Abstract
Phosphatidylcholine (PC) is the most abundant phospholipid in the membranes of the human parasite Leishmania. It is synthesized via two metabolic routes, the de novo pathway that starts with the uptake of choline, and the threefold methylation of phosphatidylethanolamine. Choline was shown to be dispensable for Leishmania; thus, the methylation pathway likely represents the primary route for PC production. Here, we have identified and characterized two phosphatidylethanolamine methyltransferases, LmjPEM1 and LmjPEM2. Both enzymes are expressed in promastigotes as well as in the vertebrate form amastigotes, suggesting that these methyltransferases are important for the development of the parasite throughout its life cycle. These enzymes are maximally expressed during the log phase of growth which correlates with the demand of PC synthesis during cell multiplication. Immunofluorescence studies combined with cell fractionation have shown that both methyltransferases are localized at the endoplasmic reticulum membrane. Heterologous expression in yeast has demonstrated that LmjPEM1 and LmjPEM2 complement the choline auxotrophy phenotype of a yeast double null mutant lacking phosphatidylethanolamine methyltransferase activity. LmjPEM1 catalyzes the first, and to a lesser extent, the second methylation reaction. In contrast, LmjPEM2 has the capacity to add the second and third methyl group onto phosphatidylethanolamine to yield (lyso)PC; it can also add the first methyl group, albeit with very low efficiency. Finally, we have demonstrated using inhibition studies with choline analogs that miltefosine and octadecyltrimethylammonium bromide are potent inhibitors of this metabolic pathway.
Keywords: Leishmania major, Phosphatidylethanolamine methyltransferase, Phosphatidylcholine biosynthesis, Choline analogs
1. Introduction
Leishmania species are protozoan parasites of medical relevance that cause a wide range of important human and animal diseases, collectively named leishmaniasis. They affect at least 10 million patients worldwide, primarily in tropical and sub-tropical areas of the world, where approximately 300 million people are at risk. This parasite cycles between the sand fly vector as a flagellated promastigote and the vertebrate host’s macrophages as a non-motile amastigote.
Phosphatidylcholine (PC) is the most abundant lipid of the Leishmania biological membrane, representing 30–40% of total cellular lipids [1,2,3]. Structurally, it contains unusually long unsaturated fatty acid chains that are believed to confer a higher degree of resistance towards host-derived oxidants [3]. PC fulfills its structural function as a main constituent of cellular membranes. However, it also serves as a reservoir of secondary messenger metabolites (e.g. PA, DAG, and lysoPC) that play critical roles in signaling pathways controlling key cellular processes including mitogenesis, cell differentiation, and gene transcription (reviewed in [4,5,6,7]). PC seems to be an essential lipid of Leishmania membranes because a decrease of PC levels to 25% or below is deleterious to the parasite [8].
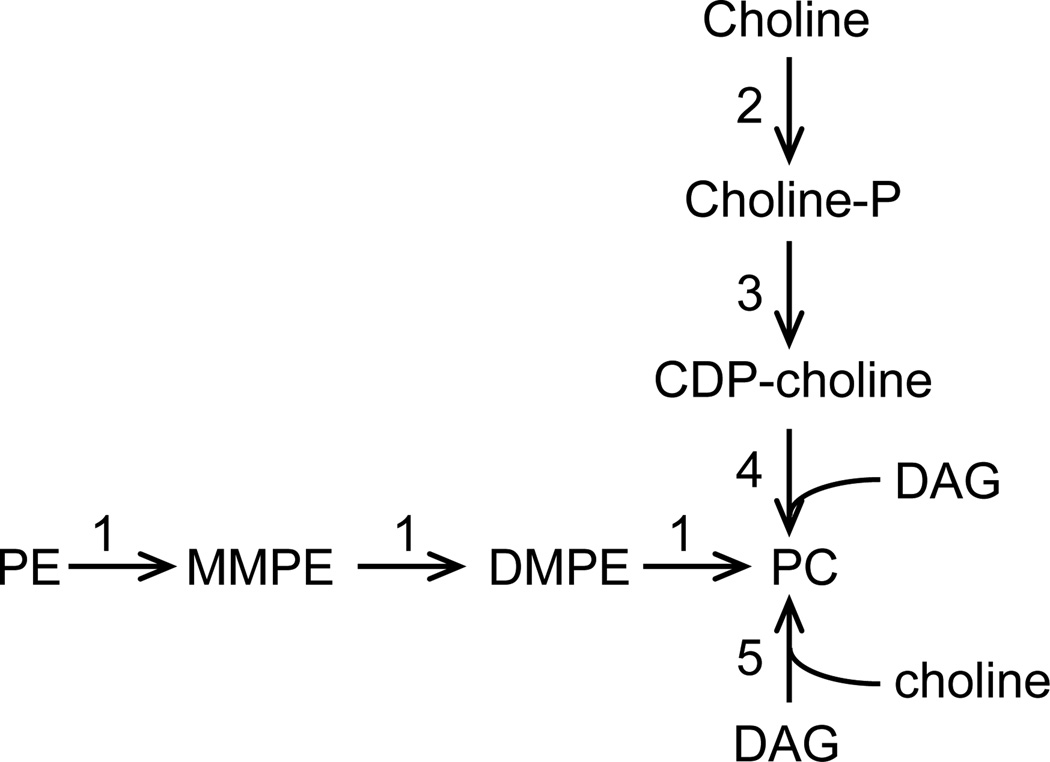
Three distinct routes lead to PC biosynthesis (Fig. 1). Firstly, the de novo pathway starts with the uptake of extracellular choline which is subsequently phosphorylated to give phosphocholine. The latter is then linked to a diacylglycerol (DAG) moiety to yield PC. Secondly, PC can be produced by the threefold methylation of phosphatidylethanolamine (PE) by one or several PE methyltransferases (PEMT) that utilize S-adenosylmethionine (SAM) as the methyl donor (reviewed in [9,10,11,12]). Lastly, in a pathway seemingly restricted to prokaryotes, PC results from the condensation of choline with CDP-DAG carried out by a PC synthase (reviewed in [11,12]). PC biosynthesis in Leishmania seems to occur via the de novo and methylation pathways (Fig. 1; [8]). Based on biochemical studies, Leishmania has the capacity to take up choline from the medium or the host [13]. Sequence analysis of the L. major genome reveals the presence of orthologs of the choline kinase (Lmjf27.1420 and Lmjf35.1470), CTP:phosphocholine cytidylyltransferase (Lmjf18.1330), and choline phosphotransferase (Lmjf18.0810); however, the enzymatic activities linked to these gene products have yet to be established [13,14]. In addition, Leishmania has the ability to convert PE into PC, suggesting that it possesses one or several PE methyltransferases [8].
Fig. 1.
General PC biosynthetic pathways. 1. PEMT; 2. choline kinase; 3. phosphocholine cytidylyltransferase; 4. choline phosphotransferase; 5. PC synthase. DAG, diacylglycerol; DM, dimethyl; MM, monomethyl; P, phosphate.
PEMT enzymes have been characterized from various organisms (reviewed in [9,11,12,15]). Eukaryotic PEMT can be divided into two classes based on substrate specificity and protein structure. In yeasts, such as Saccharomyces cerevisiae and S. pombe, class II PEMTs have an internal sequence duplication and carry out the first methylation step, forming monomethyl-PE (MMPE) from PE [15,16]. Class I PEMTs usually catalyze the last two methylation reactions to form PC from MMPE with dimethyl-PE (DMPE) as the intermediate. Additionally, yeast class I PEMTs are related to mammalian PEMT which carries out all three methylation steps. Interestingly, in S. cerevisiae, ScPEM2, a class I PEMT, can perform all three methylation reactions as the mammalian counterpart, although it performs the first methylation step with much lower efficacy than the class II PEMT enzyme, ScPEM1 [16].
Phosphocholine analogs, such as miltefosine and edelfosine, have potent anti-microbial activities, including anti-leishmanial activity. Several potential mechanisms responsible for the anti-leishmanial properties of these compounds have been proposed ([17,18,19,20]; reviewed in [21,22,23]). These potential mechanisms include interference with enzymes involved in lipid metabolism, induction of apoptosis, instigation of mitochondrial dysfunction, inhibition of cell signaling, and immunostimulation. However, their exact mechanism of action has yet to be established.
Choline is not essential for L. major growth demonstrating that the de novo pathway is dispensable for PC production in this parasite [13]. Thus, PE methylation likely represents the primary route for PC biosynthesis. To address this hypothesis, we have initiated a characterization of this metabolic pathway by identifying the PE methyltransferase enzymes and determining their substrate specificity, subcellular localization, and their inhibition by choline analogs.
2. Materials and Methods
2.1. Strains and media
Promastigotes of L. major Friedlin strain V1 (MHOM/IL/80/Friedlin) were grown in liquid M199-derived media [24]. Amastigotes were isolated from mouse footpad lesions resulting from inoculation with wild-type Leishmania as described in [24] following protocol 1697.0 approved by the Institutional Animal Care and Use Committees (IACUC) at St John’s University.
The Saccharomyces cerevisiae strains used in this study are listed in Table 1. Standard methods for yeast culture, transformation, and genomic DNA isolation were used [25]. Yeast was cultivated at 30°C in YPD rich medium (1% yeast extract, 2% Bacto peptone, and 2% glucose) or synthetic minimal medium (yeast nitrogen base, 2% glucose). The synthetic minimal medium was supplemented with histidine (30 µg/ml), uracil (30 µg/ml), leucine (100 µg/ml), methionine (100 µg/ml), or choline (10 µM) as required to maintain cell growth.
Table 1.
S. cerevisiae strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| By4741 (Sc1) | MATαhis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| scpem1Δscpem2Δ (Sc55) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr | [45] |
| scpem1Δscpem2Δ+U+L (Sc140) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U][pBEVY-L] | this work |
| scpem1Δscpem2Δ+LmjPEM1+L (Sc118) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U-LmjPEM1][pBEVY-L] | this work |
| scpem1Δscpem2Δ+ScPEM1+L (Sc50) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U-ScPEM1][pBEVY-L] | this work |
| scpem1Δscpem2Δ+U+LmjPEM2 (Sc82) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U][pBEVY-L-LmjPEM2] | this work |
| scpem1Δscpem2Δ+U+ScPEM2 (Sc174) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U][pBEVY-L-ScPEM2] | this work |
| scpem1Δscpem2Δ+LmjPEM1+LmjPEM2 (Sc84) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U-LmjPEM1][pBEVY-L-LmjPEM2] | this work |
| scpem1Δscpem2Δ+ScPEM1+ScPEM2 (Sc175) | MATα his3ΔI leu2Δ0 ura3Δ0 pem1::Kanr-pem2::Kanr [pBEVY-U-ScPEM1][pBEVY-L-ScPEM2] | this work |
For growth curves, yeast cultures were grown in synthetic minimal medium supplemented with 1 µM choline, washed twice in sterile water, and resuspended to an OD600=0.1 in minimal medium in the absence or presence of 10 µM choline. The turbidity of the cultures was monitored with a spectrophotometer as a function of time.
2.2. Molecular biology
Genomic DNA from L. major and Trypanosoma brucei was prepared as described by Acosta-Medina and Cross [26].
pXGHYG2.SS-GFP-MDDL (Ec613) was created as follows. The signal sequence of T. brucei BIP was PCR-amplified with primers O281 (5’-CCCGGGATGTCGAGGATGTGGCTGAC-3’) and O290 (5’-TCTAGAGTATGTTGTGCCGAGGTCGATG-3’) using wild-type genomic DNA as template. The GFP-MDDL encoding region was amplified with O291 (5‘-TCTAGAGTGAGCAAGGGCGAGGAG-3’) and O280 (5’-GGGCCCTTACAGATCGTCCATCTTGTACAGCTCGTCCATGC-3’) using pXG.GFP’ as template [27]. Both resulting DNA fragments were cut with XmaI and XbaI, and XbaI and ApaI, respectively, and triple ligated into the XmaI and ApaI sites of pXGHYG2 (derived from pXG.HYG [28] but bears an ApaI site downstream of the XmaI site).
Expression vectors GST-LmjPEM1 (Ec648) and GST-LmjPEM2 (Ec652) were constructed using genomic DNA from L. major FV1 as a template. Primers O352 (5’-CGAATTCTCACTGATTCTTGCGACATTC-3’) and O353 (5’-GCGGATCCATGCGCAAGCGCTACGGTAAC-3’) were applied to amplify the 3’end of LmjPEM1, while O350 (5’-CGAATTCTCACTGCTTCTTCACCGAGGC-3’) and O351 (5’-GCGGATCCCTGGTGTATCATGTGTCGAC-3’) were used to amplify the 3’end of LmjPEM2. Both PCR products were digested with BamHI and EcoRI, and ligated into the corresponding sites of pGEX-2T (GE Healthcare Bio-Science AB). The resulting heterologous proteins bear the 46 and 39 C-terminal amino acids of LmjPEM1 and LmjPEM2, respectively, fused to the C-terminus of the glutathione S-transferase.
The plasmid pBEVY-L-LmjPEM2 (Ec723) was generated by PCR-amplifying LmjPEM2 with oligonucleotides O391 (5’-CGGATCCATGACGCAGTTGCCCAC-3’) and O390 (5’-CGGATCCTCACTGCTTCTTCACCGAGGCAG-3’) using genomic DNA from L. major FV1 as a template. The obtained PCR products were digested with BamHI and cloned in sense orientation into the BamHI site of pBEVY-L [29]. pBEVY-U-LmjPEM1 (Ec714) was created as follows. LmjPEM1 was amplified with the primers O388 (5’-CGGATCCTCACTGATTCTTGCGACATTCCAG-3’) and O370 (5’-GGATCCATGTCTCTTGAAAGCGCGTC-3’) using genomic DNA from L. major FV1 as a template. The resulting PCR products were digested with BamHI and cloned in sense orientation into the BamHI site of pBEVY-U [29].
The episomes pBEVY-U-ScPEM1 (Ec886) and pBEVY-L-ScPEM2 (Ec887) were constructed using wild-type S. cerevisiae genomic DNA as a template. Primers O526 (5’-GTCTAGATGTCCAGTTGTAAAACCACTTTGTC-3’) and O532 (5’-CCTGCAGTCAAGCAAGACTATCAAGCGTTTG-3’) were applied to amplify the ScPEM1 (YGR157W) gene, while O531 (5’-GTCTAGATGAAGGAGTCAGTCCAAGAG-3’) and O533 (5’-CCTGCAGTTACATATTCTTTTTGGCCTTATCACGG-3’) were used to amplify the ScPEM2 (YJR073C) gene. Resulting DNA fragments were digested with PstI and XbaI, and ligated into the respective sites of pBEVY-U and pBEVY-L, respectively [29]. All PCR products were verified by sequencing.
2.3. Antigen production and purification
Recombinant proteins GST-LmjPEM1 and GST-LmjPEM2 were produced in the Escherichia coli strain pLysS (F− ompT hsdSB (rB−mB−) gal dcm (DE3) pLysE (CamR); Novagen); their expression was induced in the presence of 0.04 mM IPTG for 5 hr at 37°C. Both recombinant proteins were detected in inclusion bodies that were isolated by lysing the cells in phosphate buffered saline (PBS) containing 1 mM EDTA using sonication. Inclusion bodies were pelleted by centrifugation at 15,000 g and washed three times in PBS. Finally, the inclusion bodies were solubilized in 1× SDS-PAGE loading dye and the recombinant proteins separated by SDS-PAGE. The gel area containing the recombinant protein was used for immunization of two rabbits according to the NIH guidelines. This was accomplished by the company Cocalico Inc. The obtained sera were subsequently purified according to a small scale protocol described in [30]. Briefly, the antibodies were purified by binding to GST-LmjPEM1 or GST-LmjPEM2 recombinant proteins previously electroblotted onto a PVDF membrane, followed by elution with 100 mM glycine, pH 2.5. Purified anti-LmjPEM1 and anti-LmjPEM2 antibodies were used throughout this work.
2.4. Digitonin fractionation, protein quantification, Western blot, and immunofluorescence assay
Digitonin fractionation was carried out as follows. Approximately 2×108 late-log phase parasites were washed once with cold PBS. Cell pellets were resuspended in 1 ml TED buffer (20 mM TrisHCl, pH8.0, 1 mM EDTA, 1 mM dithiothreitol, protease inhibitor cocktail (Roche Life Sciences)) and 100 µl aliquots were prepared into seven tubes. Cells were permeabilized by adding digitonin (from a stock solution of 15 mg/ml in PBS) to final concentrations of 0.075, 0.15, 0.30, 0.45, 0.6, and 0.75 mg/ml and incubating at 26°C for 10 min. Supernatants and pellet fractions were separated by centrifugation at 20,800 g for 3 min at 4°C.
Protein concentration was determined according to the bicinchoninic assay with bovine serum albumin as a standard. Western blots and immunofluorescence assays were carried out as described before [24]. The purified anti-LmjPEM1 and anti-LmjPEM2 antibodies were used at 1:500 and 1:250 dilutions, respectively, for Western blots and immunofluorescence assays.
2.5. Enzymatic assay
Yeast cultures were grown to an optical density of OD600 =1.0 and washed twice in ice cold water. Leishmania parasites were harvested at mid-log (5×106/ml), late-log (2–5×107 /ml) or stationary phase of growth and washed once in cold PBS. Yeast and Leishmania cell pellets were then resuspended in lysis buffer (0.5 M sucrose, 0.1 M TrisHCl, pH7.5, 2 mM EDTA, and protease inhibitor) and lysed using beads by vortexing for 10 min at 4°C. Cell debris was separated by low speed centrifugation (1300 g) and the supernatant was used for the PEMT assay. Reactions were set up using 200 µg protein and 0.1 µCi S-[Methyl-3H]adenosyl-L-methionine (specific activity of 15 Ci/mMole; Perkin Elmer) in 0.1 M TrisHCl, pH7.5. Reactions were incubated at 30°C for 50 minutes and stopped by the addition of 2 ml of chloroform/methanol (1:1 (v/v)). Total lipids were then extracted and quantified by liquid scintillation spectrometry (Perkin-Elmer; [31]). Each experiment was performed at least twice in duplicate.
2.6. Lipid analysis
Yeast strains By4741 (wild type), scpem1Δscpem2Δ+LmjPEM1+L, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2, and scpem1Δscpem2Δ+U+LmjPEM2 were grown in minimal media lacking choline and harvested at an OD600=1–1.5. The strain scpem1Δscpem2Δ+U+L was grown in minimal medium containing 5 µg/ml choline to an OD600=0.3, washed twice in water and further incubated in minimal medium lacking choline for 10 hr. Lipids were purified according to [32] and were profiled by electrospray ionization tandem mass spectrometry using the method described by Zufferey et al. [33], except that internal standards were (with some small variation in amounts in different batches of internal standards): 0.6 nmol di12:0-PC, 0.6 nmol di24:1-PC, 0.6 nmol 13:0-lysoPC, 0.6 nmol 19:0-lysoPC, 0.3 nmol di12:0-PE, 0.3 nmol di23:0-PE, 0.3 nmol,14:0-lysoPE, 0.3 nmol 18:0-lysoPE, 0.3 nmol 14:0-lysophosphatidylglycerol (lysoPG), 0.3 nmol 18:0-lysoPG, 0.3 nmol di14:0-phosphatidic acid (PA), 0.3 nmol di20:0(phytanoyl)-PA, 0.2 nmol di14:0-phosphatidylserine (PS), 0.2 nmol di20:0(phytanoyl)-PS, 0.23 nmol 16:0–18:0-phosphatidylinositol (PI), 0.3 nmol di14:0-PG, and 0.3 nmol di20:0(phytanoyl)-PG. In addition to the scans previously described [33], a scan for PG as [M + NH4]+ in the positive mode with NL 189.0 was performed with collision energy of 20 V, declustering potential of 100 V, and exit potential of 14 V. Scans for MMPE (NL155.0) and DMPE (NL 169.0) were performed with the same instrument parameters as for the PE scans. MMPE and DMPE were determined in comparison to the PE standards without correction for response factor for these compounds as compared to PE.
2.7. Statistical analysis
Two-tailed Student's test was used for statistical analysis which was performed using GraphPad Prism Software (version 5.0). P-values ≤0.01 were considered to be statistically significant. Asterisks indicate statistically significant differences: *** p ≤0.001, and ** p ≤0.01.
3. Results and discussion
3.1. Identification of LmjPEM1 and LmjPEM2
Biochemical studies established that PC biosynthesis occurs via two pathways in Leishmania, the de novo pathway that starts with uptake of choline, and the PE methylation pathway (Fig. 1; [8]). Previous studies have shown that choline is dispensable for growth of L. major [13]. Thus, to understand the importance of the PE methylation pathway in the biosynthesis of PC in this parasite, we searched for PE methyltransferase (PEMT) gene orthologs in L. major genome using the Saccharomyces cerevisiae PEMTs ScPEM1 (gene ID YGR157W) and ScPEM2 (gene ID YJR073C) as a query. LmjPEM1 (LmjF31.3120) and LmjPEM2 (LmjF31.2290) were identified and they code for predicted proteins of 582 and 225 amino acids, respectively (Fig. 2). LmjPEM1 exhibits 32% and 28% identity, and 49% and 42% similarity to ScPEM1 and ScPEM2, respectively. Interestingly, LmjPEM1 does not display any significant similarity to human PEMT. In contrast, LmjPEM2 shows 43% and 41% identity, and 57% and 54% similarity to yeast ScPEM2 and human PEMT, respectively. TMHMM algorithm predicts four putative hydrophobic stretches (amino acids to 13 to 35, 54 to 76, 109 to 131, 167 to 189) in LmjPEM2 while eight nonpolar domains (amino acids 54 to 76, 86 to 105, 187 to 204, 235 to 257, 336 to 358, 403 to 425, 438 to 460, and 496 to 518) could be identified in LmjPEM1. In addition, LmjPEM2 has 3 conserved blocks of amino acids shared by PEMT enzymes. These domains may be involved in substrate recognition or catalysis. Notably, similar to ScPEM1, LmjPEM1 bears two of these domains and thus, may arise from internal gene duplication. Both LmjPEM1 and LmjPEM2 carry a putative C-terminal endoplasmic reticulum retrieval signal (RKNQ and VKKQ, respectively), suggesting that they reside in this organelle [34]. In contrast, mammalian counterparts and the yeast ScPEM1 lack an endoplasmic retrieval signal but not ScPEM2 (KKNM; data not shown).
Fig. 2.
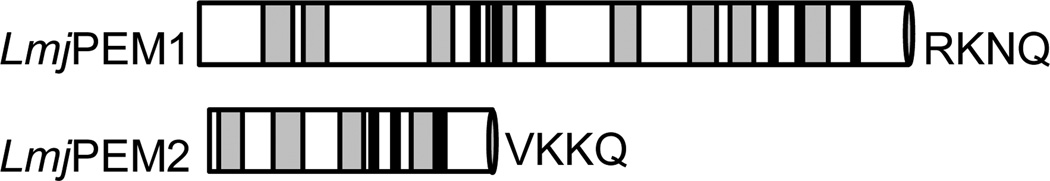
Schematic representation of the PE methyltransferases LmjPEM1 and LmjPEM2 of L. major. The grey rectangles represent the putative transmembrane spans, the black rectangles depict the conserved amino acids diagnostic of PE methyltransferases, and the grey ovals symbolize the putative endoplasmic reticulum retention motif.
3.2. LmjPEM1 and LmjPEM2 are expressed throughout the life cycle of Leishmania
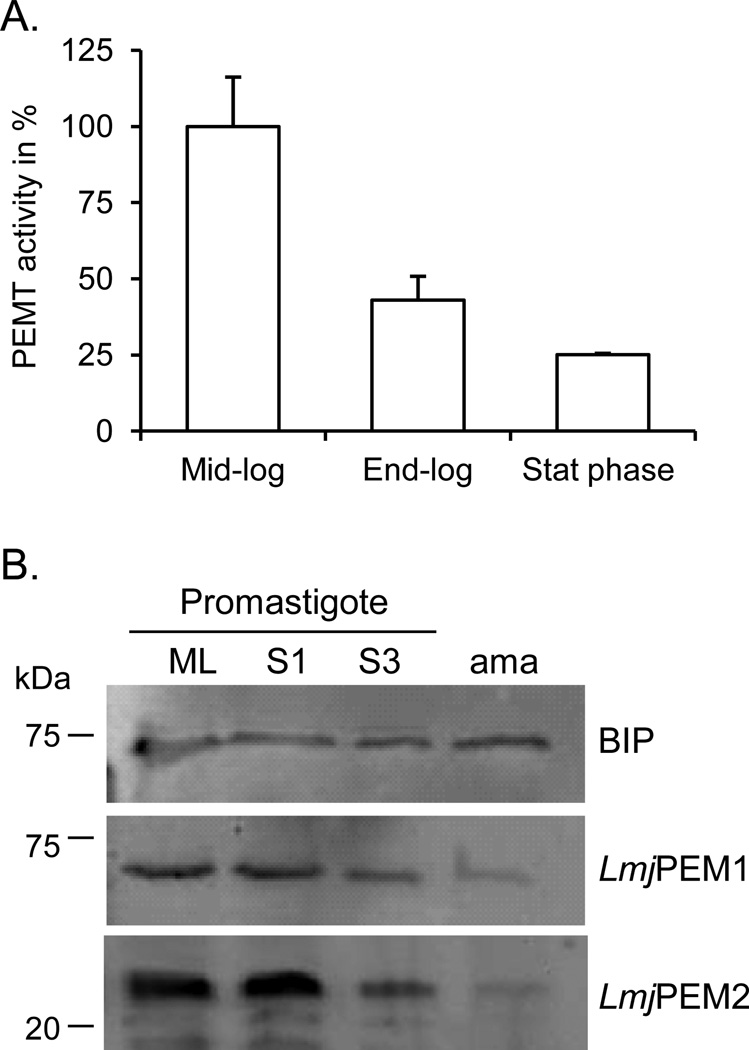
To address whether PEMT activity is regulated during the growth phase of the parasite, PEMT activity was measured with whole cell extracts derived from promastigotes harvested at different stages of growth (Fig. 3). PEMT activity was highest in mid-log parasites that are actively dividing (Fig. 3A). In contrast, PEMT activity in stationary phase parasites dropped to 25% of that of replicating cells. Western blot analyses performed with LmjPEM1 and LmjPEM2 specific antibodies confirmed these results. LmjPEM1 and LmjPEM2 levels were highest when the cells were actively growing (mid-log phase) and their expression decreased as the cells entered stationary phase (Fig. 3B). Thus, the level of expression of LmjPEM1 and LmjPEM2 seems to correlate with the demand for PC biosynthesis linked to cell growth and multiplication. These enzymes are also expressed in vertebrate cell stage amastigotes, although at lower levels, suggesting that the methylation pathway is functional and may be important for PC production in the vertebrate host.
Fig. 3.
LmjPEM1 and LmjPEM2 are expressed throughout the life cycle of Leishmania. (A) Growth phase dependent PEMT assay was performed as described in Materials and methods. The enzymatic assay was carried out twice in duplicate and a representative experiment is shown. Standard deviations are depicted. An activity of 100% corresponds to approximately 15 pmol/mgxhr. (B) Western blot with whole cell extract derived from promastigotes harvested in mid log phase [54], 1 day (S1) or 3 day (S3) stationary growth phase, and from amastigotes (ama) in the presence of anti-LmjPEM1, anti-LmjPEM2, and anti-BIP sera [39]. Approximately 2×107 cells were loaded in each lane. The protein ladder is shown on the left.
3.3. PEMT activity in Leishmania is not regulated by choline
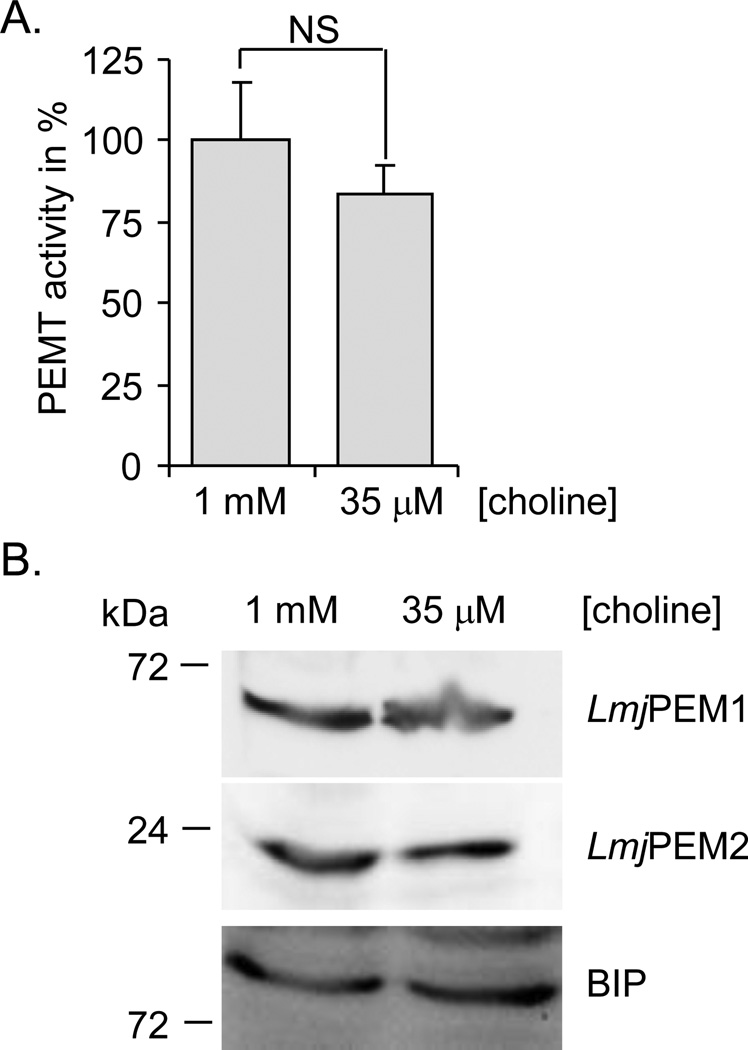
Yeast S. cerevisiae ScPEM1 and ScPEM2 have been shown to be regulated by lipid precursors, such as choline [15,35,36]. Thus, we assessed whether PEMT activity in Leishmania is regulated by choline. Cells were grown at low (35 µM) or high (1 mM) concentration of choline. Cell extracts derived from these cultures were assayed for PEMT activity and for protein expression by Western blot analysis (Fig. 4). PEMT activity was similar independently of the choline concentration present in the medium (Fig. 4A). Also, LmjPEM1 and LmjPEM2 protein levels were comparable in cells grown in low and high concentration of choline (Fig. 4B). Thus, in contrast to yeast, Leishmania does not regulate its PEMT enzymes according to choline availability.
Fig. 4.
Expression of LmjPEM1 and LmjPEM2 is choline independent. (A) PEMT assay was carried out with wild-type cells grown in the presence of low (35 µM) or high (1 mM) concentration of choline and harvested in the mid-log phase of growth. The assay was performed at least twice in duplicate and a representative experiment is shown. Standard deviations are depicted. An activity of 100% corresponds to approximately 15 pmol/mgxhr. NS denotes statistically not significant, with a p-value > 0.05. (B) Western blot analysis with cells grown in the presence of low (35 µM) or high (1 mM) concentration of choline using antibodies specific to LmjPEM1, LmjPEM2 or BIP [39] as a loading control. Approximately 2×107 cells were loaded in each lane. The protein ladder is shown on the left.
3.4. LmjPEM1 and LmjPEM2 localize to the endoplasmic reticulum membrane
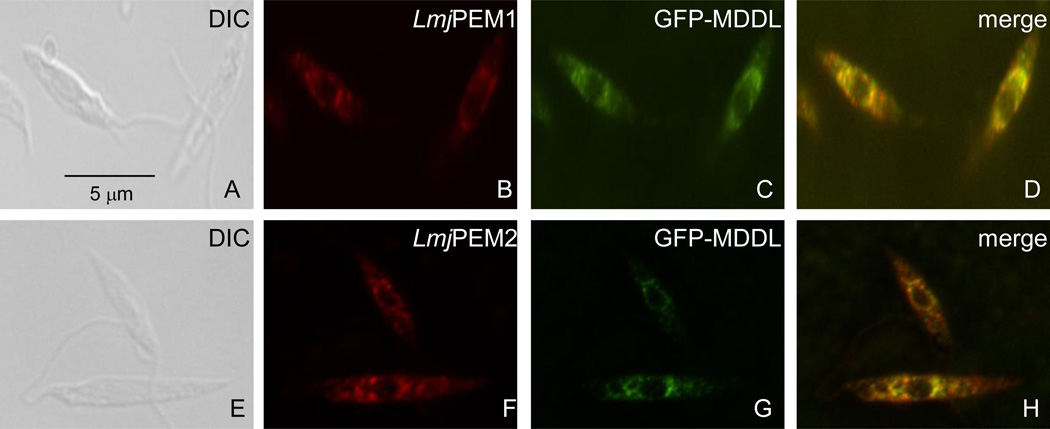
Both LmjPEM1 and LmjPEM2 bear a putative endoplasmic reticulum retention motif at their C-terminus suggesting an endoplasmic reticulum subcellular localization. Immunofluorescence assays carried out in the presence of antibodies specific to LmjPEM1 or LmjPEM2 displayed a punctate signal around the nucleus that overlapped with that of GFP-MDDL, an endoplasmic reticulum resident ([37]; Fig. 5). This demonstrates that LmjPEM1 and LmjPEM2 reside in the endoplasmic reticulum.
Fig. 5.
LmjPEM1 and LmjPEM2 localize to the endoplasmic reticulum. Immunofluorescence assays were carried out with GFP-MDDL Leishmania transformants using anti-LmjPEM1 (B) and anti-LmjPEM2 (F) antibodies. (C), (G), autofluorescence of GFP-MDDL, which is used as an endoplasmic reticulum marker [37]; (D), (H), merge of (B) and (C), and (F) and (G), respectively. The scale bar is shown.
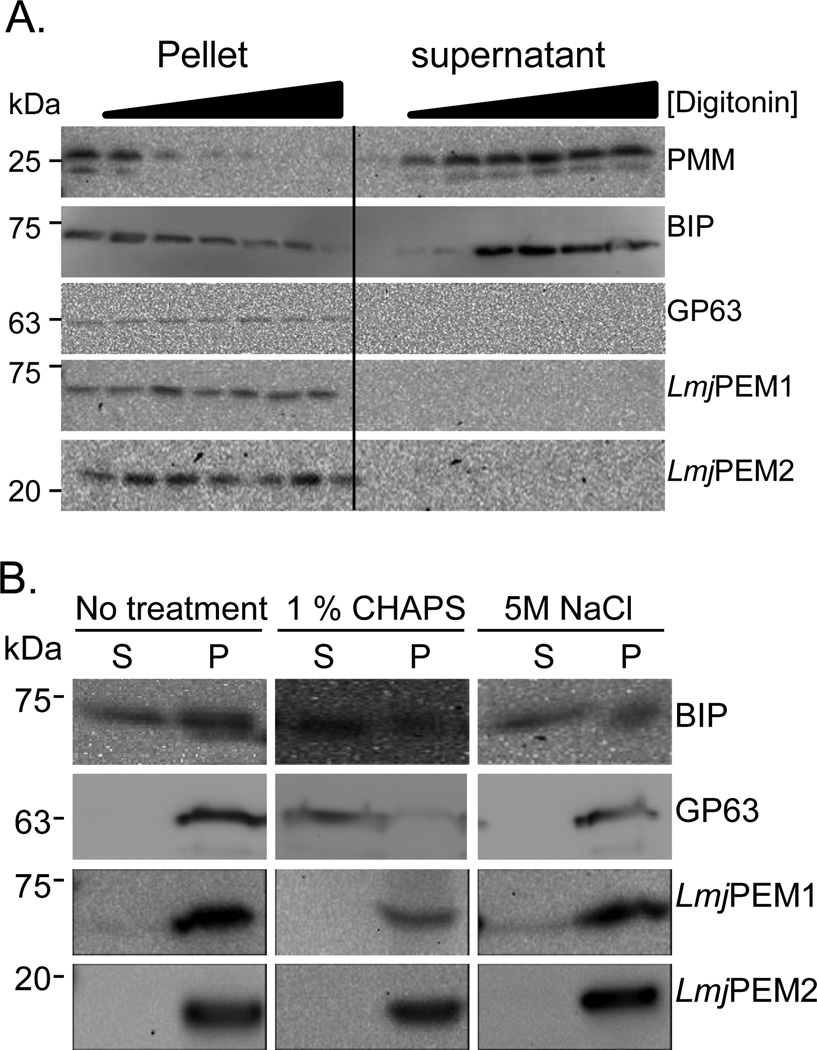
We then assessed whether LmjPEM1 and LmjPEM2 are membrane associated or integral membrane proteins as both proteins bear several hydrophobic stretches (Fig. 2). Whole cells were incubated in the presence of increasing concentration of the mild detergent digitonin, followed by separation of supernatants and pellets by centrifugation. Both fractions we re analyzed by Western blot in the presence of antisera specific to the phosphomannomutase (PMM) used as a cytosolic marker, the luminal endoplasmic reticulum marker BIP, the GPI-anchored protease GP63, LmjPEM1 and LmjPEM2 (Fig. 6A; [38,39,40]). PMM was released in the supernatant fraction at very low digitonin concentration while the luminal BIP stayed in the pellet fraction until higher concentration (0.3 mg/ml) of the detergent. The GPI-anchored integral membrane GP63 remained in the pellet even at very high digitonin concentration (0.75 mg/ml). LmjPEM1 and LmjPEM2 behaved similarly to GP63, suggesting that they are integral membrane proteins. The fact that LmjPEM1 and LmjPEM2 were not released from the pellet membrane fraction by 0.5 M NaCl or the detergent CHAPS further supports the notion that both are integral membrane proteins (Fig. 6B). The endoplasmic reticulum membrane localization of LmjPEM1 and LmjPEM2 is reminiscent of yeast orthologs ScPEM1 and ScPEM2, as well as mammalian PEMT [41,42]. It makes sense that PEMT enzymes are localized in the same organelle where PE is produced [43].
Fig. 6.
LmjPEM1 and LmjPEM2 are integral membrane proteins. (A) Whole cells were subjected to digitonin fractionation followed by Western blot analysis in the presence of antibodies specific to PMM (cytosolic marker), BIP (luminal endoplasmic reticulum marker), GP63 (GPI-anchored integral membrane protein), LmjPEM1, and LmjPEM2 [38,39,40]. (B) Whole cell extracts were incubated in the absence or presence of 1% CHAPS or 0.5 M NaCl followed by ultracentrifugation. The resulting pellets and supernatants were then analyzed by Western blot in the presence of antibodies specific to BIP, GP63, LmjPEM1, and LmjPEM2 [39,40]. (A), (B), Apparent molecular weight are shown on the left. P, pellet; S, supernatant.
3.5. LmjPEM1 and LmjPEM2 complement the choline auxotrophy phenotype of S. cerevisiae double mutant scpem1Δscpem2Δ
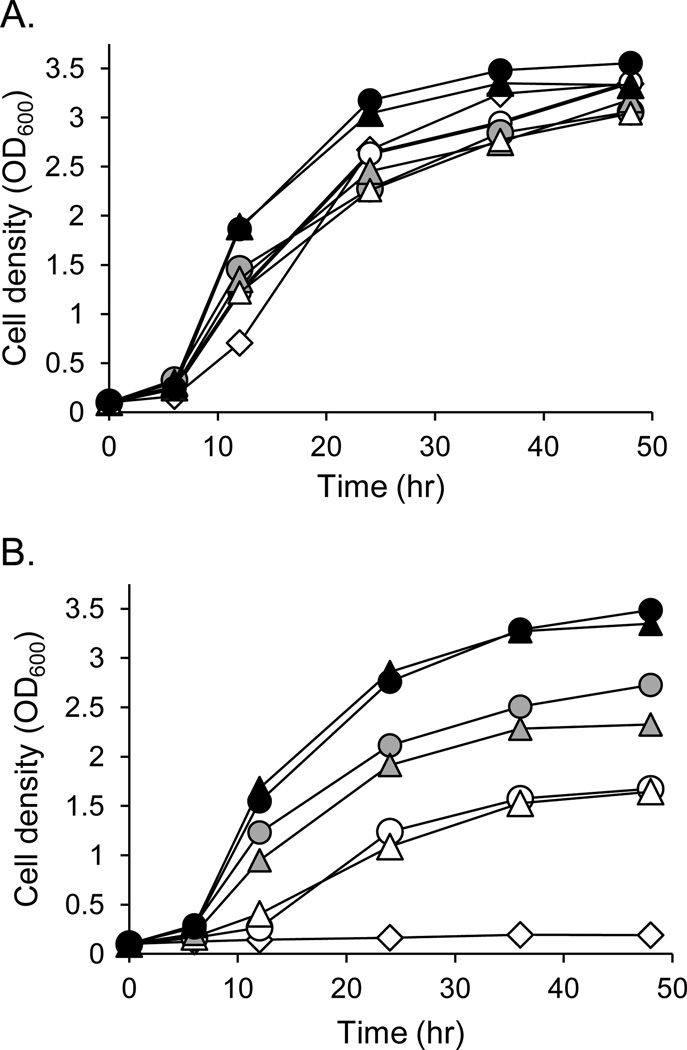
To assess whether LmjPEM1 and LmjPEM2 encode PEMT enzymes, they were expressed both individually (scpem1Δscpem2Δ+LmjPEM1+L, scpem1Δscpem2Δ+U+LmjPEM2) or together (scpem1Δscpem2Δ+LmjPEM1+LmjPEM2) in the S. cerevisiae double null mutant scpem1Δscpem2Δ that lacks endogenous PEMT activity [44,45]. An empty vector strain (scpem1Δscpem2Δ+U+L) was generated to serve as a negative control. Additionally, positive control lines expressing ScPEM1 or ScPEM2 alone (scpem1Δscpem2Δ+ScPEM1+L, scpem1Δscpem2Δ+U+ScPEM2) or together (scpem1Δscpem2Δ+ScPEM1+ScPEM2) were created. The PC biosynthetic pathways in yeast are identical to those of Leishmania. Two routes lead to PC production in yeast: i) the de novo pathway that starts from uptake of choline and ii) the methylation pathway that involves the threefold methylation of PE catalyzed by ScPEM1 and ScPEM2. Thus, abrogation of both ScPEM1 and ScPEM2 renders the double null mutant scpem1Δscpem2Δ a choline auxotroph [15,44]. These strains were grown in minimal medium in the absence or presence of 10 µM choline (Fig. 7). Growth curve assays showed that all strains grew equally well in the presence of choline (Fig. 7A). As expected, the double null mutant bearing empty vectors (scpem1Δscpem2Δ+U+L) remained a choline auxotroph (Fig. 7B). In contrast, both LmjPEM1 and LmjPEM2 individually as well as in combination had the capacity to complement the choline auxotrophy of the scpem1Δscpem2Δ strain (Fig. 7B). However, similar to the ScPEM1 transgenic, yeast expressing LmjPEM1 alone grew much slower in the absence of choline and reached a lower cell density during the stationary phase of growth than strains expressing either LmjPEM2 or ScPEM2. Also, the line scpem1Δscpem2Δ+LmjPEM1+LmjPEM2, as scpem1Δscpem2Δ+ScPEM1+ScPEM2, grew better and reached a higher cell density during the stationary growth phase than scpem1Δscpem2Δ+U+ScPEM2 or scpem1Δscpem2Δ+U+LmjPEM2 (Fig. 7B). This assay has demonstrated that both LmjPEM1 and LmjPEM2 relieve the choline auxotrophy phenotype of scpem1Δscpem2Δ and that this complementation is better achieved with LmjPEM2 and LmjPEM1 together, than with LmjPEM2 or LmjPEM1 alone.
Fig. 7.
LmjPEM1 and LmjPEM2 complement the choline auxotrophy phenotype of S. cerevisiae double null mutant scpem1Δscpem2Δ that lacks PEMT activity. Growth curves in minimal medium in the presence of 10 µM (A) or absence of choline (B). (A), (B) Open diamonds, scpem1Δscpem2Δ+U+L; open triangles, scpem1Δscpem2Δ+LmjPEM1+L; grey triangles, scpem1Δscpem2Δ+U+LmjPEM2; black triangles, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2; open circles, scpem1Δscpem2Δ+ScPEM1+L; grey circles, scpem1Δscpem2Δ+U+ScPEM2; black circles, scpem1Δscpem2Δ+ScPEM1+ScPEM2. This experiment was carried out in duplicate and was repeated at least once. A typical experiment is represented.
3.6. LmjPEM1 forms MMPE and DMPE while LmjPEM2 catalyzes all three methylation reactions to form PC
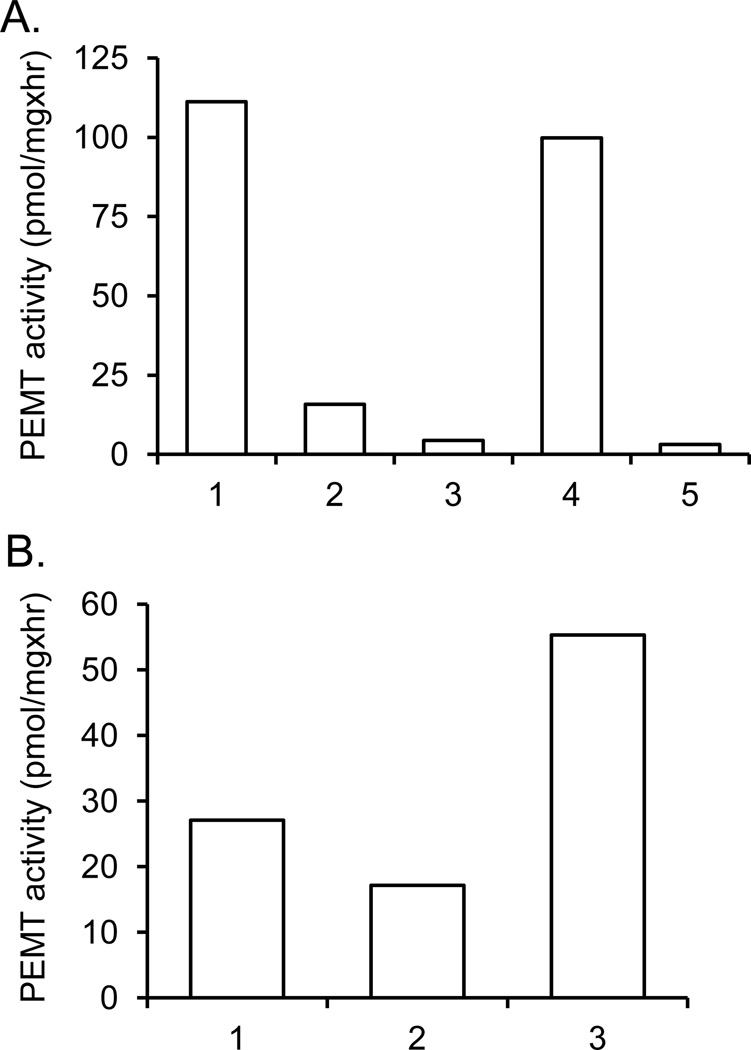
To address whether LmjPEM1 and LmjPEM2 exhibit PEMT activity, PEMT assay was performed with whole cells extract derived from yeast wild type, scpem1Δscpem2Δ+U+L, scpem1Δscpem2Δ+LmjPEM1+L, scpem1Δscpem2Δ+U+LmjPEM2 and scpem1Δscpem2Δ+LmjPEM1+LmjPEM2 (Fig. 8). As expected, the double null mutant lacked PEMT activity (Fig. 8A). LmjPEM1 showed only 15% of wild-type PEMT activity while LmjPEM2 gave even lower PEMT activity than LmjPEM1 (Fig. 8A). This suggests that PE is a better substrate for LmjPEM1 than LmjPEM2. When LmjPEM1 and LmjPEM2 were co-expressed in the same cell, PEMT activity was higher than the combined individual activities of LmjPEM1 and LmjPEM2. The same effect was observed when equal amounts of protein derived from yeast strains scpem1Δscpem2Δ+LmjPEM1+L and scpem1Δscpem2Δ+U+LmjPEM2 were mixed (Fig. 8B). This can be explained by a model where the product of either LmjPEM1 or LmjPEM2 serves as a substrate for the other enzyme, a phenomenon also observed with yeast PEMT enzymes [46].
Fig. 8.
LmjPEM1 and LmjPEM2 act as PEMT enzymes. (A), (B) PEMT assay with yeast transformants was carried out at least twice in duplicate as described in Materials and methods. A typical experiment with standard deviations is shown. (A), 1, wild type; 2, scpem1Δscpem2Δ+LmjPEM1+L; 3, scpem1Δscpem2Δ+U+LmjPEM2; 4, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2; 5, scpem1Δscpem2Δ+U+L. (B) 1, scpem1Δscpem2Δ+LmjPEM1+L; 2, scpem1Δscpem2Δ+U+LmjPEM2; 3, scpem1Δscpem2Δ+LmjPEM1+L and scpem1Δscpem2Δ+U+LmjPEM2 mixed 1:1 (v:v).
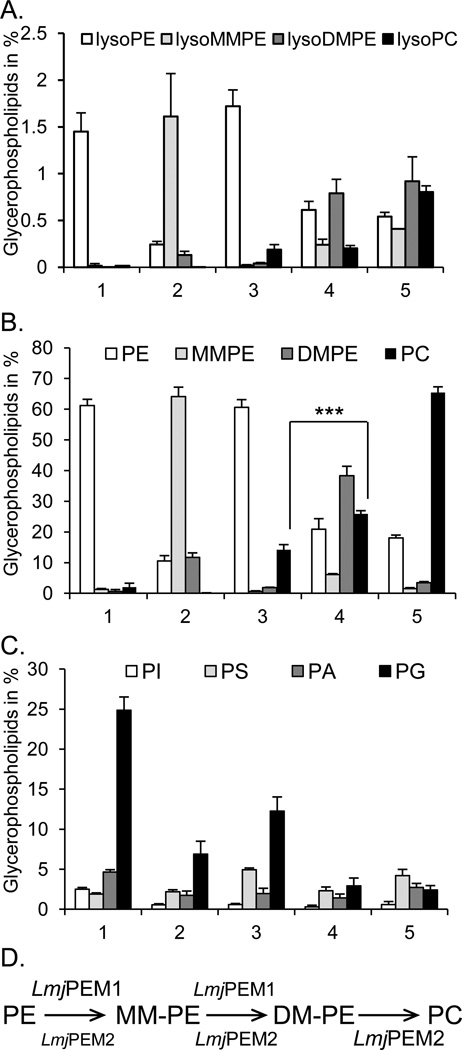
To determine the substrate specificity of LmjPEM1 and LmjPEM2, bulk lipids from yeast transformants scpem1Δscpem2Δ+U+L, scpem1Δscpem2Δ+LmjPEM1+L, scpem1Δscpem2Δ+U+LmjPEM2, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2, as well as from the wild type were isolated and analyzed by comprehensive electrospray ionization tandem mass spectrometry. Expression of LmjPEM1 lead primarily to the formation of lysoMMPE and MMPE, and to a lower extent to lysoDMPE and DMPE with concomitant decreases in lysoPE and PE levels. No lysoPC or PC was detected (Fig. 9A, B). The best lysoPE substrates for LmjPEM1 were those carrying medium length fatty acyl groups, specifically palmityl, palmitoyl, and a stearyl group (Table 2). The best diacyl-PE substrates for LmjPEM1 were PE(32:2) and PE(32:1) (Table 3). This result demonstrates that LmjPEM1 has the ability to methylate PE and lysoPE as well as MMPE and lysoMMPE, albeit with lower efficiency, and that it cannot catalyze the third methylation reaction (Fig. 9D). Alternatively, lysoMMPE and lysoDMPE may result from sn-2 deacylation of MMPE and DMPE by the action of an endogenous phospholipase A2 activity [47,48]. The scpem1Δscpem2Δ+LmjPEM1+L strain produces large amounts of MMPE and thus, can be used as an excellent source of this lipid.
Fig. 9.
LmjPEM1 and LmjPEM2 substrate specificities. Bulk lipids were purified from yeast transgenics and analyzed by electrospray ionization tandem mass spectrometry as described in Materials and method section. (A) Percentages of lysoPE, lysoMMPE, lysoDMPE, and lysoPC. (B) Percentages of PE, MMPE, DMPE, and PC. ***, P-value <0.001. (C) Percentages of glycerolipids PI, PS, PA, and PG. (D) Schematic representation of the involvement of LmjPEM1 and LmjPEM2 in PE methylation leading to PC production. (A), (B), (C), 1, scpem1Δscpem2Δ+U+L; 2, scpem1Δscpem2Δ+LmjPEM1+L; 3, scpem1Δscpem2Δ+U+LmjPEM2; 4, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2; 5, wild type. Standard deviations are shown.
Table 2.
Fatty acid composition of lysoPE, lysoMMPE, lysoDMPE, and lysoPC present in yeast. Numbers represent the percentage of total cellular glycerolipids.
| Glycerolipid | U+L | LmjPEM1+L | U+LmjPEM2 | LmjPEM1+LmjPEM2 | WT |
|---|---|---|---|---|---|
| lysoPE(16:1)* | 0.623 | 0.078 | 0.800 | 0.336 | 0.191 |
| lysoPE(16:0) | 0.563 | 0.108 | 0.674 | 0.161 | 0.066 |
| lysoPE(18:1) | 0.235 | 0.050 | 0.227 | 0.144 | 0.172 |
| lysoMMPE(16:1) | 0.014 | 0.621 | 0.010 | 0.106 | 0.019 |
| lysoMMPE(16:0) | 0.010 | 0.601 | 0.010 | 0.081 | 0.012 |
| lysoMMPE(18:2) | 0.000 | 0.196 | 0.000 | 0.029 | 0.011 |
| lysoDMPE(16:1) | 0.000 | 0.058 | 0.015 | 0.328 | 0.038 |
| lysoDMPE(16:0) | 0.000 | 0.029 | 0.012 | 0.208 | 0.022 |
| lysoDMPE(18:1) | 0.000 | 0.016 | 0.003 | 0.089 | 0.027 |
| lysoPC(16:1) | 0.010 | 0.000 | 0.093 | 0.108 | 0.334 |
| lysoPC(16:0) | 0.000 | 0.000 | 0.050 | 0.040 | 0.067 |
| lysoPC(18:1) | 0.214 | 0.000 | 0.025 | 0.036 | 0.183 |
, the first number indicates the total carbon atoms and the second number represents the total number of double bonds present in the fatty acids.
U+L, scpem1Δscpem2Δ+U+L; LmjPEM1+L, scpem1Δscpem2Δ+LmjPEM1+L; U+LmjPEM2, scpem1Δscpem2Δ+U+LmjPEM2; LmjPEM1+LmjPEM2, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2; WT, wild type.
Table 3.
Fatty acid composition of the most abundant PE, MMPE, DMPE, and PC present in yeast. Numbers represent the percentage of total cellular glycerolipids.
| Glycerolipid | U+L | LmjPEM1+L | U+LmjPEM2 | LmjPEM1+LmjPEM2 | WT |
|---|---|---|---|---|---|
| PE(30:1)* | 1.37 | 0.224 | 2.577 | 0.602 | 0.268 |
| PE(32:2) | 20.626 | 0.954 | 22.022 | 7.731 | 3.192 |
| PE(32:1) | 15.599 | 0.435 | 14.068 | 3.439 | 2.067 |
| PE(34:2) | 9.845 | 2.291 | 9.157 | 5.048 | 5.205 |
| PE(34:1) | 9.911 | 2.904 | 8.631 | 2.706 | 2.992 |
| MMPE(30:1) | 0.041 | 2.213 | 0.026 | 0.277 | 0.029 |
| MMPE(32:2) | 0.429 | 17.135 | 0.217 | 2.237 | 0.270 |
| MMPE(32:1) | 0.541 | 20.995 | 0.184 | 1.685 | 0.221 |
| MMPE(34:2) | 0.199 | 9.788 | 0.106 | 1.018 | 0.460 |
| MMPE(34:1) | 0.172 | 9.471 | 0.081 | 0.585 | 0.220 |
| DMPE(30:1) | 0.000 | 0.439 | 0.107 | 1.586 | 0.095 |
| DMPE(32:2) | 0.046 | 3.296 | 0.555 | 12.227 | 0.994 |
| DMPE(32:1) | 0.000 | 3.472 | 0.618 | 10.711 | 0.678 |
| DMPE(34:2) | 0.014 | 1.747 | 0.234 | 6.996 | 0.984 |
| DMPE(34:1) | 0.014 | 1.902 | 0.264 | 4.615 | 0.583 |
| PC(30:1) | 0.095 | 0.006 | 1.061 | 1.226 | 1.985 |
| PC(32:2) | 0.765 | 0.037 | 4.159 | 8.462 | 14.927 |
| PC(32:1) | 0.882 | 0.033 | 3.424 | 6.170 | 9.361 |
| PC(34:2) | 0.402 | 0.020 | 2.301 | 4.823 | 13.430 |
| PC(34:1) | 0.476 | 0.024 | 1.711 | 2.911 | 5.880 |
, the first number indicates the total carbon atoms and the second number represents the total number of double bonds present in both fatty acyl groups.
U+L, scpem1Δscpem2Δ+U+L; LmjPEM1+L, scpem1Δscpem2Δ+LmjPEM1+L; U+LmjPEM2, scpem1Δscpem2Δ+U+LmjPEM2; LmjPEM1+LmjPEM2, scpem1Δscpem2Δ+LmjPEM1+LmjPEM2; WT, wild type.
The strain scpem1Δscpem2Δ+U+LmjPEM2 contained minute amounts of MMPE, lysoMMPE, DMPE, lysoDMPE, a small but significant amount of PC, and similar amounts of PE as the double null mutant (Fig. 9A, B). The lysoPC species produced by LmjPEM2 are lysoPC(16:1) followed by lysoPC(16:0) and lysoPC(18;1), while the wild type generated mainly lysoPC(16:1) followed by lysoPC(18:1) and lysoPC(16:0) (Table 2). These species may be the product of LmPEM2 activity or the action of yeast phospholipase A2 [47,48]. In addition, LmjPEM2 seemed to be more specific to the length (C16) of than to the presence of double bonds in the fatty acyl group of the lysoPE, lysoMMPE, and lysoDMPE. The most abundant PC species produced by LmjPEM2 were PC(32:2) and PC(32:1) (Table 3). These data demonstrate that LmjPEM2 inefficiently adds the first methyl group to PE, but catalyses the second and third methylation reaction with higher efficacy to give PC (Fig. 9D). This also explains the lower PEMT activity of LmjPEM2 compared to that of LmjPEM1 when expressed in the yeast background scpem1Δscpem2Δ when endogenous PE was used as substrate (Fig. 8A). In addition, LmjPEM2 conferred better growth to yeast scpem1Δscpem2Δ than LmjPEM1 in medium lacking choline owing to its ability to produce PC in contrast to LmjPEM1, which cannot generate PC but only MMPE and DMPE (Fig. 9B).
Coexpression of LmjPEM1 and LmjPEM2 gave small amounts of MMPE, lysoPE, DMPE, and lysoDMPE, but more PC than the scpem1Δscpem2Δ+U+LmjPEM2 strain but less than the wild type (Fig. 9A, B). Levels of PE in the scpem1Δscpem2Δ+LmjPEM1+LmjPEM2 strain were similar to that of the wild type. It seems that LmjPEM2 does not efficiently convert all MMPE, lysoMMPE, DMPE, and lysoDMPE produced by LmjPEM1 into PC (Fig. 9A, B). This may be due to the fatty acid composition of PE and lysoPE produced by yeast versus Leishmania. In fact, yeast PC fatty acids are of medium length while Leishmania produces PC species with long to very long fatty acids (Tables 2 and 3; data not shown; [3]). It is unclear as to which subspecies of PC are produced via the methylation or the de novo pathway in Leishmania. Alternatively, the level of expression of LmjPEM2 in yeast was not high enough to efficiently support MMPE and DMPE, and lysoMMPE and lysoDMPE conversion into PC and lysoPC, respectively.
The fact that LmjPEM1 bears an internal gene duplication, and its gene product is involved in the addition of the first and second methyl groups onto PE, is very reminiscent of ScPEM1. Thus, LmjPEM1 can be classified as a class II PEMT enzyme. In contrast, LmjPEM2 lacks internal gene duplication, and catalyses all three PE methylation reactions similar to mammalian PEMTs and yeast ScPEM2; therefore, it belongs to class I PEMT.
Interestingly, the double null mutant scpem1 Δscpem2Δ produced six times more PG than the wild type, likely to compensate for the lack of PC (Fig. 9C). This effect was also seen in the LmjPEM1 and LmjPEM2 expressing lines scpem1Δscpem2Δ+LmjPEM1+L and scpem1Δscpem2Δ+U+LmjPEM2 although to a lower extent. In addition, the double null mutant generated approximately four times more PI than the wild type, and double null mutant expressing LmjPEM1 and LmjPEM2 individually or together.
To our knowledge, this is the first study to identify the PEMT enzymes in a protozoan parasite. The presence of the methylation pathway for PC biosynthesis seems to be unique to Leishmania. The related parasites Trypanosoma brucei and T. cruzi, as well as Toxoplasma gondii, Giardia lamblia, and Trichomonas vaginalis lack PEMT encoding genes. They produce PC exclusively from the de novo pathway, and thus, are choline auxotrophs [14,49]. Alternatively, Plasmodium falciparum utilizes a phosphoethanolamine N-methyltransferase to produce phosphocholine from phosphoethanolamine for PC synthesis [50,51]. In this parasite, similarly to plants, phosphocholine is then used to generate PC via the CDP-choline pathway [45,51]. Why does Leishmania have two pathways for PC biosynthesis? Leishmania may encounter a choline poor environment during its life cycle, and thus, relies on the methylation pathway for PC production. In fact, nutrient starvation occurs after digestion of the blood meal in the sand fly and triggers metacyclogenesis. This hypothesis is supported by data showing that purine starvation increased PEMT protein levels [52].
3.7. LmjPEM1 and LmjPEM2 inhibition by choline analogs
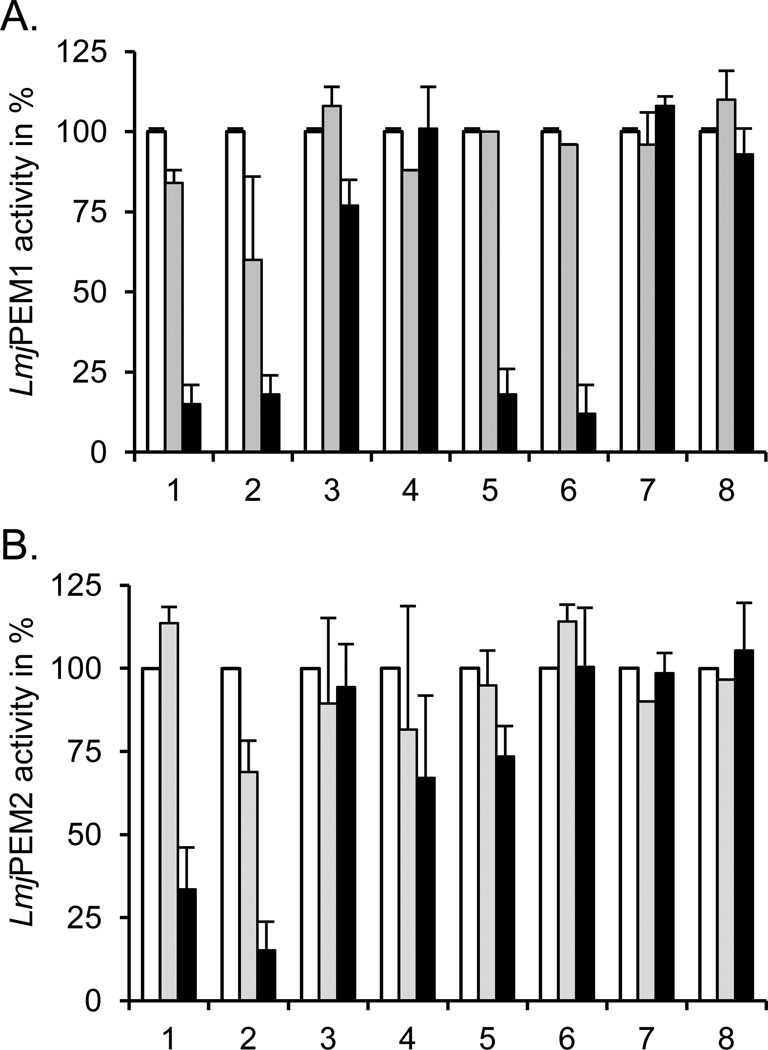
Previous studies have demonstrated that miltefosine, a new drug used for the treatment of leishmaniasis, led to decreased PC production that is accompanied by concomitant increases in PE levels, suggesting that enzymes involved in the methylation of PE may be inhibited by this drug [20]. Therefore, we examined whether LmjPEM1 and/or LmjPEM2 are inhibited by miltefosine, while also testing choline and phosphocholine analogs (Fig. 10). LmjPEM1 activity was best inhibited by hexadecyltrimethylammonium bromide, followed by miltefosine, octadecyltrimethylammonium bromide, and octadecylphosphocholine, while choline had a very small effect. Phosphocholine, ethanolamine, and the ethanolamine analog hydroxyethylhydrazine did not affect LmjPEM1 activity at all (Fig. 10A). LmjPEM2 was less sensitive to the compounds tested than LmjPEM1. However, it was efficiently inhibited by hexadecyltrimethylammonium and octadecyltrimethylammonium bromide (Fig. 10B). Phosphocholine and miltefosine impacted LmjPEM2 activity minimally, while choline, octadecylphosphocholine, ethanolamine, and hydroxyethylhydrazine did not diminish its activity. The fact that higher concentrations of drugs are needed to inhibit LmjPEM1 and LmjPEM2 indicates that these PEMTs are likely not the primary target of these drugs.
Fig. 10.
LmjPEM1 (A) and LmjPEM2 (B) inhibition by choline analogs. PEMT assay with yeast strains scpem1Δscpem2Δ+LmjPEM1+L (A) and scpem1Δscpem2Δ+U+LmjPEM2 (B) was carried out at least twice in duplicate as described in Materials and methods in the absence or presence of 10 or 100 µM of various compounds. An activity of 100% corresponds to approximately 100 pmol/mgxhr and standard deviations are depicted. 1, octadecyltrimethylammonium bromide; 2, hexadecyltrimethylammonium bromide; 3, choline; 4, phosphocholine; 5, miltefosine; 6, octadecylphosphocholine; 7, ethanolamine; 8, hydroxyethylhydrazine. (A), (B), open bars, no drug; grey bars, 10 µM of compound; black bars, 100 µM of drug.
Surprisingly, hydroxyethylhydrazine, a specific inhibitor of S. cerevisiae ScPEM1 and ScPEM2 enzymes, had no impact on either LmjPEM1 or LmjPEM2 activity; this may reflect structural differences despite the high level of similarity [53]. Octadecyltrimethylammonium bromide was shown to be a very potent anti-leishmanial drug that also inhibits choline transport [13]. Therefore, this drug may kill the parasite by inhibiting PC biosynthesis by blocking both the de novo and methylation pathways.
In conclusion, this study established, for the first time in a protozoan parasite, that in Leishmania, the PE methylation pathway consists of two enzymes, LmjPEM1 and LmjPEM2 which localize at the endoplasmic reticulum membrane. LmjPEM1 adds the first and second methyl group onto PE and lysoPE, while LmjPEM2 adds the second and third methyl group to give PC and lysoPC, respectively. Future studies should investigate the role of LmjPEM1 and LmjPEM2 in parasite biology by creating null mutants. Importantly, our results have demonstrated that LmjPEM1 and LmjPEM2 are inhibited by the choline analogs miltefosine and hexadecyltrimethylammonium bromide, indicating that they could represent novel targets for anti-leishmanial therapies.
Highlights.
-
-
LmjPEM1 and LmjPEM2 are expressed throughout the life cycle of Leishmania
-
-
LmjPEM1 and LmjPEM2 localize to the endoplasmic reticulum membrane
-
-
LmjPEM1 adds the first and the second methyl group to phosphatidylethanolamine
-
-
LmjPEM2 methylates thrice phosphatidylethanolamine to give phosphatidylcholine
-
-
LmjPEM1 and LmjPEM2 are inhibited by hexadecyl- and octadecyltrimethylammonium
Acknowledgments
We thank Brian Ransom, Jennifer Page, and Subbhalakshmi Dhalladoo for their excellent technical help. We would also like to thank Dr. C. BenMamoun for yeast strains, and Drs. J. Bangs, L. Kedzierski, and G. McGuire for their generous gifts of antibodies. We are grateful to Ivana Vancurova and Brent Berger for editing the manuscript. This project was supported by the American Heart Association award 0630001N, the NIH grant P20 RR016475 from the INBRE Program of the National Center for Research Resources and the NIH grant ARRA 1 R03 AI078145 to RZ. The lipid profile data were acquired at Kansas Lipidomics Research Center (KLRC). Instrument acquisition and method development at KLRC were supported by NSF grants MCB 0455318, MCB 0920663, DBI 0521587, DBI 1228622, Kansas INBRE (P20 GM103418 from the National Institute of General Medical Sciences), NSF EPSCoR grant EPS-0236913, Kansas Technology Enterprise Corporation, and Kansas State University.
Abbreviations
- DAG
diacylglycerol
- DMPE
dimethyl-phosphatidylethanolamine
- MMPE
monomethyl-phosphatidylethanolamine
- PBS
phosphate buffered saline
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PEMT
PE methyltransferase
- PA
phosphatidic acid
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- SAM
S-adenosylmethionine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beach DH, Holz GG, Jr, Anekwe GE. Lipids of Leishmania promastigotes. J Parasitol. 1979;65:201–216. [PubMed] [Google Scholar]
- 2.Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Beverley SM. Phospholipid and sphingolipid metabolism in Leishmania. Mol Biochem Parasitol. 2010;170:55–64. doi: 10.1016/j.molbiopara.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 5.Prokazova NV, Zvezdina ND, Korotaeva AA. Effect of lysophosphatidylcholine on transmembrane signal transduction. Biochemistry (Mosc) 1998;63:31–37. [PubMed] [Google Scholar]
- 6.Liu Y, Su Y, Wang X. Phosphatidic acid-mediated signaling. Adv Exp Med Biol. 2013;991:159–176. doi: 10.1007/978-94-007-6331-9_9. [DOI] [PubMed] [Google Scholar]
- 7.Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol. 2013;48:20–38. doi: 10.3109/10409238.2012.735643. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann H, Gercken G. Synthesis of phospholipids in Leishmania donovani. Hoppe Seylers Z Physiol Chem. 1980;361:1735–1742. doi: 10.1515/bchm2.1980.361.2.1735. [DOI] [PubMed] [Google Scholar]
- 9.Vance DE. Phospholipid methylation in mammals: from biochemistry to physiological function. Biochim Biophys Acta. 2014;1838:1477–1487. doi: 10.1016/j.bbamem.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Aktas M, Wessel M, Hacker S, Klusener S, Gleichenhagen J, et al. Phosphatidylcholine biosynthesis and its significance in bacteria interacting with eukaryotic cells. Eur J Cell Biol. 2010;89:888–894. doi: 10.1016/j.ejcb.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Geiger O, Lopez-Lara IM, Sohlenkamp C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim Biophys Acta. 2013;1831:503–513. doi: 10.1016/j.bbalip.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Zufferey R, Mamoun CB. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol Biochem Parasitol. 2002;125:127–134. doi: 10.1016/s0166-6851(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 14.Lykidis A. Comparative genomics and evolution of eukaryotic phospholipid biosynthesis. Prog Lipid Res. 2007;46:171–199. doi: 10.1016/j.plipres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Kanipes MI, Henry SA. The phospholipid methyltransferases in yeast. Biochim Biophys Acta. 1997;1348:134–141. doi: 10.1016/s0005-2760(97)00121-5. [DOI] [PubMed] [Google Scholar]
- 16.Kodaki T, Yamashita S. Yeast phosphatidylethanolamine methylation pathway. Cloning and characterization of two distinct methyltransferase genes. J Biol Chem. 1987;262:15428–15435. [PubMed] [Google Scholar]
- 17.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 18.Paris C, Loiseau PM, Bories C, Breard J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2004;48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieder T, Orfanos CE, Geilen CC. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J Biol Chem. 1998;273:11025–11031. doi: 10.1074/jbc.273.18.11025. [DOI] [PubMed] [Google Scholar]
- 20.Rakotomanga M, Blanc S, Gaudin K, Chaminade P, Loiseau PM. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2007;51:1425–1430. doi: 10.1128/AAC.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbina JA. Mechanisms of action of lysophospholipid analogues against trypanosomatid parasites. Trans R Soc Trop Med Hyg. 2006;100(Suppl1):S9–S16. doi: 10.1016/j.trstmh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Barratt G, Saint-Pierre-Chazalet M, Loiseau PM. Cellular transport and lipid interactions of miltefosine. Curr Drug Metab. 2009;10:247–255. doi: 10.2174/138920009787846332. [DOI] [PubMed] [Google Scholar]
- 23.Croft SL, Seifert K, Duchene M. Antiprotozoal activities of phospholipid analogues. Mol Biochem Parasitol. 2003;126:165–172. doi: 10.1016/s0166-6851(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 24.Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, et al. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology in Methods in Enzymology. San Diego: Academic Press; 1991. [PubMed] [Google Scholar]
- 26.Medina-Acosta E, Cross GA. Rapid isolation of DNA from trypanosomatid protozoa using a simple 'mini-prep' procedure. Mol Biochem Parasitol. 1993;59:327–329. doi: 10.1016/0166-6851(93)90231-l. [DOI] [PubMed] [Google Scholar]
- 27.Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 28.Freedman DJ, Beverley SM. Two more independent selectable markers for stable transfection of Leishmania. Mol Biochem Parasitol. 1993;62:37–44. doi: 10.1016/0166-6851(93)90175-w. [DOI] [PubMed] [Google Scholar]
- 29.Miller CA, 3rd, Martinat MA, Hyman LE. Assessment of aryl hydrocarbon receptor complex interactions using pBEVY plasmids: expression vectors with bi-directional promoters for use in Saccharomyces cerevisiae. Nucleic Acids Res. 1998;26:3577–3583. doi: 10.1093/nar/26.15.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 32.Schneiter R, Daum G. Extraction of yeast lipids. Methods Mol Biol. 2006;313:41–45. doi: 10.1385/1-59259-958-3:041. [DOI] [PubMed] [Google Scholar]
- 33.Zufferey R, Al-Ani GK, Dunlap K. Leishmania dihydroxyacetonephosphate acyltransferase Lm DAT is important for ether lipid biosynthesis but not for the integrity of detergent resistant membranes. Mol Biochem Parasitol. 2009;168:177–185. doi: 10.1016/j.molbiopara.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill CC JE, McGraw P, Summers E, Henry SA. In: Biosynthesis and role of phospholipids in yeast membranes. Kuhn APT PJ, Jung MJ, Goosey MW, Copping LG, editors. Berlin: Heidelberg; 1990. [Google Scholar]
- 36.Gaynor PM, Gill T, Toutenhoofd S, Summers EF, McGraw P, et al. Regulation of phosphatidylethanolamine methyltransferase and phospholipid methyltransferase by phospholipid precursors in Saccharomyces cerevisiae. Biochim Biophys Acta. 1991;1090:326–332. doi: 10.1016/0167-4781(91)90197-t. [DOI] [PubMed] [Google Scholar]
- 37.Mullin KA, Foth BJ, Ilgoutz SC, Callaghan JM, Zawadzki JL, et al. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol Biol Cell. 2001;12:2364–2377. doi: 10.1091/mbc.12.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garami A, Mehlert A, Ilg T. Glycosylation defects and virulence phenotypes of Leishmania mexicana phosphomannomutase and dolicholphosphate-mannose synthase gene deletion mutants. Mol Cell Biol. 2001;21:8168–8183. doi: 10.1128/MCB.21.23.8168-8183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- 40.Schneider P, Ferguson MA, McConville MJ, Mehlert A, Homans SW, et al. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990;265:16955–16964. [PubMed] [Google Scholar]
- 41.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, et al. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui Z, Vance JE, Chen MH, Voelker DR, Vance DE. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J Biol Chem. 1993;268:16655–16663. [PubMed] [Google Scholar]
- 43.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol Biol Cell. 2002;13:3148–3161. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodaki T, Yamashita S. Characterization of the methyltransferases in the yeast phosphatidylethanolamine methylation pathway by selective gene disruption. Eur J Biochem. 1989;185:243–251. doi: 10.1111/j.1432-1033.1989.tb15109.x. [DOI] [PubMed] [Google Scholar]
- 45.Pessi G, Choi JY, Reynolds JM, Voelker DR, Mamoun CB. In vivo evidence for the specificity of Plasmodium falciparum phosphoethanolamine methyltransferase and its coupling to the Kennedy pathway. J Biol Chem. 2005;280:12461–12466. doi: 10.1074/jbc.M414626200. [DOI] [PubMed] [Google Scholar]
- 46.Janssen MJ, de Jong HM, de Kruijff B, de Kroon AI. Cooperative activity of phospholipid-N-methyltransferases localized in different membranes. FEBS Lett. 2002;513:197–202. doi: 10.1016/s0014-5793(02)02298-6. [DOI] [PubMed] [Google Scholar]
- 47.Wagner S, Paltauf F. Generation of glycerophospholipid molecular species in the yeast Saccharomyces cerevisiae. Fatty acid pattern of phospholipid classes and selective acyl turnover at sn-1 and sn-2 positions. Yeast. 1994;10:1429–1437. doi: 10.1002/yea.320101106. [DOI] [PubMed] [Google Scholar]
- 48.Yost RW, Grauvickel SJ, Cantwell R, Bomalaski JS, Hudson AP. Yeast mitochondria (Saccharomyces cerevisiae) contain Ca(2+)-independent phospholipase A1 and A2 activities: effect of respiratory state. Biochem Int. 1991;24:199–208. [PubMed] [Google Scholar]
- 49.Ramakrishnan S, Serricchio M, Striepen B, Butikofer P. Lipid synthesis in protozoan parasites: a comparison between kinetoplastids and apicomplexans. Prog Lipid Res. 2013;52:488–512. doi: 10.1016/j.plipres.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dechamps S, Wengelnik K, Berry-Sterkers L, Cerdan R, Vial HJ, et al. The Kennedy phospholipid biosynthesis pathways are refractory to genetic disruption in Plasmodium berghei and therefore appear essential in blood stages. Mol Biochem Parasitol. 2010;173:69–80. doi: 10.1016/j.molbiopara.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Pessi G, Kociubinski G, Mamoun CB. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci USA. 2004;101:6206–6211. doi: 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin JL, Yates PA, Soysa R, Alfaro JF, Yang F, et al. Metabolic reprogramming during purine stress in the protozoan pathogen Leishmania donovani. PLoS Pathog. 2014;10:e1003938. doi: 10.1371/journal.ppat.1003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikawa J, Yamashita S. 2-hydroxyethylhydrazine as a potent inhibitor of phospholipid methylation in yeast. Biochim Biophys Acta. 1983;751:201–209. [PubMed] [Google Scholar]
- 54.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]