casetta et al., 2012) whereas in Ethiopia there was a higher rate in men (72/100,000 vs. 57/100,000) and in Turkey the rate was higher in women (42.2/100,000 vs. 33.5/100,000; Celikkas et al., 2010). Although differences in the way these studies are conducted around the world help explain these different results, it seems likely that pooling patients with distinct types of epilepsy, and comparing studies with a different composition of subjects will contribute to diverse epidemiological findings.
Other characteristics of clinical epilepsy besides epidemiology make a better case that sex differences exist. Several aspects of temporal lobe epilepsy (TLE) appear to differ in men and women, such as auras, which are more common in females (Janszky et al., 2004), and differences between men and women exist in lateralization and generalization of seizures (Janszky et al., 2004). Voxel based morphometry shows abnormalities in men with TLE that are frontal whereas they are more often temporal in females with TLE (Santana et al., 2014). Extratemporal hypometabolism has been reported to be more common in men than women with TLE using neuroimaging (Savic and Engel, 1998; Nickel et al., 2003). Despite these findings, there still are many aspects of epilepsy where sex differences are understudied and as yet unclear.
Most investigators relate sex differences in epilepsy to actions of estrogens, progestins and androgens. A conceptual framework has guided the views about these hormones and their actions into two fundamental principles. First, estrogens and androgens impart sex differences in the brain early in life by programming or “organizing” the nervous system to become sexually dimorphic. Second, these hormones are important after puberty to “activate” the programmed circuitry. Together the processes of organization and activation lead to sex differences in adult behavior (McCarthy et al., 2012; Kight and McCarthy, 2014).
This framework for explaining sex differences in the brain and behavior provides potential explanations for sex differences in epilepsy. For example, regarding neurodevelopmental causes of epilepsy, sex differences in GABAergic signaling that occur in early life have been implicated (Galanopoulou 2008a, 2008b; Briggs and Galanopoulou, 2011; Akman et al., 2014). Regarding the predominance of malformation-associated epilepsy in males described above, differences in the organizational phase of neurodevelopment seem likely to be responsible, i.e., making males more susceptible to a type of malformation than females. The cause of a male dominance in a specific type of malformation is not clear, but if the organizational time of life is important, it is likely to be related to the normal neonatal surge in androgens in the male that is metabolized to 17β-estradiol, because the rise in neonatal 17β-estradiol causes masculinization of the brain and behavior (McCarthy et al., 2012). Sex differences in seizures and epilepsy support this idea. For example, studies in rodents have shown that freeze lesions early in life cause males to have more vulnerability to induction of epilepsy later in life, and this effect could be mimicked by androgenization of females during the perinatal period (Desgent et al., 2012). It was suggested that increased corticosterone mediates the effect of perinatal androgens to increase seizure susceptibility later in life in males (Desgent et al., 2012), which is consistent with the observation that prenatal stress associated with a rise in corticosterone often increases the likelihood of seizures postnatally (Edwards et al., 2002; Sadaghiani and Saboory, 2010). Another example of the regulation of seizure susceptibility by perinatal gonadal hormones is the emergence of sexual dimorphism in the substantia nigra during early postnatal life in the rodent, discussed elsewhere in this Special issue (Giorgi et al., 2014).
Sex differences that would be likely to influence clinical epilepsy are not only related to neurodevelopmental causes. For example, sexually-differentiated patterns of drug metabolism are well-documented (Pigeolet et al., 2007; Marino et al., 2012; Ibarra et al., 2013), yet, surprisingly, clear sex differences have not emerged for most ASDs. Again, gonadal steroids are highly influential based on studies of estrogens, progestins and androgens. However, sex differences are less clear.
In laboratory animal models, ASDs are often not included in the study, allowing an opportunity to identify fundamental sex differences in seizures and epilepsy. Yet sex differences in animals are not well established, with a few notable exceptions such as the substantia nigra, as mentioned above.
Below, we address preclinical data in rodents, provide examples of sex differences, and discuss the difficulties in clarifying sex differences. We also suggest a method to gain better insight into sex differences in neuropathology, and how inconsistencies can be avoided. First, however, we explain our use of terms.
II. TERMINOLOGY
A. General terminology
The terms that are used to discuss sex differences are often used differently from one investigator to another, so we first will define terms as they are used in the present manuscript. Gender refers to the sexual preference of humans which is typically consistent with biological sex (men with male gonads and other male reproductive organs vs. women with female gonads, etc.). Further discussion of the important distinctions between gender and sex is provided in an excellent recent review of these terms by experts in the field (Becker et al., 2005).
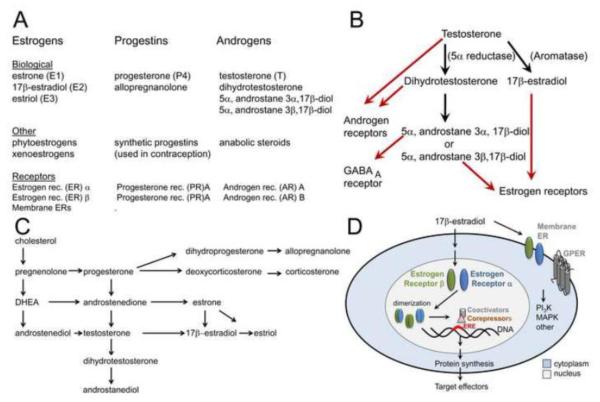
The relationship between the estrogens, progestins, and androgens is illustrated in Figure 1A. The three principal naturally-occurring estrogens in mammals are 17β-estradiol (E2), estrone (E1) and estriol (E3): more information about this terminology is provided elsewhere (Blaustein, 2008). 17β-estradiol is used as an example to discuss the effects of estrogens below, because it mediates many of the neurobiological effects of estrogens and is the predominant physiologically active estrogen in most species. Progestins include progesterone and synthetic progestins such as those used in oral contraceptives (Figure 1A-C). Progesterone and its 5α-reduced metabolite dihydroprogesterone (DHP) have biological effects but they are not as commonly studied in epilepsy research as the reduced metabolite of DHP, 3α-hydroxy-5α-pregnan-20-one (THP, or allopregnanolone). Allopregnanolone is a neurosteroid, which binds to GABAA receptors to enhance the effects of GABA, and at high concentrations, can directly activate the receptor (for review see Reddy, 2013).
Figure 1. Estrogens, progestins, and androgens.
A. The major estrogens, progestins and androgens that are synthesized in the body (Biological) or not (Other) are listed, as well as receptors. Common abbreviations are in parentheses.
B. Major metabolic pathways of testosterone are shown (black) and receptors for the metabolites (red).
C. Major pathways of biosynthesis of estrogens, progesterone, and androgens are shown.
D. A schematic of a cell shows the receptors for estrogens and the general route of nuclear receptor activation of target genes. GPER, G-protein coupled estrogen receptor. ERE, estrogen response element.
Androgens refer to multiple ligands that act on androgen receptors (ARs), including testosterone and its metabolite, dihydrotestosterone (DHT). It is important to recognize that testosterone can be metabolized either to DHT or 17β-estradiol, thereby exerting both androgenic or estrogenic effects, depending on the enzymes present in the target tissues (Figure 1B-C). DHT is further metabolized to 5α-androstane-3α,17β-diol or 5α-androstane-3β,17β-diol (Figure 1B-C). The 3α androstanediol isomer is a neurosteroid which, like allopregnanolone, enhances the effects of GABA at GABAA receptors. The 3β androstanediol isomer can act as an agonist at the ERβ estrogen receptor (Figure 1A-C; Handa et al., 2008).
Receptors for all three steroid classes – estrogens, progestins, and androgens - include multiple membrane-associated and cell nuclear receptors (Figure 1A,D). For estrogens, both of the nuclear receptor subtypes (ERα and ERβ) can also localize to the plasma membrane, where they can activate a number of signal transduction cascades (Figure 1D). In addition, estradiol can also activate a G-protein coupled membrane estrogen receptor (GPER-1) with actions on adenylate cyclase, as well as other signaling mechanisms (Filardo and Thomas, 2012). For progestins, the principal target receptors are PR (two isoforms, PR A and PR B, differing only in the length of the N terminal amino acid chain) or the GABAA receptor (Figure 1A). For androgens, there are two ARs (AR A and AR B, differing, like the progestin receptors, in the length of the N-terminus; Figure 1A). All steroid hormones are lipid soluble, so they can act at the plasma membrane or cross the membrane to bind to nuclear receptors. Activated nuclear receptors act as transcription factors for target genes (Figure 1D). Thus, membrane receptors subserve rapid actions (seconds – minutes) while nuclear receptors generally mediate effects that require more time.
B. The menstrual cycle of women and the estrous cycle of rodents
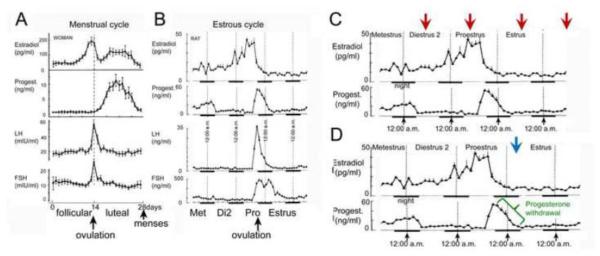
In many mammals, the term estrous cycle refers to the physiological changes that accompany the ovarian cycle. In women, these events are termed the menstrual cycle. In laboratory rodents, such as rats and mice, the estrous cycle lasts approximately 4 days rather than the 28 days of the menstrual cycle (Figure 2A-B). These four days include a day called proestrus, where estradiol levels in the serum increase rapidly, reaching their peak in the morning. If the start of the light:dark cycle is 7:00 a.m., the peak of the proestrous surge is mid-morning (data for the rat shown in Figure 2B; Freeman, 2006). Ovulation occurs later on the day of proestrus, and mating typically occurs that evening. Progesterone increases rapidly at ovulation, peaking at approximately midnight before declining dramatically during the early hours of the following day, estrus. Subsequently there are two days of diestrus when serum hormone levels are much lower, although smaller fluctuations occur. The first day of diestrus is called diestrus 1 (or metestrus), and the second day is called diestrus 2.
Figure 2.
A. Measurements of 17β-estradiol, progesterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) during the menstrual cycle.
B. The estrous cycle of the Sprague-Dawley rat. A and B are adapted from (Scharfman and MacLusky, 2006b).
C. The times of the estrous cycle that are commonly used to compare the effects of 17β-estradiol when serum levels peak (proestrous morning) and when they are at low levels (diestrous 2 morning, estrous morning, metestrous morning).
D. The time of the estrous cycle when serum levels of progesterone rapidly decline from their peak values is designated in green. A suggested time to assay effects of progesterone withdrawal is indicated by a blue arrow.
Although ovarian cycles in women are considerably longer, the different phases of menstrual and estrous cycles are comparable. The second part of diestrus 2 and the morning of proestrus are analogous, hormonally, to the late follicular phase in women. Early estrous morning is analogous to the perimenstrual period in women. Thus, there are similarities in the sequence of fluctuations in serum levels of estrogens prior to ovulation in rodents and women, and the species are also similar in that rapid fluctuations in serum levels of progesterone during and immediately after ovulation. The main exception to the species similarity is that the secondary surge in serum levels of estrogens that occurs during the mid-luteal phase in women (i.e. approximately 21 days) does not have a counterpart in the rodent (Figure 2A-B).
One of the points of confusion in the rodent literature is the term ‘cycle stage,’ because it is often used synonymously with the cycle day (e.g., proestrus), and that can become confusing because the hormonal milieu changes rapidly between the morning and evening of proestrus. It also is important to be careful in comparing rodent and human ovarian cycles. For example, the human follicular and luteal phases comprise the first and second half of the 28-day cycle, respectively whereas the analogous times in the rodent do not correspond to half the estrous cycle (Figure 2A-B). Instead, the time that is analogous to the follicular phase begins on estrous morning (after the decline in serum levels of progesterone), and ends with ovulation approximately 3 days later.
A final consideration is that in rodent endocrinology, the estrous cycle is commonly used to study effects of serum levels of 17β-estradiol by comparing times of the estrous cycle when these hormones are high or low, while the time of day is kept constant to minimize contributions from circadian rhythms. This is usually done by comparing mid-morning of each of the four days as shown in Figure 2C. The approach allows one to compare the time when serum levels of estradiol are at their peak (mid-morning of proestrus) vs. baseline (mid-morning of other 3 days of the estrous cycle). However, in practice, studies are not always done this way: some, for example, use tissue from rats taken during the afternoon of proestrus, when the effects of circadian hormone release may be different from mid-morning, a longer period has elapsed for expression of changes in estrogen-activated gene transcription, and progesterone levels in the serum have begun to rise (Figure 2B). Such approaches may not yield the same results as experiments performed earlier on the day of proestrus.
C. Seizures, epileptogenesis, and epilepsy
Regarding terms relevant to preclinical epilepsy, a seizure is a symptom of the disease epilepsy where seizures are recurrent and spontaneous. Epileptogenesis is the transformation of the normal condition, without seizures, into a condition where epilepsy exists. The exception to these definitions is reflex epilepsy, where seizures are provoked by a stimulus such as a flashing light (Scharfman, 2014).
In rodents, experimental seizures are often induced by drugs or electrical stimulation. Some drugs, such as pentylenetetrazol (PTZ) produce seizures with convulsive behaviors that are unlike seizures produced by drugs such as pilocarpine which can induce status epilepticus (SE), a state of continuous seizures that lasts for hours before self-terminating. SE in the rodent often initiates a pattern of brain damage and other changes in the brain that initiate epileptogenesis, leading within days or weeks to epilepsy (Scharfman, 2014b). PTZ does not induce SE. Kainic acid induces SE but is significantly different from pilocarpine because it initially produces numerous wet dog shakes (Scharfman et al., unpublished) and more severe neuronal loss where kainic acid receptors are concentrated, such as area CA3. All of these experimental models of seizures can yield data that can distinguish males and females, such as latency to the first seizure or SE, latency to the first convulsive seizure, and the duration of induced seizures. However, because of their differences, sex differences in response to one drug are hard to compare to sex differences in response to another drug.
III. SEIZURES, EPILEPTOGENESIS AND EPILEPSY IN RODENTS
A. Seizures
1) The importance of experimental conditions
Numerous publications suggest that sex differences in seizures exist in laboratory animals. However, the results differ greatly depending on the method used to induce seizures, the measurement of seizures (latency to onset, severity, duration), species, age, and the time of the estrous cycle that is chosen to study females; the result is that sometimes females are more severely affected, but in other studies males are more affected. (Scharfman and MacLusky, 2006b).
Some of the reasons for these diverse findings are that the variables matter more than one might think. An example is provided by our own studies of the chemoconvulsants pilocarpine and kainic acid in adult rats. In 2005 we reported that pilocarpine had a similar effect when administered mid-morning of diestrus 1, diestrus 2, or proestrus (Scharfman et al., 2005). We found that mid-morning of estrus was the only time when differences in the effects of pilocarpine could be discriminated. This was surprising given the substantial differences in the levels of hormones such as estradiol in the different mornings of the estrous cycle. A potential explanation is that severe seizures such as pilocarpine-induced status epilepticus are such severe electrical events that fluctuations in serum levels of estradiol in the normal intact rat do not have a modulatory effect - but more subtle seizure-like events might be modulated, if we had studied them. Indeed most studies of estradiol are made in the ovariectomized rat and physiological replacement is usually not tested. When intact rats are studied all cycle stages are not typically included. This leads to difficulty discriminating differences between intact rats and gonadectomized rats injected either with vehicle or hormone.
At mid-morning of estrus, in contrast to other cycle stages, we did find that there was a striking effect of cycle stage in the intact rat: a resistance of female rats to pilocarpine-induced SE in that few of the estrous females that were tested entered into SE compared to other females (Scharfman et al., 2005). Notably males were not tested. Thus, even when many groups of intact females are studied, an additional group of intact male rats may not be added. If a group of males is added, however, body weight and age become hard to match to females. Thus, if one uses female and male rodents at the same age to study sex differences, body weight will differ. Moreover, the location of body weight is mostly abdominal fat, although there may also be significant differences in subcutaneous fat distribution. Drugs vary a great deal in terms of their lipophilicity, potentially affecting their pharmacokinetics, so differences in seizures could develop that reflect body composition and injection site rather than sex differences per se. Directly examining body weight as a variable may help to sort such issues out (Scharfman et al., 2005), but particularly when comparing males and females, body weight per se may not adequately control for differences in body composition.
Several other aspects of our 2005 (Scharfman et al., 2005) study are important to note because they illustrate the importance of experimental conditions. One aspect that is notable is the choice of the time of day to study intact female rats on the day of estrus. We chose mid-morning, but if we had chosen an earlier time of that day, the results could have been very different because progesterone levels in the serum fall during the early morning of estrus after a sharp decline from a peak during the evening of the previous day). This rapid decline leads to a phenomenon called progesterone withdrawal that we could have missed because experiments were not conducted early enough on estrous morning (Figure 2D). Progesterone withdrawal is potentially important based on studies that modeled progesterone withdrawal by administering progesterone after ovariectomy and then inducing withdrawal pharmacologically (Reddy et al., 2001; Smith, 2002; Reddy and Rogawski, 2012; Reddy, 2013). It has been proposed that the increase in excitability caused by progesterone withdrawal explains the rise in seizures that often occurs during the perimenstrual period in women with epilepsy (Reddy et al., 2001; Rogawski, 2003; Reddy, 2009; Reddy and Rogawski, 2009). The majority of studies using the models of progesterone withdrawal emphasize one mechanism for progesterone withdrawal-induced seizure susceptibility: a change in the action of allopregnanolone and GABAA receptor subunit composition (Maguire et al., 2005; Smith et al., 2007; Reddy, 2013). Studies from our own laboratory have also shown that BDNF levels are increased at the time of the estrous cycle that could be considered analogous to the perimenstrual period, and this would be another mechanism for increased excitability that could also depend on precise timing of experiments (Scharfman et al., 2003; Scharfman and MacLusky, 2008; McNamara and Scharfman, 2012). The two mechanisms may, in fact, be related because BDNF signaling is implicated in the regulation of GABAA receptors and GABAergic inhibition (Seil, 2003; Gottmann et al., 2009; Grabenstatter et al., 2012). In summary, bringing the predictions of models to the intact animal is a difficult proposition because timing of the analysis is likely to be critical. Circadian rhythms in other hormones (particularly the glucocorticoids which are metabolized via pathways analogous to those responsible for metabolizing progesterone, also resulting in neurosteroids with effects at GABAA receptors) further complicate the situation.
It is also important to be careful when comparing in vitro vs. in vivo methods to study sex differences because there could be an important distinction: the brain may have higher levels of allopregnanolone in vivo than in vitro conditions (where neurosteroids could be washed out), and this could lead to relative seizure resistance in vivo but not in vitro. Indeed, our in vivo study may have been conducted at an appropriate time of estrous morning since slices prepared at that time showed hyperexcitability (Scharfman et al., 2003). As discussed elsewhere (Scharfman et al., 2005), high levels of allopregnanolone may have washed out in vitro, revealing the underlying hyperexcitability. On the other hand, the high levels of allopregnanolone may have been maintained in the brain in vivo, leading to seizure resistance.
An additional consideration is the choice of drug to induce seizures or the animal model of epilepsy. Agents such as pentylenetetrazol may test seizure networks that are more or less influenced by sex than a drug such as pilocarpine. A final consideration in the context of animals with epilepsy is that the GABAA receptor subunit where allopregnanolone normally binds may be reduced in chronic epilepsy, leading to an insensitivity to allopregnanolone, and other neurosteroids (Joshi et al., 2013). Therefore, our studies in normal animals may not predict the effects observed in epileptic animals.
Thus, at least four variables are important: 1) time of day when experiments are conducted in the intact female rat, 2) whether experiments are conducted in vivo or in vitro, 3) the seizure or epilepsy model, and 4) use of control vs. epileptic animals.
2) A case study: pilocarpine vs. kainic acid
As mentioned above, sex differences in animal models of seizures are not always clear because seizures and epilepsy are induced in animals in different ways, interacting with sex differences in the CNS in diverse ways. A good example is the sex difference in eliciting SE, which our studies in rats suggest will depend on the way SE is induced. Moreover, even if one chooses the method that employs a similar method (a systemic injection of a convulsant), the sex difference appears to depend on the choice of the chemoconvulsant.
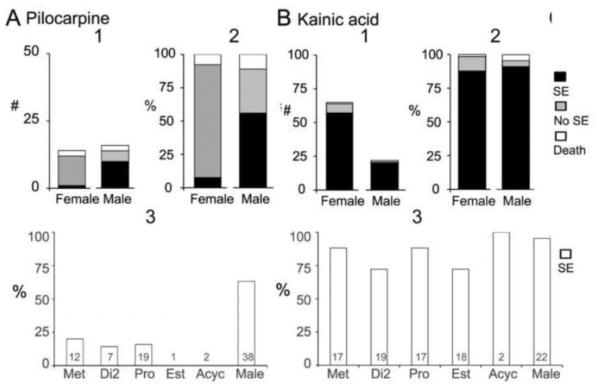
The most common way to induce SE is by systemic chemoconvulsant injection, either pilocarpine, a muscarinic cholinergic agonist, or kainic acid, the prototypical agonist at the kainic acid subtype of glutamate receptor. Both of these chemoconvulsants are used to elicit SE, and also to induce epilepsy in the weeks after SE. As shown in Figure 3, there is a striking resistance of adult female rats to pilocarpine-induced SE compared to males but this is not true for kainic acid-induced SE.
Figure 3. Sex differences in the response to pilocarpine and kainic acid.
A. Pilocarpine injection (380 mg/kg, s.c.) to adult female and male rats produced animals with status epilepticus (SE, black), seizures but not SE (grey), and SE that led to death from a severe seizure (white). Males had SE more often than females whether the animals that died were included or not (Fisher’s exact tests, p<0.05). 1. Numbers of rats. 2. Percent of rats. 3. The percent of animals that had SE is plotted in relation to the cycle stage at the time of pilocarpine administration. Sample sizes are at the base of the bar. Note that for estrous and acyclic animals (Acyc), SE did not occur.
B. Kainic acid (12 mg/kg, s.c.) to adult female and male rats led to SE more often than pilocarpine and sex differences were not evident. 1. Numbers of rats. 2. Percentage of rats. 3. The percent of animals that had SE are plotted in relation to their cycle stage at the time of kainic acid injection. There were not significant differences (one-way ANOVA, p>0.05).
In these experiments, adult female Sprague-Dawley rats were tested at an age when puberty is over (typically 45-50 days is the age when regular estrous cycles are exhibited) and 10-11 a.m. of each morning of the estrous cycle was examined to keep time of day consistent. Thus, four groups of intact rats were compared to intact males. The four times of the estrous cycle were chosen because they allow a comparison of females with low and high serum levels of estradiol. On each of the mornings of the estrous cycle, serum levels of progesterone are relatively low (compared to their peak on the evening of proestrus), which allows potential insight into the effects of high vs. low serum levels of estradiol while progesterone is low in the serum (Figure 1). Males were tested at several ages (1.5-3 months) and results did not appear to differ among these ages. As shown in Figure 3, females rarely entered SE compared to males. Females did exhibit convulsive behaviors, and these ranged between stages 1 and 5 on the Racine scale (Racine, 1972). However, the convulsive behaviors never became continuous (lasting 3 min or more, which was used to define SE; for further details see Scharfman et al., 2005). Even when a higher dose of pilocarpine was administered (400 mg/kg), females had few convulsive seizures, and rarely had SE.
The sex difference in response to pilocarpine is consistent with the resistance of females to lithium-pilocarpine (Persinger et al., 1988) which is also used to elicit SE. In this procedure, lithium pretreatment is followed by a lower dose of pilocarpine than is typically used when pilocarpine is administered alone. Our results are also consistent with a study where the muscarinic antagonist atropine was infused into the septum to induce spiking and had a reduced effect in females compared to males (Herink, 1998). Resistance of females to cholinergic drugs was also shown in DBA/2J female mice compared to males, when audiogenic seizures were tested. In that study, thiamine tetrahydrofurfuryl disulfide, a drug that has a cholinergic mechanism of action, influenced male seizures but not female seizures (Lonsdale, 1982). Other data also support a relative resistance of females to the effects of pilocarpine on behavior, such as the ability of pilocarpine to stimulate drinking (Fregly, 1980). The mechanism of resistance to muscarinic cholinergic agonists in females may be related to 17β-estradiol, because 17β-estradiol treatment of ovariectomized rats reduces muscarinic receptors in hippocampus (Cardoso et al., 2010). Ovariectomy followed by 17β-estradiol replacement also reduces hippocampal concentrations of [3]inositol phosphate, which is normally increased by muscarinic receptor activation (Pereira et al., 2008). 17β-estradiol also impairs the effects of the muscarinic antagonist scopolamine to impair memory in adult rats (Rodgers et al., 2010). In rat cortex, the effects of estradiol appears to be similar to the effects on hippocampus, because the number and affinity of muscarinic receptors decrease in response to estrogens in ovariectomized rats (van Huizen et al., 1994). Muscarinic receptors are lower on estrus relative to diestrus (van Huizen et al., 1994), consistent with the observation that pilocarpine-induced SE is rare on estrous morning compared to diestrous morning (Scharfman et al., 2005). In contrast, kainic acid-induced SE is robust on estrous morning, indistinguishable from males and there is no statistical difference in cycle stages (Figure 3). The severity of seizures has not been studied using EEG however, so further studies may reveal cycle stage or sex differences in kainic acid -SE using EEG. Thus, suppression of muscarinic receptors is likely to be responsible for the low incidence of pilocarpine-induced SE in females.
There also were sex differences in morbidity after SE using pilocarpine. Thus, those females that had pilocarpine-SE had limited grooming, movement and feeding or drinking the next day. When pre-treated with the serum estrogen response modulator (SERM) raloxifene, 30 min prior to pilocarpine injection, the outcome the next day was improved in that animals were exhibiting normal exploratory behavior and food/water intake. Notably, the seizures that were induced seemed as robust with raloxifene pretreatment as without although this assessment was based on behavior not EEG and EEG provides greater resolution of seizures that quantification of convulsive behaviors (Scharfman et al., 2009).
There are several reasons that can potentially explain the beneficial effects of raloxifene such as protection of hypothalamic neurons that are usually vulnerable to excitotoxicity during SE (further discussed in Scharfman et al., 2009). In contrast to females, males that had pilocarpine-SE had some morbidity and mortality the day after SE, but less than females. Surprisingly, when pre-treated with raloxifene, SE occurred more often in males (no raloxifene, 5/9 males had SE or xx %; raloxifene, 5/5 or 100%) and mortality occurred in the next 24 hrs (no raloxifene, 0/5 males with SE died; raloxifene, 3/5 males with SE died).Thus, raloxifene pre-treatment appeared to benefit the female but exacerbate seizures and cause mortality in the male. In the future, EEG will be important to follow up on these initial findings because without EEG it is hard to be sure electrical activity was more severe; one only infers that is the case from a greater incidence of SE.
These data are relevant to the general issue of sex differences in epilepsy, because they show that the benefits of a drug for one sex are not necessarily beneficial for the other sex. The implication is that preclinical studies of drugs to treat epilepsy should be tested in both sexes.
B. Epileptogenesis and epilepsy
1) Genetic forms of epilepsy
Women are more affected in some genetic epilepsies such as IGE (McHugh and Delanty, 2008). One cause is an X-linked gene. For example, Rett syndrome predominantly affects females, which is primarily caused by mutations in the X-linked gene mecp2. In other epilepsy syndromes that are genetic, sex differences exist for other reasons such as related a mutation that affects a characteristic that is already sexual dimorphic or influenced by gonadal hormones. One example that is relevant to IGE and photosensitive epilepsy (Graham and Jeavons, 1999; Panayiotopoulos, 2005) is modulation of a common EEG pattern - 3 Hz spike and wave rhythm. In some animal models of absence epilepsy, such as cholesterol synthesis inhibitor (CSI) AY9944 treatment of Long-Evans rats (Persad et al., 2002; Li et al., 2007) there also is a common spike and wave rhythm (typically 6-10 Hz rather than 3 Hz) and predominance in females. In our studies of normal Sprague-Dawley rats, females exhibited spike wave discharges spontaneously at approximately 3 months of age, much earlier than males (Pearce et al., 2014). Those data also suggest an influence of sex, with females more affected. However, the predisposition for spike-wave in females is not completely clear, because females are not the predominate sex in the Wag/Rij animal model of absence epilepsy (Coenen and Van Luijtelaar, 1987).
Nevertheless, it is interesting to consider spike-wave discharges and the possibility of a weak predisposition in females that may occur normally. Reasons for this predisposition would be likely to be related to mechanisms that underlie thalamocortical oscillations because of the established relationship between these oscillations and spike-wave discharges. For example, the hyperpolarization-activated current Ih in thalamic relay cells is critical to thalamocortical rhythms (McCormick and Pape, 1990; Yue and Huguenard, 2001; Poolos, 2006). Ih has also been implicated in the Wag/Rij animal model of absence seizures (Budde et al., 2005; Shridde et al., 2006). Since estradiol increases Ih (in hypothalamus; Piet et al., 2013) and ovariectomy reduces Ih (in visceral ganglia; Qiao et al., 2013), it seems likely that 17β-estradiol would increase thalamocortical oscillations by increasing Ih. Estradiol also modulates T-type calcium currents (Kelly et al., 2013), which is relevant because one of the mutations contributing to spike-wave discharges in rats has been identified as a T-type calcium channel (Powell et al., 2008, 2009). This is just one of many examples of mechanistic directions in preclinical sex difference research that are understudied, which is unfortunate because they could ultimately lead to improved therapeutic strategies.
2) Acquired epileptogenesis and epilepsy
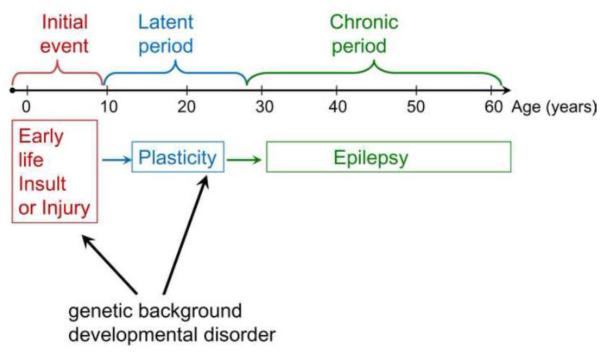
Acquired epilepsy is defined here as a type of epilepsy that is initiated by a brain insult or injury. In acquired TLE, years or decades may occur before seizures begin whereas in animal models of acquired TLE the timeframe is days, weeks or months. Epileptogenesis is thought to occur during this time span, initiated by the insult or injury and caused by changes in the brain in response to the injury, which may vary due to genetic predisposition (Figure 4). In the next section we consider sex differences in the response to insult, called the precipitating or initial event, and consider epileptogenesis afterwards.
Figure 4. Acquired epileptogenesis.
A schematic illustrates the phases of acquired epileptogenesis in temporal lobe epilepsy. An early life insult or injury leads to many years where changes occur in the brain that involve plasticity. Ultimately these changes lead to spontaneous recurrent seizures, i.e., epilepsy. Superimposed on this process are the influences of genes and any concurrent developmental disorder that may exist, such as an area of cortex where there is a malformation.
a. Sex differences in brain injury
It is often stated that males have a greater predisposition to behaviors that involve physical risk, and therefore are more likely to sustain insults or injuries that lead to acquired epilepsy. It is also commonly assumed that women are protected from injury by estrogens and progesterone. However, it is not clear from the published literature if women have a lower risk of acquired epilepsy. The reason may be that males do not actually have more of the type of brain injury that leads to epilepsy (which is currently not defined). Or women may actually not be protected from epileptogenesis due to brain injury, even if there are some protective effects of estrogens on other aspects of health (Brinton, 2008; Scharfman and MacLusky, 2008; Bath et al., 2013).
Recently we examined a phenomenon that may shed light on a reason women may develop acquired epilepsy in robust numbers. At the same time, we found a method that is a sensitive assay of neurons that are damaged but are not yet dead. This is notable because currently only a few stains, such as fluorojade, are typically used to assay damaged neurons in research using animal models of epilepsy. Additional methods would potentially be helpful in characterizing neuropathology.
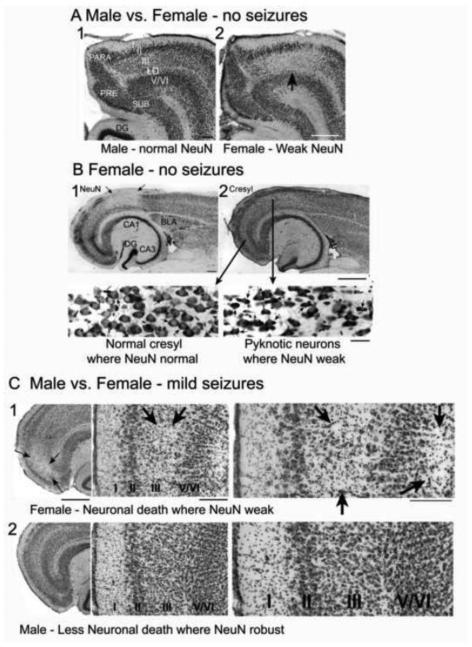
We found that the neuronal antigen NeuN, a marker of a nuclear protein found in almost all neurons, was sexually dimorphic in the normal adult brain. In females, but not males, there was a loss of antigenicity in several limbic regions of females after puberty (Figure 5A). The limbic areas of the female that showed weak NeuN expression included the entorhinal, frontal, perirhinal, retrosplenial and olfactory cortices. Loss of expression occurred in patches rather than respecting laminar borders or cytoarchitectonic boundaries of subregions. The neurons that had weak expression of NeuN also showed pkynosis using cresyl violet as a stain, which historically is considered to be a sign of cellular damage. These findings were made in rats or mice and were exacerbated by age and loss of neurotrophic support (Duffy et al., 2011). Prior studies have documented that diverse insults and injury, ranging from age to soman toxicity, can lead to phosphorylation of NeuN and as a result, reduction of immunodetection using an antibody raised against NeuN (for detailed discussion, see (Duffy et al., 2011). Presumably there is a loss of NeuN immunoreactivity because the antibody to NeuN does not recognize the conformational change in the protein that has been phosphorylated.
Figure 5. Sex differences in the entorhinal cortex.
A. A horizontal section of the entorhinal cortex of a male (1) and female (2) rat shows normal immunostaining with an antibody that marks a neuronal nuclear antigen (NeuN) in the male but in the female, there is light staining in layer III (arrow). LD= lamina dissecans. DG= dentate gyrus. PRE= presubiculum. SUB = subiculum. PARA = parasubiculum. Calibration, 250 μm.
B. In a female, a horizontal section shows an area of weak NeuN (1) and cresyl violet staining in an adjacent section (2). Arrows point to the area of weak NeuN in 1 and insets from areas with normal cresyl where NeuN staining was robust (left) or pkynotic neurons where NeuN staining was weak (right). Calibration, 250 μm (top) and 30 μm (insets).
C. Pilocarpine injection (380 mg/kg) was used to elicit brief seizures, which were truncated by pentobarbital (20 mg/kg) after 5-10 min. In these animals, perfusion 3 days later was followed by NeuN and cresyl violet staining of horizontal sections. 1. In the females (n=3), an area of cell loss was evident in the entorhinal cortex (arrows). At high power (right), both the superficial and deep layers appeared to have cell loss (arrows). 2. In the males (n=5), there was no detectable area of cell loss. Thus, female entorhinal cortex was more vulnerable to cell death after a brief series of seizures than males. Calibration, 250 μm (left), 100 μm (center and right).
These data are intriguing because they suggest an inherent difference in neurons of many areas of the cortex in females compared to males. They are reminiscent of suggestions that areas such as the entorhinal and retrosplenial cortex in females are vulnerable to the NMDA antagonist MK-801 (de Olmos et al., 2008; Bender et al., 2010). They also suggest a sex difference that might be relevant to disease. Psychiatric disease is discussed with respect to the data from studies of MK-801 administration by Feinstein and Kritzer (2013).
Based on the assumption that weak NeuN immunoreactivity indicated a vulnerability of neurons, we asked if a second ‘hit’ (the first hit being the initial stress causing weak NeuN expression) would cause the areas with weak NeuN expression to undergo neuronal loss. Furthermore, we compared females to males, which normally do not show the weak expression of NeuN as mentioned above. If weak NeuN expression were a contributing factor to the consequences of the second ‘hit’ then sex differences would be expected. Therefore we injected a relatively low dose of pilocarpine, 300 mg/kg, to intact adult female and male rats. We then examined these regions using NeuN immunohistochemistry and cresyl violet staining 3 days later, a time that typically is ideal to study neuronal loss after pilocarpine-induced SE.
We found that there was neuronal loss in the regions that showed weak NeuN expression in females (Figure 5C). Males did not sustain detectable neuronal loss in these regions, or elsewhere, probably because of the low dose of pilocarpine we used. The data suggest that females may have an inherent vulnerability that predisposes them to brain damage in select brain regions that are distinct from males. This vulnerability is quite remarkable because it was induced using pilocarpine, where females normally exhibit an insensitivity (described above). The data could explain why females have effects after brain injury that differ from males, and in TLE, more auras, because they involve limbic / frontal regions where the effect occurred (described above). Thus, assays such as expression of NeuN could be useful in the future.
b. Sex differences in the response to brain injury
Regrettably, there are several questions that have not yet been answered about sex differences in epileptogenesis following a brain injury. One of the reasons there is little that is known is that females are rarely studied and compared to males. Moreover, studies of females and males often do not include females at all stages of the estrous cycle. It is difficult to conduct these studies because of the cost to use many experimental groups instead of 1 group of males.
Moreover, when female rodents have convulsive seizures there usually is a cessation of estrous cycles. This becomes an additional experimental variable that is hard to control. In kindling, for example, females often develop persistent estrus (Edwards et al., 1999) where a high level of estrogen develops chronically. Irregular estrous cycles typically occur in models of TLE that use SE as the initial insult (Amado and Cavalheiro, 1998; Scharfman et al., 2008). Polycystic ovaries and increased androgen levels can develop, adding additional experimental factors (Scharfman et al., 2008). Although interesting because women with epilepsy can develop polycystic ovaries and increased androgen levels, it is difficult to use laboratory animals to explore sex differences in epileptogenesis because inducing convulsive seizures causes a loss or dramatic alteration in normal gonadal hormones.
IV. WHY ARE EXPERIMENTAL CONDITIONS SO IMPORTANT TO RESEARCH STUDIES ABOUT SEX DIFFERENCES?
A. Pharmacology vs. physiology
One reason that sex differences that have been reported previously seem inconsistent (for review, see Scharfman and MacLusky 2006) is related to the fact that the experiments in laboratory animals often study pharmacological effects after castration, and the conditions of these experiments are not the same. The timing and dose/preparation of steroids make a very large difference to effects. In addition, the ovaries are not the only source. The brain makes estrogens, progestins and androgens; regarding estrogens, there are striking effects of the estrogen synthesis within the brain (Prange-Kiel and Rune, 2006; Fester et al., 2011). In addition, the dose-response curve for hormonal effects is nonlinear. Effects that occur within minutes of estrogen exposure may not persist even if the ligand concentration is maintained, because ERs internalize in response to prolonged exposure to agonist (Bondar et al., 2009; Dominguez and Micevych, 2010). The response to estrogen also is sensitive to prior conditions. As discussed elsewhere (Scharfman et al., 2007), the slow rise in serum levels of 17β-estradiol that occurs during diestrus 2 of the rat ovarian cycle may "prime" the brain for the rapid surge in 17β-estradiol the next morning, proestrous morning. The "priming" may be critical for a maximal effect of the proestrous morning surge in 17β-estradiol. Yet 'priming' is not usually conducted in studies of estrogen action.
Another issue is that ovariectomy is followed by rapid changes in estrogen responsiveness in the rodent, so if experiments differ in the delay between ovariectomy and estrogen replacement, they are hard to compare. In our experiments that examined the effects of estrogen on afferent input to area CA3 of the hippocampus, we found that ovariectomized rats treated with estradiol had a large effect when treated 4-7 days after ovariectomy, a lesser effect if the delay was 10-20 days, and hardly any effect if the delay was >20 days following ovariectomy (Scharfman et al., unpublished). In additional experiments that also examined area CA3 electrophysiologically, a dose-regimen that precisely simulated the preovulatory surge led to a very large increase in the afferent response of CA3 neurons, but a slight change in the dose-regimen changed the effect dramatically, leading to rhythmic field potential reminiscent of epileptiform activity (Scharfman et al., 2007).
B. Stress
When low concentrations of hormones that simulate in vivo concentrations were used in prior experiments, it was possible to simulate changes occurring in the intact rat, but there were several factors that could reduce the ability to detect effects. The implication is that hormonal modulation of seizures that recapitulates effects of hormones in the intact rodent can be observed experimentally, but detection is very sensitive to experimental conditions. Others have also noted that variables that are often not considered to be major contributing factors, such as diet, can have dramatic affects on the ability to detect the influence of estradiol on behavior. One reason appears to be the levels of phytoestrogens such as soy in the diet (Luine et al., 2006). The light:dark cycle is also a variable that is not considered to be extremely important but if it is not regular, the estrous cycle is disrupted in rodents (Singh, 2005).
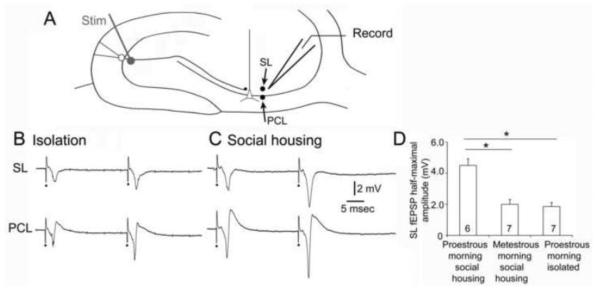
The recent history of social housing is another variable that appears to be surprisingly important. Based on our own studies of rats, we found that there were robust distinctions between animals that are socially housed and animals that have had their cage mates removed up to 24 hrs earlier. The isolated animal appears to maintain its estrous cycle based on vaginal cytology, but lose its hippocampal response to changes during the cycle. A similar finding was made when ovariectomized rats were examined after low doses of estradiol vs. vehicle; animals that were housed with another rat exhibited an effect of estradiol but not if they had been isolated from their cage mates within 24 hrs prior to use. In these experiments, slices were prepared and recordings were made in area CA3 to compared the responses to mossy fiber stimulation (Figure 6). For these experiments, animals were either housed with 1-2 other rats until euthanasia, or animals were separated, placed alone in a cage, and brought to the laboratory for euthanasia. Even if animals were treated with estradiol, there was a markedly diminished response to mossy fiber stimulation, making estradiol-reatment similar to vehicle-treatment (Figure 6). The results are consistent with findings of others showing that behavioral stress decreases spine synapses on hippocampal neurons in area CA3 (McEwen, 1999). The implication of the results is that acute stress decreases the responses of area CA3 neurons to afferent input, and in so doing, blocks the ability to detect differences that are due to gonadal hormones such as estradiol. An impairment by stress of the acute effects of progesterone administration has been described in Wag/Rij rats (Tolmacheva and van Luijtelaar, 2007). If one were to generalize from these observations to humans, one would predict that an individual who is behaviorally stressed may not show the same cycle-dependent effects as an individual who is not stressed. Behavioral stress is relevant because individuals who are brought to a clinical laboratory for evaluation of seizures and may be stressed by that examination and therefore exhibit diminished hormone-dependent effects. If they could be examined in the comfort of their own home, one would predict that hormone-dependent effects on seizures would be detected more readily. In the future, digital devices that can be maintained in the home could be used to circumvent this potential problem.
Figure 6. Social isolation blocks the positive influence of 17β-estradiol on responses to mossy fiber stimulation in area CA3.
A. Schematic of the location of stimulating (stim) and recording (record) electrodes in the hippocampal slice used for the recordings in B. One recording electrode was placed in stratum lucidum (SL) to record the response to stimulation in the subgranular zone to activate granule cell axons, the mossy fibers (MFs). It was then moved to the pyramidal cell layer to record the response of pyramidal cells to the same stimulus, adjacent to the axon hillock where action potentials are generated. The sum of the action potentials in the area around the recording electrode is a negative deflection called a population spike.
B. Responses to 2 stimuli (40 msec apart) are shown for the SL and PCL recording sites for a slice from a female rat that had been isolated in a cage for 24 hrs. This animal was ovariectomized and treated with 17β-estradiol as described elsewhere (Scharfman et al., 2007). It was euthanized and slices were prepared at the time of the peak serum level of 17β-estradiol. Stimuli are marked by the dots. All stimuli are half-maximal.
C. Responses to 2 half-maximal stimuli are shown for a slice from a female animal that was housed with 2 other female rats (socially housed) until euthanasia. It was ovariectomized and treated with 17β-estradiol with the same delay between surgery and treatment as the isolated rat, and same dose-regimen of 17β-estradiol. These examples show similar response morphology but smaller amplitudes in the isolated animal.
D. Comparison of MF responses in isolated and socially-housed rats that were euthanized mid-morning of proestrus or socially-housed rats that were euthanized mid-morning of metestrus. The normally large evoked responses on proestrous morning in socially-housed rats were not observed in isolated rats Isolated rats were similar in their responses to socially-housed rats that were euthanized on metestrous morning, when responses are normally small compared to proestrous morning (Scharfman et al., 2003). * = one-way ANOVA followed by Student’s t-tests, p<0.05.
C. The complexity of hormone action and epilepsy research
Another important issue is that hormonal effects in the brain are extremely complex - as are seizures and epilepsy. Most hormones have more than one biological effect, and the net effect can be less obvious than each effect observed in isolation in vitro. Epilepsy research is also complex in that a study of seizures may show an effect of a hormone but the results may not predict effects in chronic epilepsy.
Even if one just considers 17β-estradiol and only the hippocampus, the complexity of hormone action is readily appreciated. There are a diversity of effects as discussed in excellent reviews elsewhere (Mendez et al., 2005; de Lacalle, 2006; Prange-Kiel and Rune, 2006; Foy et al., 2008; Spencer et al., 2008; Smith et al., 2009; Barha and Galea, 2010) which document that estradiol has effects on structure, glutamatergic transmission, GABAergic neurons and astrocytes. Actions differ in development, adulthood, and aging. Rapid, direct actions are followed by secondary effects at diverse target genes, which in turn can have their own complex actions. Regarding seizures and epilepsy, 17β-estradiol on the one hand exhibits protective effects, but on the other hand can increase excitability. The first effect would tend to reduce epileptogenicity but the second would increase it. The mechanisms are complex because there are a number of biological effectors, so one cannot easily remove one and then perform experiments in a more controlled fashion. For example, there is evidence that 17β-estradiol increases the levels of brain-derived neurotrophic factor (BDNF) in hippocampus of the rat (Scharfman and MacLusky, 2006a), which has been shown to have both protective actions and increase hippocampal excitability (Scharfman and Maclusky, 2005). BDNF also influences neuropeptide Y (Wirth et al., 2005; Scharfman and MacLusky, 2006a), as does 17β-estradiol (Veliskova and Velisek, 2007; Ledoux et al., 2009) and neuropeptide Y has many effects that could influence seizures and epilepsy (Scharfman and Gray, 2006; Sperk et al., 2007). Together, actions of BDNF and neuropeptide Y make effects of 17β-estradiol hard to predict.
Androgens similarly have diverse effects, and the net effect is unlikely to be only pro- or anticonvulsant (Harden and MacLusky, 2005; Frye, 2010). As shown in Figure 1, the prototypical androgen, testosterone, is metabolized either to 17β-estradiol or dihydrotestosterone (DHT). Therefore, testosterone can have the effects of 17β-estradiol in the brain, or the effects of DHT at androgen receptors. Furthermore, DHT has two metabolites, one that enhances the actions of GABA at GABAA receptors, and another which can act like estradiol at estrogen receptor β (ERβ). As a result of these diverse actions, testosterone can have multiple effects that can be both pro- and anticonvulsant.
Effects of one gonadal steroid can also antagonize another. For example, progesterone synergizes with 17β-estradiol to produce the surge in luteinizing hormone on proestrus (Mann and Barraclough, 1973). However, the actions of the neurosteroid metabolite of progesterone, allopregnanolone, are generally inhibitory and therefore can oppose the pro-excitatory effects of 17β-estradiol. Furthermore, actions of a hormone in one brain nucleus may oppose the action in another part of the brain.
D. Females vary in the timing of their hormone-dependent changes
One problem that arises in evaluating sex differences is that there is variability in the females even at the same time of the estrous cycle. Unless sample size is extremely large, the variability can make sex differences hard to establish statistically. The reason for the variability is not only experimental conditions (described above) but biological.
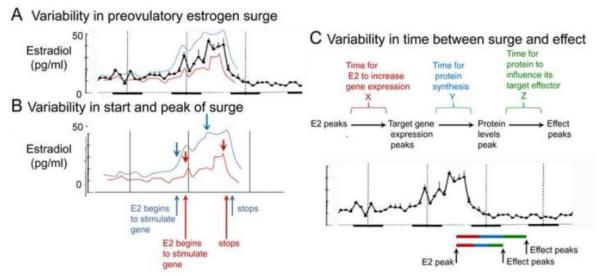
The underlying biological variability of the female estrous cycle is shown schematically in Figure 7. Analogous variability is likely to occur for the female menstrual cycle. There is variation that has been measured in the levels of serum hormones and it is particularly large during the preovulatory surge compared to other times of the estrous cycle. The implication is that one female may experience a rise in serum estradiol considerably later, and/or during the diestrus 2-proestrus transition than another female. One cycle of a given female may be different than the next. The effects of estradiol may vary as a result. In some cycles, effects could already be robust early during the preovulatory surge, but not in the next (Figure 7A). This leads to variation across cycles and across females that is likely to make it hard to detect consistent effects - even when experimental conditions are constant.
Figure 7. Variability in the effects of the preovulatory surge of serum estradiol in the rat.
A. Values for serum estradiol show that variance increases during the preovulatory surge, which can lead to relatively high (blue) or low (red) values from one cycle to the next, or one rat to another.
B. Arrows illustrate where the start and peak of the preovulatory estrogen surge would be in the example in blue and red. The variability in the start of the surge and its peak could change the time and degree that a given effect is influenced by 17β-estradiol.
C. A schematic illustrates the different phases of the process where 17β-estradiol causes an effect during the estrous cycle. During each phase is a variance in duration that the phase may have. At the bottom, two examples are shown where the durations are relatively long or short. When relatively long, the peak effect may not occur until the start of the night on estrus. When relatively short, the peak effect may occur much earlier, at the start of estrous day. For these reasons and others, effects of the estrous cycle may be too variable to be detected, leading to the assumption that sex differences are not present.
There also is like to be variation in the timing of 'downstream' effects resulting from the preovulatory surge such as the effects that require a sequential pattern of gene activation. For example, when serum levels of estradiol rise, growth responses result from actions of estradiol at target genes. Some of the genes may have additional targets that require time to produce an effect, such as the actions of which initiate spine plasticity, axonal sprouting and synaptogenesis (Figure 7B). If each time a signaling cascade is activated the duration to the peak effect is somewhat variable, the sum of the variances will lead to a very large net variance (Figure 7B). In the case of a complex growth response, the peak effect might vary extensively from cycle to cycle, or subject to subject (Figure 7C).
The implication is that a woman might have a different duration between the time of a peak serum level (or estrogen or progesterone) to the time of the pro- or anticonvulsant effect of estradiol or progesterone. This variance may lead to a need to survey many times of the ovarian cycle repetitively to find a robust effect of the cycle on seizures. This is not usually possible in a preclinical study or clinical research.
VII. CONCLUSIONS
In summary, there are important sex-dependent differences that influence seizures and epilepsy, and they can easily be missed by pooling males and females. Studies are likely to obtain different results unless experimental conditions are carefully controlled, with attention to age, body weight, time of day, housing, diet, and the type of seizure that is studied. Even if conditions are constant, biological variability will require large sample sizes to detect significant differences.
However, without such studies, treatment of patients with epilepsy will be adversely affected. For example, a drug that acts on GABAA receptors may always have effects in males, but not be as consistent in women because at the end of the luteal phase of her menstrual cycle there are changes in GABAA receptor subunits (Maguire et al., 2005; Smith et al., 2007; Ferando and Mody, 2012). Substantial sex differences may exist in drugs that act on muscarinic receptors. There also are dangers in withholding treatments such as estrogen or androgen therapy. For example, if women with epilepsy are postmenopausal, estrogens may be helpful to support bone and mood. However, they may not be administered because of a suspicion that estrogens will increase the likelihood of seizures. What can be done to advance our ability to treat women (and men) optimally? A simple but difficult answer, especially in the present economic climate: more support for research in sex differences with careful and comprehensive approaches - both clinical and basic research.
Acknowledgements
Supported by R01 NS-37562, R01 DA-008259, R42 NS-064474, R21 MH-084215 and the New York Office of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akman O, Moshé SL, Galanopoulou AS. Sex-specific consequences of early life seizures. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2014.05.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–274. doi: 10.1016/s0920-1211(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: Effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Benamer HT, Grosset DG. A systematic review of the epidemiology of epilepsy in arab countries. Epilepsia. 2009;50:2301–2304. doi: 10.1111/j.1528-1167.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- Bender C, Rassetto M, de Olmos JS, Lorenzo A. Involvement of AMPA/kainate-excitotoxicity in MK-801-induced neuronal death in the retrosplenial cortex. Neuroscience. 2010;169:720–732. doi: 10.1016/j.neuroscience.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. An estrogen by any other name. Endocrinology. 2008;149:2697–2698. doi: 10.1210/en.2008-0396. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-α trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs SW, Galanopoulou AS. Altered GABA signaling in early life epilepsies. Neural Plast. 2011 doi: 10.1155/2011/527605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: Therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Caputi L, Kanvshkova T, Staak R, Abrahamczik C, Munsch T, Pape HC. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci. 2005;25:9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burneo JG, Tellez-Zenteno J, Wiebe S. Understanding the burden of epilepsy in Latin America: A systematic review of its prevalence and incidence. Epilepsy Res. 2005;66:63–74. doi: 10.1016/j.eplepsyres.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FM. Effects of 17β-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor a in rat hippocampus. Eur J Pharmacol. 2010;634:192–200. doi: 10.1016/j.ejphar.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Casetta I, Pugliatti M, Faggioli R, Cesnik E, Simioni V, Bencivelli D, De Carlo L, Granieri E. Incidence of childhood and adolescence epilepsy: A community-based prospective study in the province of Ferrara and in Copparo, Italy, 1996-2005. Eur J Neurol. 2012;19:312–316. doi: 10.1111/j.1468-1331.2011.03506.x. [DOI] [PubMed] [Google Scholar]
- Celikkas E, Erdinc OO, Metintas S, Fidan HS, Arikan I, Kalyoncu C, Ozdemir G. Incidence of epilepsy in a defined area of Central Anatolia, Turkey, after 15 years of age. Neuroepidemiology. 2010;35:221–225. doi: 10.1159/000319464. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL. The WAG/Rij rat model for absence epilepsy: Age and sex factors. Epilepsy Res. 1987;1:297–301. doi: 10.1016/0920-1211(87)90005-2. [DOI] [PubMed] [Google Scholar]
- de Lacalle S. Estrogen effects on neuronal morphology. Endocrine. 2006;29:185–190. doi: 10.1385/ENDO:29:2:185. [DOI] [PubMed] [Google Scholar]
- de Olmos S, Bueno A, Bender C, Lorenzo A, de Olmos J. Sex differences and influence of gonadal hormones on MK-801-induced neuronal degeneration in the granular retrosplenial cortex of the rat. Brain Struct. Funct. 2008;213:229–238. doi: 10.1007/s00429-008-0186-0. [DOI] [PubMed] [Google Scholar]
- Desgent S, Duss S, Sanon NT, Lema P, Levesque M, Hebert D, Rebillard RM, Bibeau K, Brochu M, Carmant L. Early-life stress is associated with gender-based vulnerability to epileptogenesis in rat pups. PLoS One. 2012;7:e42622. doi: 10.1371/journal.pone.0042622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor α levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy AM, Schaner MJ, Wu SH, Staniszewski A, Kumar A, Arevalo JC, Arancio O, Chao MV, Scharfman HE. A selective role for ARMS/kidins220 scaffold protein in spatial memory and trophic support of entorhinal and frontal cortical neurons. Exp Neurol. 2011;229:409–420. doi: 10.1016/j.expneurol.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, Ng MM, Asa S, MacLusky NJ. Limbic seizures alter reproductive function in the female rat. Epilepsia. 1999;40:1370–1377. doi: 10.1111/j.1528-1157.1999.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Dortok D, Tam J, Won D, Burnham WM. Prenatal stress alters seizure thresholds and the development of kindled seizures in infant and adult rats. Horm Behav. 2002;42:437–447. doi: 10.1006/hbeh.2002.1839. [DOI] [PubMed] [Google Scholar]
- Feinstein I, Kritzer MF. Acute NMDA hypofunction induced by MK-801 evokes sex-specific changes in behaviors observed in open-field testing in adult male and proestrus female rats. Neuroscience. 2013;228:200–214. doi: 10.1016/j.neuroscience.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, Mody I. GabaA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia. 2012;53(Suppl 9):89–101. doi: 10.1111/epi.12038. [DOI] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345:285–294. doi: 10.1007/s00441-011-1221-7. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, gper-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Diaz Brinton R, Thompson RF. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008;15:589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle in the rat. In: Knobil E, Nieill J, editors. The Physiology of Reproduction. Academic Press; New York: 2006. pp. 2327–2388. [Google Scholar]
- Fregly MJ. Attenuation of pilocarpine-induced drinking by chronic treatment with estrogens. Proc Soc Exp Biol Med. 1980;164:178–183. doi: 10.3181/00379727-164-40844. [DOI] [PubMed] [Google Scholar]
- Frye CA. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia. 2010;51(Suppl 3):135–140. doi: 10.1111/j.1528-1167.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. GABA(A) receptors in normal development and seizures: friends or foes? Curr Neuropharmacol. 2008a;6:1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008b;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi FS, Galanopoulou AS, Moshé SL. Sex dimorphism in seizure-controlling networks. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2014.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Russek SJ, Brooks-Kayal AR. Molecular pathways controlling inhibitory receptor expression. Epilepsia. 2012;53(Suppl 9):71–78. doi: 10.1111/epi.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FA, Jeavons PM. Photosensitive epilepsy. Wiley; New York: 1999. [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden C, MacLusky NJ. Aromatase inhibitors as add-on treatment for men with epilepsy. Expert Rev Neurother. 2005;5:123–127. doi: 10.1586/14737175.5.1.123. [DOI] [PubMed] [Google Scholar]
- Herink J. Atropine-induced convulsions in the septohippocampal system. I. Effects of cannula position and sex. Acta Medica (Hradec Kralove) 1998;41:131–134. [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: A population-based study in rochester, minnesota. Neurology. 2011;76:23–27. doi: 10.1212/WNL.0b013e318204a36a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra M, Vazquez M, Fagiolino P, Derendorf H. Sex related differences on valproic acid pharmacokinetics after oral single dose. J Pharmacokinet Pharmacodyn. 2013;40:479–486. doi: 10.1007/s10928-013-9323-3. [DOI] [PubMed] [Google Scholar]
- Janszky J, Schulz R, Janszky I, Ebner A. Medial temporal lobe epilepsy: Gender differences. J Neurol Neurosurg Psychiatry. 2004;75:773–775. doi: 10.1136/jnnp.2003.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Kapur J. Gabaergic transmission in temporal lobe epilepsy: The role of neurosteroids. Exp Neurol. 2013;244:36–42. doi: 10.1016/j.expneurol.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Zhang C, Qiu J, Ronnekleiv OK. Pacemaking kisspeptin neurons. Exp Physiol. 2013;97:1545–1543. doi: 10.1113/expphysiol.2013.074559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kight KE, McCarthy MM. Using sex differences in the developing brain to identify nodes of influence for seizure susceptibility and epileptogenesis. Neurobiol. Dis. 2014 doi: 10.1016/j.nbd.2014.05.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Lee SY, Chung SE, Cheong HK, Jung KY, Korean Epilepsy S. Clinical characteristics of patients with treated epilepsy in korea: A nationwide epidemiologic study. Epilepsia. 2014;55:67–75. doi: 10.1111/epi.12469. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;29:1457–1468. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Huguenard JR, Fisher RS. Gender and age differences in expression of gabaa receptor subunits in rat somatosensory thalamus and cortex in an absence epilepsy model. Neurobiol Dis. 2007;25:623–630. doi: 10.1016/j.nbd.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. Effect of thiamine tetrahydrofurfuryl disulfide on audiogenic seizures in DBA/2J mice. Dev Pharmacol Ther. 1982;4:28–36. doi: 10.1159/000457388. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mann DR, Barraclough CA. Role of estrogen and progesterone in facilitating lh release in 4-day cyclic rats. Endocrinology. 1973;93:694–699. doi: 10.1210/endo-93-3-694. [DOI] [PubMed] [Google Scholar]
- Marino SE, Birnbaum AK, Leppik IE, Conway JM, Musib LC, Brundage RC, Ramsay RE, Pennell PB, White JR, Gross CR, Rarick JO, Mishra U, Cloyd JC. Steady-state carbamazepine pharmacokinetics following oral and stable-labeled intravenous administration in epilepsy patients: Effects of race and sex. Clin Pharmacol Ther. 2012;91:483–488. doi: 10.1038/clpt.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: The not so inconvenient truth. J Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McHugh JC, Delanty N. Epidemiology and classification of epilepsy: Gender comparisons. Int Rev Neurobiol. 2008;83:11–26. doi: 10.1016/S0074-7742(08)00002-0. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Scharfman HE. Temporal lobe epilepsy and the BDNF receptor, trkb. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th. Oxford University Press; New York: 2012. [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Interdependence of oestrogen and insulin-like growth factor-1 in the brain: Potential for analysing neuroprotective mechanisms. J Endocrinol. 2005;185:11–17. doi: 10.1677/joe.1.06058. [DOI] [PubMed] [Google Scholar]
- Nickel J, Jokeit H, Wunderlich G, Ebner A, Witte OW, Seitz RJ. Gender-specific differences of hypometabolism in MTLE: Implication for cognitive impairments. Epilepsia. 2003;44:1551–1561. doi: 10.1111/j.0013-9580.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- Ortiz-Gonzalez XR, Poduri A, Roberts CM, Sullivan JE, Marsh ED, Porter BE. Focal cortical dysplasia is more common in boys than in girls. Epilepsy Behav. 2013;27:121–123. doi: 10.1016/j.yebeh.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos CP. The Epilepsies: Seizures, Syndromes and Management. Bladon Medical Publishing; Oxfordshire: 2005. Reflex seizures and reflex epilepsies. [PubMed] [Google Scholar]
- Pereira RT, Porto CS, Godinho RO, Abdalla FM. Effects of estrogen on intracellular signaling pathways linked to activation of muscarinic acetylcholine receptors and on acetylcholinesterase activity in rat hippocampus. Biochem Pharmacol. 2008;75:1827–1834. doi: 10.1016/j.bcp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Persad V, Cortez MA, Snead OC., 3rd A chronic model of atypical absence seizures: Studies of developmental and gender sensitivity. Epilepsy Res. 2002;48:111–119. doi: 10.1016/s0920-1211(01)00319-9. [DOI] [PubMed] [Google Scholar]
- Persinger MA, Makarec K, Bradley JC. Characteristics of limbic seizures evoked by peripheral injections of lithium and pilocarpine. Physiol Behav. 1988;44:27–37. doi: 10.1016/0031-9384(88)90342-3. [DOI] [PubMed] [Google Scholar]
- Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current Ih is mediated by circulating 17β-estradiol in preoptic area kisspeptin neurons. J Neurosci. 2013;33:10828–10839. doi: 10.1523/JNEUROSCI.1021-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeolet E, Jacqmin P, Sargentini-Maier ML, Stockis A. Population pharmacokinetics of levetiracetam in japanese and western adults. Clin Pharmacokinet. 2007;46:503–512. doi: 10.2165/00003088-200746060-00004. [DOI] [PubMed] [Google Scholar]
- Poolos NP. H-channel dysfunction in generalized epilepsy: It takes two. Epilepsy Curr. 2006;6:88–90. doi: 10.1111/j.1535-7511.2006.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, Foote SJ, Snutch TP, O'Brien TJ. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–80. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KL, Kyi M, Reid CA, Paradiso L, D'Abaco GM, Kaye AH, Foote SJ, O'Brien TJ. Genetic absence epilepsy rats from Strasbourg have increased corticothalamic expression of stargazin. Neurobiol Dis. 2008;31:261–265. doi: 10.1016/j.nbd.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Qiao GF, Qian Z, Sun HL, Xu WX, Yan ZY, Liu Y, Zhou JY, Zhang HC, Wang LJ, Pan XD, Fu Y. Remodeling of hyperpolarization-activated current, Ih, in ah-type visceral ganglion neurons following ovariectomy in adult rats. PLoS One. 2013;8:e71184. doi: 10.1371/journal.pone.0071184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of hormones and neurosteroids in epileptogenesis. Front. Cell. Neurosci. 2013;7:1–20. doi: 10.3389/fncel.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroids - endogenous regulation of seizure susceptibility and role in the treatment of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th Oxford University Press; New York: 2012. pp. 982–1000. [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Progesterone, neurosteroids, and the hormonal basis of catamenial epilepsy. Ann Neurol. 2003;53:288–291. doi: 10.1002/ana.10534. [DOI] [PubMed] [Google Scholar]
- Sadaghiani MM, Saboory E. Prenatal stress potentiates pilocarpine-induced epileptic behaviors in infant rats both time and sex dependently. Epilepsy Behav. 2010;18:166–170. doi: 10.1016/j.yebeh.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Santana MT, Jackowski AP, Britto Fdos S, Sandim GB, Caboclo LO, Centeno RS, Carrete H, Jr., Yacubian EM. Gender and hemispheric differences in temporal lobe epilepsy: A VBM study. Seizure. 2014;23:274–279. doi: 10.1016/j.seizure.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Savic I, Engel J., Jr. Sex differences in patients with mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:910–912. doi: 10.1136/jnnp.65.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Epilepsy as an example of neural plasticity. Neuroscientist. 2002;8:154–173. doi: 10.1177/107385840200800211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Epilepsy. In: Zigmond M, editor. The Neurobiology of Brain Disorders. Elsevier; New York: 2014. [Google Scholar]
- Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196:73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Gray WP. Plasticity of neuropeptide Y in the dentate gyrus after seizures, and its relevance to seizure-induced neurogenesis. EXS. 2006:193–211. doi: 10.1007/3-7643-7417-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Kim M, Hintz TM, MacLusky NJ. Seizures and reproductive function: Insights from female rats with epilepsy. Ann Neurol. 2008;64:687–697. doi: 10.1002/ana.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: Coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006a;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006b;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen-growth factor interactions and their contributions to neurological disorders. Headache. 2008;48(Suppl 2):S77–89. doi: 10.1111/j.1526-4610.2008.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Malthankar-Phatak GH, Friedman D, Pearce P, McCloskey DP, Harden CL, Maclusky NJ. A rat model of epilepsy in women: A tool to study physiological interactions between endocrine systems and seizures. Endocrinology. 2009;150:4437–4442. doi: 10.1210/en.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Witter MP, Schwarcz R. The parahippocampal region. Implications for neurological and psychiatric diseases. Introduction. Ann N Y Acad Sci. 2000;911:ix–xiii. [PubMed] [Google Scholar]
- Schwarcz R, Bertram EH, Scharfman HE. Temporal lobe epilepsy: Renewed emphasis on extrahippocampal areas. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: Fifth generation of progress. American College of Neuropsychopharmacology; 2002. pp. 1844–1857. [Google Scholar]
- Seil FJ. Trkb receptor signaling and activity-dependent inhibitory synaptogenesis. Histol Histopathol. 2003;18:635–646. doi: 10.14670/HH-18.635. [DOI] [PubMed] [Google Scholar]
- Schridde U, Strauss U, Bráuer AU, van Luijtelaar G. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci. 2013;23:3346–3358. doi: 10.1111/j.1460-9568.2006.04865.x. [DOI] [PubMed] [Google Scholar]
- Singh KB. Persistent estrus rat models of polycystic ovary disease: An update. Fertil Steril. 2005;84(Suppl 2):1228–1234. doi: 10.1016/j.fertnstert.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, Free SL, Thom M, Everitt AE, Fish DR, Shorvon SD. Evidence for nodular epileptogenicity and gender differences in periventricular nodular heterotopia. Neurology. 1999;52:336–341. doi: 10.1212/wnl.52.2.336. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, McMahon LL. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34(Suppl 1):S130–142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS. Withdrawal properties of a neuroactive steroid: Implications for GABA(A) receptor gene regulation in the brain and anxiety behavior. Steroids. 2002;67:519–528. doi: 10.1016/s0039-128x(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the α4 and δ subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia. 1999;40:631–636. doi: 10.1111/j.1528-1157.1999.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Taylor I, Hodgson B, Scheffer IE, Mulley J, Berkovic SF, Dibbens L. Is photosensitive epilepsy less common in males due to variation in X chromosome photopigment genes? Epilepsia. 2007;48:1807–1809. doi: 10.1111/j.1528-1167.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- Tolmacheva EA, van Luijtelaar G. The role of ovarian steroid hormones in the regulation of basal and stress induced absence seizures. J Steroid Biochem Mol Biol. 2007;104:281–288. doi: 10.1016/j.jsbmb.2007.03.017. [DOI] [PubMed] [Google Scholar]
- van Huizen F, March D, Cynader MS, Shaw C. Muscarinic receptor characteristics and regulation in rat cerebral cortex: Changes during development, aging and the oestrous cycle. Eur J Neurosci. 1994;6:237–243. doi: 10.1111/j.1460-9568.1994.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L. β-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AS, Kerschbaumsteiner K, Stelzhammer B, Meindl M, Kaaya J, Schmutzhard E. Prevalence, incidence, and clinical characteristics of epilepsy--a community-based door-to-door study in northern tanzania. Epilepsia. 2009;50:2310–2313. doi: 10.1111/j.1528-1167.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- Wirth MJ, Patz S, Wahle P. Transcellular induction of neuropeptide y expression by NT4 and BDNF. Proc Natl Acad Sci U S A. 2005;102:3064–3069. doi: 10.1073/pnas.0404712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue BW, Huguenard JR. The role of h-current in regulating strength and frequency of thalamic network oscillations. Thalamus Relat Syst. 2001;1:95–103. doi: 10.1016/S1472-9288(01)00009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]