Abstract
Objective
To determine the population-based incidence of leukocytoclastic vasculitis (LCV).
Patients and Methods
Retrospective population-based study of all Olmsted County, Minnesota, residents with a skin biopsy–proven diagnosis of LCV from January 1, 1996, through December 31, 2010.
Results
A total of 84 patients (mean age at diagnosis, 48.3 years) with newly diagnosed, skin biopsy–proven LCV (43 women, 41 men) were identified. The incidence rate (age and sex adjusted to the 2000 US white population) was 4.5 per 100,000 person-years (95% confidence interval, 3.5–5.4). The incidence of LCV increased significantly with age at diagnosis (P<.001) and did not differ between female and male patients. Subtypes of LCV were cutaneous small-vessel vasculitis (CSVV), 38 patients (45%); IgA vasculitis, 25 (30%); urticarial vasculitis, 10 (12%); cryoglobulinemic vasculitis, 3 (4%); and antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, 8 (10%). LCV was idiopathic in 29 of 38 CSVV patients (76%) and 24 of 25 IgA vasculitis patients (96%). Thirty-nine of 84 patients (46%) had systemic involvement, with the renal system most commonly involved (17/39; 44%). Twenty-four of 80 patients (30%) with follow-up data available had recurrent disease. Compared with the Minnesota white population, observed survival in the incident LCV cohort was significantly poorer than expected (P<.001), including the subset of patients with idiopathic CSVV (P=.03).
Conclusion
The incidence of LCV was higher than that in previously published studies. Idiopathic LCV was more common in our population-based cohort than previously described. Overall survival was significantly poorer and should be explored further in future studies.
Keywords: ANCA-associated vasculitis, cryoglobulin, cutaneous vasculitis, epidemiology of vasculitis, Henoch-Schönlein purpura, IgA vasculitis, incidence of vasculitis, leukocytoclastic vasculitis, small vessel vasculitis, urticarial vasculitis
Introduction
Leukocytoclastic vasculitis (LCV) refers to a histopathologic finding of neutrophilic inflammation of postcapillary venules. Diverse clinical entities can lead to LCV in the skin, yet few studies have been conducted to determine the incidence of LCV. Therefore, our primary aim was to detect the incidence of LCV in Olmsted County, Minnesota.
Numerous classification and nomenclature systems characterize and subdivide the different vasculitides that have LCV as a finding on cutaneous histopathology.1–3 Given this, we also aimed to formulate a nomenclature system to organize various subtypes of cutaneous LCV in a clinically relevant fashion. We also sought to analyze the etiologies of various cutaneous entities that lead to LCV in the skin and to calculate the overall survival of patients with LCV.
Patients and Methods
The study was approved by both the Mayo Clinic and Olmsted Medical Center Institutional Review Boards, Rochester, Minnesota. We used the Rochester Epidemiology Project (REP) database to retrieve the medical records of all the skin biopsy–proven cases of LCV between January 1, 1996, and December 31, 2010.
The REP was founded in 1966 and is a comprehensive database that links the medical records of Olmsted County, Minnesota, residents.4 It is a computerized index system that contains medical diagnoses that have been made for each resident at different medical facilities in Olmsted County, including outpatient clinics, inpatient hospitals, and nursing homes or at autopsies.4 Patients who denied research authorization were excluded from the study.
Inclusion Criteria
LCV was histopathologically defined as predominantly polymorphonuclear neutrophilic infiltrate, primarily affecting superficial postcapillary venules, with fibrinoid deposits in and around the vessel wall, endothelial swelling, and extravasation of red blood cells.5 We included all incident cases of LCV (ie, first lifetime diagnosis of LCV) in which a final diagnosis of cutaneous vasculitis was rendered by the clinician and the diagnosis was confirmed by LCV on skin biopsy. Cases of LCV secondary to other cutaneous pathologies (eg, secondary LCV changes in the skin surrounding cutaneous ulcers due to other causes) and cases demonstrating LCV on skin biopsies of conditions not traditionally classified as forms of vasculitis (eg, granuloma faciale) were excluded from the study. The patients had to be residents of Olmsted County, Minnesota, for at least 1 year before and 1 year after the date of diagnosis, which was defined as the date the skin biopsy was obtained.
Search Strategy
We used the following search terms to generate a list of patients from the REP: vasculitis, not otherwise specified; cutaneous vasculitis; leukocytoclastic vasculitis; cutaneous small-vessel vasculitis (CSVV); Henoch-Schönlein purpura (HSP); urticarial vasculitis (UV); erythema elevatum diutinum; cryoglobulinemic vasculitis; acute hemorrhagic edema of childhood; microscopic polyangiitis; Wegener granulomatosis; Churg-Strauss syndrome; and connective tissue disease–associated vasculitis. We then excluded cases that did not have histopathologic confirmation of LCV on a skin biopsy.
Nomenclature
We divided LCV on histopathology into the following 5 categories and definitions:
CSVV, which represented cutaneous vasculitis limited primarily to the skin and did not represent a distinct clinical subtype of CSVV (eg, IgA vasculitis, UV, cryoglobulinemic vasculitis) and was equivalent to leukocytoclastic angiitis or hypersensitivity vasculitis, according to the inclusion criteria of Fiorentino6 and Russell and Gibson.5
IgA vasculitis, defined by a finding of IgA deposition as the predominant immunoglobulin on direct immunofluorescence (DIF) microscopy.
UV, defined as clinical morphology of urticarial lesions and a finding of LCV on histopathology.
Cryoglobulinemic vasculitis, defined as having LCV on histopathology and positive cryoglobulins (type 2 or 3 in blood).
Antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, marked by positive myeloperoxidase antibodies or proteinase 3 antibodies or positive perinuclear ANCA or cytoplasmic ANCA.
The 5 LCV subtypes in our study were additionally categorized according to the predominant lesional morphology of their traditional clinical presentation: 1) palpable purpura (ie, CSVV, IgA vasculitis, cryoglobulinemic vasculitis, and ANCA-associated vasculitis) and 2) urticaria-like lesions (ie, UV).
We further attributed various etiologic associations found to be implicated in the causation of the different subtypes of cutaneous vasculitis. These included the following: idiopathic, drug associated, infection associated, malignancy associated, and autoimmune connective tissue disease (ACTD) associated.
Collected Data
Collected data included patient demographics such as age at diagnosis and sex; date and results of skin biopsy; DIF microscopy results; laboratory results; associated systemic symptoms or conditions; clinical follow-up, including any recurrent episodes of LCV; treatment of vasculitis; and patient outcomes.
Statistical Analysis
Continuous variables were summarized with means, standard deviations (SDs), medians, and ranges; categorical variables were summarized with frequency counts and percentages. Overall survival was estimated using the Kaplan-Meier method. The duration of follow-up was calculated from the date of diagnosis to the date of death or last follow-up. Overall survival was compared with the survival expected in the Minnesota white population based on age at diagnosis, sex, and year of diagnosis using the cohort method.7 Overall survival was compared between the recurrent and nonrecurrent LCV groups using a log-rank test. Incidence rates per 100,000 person-years were calculated using incident cases of LCV as the numerator and age- and sex-specific estimates of the population of Olmsted County, Minnesota, as the denominator. The populations at risk for 1996 through 2000 were estimated using the census data from 1990 and 2000, with linear interpolation for intercensal years. The populations at risk for 2001 through 2010 were obtained from the US Intercensal Estimates (www.census.gov). Because the population of Olmsted County is nearly all white, incidence rates were directly age and sex adjusted to the structure of the 2000 US white population. Incident cases were grouped into intervals based on age at diagnosis (0–19, 20–29, 30–39, 40–49, 50–59, 60–69, ≥70 years) and on year of diagnosis (1996–2000, 2001–2005, 2006–2010). The relationships of age at diagnosis, sex, and year of diagnosis with incidence of LCV were assessed by fitting Poisson regression models using the SAS procedure GENMOD. Statistical analyses were performed using the SAS software package (SAS Institute Inc, Cary, North Carolina). All tests were 2 sided and P values less than .05 were considered statistically significant.
Results
Patient Population
Between January 1, 1996, and December 31, 2010, a total of 405 patients were identified from the REP as having a coded diagnosis that included one or more of the terms used to conduct the initial query of the REP. After exclusion of patients who did not have skin histopathologic confirmation of the diagnosis or who denied research authorization, the final analysis comprised 84 patients with a new diagnosis of skin biopsy–proven LCV.
Mean age at diagnosis for the 84 incident cases of LCV was 48.3 years (SD, 18.8 years; median, 48.5 years; range, 5–93 years). Six of the 84 patients were younger than 18 years. There were 41 men (49%) and 43 women (51%). Other pertinent patient characteristics are outlined in Tables 1–3.
Table 1.
Clinical Characteristics of 84 Patients With Leukocytoclastic Vasculitis
| Characteristic | No. (%) of Patientsa |
|---|---|
| Age at diagnosis, y | |
| Mean±SD | 48.3±18.8 |
| Median (range) | 48.5 (5–93) |
| Sex | |
| Male | 41 (49) |
| Female | 43 (51) |
| LCV subtype | |
| CSVV | 38 (45) |
| IgA | 25 (30) |
| UV | 10 (12) |
| ANCA associated | 8 (10) |
| Cryoglobulinemic | 3 (4) |
| Lesion morphologyb | |
| Palpable purpura | 60 (71) |
| Petechial | 14 (17) |
| Urticarial | 11 (13) |
| Ulcers | 8 (10) |
| Purpura | 8 (10) |
| Pustules | 3 (4) |
| Bullae or vesicles | 3 (4) |
| Nodules | 2 (2) |
| Distributionb | |
| Lower extremity | 82 (98) |
| Upper extremity | 28 (33) |
| Abdomen | 19 (23) |
| Back | 11 (13) |
| Chest | 9 (11) |
| Recurrent episodes (n=80) | |
| Yes | 24 (30) |
| No | 56 (70) |
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; CSVV, cutaneous small-vessel vasculitis; LCV, leukocytoclastic vasculitis; UV, urticarial vasculitis.
Values are number (percentage) unless indicated otherwise.
Patient may be listed in more than 1 category.
Table 3.
Test Results and Treatment of 84 Patients With Leukocytoclastic Vasculitis
| Characteristic | No. (%) of Patients |
|---|---|
| DIF (n=75) | |
| Negative | 21 (28) |
| Positive | 54 (72) |
| Laboratory values | |
| ESR (n=66) | |
| Normal | 43 (65) |
| High | 23 (35) |
| CBC (n=78) | |
| Normal | 41 (53) |
| Abnormal | 37 (47) |
| Anemia | 25 (32) |
| Urinalysis (n=66) | |
| Normal | 25 (38) |
| Abnormal | 41 (62) |
| Hematuria and proteinuria | 25 (38) |
| Hematuria | 11 (17) |
| Proteinuria | 5 (8) |
| Treatment (n=47)a | |
| Prednisone | 43 (91) |
| Dapsone | 7 (15) |
| Azathioprine | 6 (13) |
| Colchicine | 6 (13) |
| Methotrexate | 5 (11) |
| Mycophenolate mofetil | 5 (11) |
| Cyclophosphamide | 3 (6) |
| Hydroxychloroquine | 3 (6) |
| NSAID | 1 (2) |
Abbreviations: CBC, complete blood cell count; DIF, direct immunofluorescence; ESR, erythrocyte sedimentation rate; NSAID, nonsteroidal anti-inflammatory drug.
Each treated patient may have received more than 1 drug.
Epidemiology of LCV
As shown in Table 4, the overall age- and sex-adjusted incidence of LCV, including all subtypes, was 4.5 per 100,000 person-years (95% confidence interval [CI], 3.5–5.4); age-adjusted incidence was 4.4 per 100,000 person-years (95% CI, 3.1–5.7) for female patients compared with 4.8 per 100,000 person-years (95% CI, 3.3–6.2) for male patients (P=.81). Incidence of LCV increased significantly with age at diagnosis (P<.001); the increase in incidence with age did not differ significantly between male and female patients (P=.12). There was not a statistically significant trend in incidence by year of diagnosis (P=.33). The overall age- and sex-adjusted incidence rates per 100,000 person-years for the CSVV subtypes are enumerated in Table 4.
Table 4.
Incidence of Leukocytoclastic Vasculitis in Olmsted County, Minnesota, 1996–2010
| Female Patients | Male Patients | Total | ||||
|---|---|---|---|---|---|---|
| Age at diagnosis, y | No. | Rate (95% CI)a | No. | Rate (95% CI)a | No. | Rate (95% CI)a |
| 0–19 | 6 | 2.2 (0.8–4.8) | 1 | 0.3 (0.0–1.9) | 7 | 1.2 (0.5–2.6) |
| 20–29 | 3 | 2.2 (0.5–6.5) | 6 | 4.7 (1.7–10.2) | 9 | 3.4 (1.6–6.5) |
| 30–39 | 4 | 2.7 (0.7–6.9) | 3 | 2.0 (0.4–5.8) | 7 | 2.3 (0.9–4.8) |
| 40–49 | 10 | 6.7 (3.2–12.3) | 10 | 6.8 (3.3–12.6) | 20 | 6.8 (4.1–10.5) |
| 50–59 | 10 | 8.4 (4.0–15.4) | 7 | 6.2 (2.5–12.7) | 17 | 7.3 (4.3–11.7) |
| 60–69 | 7 | 9.3 (3.7–19.2) | 7 | 10.2 (4.1–21.0) | 14 | 9.7 (5.3–16.3) |
| ≥70 | 3 | 3.1 (0.6–9.1) | 7 | 10.9 (4.4–22.5) | 10 | 6.2 (3.0–11.5) |
| Year of diagnosis | No. | Rate (95% CI)b | No. | Rate (95% CI)b | No. | Rate (95% CI)c |
| 1996–2000 | 13 | 4.4 (2.0–6.8) | 8 | 3.2 (0.9–5.6) | 21 | 3.7 (2.1–5.3) |
| 2001–2005 | 13 | 4.0 (1.8–6.1) | 16 | 5.4 (2.7–8.1) | 29 | 4.6 (2.9–6.3) |
| 2006–2010 | 17 | 4.8 (2.5–7.0) | 17 | 5.3 (2.8–7.9) | 34 | 5.0 (3.3–6.7) |
| LCV subtyped | No. | Rate (95% CI)b | No. | Rate (95% CI)b | No. | Rate (95% CI)c |
| All CSVV | 19 | 1.9 (1.1–2.8) | 19 | 2.3 (1.3–3.4) | 38 | 2.1 (1.4–2.7) |
| Idiopathic CSVV | 16 | 1.6 (0.8–2.4) | 13 | 1.6 (0.7–2.6) | 29 | 1.6 (1.0–2.1) |
| IgA vasculitis | 11 | 1.1 (0.4–1.7) | 14 | 1.6 (0.7–2.4) | 25 | 1.3 (0.8–1.8) |
| Urticarial vasculitis | 9 | 0.9 (0.3–1.5) | 1 | 0.1 (0.0–0.3) | 10 | 0.5 (0.2–0.8) |
| ANCA-associated | 3 | 0.3 (0.0–0.7) | 5 | 0.5 (0.1–1.0) | 8 | 0.4 (0.1–0.7) |
| All patients | 43 | 4.4 (3.1–5.7) | 41 | 4.8 (3.3–6.2) | 84 | 4.5 (3.5–5.4) |
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; CI, confidence interval, CSVV, cutaneous small-vessel vasculitis; LCV, leukocytoclastic vasculitis.
Incidence per 100,000 person-years.
Incidence per 100,000 person-years age-adjusted to 2000 US white population.
Incidence per 100,000 person-years age- and sex-adjusted to 2000 US white population.
Age- and sex-adjusted incidence was not calculated for cryoglobulinemic vasculitis because this subgroup included only 3 patients.
Etiology and Subtypes of LCV
As shown in Table 1, the most common subtype of LCV in our population was CSVV (38 patients [45%]), followed by IgA vasculitis (25 patients [30%]). In both these subtypes, the most common etiology of LCV was idiopathic, accounting for 29 of 38 patients with CSVV (76%) and 24 of 25 patients with IgA vasculitis (96%). Other etiologic associations for all the subtypes of LCV are shown in Table 5.
Table 5.
Summary of Subtypes and Etiologies of Leukocytoclastic Vasculitis
| Subtype | No. (%) |
|---|---|
| Palpable purpura (predominant) | |
| CSVV (n=38) | |
| Idiopathic | 29 (76) |
| ACTDa | 2 (5) |
| Infectionb | 6 (16) |
| Drug reactionc | 1 (3) |
| IgA vasculitis (n=25) | |
| Idiopathic | 24 (96) |
| Infectiond | 1 (4) |
| ANCA-associated vasculitis (n=8) | |
| Microscopic polyangiitis | 1 (12.5) |
| Granulomatosis with polyangiitis | 2 (25) |
| Eosinophilic granulomatosis with polyangiitis | 2 (25) |
| p-ANCA, NOS | 2 (25) |
| c-ANCA, NOS | 1 (12.5) |
| Cryoglobulinemic vasculitis (n=3) | |
| ACTDe | 1 (33) |
| Infectionf | 2 (67) |
| Urticaria-like lesions | |
| Urticarial vasculitis (n=10) | |
| Normocomplementemic | 8 (80)g |
| Idiopathic | 6 (75) |
| Infectionh | 1(12.5) |
| Drug reactioni | 1 (12.5) |
| Hypocomplementemic | 2 (20) |
| ACTDj | 2 (100) |
Abbreviations: ACTD, autoimmune connective tissue disease; ANCA, antineutrophil cytoplasmic antibody; c-ANCA, cytoplasmic ANCA; CSVV, cutaneous small-vessel vasculitis; NOS, not otherwise specified; p-ANCA, perinuclear ANCA.
Sjögren syndrome and systemic lupus erythematosus in 1 patient each.
Hepatitis C in 2 patients and hepatitis B and C, parvovirus, pneumonia, and streptococcal infection in 1 patient each.
Amoxicillin.
Streptococcal infection.
Rheumatoid arthritis.
Hepatitis C.
Complements were not checked in 2 patients.
Hepatitis C.
Epoetin alfa.
Systemic lupus erythematosus and mixed connective tissue disease in 1 patient each.
Systemic Involvement in LCV and Frequency of Recurrent Disease
LCV was accompanied by systemic involvement in 39 of 84 patients (46%). Organ systems affected during episodes of LCV are shown in Table 2. The following subtypes of LCV were present in the 39 patients with systemic involvement: IgA vasculitis, 18 patients; CSVV, 11 patients; ANCA-associated vasculitis, 6 patients; UV, 2 patients; cryoglobulinemic vasculitis, 2 patients.
Table 2.
Systemic Involvement of 84 Patients With Leukocytoclastic Vasculitis
| Characteristic | No. (%) of Patients |
|---|---|
| Systemic involvement | |
| Yes | 39 (46) |
| No | 45 (54) |
| Type of systemic involvement (n=39)a | |
| Renal | 17 (44) |
| Musculoskeletal | 14 (36) |
| Gastrointestinal | 12 (31) |
| Constitutional | 10 (26) |
| Otorhinolaryngologic | 3 (8) |
| Pulmonary | 2 (5) |
| Neurologic | 1 (3) |
Each patient may be listed in more than 1 category.
Follow-up data regarding recurrent episodes of LCV were available for 80 of the 84 patients. Among these, recurrence was noted in 24 patients (30%), while 56 (70%) had an isolated episode of LCV. The following subtypes of LCV were present in the 24 patients with recurrent LCV: IgA vasculitis, 8 patients (idiopathic); CSVV, 6 (idiopathic, 2; infection, 2 [hepatitis C; hepatitis B and C]; ACTD, 2 [Sjögren syndrome; systemic lupus erythematosus]); UV, 5 (normocomplementemic, 3 [idiopathic]; hypocomplementemic, 2 [hepatitis C; mixed connective tissue disease]); ANCA-associated vasculitis, 4 (eosinophilic granulomatosis with polyangiitis, 2; granulomatosis with polyangiitis, 1; ANCA–not otherwise specified, 1); cryoglobulinemic vasculitis, 1 (hepatitis C). The mean duration of disease (from the time of diagnosis to either 1) resolution of vasculitis or 2) date of last follow-up or death) for the 24 patients with recurrent episodes of LCV was 2.0 years (SD, 3.8 years; median, 0.3 years; range, 0–14.7 years).
Disease Course and Survival
Follow-up data regarding the last follow-up date after initial diagnosis of LCV or patient death were available for all 84 patients. Treatments administered for LCV are outlined in Table 3.
At last follow-up, 15 patients had died at a mean of 2.7 years after diagnosis (median, 1.6 years; range, 5 days to 9.5 years). Causes of death in the 15 patients are listed in the Box. The mean duration of follow-up for the 69 patients who were still alive at last follow-up was 5.8 years (median, 5.4 years; range, 16 days to 16.1 years). Estimated overall survival rates (95% CI, number still at risk) at 1, 3, 5, 7, and 10 years after diagnosis were 94% (89%–99%; 72), 87% (79%–95%; 54), 85% (77%–94%; 40), 78% (68%–89%; 23), and 72% (58%–89%; 11), respectively. In comparison, the expected survival rates at these time points in the Minnesota white population were 99%, 97%, 95%, 92%, and 89%, respectively. As demonstrated in Figure 1, the survival observed in the incident LCV cohort was significantly poorer than expected (P<.001).
Box. Causes of Death in 15 Patients With Leukocytoclastic Vasculitis.
| Acute cardiorespiratory failure, chronic obstructive pulmonary disease |
| Aspiration pneumonia secondary to dysphagia from cerebrovascular accident |
| Congestive heart failure, renal failure, reactive airway disease |
| Kidney failure due to hypertensive nephrosclerosis, cerebrovascular disease |
| Malignancy-related (n=4): metastatic squamous cell carcinoma of the lung, metastatic endometrial carcinoma with small bowel obstruction, mesothelioma of pleura and pericardium, metastatic esophageal adenocarcinoma |
| Multifactorial kidney failure |
| Necrotizing pancreatitis and purulent peritonitis |
| Respiratory failure, congestive heart failure, chronic hypersensitivity pneumonitis |
| Respiratory infection (viral) in setting of aortic stenosis and congestive heart failure |
| Unknown (n=3) |
Figure 1. Expected and Observed Survival.
Observed survival in the incident leukocytoclastic vasculitis cohort was decreased compared with the expected survival in the Minnesota white population.
Overall survival rates for the most common LCV subtypes, including all CSVV (n=38), idiopathic CSVV (n=29), IgA vasculitis (n=25), UV (n=10), and ANCA-associated (n=8), were also calculated and compared with the survival expected in the Minnesota white population. Estimated overall survival rates (95% CI; number still at risk) at 5 years after diagnosis were 79% (65%–96%; 17) for all CSVV (P=.009 compared with the Minnesota white population); 79% (64%–97%; 13) for idiopathic CSVV (P=.03); 91% (81%–100%; 13) for IgA vasculitis (P=.39); 80% (59%–100%; 5) for UV (P<.001); and 88% (67%–100%; 3) for ANCA-associated vasculitis (P<.001). Expected survival for the cryoglobulinemic vasculitis group was not calculated because of the small sample size.
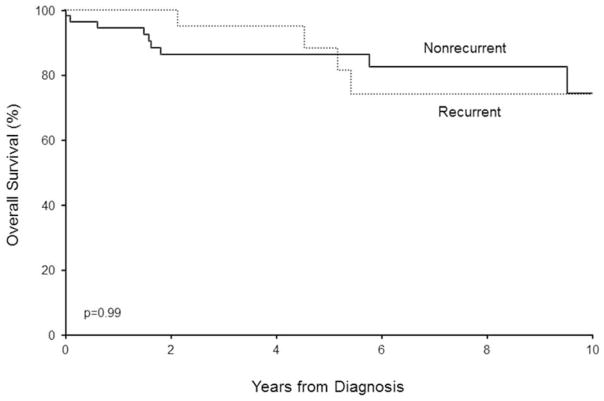
The overall survival rate (95% CI; number still at risk) at 5 years after diagnosis for the 24 patients with recurrent episodes, regardless of LCV subtype, was 88% (74%–100%; 13), which was significantly poorer than expected in the Minnesota white population (P=.04). There was no difference in overall survival between the recurrent LCV group and the nonrecurrent LCV group (P=.99, log-rank test; estimated overall 5-year survival rate in the nonrecurrent LCV group was 86% [77%–96%; 27]) (Figure 2).
Figure 2. Survival in Recurrent LCV Compared to Nonrecurrent LCV.
There was no difference in overall survival between the recurrent LCV group and the nonrecurrent LCV group. LCV indicates leukocytoclastic vasculitis.
Discussion
Patient Population and Epidemiology of LCV
Our study may be the first population-based study of histopathologically defined LCV. We found no statistical difference in the incidence of LCV in male and female patients. Chua et al8 reported that female patients outnumbered male patients 2:1 in their retrospective analysis of cutaneous vasculitis. However, other studies have demonstrated that the disorder is more common in men.9 Our study also found a statistically significant increase in the incidence of LCV with increased age at diagnosis.
The incidence of LCV has been estimated to be between 15 and 30 cases per million population per year,10 which is lower than the incidence of 45 cases per million population per year noted in our patients. Table 6 summarizes other studies that have estimated the incidence of LCV.
Table 6.
Results of Current and Previous Studies That Estimated the Incidence of Leukocytoclastic Vasculitis
| Study | Study Location | Duration of Study, y | Population Based | Incidence (Cases per Million Population per Year) | Miscellaneous |
|---|---|---|---|---|---|
| Current study | Olmsted County, Minnesota | 15 | Yes | 45 | Only cutaneous biopsy-proven cases were included |
| Watts et al, 199811 | Single district hospital, United Kingdom | 4 | No | 38.6 (“Cutaneous vasculitis”) | Incidence was 15.4 cases per million population per year for “cutaneous leukocytoclastic angiitis” |
| Garcia-Porrua and Gonzalez-Gay, 199912 | Primary referral center in a region of northwest Spain | 9 | No | 29.7 | Incidence calculated for cases of “hypersensitivity vasculitis” |
The most common subtype of LCV in our study was CSVV, with an incidence of 2.1 per 100,000 person-years, followed by IgA vasculitis, with an incidence of 1.3 per 100,000 person-years. The incidence of IgA vasculitis has been previously estimated in many studies; however, most have aimed at calculating the incidence of HSP, which is estimated to be between 3 and 26.7 per 100,000 person-years for children and infants and between 0.8 and 1.8 per 100,000 person-years for adults.13 Our study may have underestimated the true incidence of IgA vasculitis because we included only cases that were biopsy proven, and skin biopsies are often deferred in children in whom the diagnosis is made clinically. Also, for our study, IgA vasculitis was defined by the predominance of IgA on DIF; however, many studies estimating the incidence of HSP do not require that the patient evaluation include DIF.14
Etiologies and Subtypes of LCV
CSVV and IgA vasculitis were the most common subtypes of LCV in our population-based patient cohort. This frequency has implications for the most common LCV subtypes that are likely to be encountered by a dermatologist in a community-based setting. In both CSVV and IgA vasculitis, the most common etiologic association was idiopathic. It has been estimated that in 45% to 55% of cases of LCV a cause remains unknown.10,15 Other common causes of LCV include infection, ACTD, drug reactions, and malignancy. Our study had more cases that were idiopathic compared with the literature, and no cases were associated with malignancy. Fewer cases were attributed to medications than previously reported studies.16–19 A possible explanation for the larger proportion of idiopathic cases in our study could be that not all laboratory evaluations were obtained in each patient, making it difficult to ascertain an exact cause in some patients. Furthermore, we may have had fewer cases associated with malignancy or ACTD, given that our study was population-based (ie, only patients who were residents of Olmsted County, Minnesota [which is located in southeastern Minnesota approximately 80 miles from Minneapolis-St. Paul and has a population of approximately 125,000 residents], for 1 year before and 1 year after the diagnosis of LCV were included in the study; and all other patients with LCV who resided outside Olmsted County and were referred to Mayo Clinic were excluded from our study), whereas most of the other literature on LCV is derived from studies at tertiary referral centers.
Approximately 10% of the patients in our study had ANCA-associated vasculitis. This highlights that ANCA-associated vasculitides can manifest as LCV, emphasizing the importance of assessing for this entity in patients who have skin findings that histopathologically demonstrate LCV.
Systemic Involvement and Frequency of Recurrent Disease
As previously stated, 46% of our patients had systemic involvement, noted in all the different subtypes of CSVV. While IgA vasculitis commonly manifested systemic involvement (18/25 patients; 72%), systemic features were also observed in nearly 30% of patients with CSVV (11/38 patients). Ekenstam and Callen16 found in their analysis of 82 patients that 50% of patients had systemic complaints, although in their study, arthralgias were more common than renal involvement. Tai et al15 found that extracutaneous complaints were noted in 39.8% of 93 patients in their series. Given that renal involvement was seen in 17 of our patients (20% of the total patient population), a urinalysis and creatinine determination are essential tests for the evaluation of any patient with cutaneous vasculitis. Similar to previous studies, other organ systems that were found to be involved in our study were musculoskeletal, gastrointestinal, pulmonary, otorhinolaryngologic, and neurologic.6,16
Our study showed that 30% of patients had more than 1 episode of LCV. Recurrent episodes of LCV were observed across all 5 subtypes of LCV in our study and were associated with varied etiologies (including infection, ACTD, and idiopathic). Tai et al15 concluded in their retrospective analysis of 93 patients that 24.8% had either symptoms lasting 3 months or longer or evidence of recurrent symptoms. Ekenstam and Callen16 found that 16% of patients had relapsing disease, which they defined as 2 or more episodes of LCV lasting less than 3 months each. Other studies have also noted that there is a subgroup of patients with cutaneous vasculitis who have chronic or recurrent disease; however, the percentage of those with recurrent disease has not been estimated.17,20 The finding that 30% have more than 1 episode is important in counseling patients about the long-term prognosis of the disease. Furthermore, because nearly one-third of patients may have more than 1 episode of LCV (and episodes may occur over a period of years), intermittent or ongoing treatment may be necessary, particularly in those with recurrent disease.
Disease Course and Survival
Our study demonstrated that survival rates were significantly decreased in the overall LCV cohort compared with the expected survival for the Minnesota white population. Similarly, a statistically significant decrease in survival was seen in the following LCV subgroups: all CSVV, idiopathic CSVV, UV, and ANCA-associated vasculitis. Despite the frequent demonstration of systemic involvement in patients with IgA vasculitis, there was not a statistically significant decrease in survival in this subgroup compared with the Minnesota white population. Our study also demonstrated a statistically significant decrease in overall survival in patients with recurrent episodes of LCV.
In our study, 15 patients had died at last follow-up compared with only 2 of 82 patients in the series reported by Ekenstam and Callen.16 Previous studies have also noted a poor prognosis; for instance, in a study by McCombs in 1965,21 there were 18 deaths in 72 patients, and Winkelmann and Ditto22 reported 4 deaths among 38 patients. In these 2 studies, however, most patients had underlying systemic vasculitis implicated in the cause of death. Decreased survival might be expected in patients with systemic vasculitis such as ANCA-associated vasculitis or in those with cutaneous vasculitis due to a systemic condition (such as UV secondary to ACTD). However, in our study, survival was decreased even in patients who did not have systemic features of vasculitis or a known cause of their LCV (such as the subgroup with idiopathic CSVV). None of the 15 patients died from causes directly attributable to LCV. Only 1 patient was on prednisone for LCV (and none were on another systemic immunosuppressive agent for LCV) at the time of death, and in general, we did not observe higher rates of infectious or cardiovascular disease–related deaths in our LCV cohort. Four patients died due to malignancies that were temporally unrelated to their LCV. There was no difference in overall survival between patients with recurrent LCV and nonrecurrent LCV, suggesting that deaths in our patient cohort were likely unrelated to consequences of systemic immunosuppressive therapy. The reasons for poorer survival in the LCV cohort need to be further explored.
Limitations
Our study draws its strengths from being the first population-based study of LCV and from including a large number of patients over a 15-year time span. Our analysis was retrospective in nature, and therefore, not all patients had exactly the same work-up performed, making it difficult to attribute causation for LCV in several patients and therefore potentially overestimating the number of idiopathic cases. Although periodic reviews of systems and laboratory tests were generally performed as indicated during follow-up visits, not all patients with LCV were serially followed over time by both dermatology and rheumatology; therefore, it is possible that some patients classified as having idiopathic CSVV may have evolved to a different subtype of LCV (eg, ANCA-associated vasculitis) or eventually had a cause of LCV found (eg, malignancy or ACTD) if serial follow-up in both dermatology and rheumatology had been ensured for all patients. Given the retrospective nature of our study and the variability of information within the medical records, we were unable to quantitatively determine in all patients with recurrent LCV the mean number of LCV episodes and the mean dose of immunosuppressive therapy at the time of recurrent LCV. Since Olmsted County, Minnesota, has a predominantly white population, our findings may not be generalizable to other populations with a more heterogeneous composition. We restricted our study cohort to biopsy-proven cases of LCV. This criterion may have underestimated the overall incidence of LCV since many cases, particularly milder ones and those in children, may have been diagnosed on the basis of clinical findings alone. Also, our strict inclusion criteria for IgA vasculitis may have underestimated the incidence of patients with LCV with clinical features of Henoch-Schönlein purpura but who did not have IgA deposition on DIF microscopy (such patients were classified as having CSVV rather than IgA vasculitis in our study). However, our strict inclusion criterion of biopsy-proven LCV helped mitigate potential differences of the various classification and nomenclature systems used in previous studies.
Conclusion
In summary, our study showed that the incidence of LCV is 45 cases per million, which is higher than what has been previously published in the literature. To our knowledge, this is the first population-based study of LCV, thus adding essential knowledge to our current understanding of this disease entity. Idiopathic LCV was more common in our population-based cohort than previously described. Systemic involvement was present in nearly one-half of patients, and approximately 30% of patients with LCV had recurrent disease. Overall survival was significantly poorer in our LCV cohort, even without the presence of an identifiable cause and in the absence of known underlying systemic vasculitis.
Acknowledgments
Funding/Support: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676.
We thank David F. Fiorentino, MD, Department of Dermatology, Stanford University, and Richard D. Sontheimer, MD, Department of Dermatology, University of Utah, for their helpful comments regarding the design of the study. They received no compensation.
Abbreviations
- ACTD
autoimmune connective tissue disease
- ANCA
antineutrophil cytoplasmic antibody
- CI
confidence interval
- CSVV
cutaneous small-vessel vasculitis
- DIF
direct immunofluorescence
- HSP
Henoch-Schönlein purpura
- LCV
leukocytoclastic vasculitis
- REP
Rochester Epidemiology Project
- SD
standard deviation
- UV
urticarial vasculitis
Footnotes
Financial Disclosure: None reported.
Conflict of interest: None.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunder GG, Arend WP, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis: introduction. Arthritis Rheum. 1990;33(8):1065–1067. doi: 10.1002/art.1780330802. [DOI] [PubMed] [Google Scholar]
- 2.Basu N, Watts R, Bajema I, et al. EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann Rheum Dis. 2010;69(10):1744–1750. doi: 10.1136/ard.2009.119032. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45(1):3–13. doi: 10.1111/j.1365-4632.2005.02898.x. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino DF. Cutaneous vasculitis. J Am Acad Dermatol. 2003;48(3):311–340. doi: 10.1067/mjd.2003.212. [DOI] [PubMed] [Google Scholar]
- 7.Therneau TM, Sicks JD, Bergstralh EJ, Offord J Department of Health Science Research. Technical Report Series No. 52: Expected Survival Based on Hazard Rates. Rochester, MN: Mayo Clinic; 1994. [Google Scholar]
- 8.Chua SH, Lim JT, Ang CB. Cutaneous vasculitis seen at a skin referral centre in Singapore. Singapore Med J. 1999;40(3):147–150. [PubMed] [Google Scholar]
- 9.Garcia-Porrua C, Gonzalez-Gay MA, Lopez-Lazaro L. Drug associated cutaneous vasculitis in adults in northwestern Spain. J Rheumatol. 1999;26(9):1942–1944. [PubMed] [Google Scholar]
- 10.Chung L, Kea B, Fiorentino DF. Cutaneous vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2. Vol. 1. St. Louis, MO: Mosby Elsevier; 2008. pp. 347–367. [Google Scholar]
- 11.Watts RA, Jolliffe VA, Grattan CE, Elliott J, Lockwood M, Scott DG. Cutaneous vasculitis in a defined population: clinical and epidemiological associations. J Rheumatol. 1998;25(5):920–924. [PubMed] [Google Scholar]
- 12.Garcia-Porrua C, Gonzalez-Gay MA. Comparative clinical and epidemiological study of hypersensitivity vasculitis versus Henoch-Schönlein purpura in adults. Semin Arthritis Rheum. 1999;28(6):404–412. doi: 10.1016/s0049-0172(99)80006-7. [DOI] [PubMed] [Google Scholar]
- 13.Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25(2):171–178. doi: 10.1097/BOR.0b013e32835d8e2a. [DOI] [PubMed] [Google Scholar]
- 14.Penny K, Fleming M, Kazmierczak D, Thomas A. An epidemiological study of Henoch-Schönlein purpura. Paediatr Nurs. 2010;22(10):30–35. doi: 10.7748/paed2010.12.22.10.30.c8135. [DOI] [PubMed] [Google Scholar]
- 15.Tai YJ, Chong AH, Williams RA, Cumming S, Kelly RI. Retrospective analysis of adult patients with cutaneous leukocytoclastic vasculitis. Australas J Dermatol. 2006;47(2):92–96. doi: 10.1111/j.1440-0960.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 16.Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis: clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120(4):484–489. doi: 10.1001/archderm.120.4.484. [DOI] [PubMed] [Google Scholar]
- 17.Callen JP, af Ekenstam E. Cutaneous leukocytoclastic vasculitis: clinical experience in 44 patients. South Med J. 1987;80(7):848–851. doi: 10.1097/00007611-198707000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez NP, Van Hale HM, Su WP. Clinical and histopathologic spectrum of necrotizing vasculitis: report of findings in 101 cases. Arch Dermatol. 1985;121(2):220–224. [PubMed] [Google Scholar]
- 19.Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M. Cutaneous vasculitis in children and adults: associated diseases and etiologic factors in 303 patients. Medicine (Baltimore) 1998;77(6):403–418. doi: 10.1097/00005792-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Cupps TR, Springer RM, Fauci AS. Chronic, recurrent small-vessel cutaneous vasculitis: clinical experience in 13 patients. JAMA. 1982;247(14):1994–1998. [PubMed] [Google Scholar]
- 21.McCombs RP. Systemic “allergic” vasculitis: clinical and pathological relationships. JAMA. 1965;194(10):1059–1064. [PubMed] [Google Scholar]
- 22.Winkelmann RK, Ditto WB. Cutaneous and visceral syndromes of necrotizing or “allergic” angiitis: a study of 38 cases. Medicine (Baltimore) 1964;43:59–89. doi: 10.1097/00005792-196401000-00003. [DOI] [PubMed] [Google Scholar]