SUMMARY
The DNA base 5-hydroxymethylcytosine (5hmC) is produced by enzymatic oxidation of 5-methylcytosine (5mC) by 5mC oxidases (the Tet proteins). Since 5hmC is recognized poorly by DNA methyltransferases, DNA methylation may be lost at 5hmC sites during DNA replication. In addition, 5hmC can be oxidized further by Tet proteins and converted to 5-formylcytosine and 5-carboxylcytosine, two bases that can be removed from DNA by base excision repair. The completed pathway represents a replication-independent DNA demethylation cycle. However, the DNA base 5hmC is also known to be rather stable and occurs at substantial levels, for example in the brain, suggesting that it represents an epigenetic mark by itself that may regulate chromatin structure and transcription. Focusing on a few well-studied tissues and developmental stages, we discuss the opposing views of 5hmC as a transient intermediate in DNA demethylation and as a modified DNA base with an instructive role.
Introduction
DNA cytosine methylation is a well-known epigenetic modification that occurs predominantly at CpG dinucleotide sequences in mammals. The methylation state of CpG sites, being either unmethylated or methylated, has long been thought to be quite stable and is generally maintained during cell division by DNA methyltransferase enzymes that transfer methyl groups from S-adenosylmethionine onto the cytosine ring shortly after DNA replication [1, 2]. However, during certain stages of development and cell differentiation, methylation may be gained or lost at specific genes or may change even genome-wide. Researchers have long been fascinated by a poorly understood mechanism of DNA demethylation that would remove methyl groups from DNA in an enzymatic reaction that takes place in the absence of any DNA replication. Such a pathway has been known to occur for almost 30 years [3] but its mechanism and enzymology have remained enigmatic and controversial [4, 5].
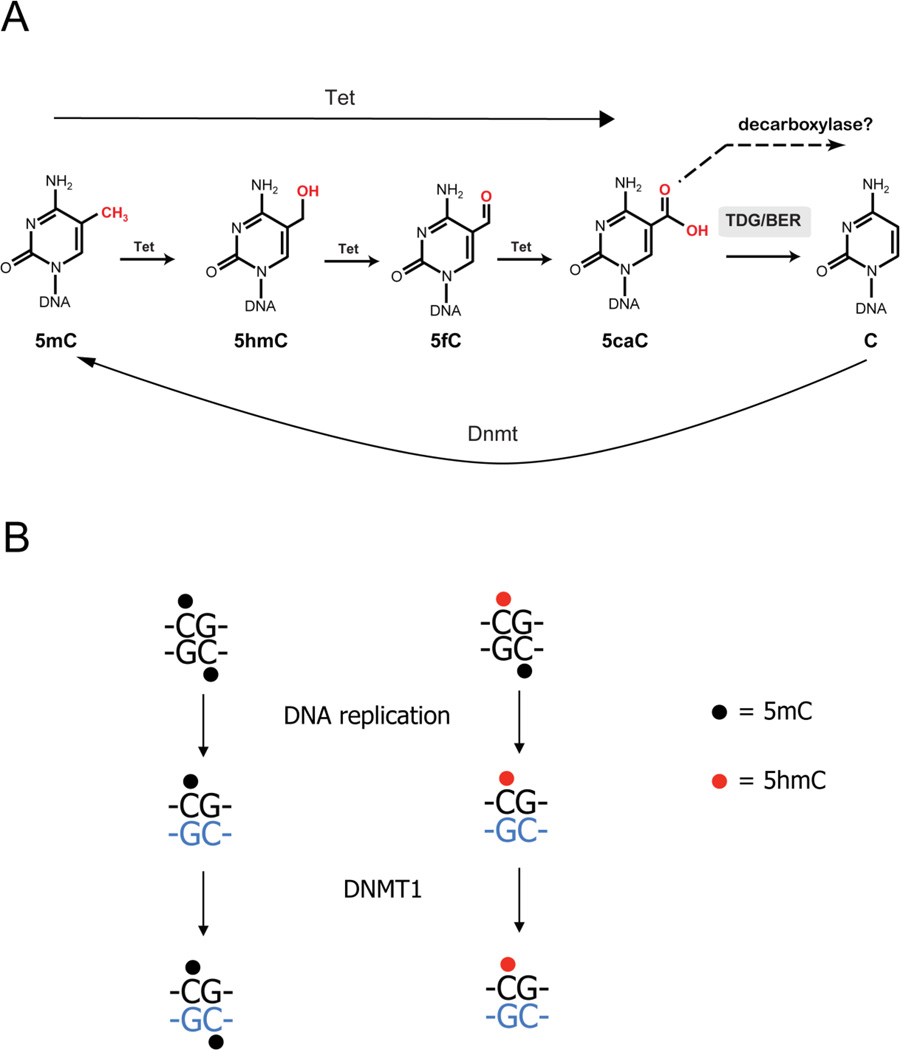
In 2009, a known product of oxidative damage to 5-methylcytosine (5mC), 5-hydroxymethylcytosine (5hmC), was ‘rediscovered’ and characterized as a relatively abundant enzymatically derived modification of 5mC in mammalian DNA [6, 7]. The protein Ten-Eleven-Translocation 1 (Tet1), previously implicated in chromosomal translocations in leukemia [8, 9], was shown to catalyze the oxidation of 5mC to 5hmC in a reaction that depends on the cofactors Fe(II) and 2-ketoglutarate [7]. Following the discovery of Tet1, the related proteins Tet2 and Tet3 were also shown to have the same enzymatic activity [10]. Tet proteins can oxidize 5mC to 5hmC and the oxidation reaction can proceed to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) [11, 12]. Furthermore, the pathway is continued by thymine-DNA glycosylase (TDG), which recognizes and excises 5caC (and also 5fC) from DNA [11]. Base excision repair is then engaged for processing of the resulting abasic site resulting eventually in appearance of unmodified cytosine and completion of a DNA methylation-demethylation cycle (see Figure 1) [11, 13, 14]. Although 5caC is chemically quite stable, one other potential mechanism for removal of 5caC is enzymatic decarboxylation. However, no such decarboxylase enzyme has been identified so far.
Figure 1. Active and passive DNA demethylation through 5-hydroxymethylcytosine (5hmC).
A. Active DNA demethylation is catalyzed by TET-protein-mediated 5mC oxidation and base excision repair.
B. Passive DNA demethylation is promoted by inhibition of DNMT1 at 5hmC-containing hemi-modified DNA strands.
As outlined in Figure 1A, 5hmC may be an intermediate in an active, replication-independent DNA demethylation process. Moreover, when present in DNA, 5hmC is a strong inhibitor of the DNA methyltransferase maintenance methylation reaction catalyzed by DNMT1 [15, 16], leading to passive DNA demethylation over subsequent replication cycles (Figure 1B). However, research over recent years also revealed that this DNA base appears to play its own role in epigenetic gene control. As we will discuss below, 5hmC is strongly associated with genes and regulatory elements in the genome, is abundant in brain, ES cells, primordial germ cells, and in fertilized oocytes. 5hmC is depleted in many types of human cancer [17], a topic that will not be discussed here in further detail but has been reviewed recently [18, 19] [see also review by Ficz and colleagues, this issue]. The fact that 5hmC can be established by three different Tet enzymes, Tet1, Tet2 and Tet3, which are differentially expressed during development and tissue formation and target diverse genomic regions such as gene body, promoters or enhancers, suggests that 5hmC may play a multifaceted role in genome biology. In this review, we will try to elucidate the dilemma as to whether 5hmC is just an intermediate product of the DNA demethylation process or is by itself an integral part of the epigenetic code.
5hmC in the brain
In 2009, the laboratory of Nathaniel Heintz described a surprisingly high level of 5hmC, 0.6% and 0.2% of total nucleotides, in Purkinje and granule cell neurons of the brain, respectively [6]. This work and a simultaneous report from the laboratory of Anjana Rao showing that Tet1 is able to oxidize 5mC to 5hmC [7] marked the beginning of the 5hmC era. The mammalian brain carries the highest level of 5hmC among all tissues analyzed so far reaching up to roughly 1% of all cytosine bases in the human brain cortex [20, 21]. This base is most abundant in neurons; for example in cerebellar Purkinje cells 5hmC reaches almost 40% of 5mC levels [6]. In contrast to 5mC, 5hmC specifically occurs in the CpG dinucleotide sequence context whereas 5mC is also commonly found at non-CpG sequences in the brain [22]. Considering these numbers, one would be inclined to assume that 5hmC must be a stable modification. Otherwise, if 5hmC represented a transient DNA modification in the brain, then its turnover rate would need to be very slow.
5hmC is accumulating in human and mouse brains during the life span, from neural progenitors through young neurons in the fetal brain, and further during aging of the brain after birth [23–25]. In mice, the accumulation of 5hmC in brain tissues continues with a slower rate at six weeks of age. In contrast to 5hmC, the level of 5mC seems to be decreasing during human brain aging [22]. It is important to note that a simultaneous loss of 5mC and accumulation of 5hmC were shown for the same genomic loci during primary synaptogenesis and during neuronal differentiation [23, 25]. In frontal cortex DNA, genic regions of highly expressed neuron-specific genes gain 5hmC between the fetus stage and 10 weeks of age [22]. However, detailed analysis of 5hmC by TAB-sequencing at nucleotide resolution revealed that the sum of methylated and hydroxymethylated cytosines remains the same for the majority of DNA regions during neuronal differentiation [23]. This fact suggests that 5hmC may partially replace 5mC in the brain and indicates a particular stability of 5hmC in this tissue. The concept of 5hmC stability in brain is clearly supported by presence of a very significant level of 5hmC among all modified cytosine bases and by 5hmC accumulation during aging [see also review by Sun and colleagues, this issue].
If 5hmC is a stable epigenetic mark, then it is logical to propose that 5hmC plays a regulatory function in the brain, where 5hmC levels are the highest, and possibly also in other tissues. Such a role may require the existence of specific 5hmC-binding proteins or 5hmC readers. Indeed, several putative 5hmC readers were identified recently [26, 27]. The best studied among them is methyl-CpG-binding protein 2 (MeCP2), mutations of which are causatively associated with the neurodevelopmental disorder Rett syndrome [28]. MeCP2 is able to bind 5hmC and 5mC with similar affinities [26]. MeCP2 may activate transcription in brain since absence of MeCP2 in knockout mice resulted predominantly in gene repression whereas MeCP2 overexpression mostly caused activation of the same brain-associated genes [29]. MeCP2 has been linked to increased chromatin accessibility, which also positively correlates with 5hmC levels and which is reduced in 5hmC-rich regions in MeCP2 KO mouse cerebellum [26]. Therefore, a 5hmC-binding protein exists in brain and appears to promote transcription and chromatin accessibility. The situation is far from clear, however, since numerous prior studies had characterized MeCP2 as a 5mC-binding transcriptional repressor [30]. It will be important to determine whether the 5mC- or the 5hmC-binding properties of MeCP2 are more important for preventing Rett syndrome.
Remarkably, according to different profiling methods, in brain, the majority of 5hmC is accumulating in intragenic regions of brain-specific and neuronal differentiation-associated genes where it positively correlates with gene transcript levels [20, 23–25, 31]. For instance, gene activation is linked to accumulation of intragenic 5hmC during neuronal differentiation in the developing mouse brain [23]. In concordance with the finding that 5hmC is predominantly an active epigenetic mark in neuronal tissue, nuclear staining with anti-5hmC antibodies indicates a clear euchromatin-specific pattern in contrast to the 5mC mark, which is concentrated in silent heterochromatin [23, 26, 32] [see also review by Wen and Tang, this issue].
The possible transcription-promoting function of 5hmC was demonstrated in experiments using manipulation of TET proteins. All three TET proteins have been associated with brain development and function. During fetal development (E11.5-E15.5) Tet3 is the most abundant Tet gene expressed, found in young neurons of the cortex where Tet1 is almost undetectable [23]. Knockdown of TET3 and TET2 by shRNA in vivo negatively affects neuronal migration in the cortex and overexpression promotes this process [23]. Conditional overexpression of Tet3 in mouse mature olfactory sensory neurons revealed that 5hmC accumulation is associated with gene activation in this cell type [33]. Moreover, it was shown that learning processes are associated with TET3 activation and 5hmC redistribution in the cortex. When Tet3 was knocked down in mice by shRNA, this event was associated with a decrease of 5hmC level and learning failure [34]. Tet1 KO mice show phenotypes of abnormal hippocampal long-term depression, affected memory and poor learning [35, 36] accompanied by downregulation of multiple neuronal activity-regulated genes [36] and genes involved in adult neural progenitor cell proliferation [35]. These data suggest that the mark, 5hmC, and its “writers”, the TET proteins may play an important role in brain function and development by regulating transcript levels of key neuron-specific genes. Such findings, taken together with the facts that 5hmC levels are so substantial in the brain and a specific reader, MeCP2 exists in this tissue, would imply that this base is a stable DNA mark and has a specific epigenetic regulatory function in neurons.
Differentially 5hmC-marked regions (DhMRs) exhibit dynamically changing 5hmC levels during aging [22, 25]. For instance, some genes that will become activated later in development, are marked by 5hmC at their regulatory regions in fetal brain [22] suggesting a type of long-term plasticity that depends on 5hmC. However, although 5hmC may mark dormant genomic elements for future DNA hypomethylation and gene activation, it would not be quite appropriate to refer to this marking as a ‘transient’ or ‘intermediary’ state in the DNA demethylation pathway because of the long time span involved.
Similarly to 5mC [37] 5hmC levels also correlate with transcript levels in gene bodies [20, 23, 24]. It is not known whether and how 5hmC enrichment along gene bodies promotes transcription. Based on these recent findings, 5hmC may be more potent than 5mC in opposing intragenic transcription initiation. Interestingly, a surprising transcription-correlated 5hmC bias exists towards the sense DNA strand and a 5mC bias towards the antisense strand is found within gene bodies [38]. Highly expressed genes showed the strongest bias. One possibility is that intragenic 5hmC, in particular when present on the sense strand, opposes spurious intragenic or anti-sense transcription. Different reports indicate that 5hmC is more strongly enriched in exons and frequently is observed at exon/intron boundaries similar to 5mC [25, 38–40]. Interestingly, DNA methylation positively affects both MeCP2 binding and exon usage during splicing [41]. Taking into account that MeCP2 binds with similar affinity to 5hmC and 5mC, we can hypothesize that the 5hmC level may regulate splicing through MeCP2 in the brain where 5mC is substituted at least partially by 5hmC in gene bodies.
In contrast to intragenic 5hmC that marks active genes, 5hmC at transcription start sites (TSS) often corresponds to an inactive/repressed state of the linked gene [22, 23, 38, 42, 43]. In addition, 5hmC is often associated with adult-specific (poised) enhancers in fetal brain and disappears upon enhancer activation. Therefore, 5hmC may play a dual role in brain by marking inactive, poised enhancers and by promoting transcriptional activity when present within gene bodies. Given that different TET proteins are associated with specific genomic 5hmC patterning in ES cells -- for instance, TET1 is associated with 5hmC at promoters and TET2 is correlated with genic 5hmC [44] -- we can hypothesize that genic and enhancer-associated 5hmC are also established by different members of the TET family in brain.
However, despite of all these data suggesting a specific regulatory role of 5hmC in the brain, the true stability of 5hmC in this tissue remains unknown. There still is the possibility that 5hmC is an intermediate product of DNA demethylation processes and that the resulting unmethylated cytosines are quickly re-methylated and then again oxidized, perhaps as part of memory or learning-related processes, or perhaps even during each round of transcription. This model would be consistent with the unusually high levels of DNMT3A protein found in the nuclei of human and mouse brain [45–47] and with the finding that TDG is associated with DNMT3A [48]. If such a continuous cycling scenario were the case, going through every stage of Figure 1A at an even pace, then levels of 5caC and 5fC should also be high and should be comparable to those of 5hmC in the brain. However, 5fC and 5caC occur at levels orders of magnitude lower than those of 5hmC in brain, as determined by very sensitive methods [12, 49, 50]. In addition, single-stranded DNA breaks generated by TDG during the DNA demethylation and repair process should be highly represented in this tissue. Brain is indeed characterized by presence of ssDNA breaks, which were previously linked to ongoing oxidative DNA damage in this tissue [51]. However, presence of ssDNA breaks negatively interferes with transcription [52]. On the other hand, a study of RNA polymerase II activity revealed that presence of 5hmC in a DNA template does not affect transcription, in contrast to 5fC and 5caC, which block polymerase elongation [53]. In summary, there is currently no direct evidence that a continuously ongoing methylation-demethylation cycle exists in the brain, or in any other tissue, although this possibility cannot formally be excluded at this time.
One unresolved issue is why the enzymatic oxidation of 5mC in vivo seems to stop in most instances at 5hmC, even though the TET proteins are fully capable of producing 5caC in vitro [11, 12]. There are at least two possible explanations. First, the activity of TET proteins in vivo may be more strictly regulated disallowing further oxidation of 5hmC except when true demethylation of the target sequence is to be achieved. Second, 5fC and 5caC are also effectively produced in vivo but are quickly removed by TDG or by other processes, perhaps because the cellular machinery views these modified bases as a form of DNA damage. In the latter situation, one would expect the target sequence to become fully demethylated almost instantaneously (which is not the case, for example in brain) unless the sequences immediately undergo re-methylation.
5hmC in zygotes
Two rounds of genome-wide, potentially active DNA demethylation events are executed during early development in mammals. Loss of 5mC occurs in primordial germ cells (PGCs) and in the male pronucleus of fertilized oocytes [54]. Replication-dependent dilution of 5mC in preimplantation embryos was first noticed by Rougier et al. [55]. Perhaps the best-documented example of active DNA demethylation is found almost immediately after fertilization when methylation seems to be removed exclusively from the paternal genome [56]. This ‘loss’ of 5mC, however, largely coincides with a conversion of 5mC into 5hmC [57–59]. After fertilization, the paternal pronucleus is characterized by a dramatic loss of 5mC staining beginning at the pronuclear stage 3 (PN3) and by appearance of the 5hmC mark. In contrast, the female pronucleus remains enriched with 5mC [57–59]. TET3 is by far the predominant TET activity present in oocytes and zygotes and its inactivation inhibits 5mC conversion to 5hmC in the male pronucleus [57–59]. The oocyte-specific protein PGC7 (DPP3A), binds to the H3K9me2 histone mark, and protects the maternal pronucleus from TET3-mediated oxidation. This is, at least in part, responsible for the asymmetry between maternal and paternal pronuclei [60, 61]. How the 5hmC is eventually processed in zygotes or early cleavage-stage embryos is still not entirely clear. Using immunostaining experiments with anti-5hmC and anti-5mC antibodies, it was found that 5hmC persists specifically on one set of chromosomes throughout the first mitotic division and is still seen in 2- and 4-cell embryos [57] suggesting that 5hmC is s stable DNA modification in zygotes. The gradual loss of 5hmC in preimplantation embryos appears to be largely replication-dependent [62] although one needs to keep in mind that immunostaining is not quantitative. It was shown that similar to 5hmC, 5fC and 5caC are also associated predominantly with the paternal pronucleus and become diluted during replication [63]. These findings argue against a genome-wide, active base removal process that produces completely demethylated cytosines in zygotes. Intuitively, active DNA demethylation involving excision repair of 5fC or 5caC by TDG, for example, should be an unlikely mechanism at this developmental stage, because the first cell of the emerging organism should not undergo genome-wide strand break repair on both DNA strands, a process invariably associated with the risk of forming lethal DNA double strand breaks.
Methylation levels in sperm are higher than those in oocytes [64–66] suggesting that there is a need for more rapid demethylation of the paternal genome in order to achieve a balance of parental chromosome methylation and eventually reach a ground state of low methylation in the inner cell mass. Of note, reprogramming factors reside predominantly in the male pronucleus [67]. A number of studies have demonstrated the presence of unmethylated cytosines at different pronuclear stages of zygotes and in 2-cell embryos and often the assumption has been made that such data reflect active DNA demethylation [57, 58, 65, 68, 69]. However, one cannot exclude the possibility that these unmethylated cytosines are the result of DNA replication processes or that they reflect 5caC, which behaves identical to cytosine in bisulfite sequencing reactions. On the other hand, if full replication-independent conversion to cytosine can be convincingly demonstrated for sperm-derived genomic loci, then 5hmC found in the paternal pronucleus could be seen as a transient modification at those sequences leading to active demethylation. In spite of recent data that the maternal genome also may undergo active DNA demethylation [65], given the prevailing evidence, it is likely that a combination of active and passive demethylation processes occurs in early embryos and that the paternal genome uses an active in addition to a passive DNA demethylation pathway. Independent of the exact mechanism, 5hmC may be viewed as a transient DNA base that initiates DNA demethylation in the sperm-derived genome of zygotes.
5hmC in primordial germ cells
Following global zygotic DNA demethylation, a major wave of de novo DNA methylation occurs from 3.5 to 6.5 dpc during embryo development [64, 70]. This highly methylated state is retained in the soma but global erasure of 5mC takes place in the embryonic germ line as part of the reprogramming of soma into gametes [71]. Recent studies focused on whether this demethylation involves 5hmC. The progenitors of PGCs emerge from proximal epiblast cells with characteristic expression of PRDM1 (BLIMP1) at 6.25 dpc and PRDM14 at 6.5 dpc [72]. Immunostaining of embryo sections showed that at 8.0 dpc DNA methylation is comparable in PGCs to neighboring somatic cells at the base of the allantois whereas migrating PGCs at 8.0 dpc and 9.5 dpc have already substantially reduced 5mC staining, which appears even further reduced at 12.5 dpc in the gonads [73]. Genome-wide mapping of DNA methylation using MeDIP-seq and whole genome bisulfite sequencing (WGBS) methods confirmed that 5mC loss occurs in two steps. A fast, global demethylation before 9.5 dpc is followed by a slow, locus-specific demethylation in the late migrating and gonadal PGCs [74, 75]. This demethylation occurs in connection with resetting the epigenome towards the germ lineage [74–78] which includes the erasure of parental-allele-specific DNA methylation marks at differentially methylated regions of imprinted genes [79–83], and in turn, erasing imprinted gene expression [81, 84, 85]. Global demethylation is complete by 13.5 dpc in both sexes with a small set of escapee loci and IAP repeats that never get fully demethylated [74–76, 80].
Bisulfite sequencing showed that erasure of methylation at imprinted differentially methylated regions (DMRs) occurs with variable kinetics and is a late event as substantial DNA methylation can be detected at DMRs at 11.5 dpc [80]. The demethylation at DMRs was already initiated in migrating PGCs and reached completion in the gonadal PGCs [83, 86]. DMR demethylation was never faster than the theoretical value calculated based on the precisely measured doubling time of PGCs, which is 12.6 hours between 9.5 dpc and 12.5 dpc, suggesting that DNA demethylation in PGCs occurs largely by a passive dilution mechanism [86]. Support for this model comes from the fact that UHRF1 (Np95), a cofactor of the maintenance methyltransferase DNMT1, is excluded from PGC nuclei and the de novo DNMTs are not expressed in PGCs (DNMT3A) or excluded from the nucleus (DNMT3B) [73, 75, 86, 87]. However, demethylation at DMRs appeared in a mosaic pattern. It was not disturbed at the H19 DMR when replication was blocked by aphidicolin, but was reduced when the BER pathway was blocked by a PARP inhibitor, 3-AB, suggesting an actively targeted process [83]. In addition, hairpin bisulfite sequencing results revealed that demethylation occurs faster and slower than the theoretical passive and fully active demethylation values, respectively; therefore, it likely involves both mechanisms [75].
Several lines of evidence suggest that DNA demethylation in PGCs involves oxidation of 5mC to 5hmC. Tet1 and Tet2 but not Tet3 RNA are detectable in PGCs [58, 86–88]. Single cell analysis showed that Tet1 was transcribed in PGCs at 9.25 dpc and in each Dppa3+ PGC at 11.5 dpc [89]. Tet2 was heterogeneously expressed at 9.25 and 10.25 but it was expressed in almost all Dpp3a+ PGC at 11.5 dpc. According to immunostaining of PGCs, TET1 and TET2 proteins are highly expressed and localized in the nucleus in PGCs at 9.5–11.5 dpc. Immunostaining of PGCs showed that 5hmC staining transiently peaks in PGCs between 9.5–10.5 dpc (between 10.5–11.5 dpc when stained in embryo sections and exhibiting an increasingly dotted pattern) and subsequently gradually diminishes, suggesting that 5hmC represents an intermediate in PGC demethylation [88, 90]. It was reported, based on MeDIP-seq and hMeDIP-seq experiments that loss of 5mC at exons paralleled 5hmC enrichment, suggesting that a 5mC to 5hmC conversion took place at exons in PGCs [88]. Glu-PCR showed 5hmC enrichment at the Dazl promoter, which is known to require DNA methylation for its repression and is demethylated in PGCs. Meiosis was impaired in Tet1−/− PGCs and corresponded to failed demethylation/activation of a subset of meiosis-specific genes, Sycp1, Sycp3 and Mael. Exons, introns, LTRs and IAPs were also affected [91]. These studies collectively suggested a functional role for TET1 and perhaps TET2 in PGC demethylation. Even though Tet1−/− PGCs displayed reduced 5hmC staining, this was not accompanied by increased global 5mC levels as detected by immunostaining or WGBS, suggesting that oxidation of 5mC by TET1 is not responsible for erasing the bulk of DNA methylation in PGCs [91]. The same result was reported for Tet1−/−Tet2−/− double knockout (DKO) PGCs using immunohistochemistry of ovaries and testes at 13.5 dpc [88]. In agreement with this finding, mass spectrometry showed that global DNA methylation was unchanged in DKO sperm.
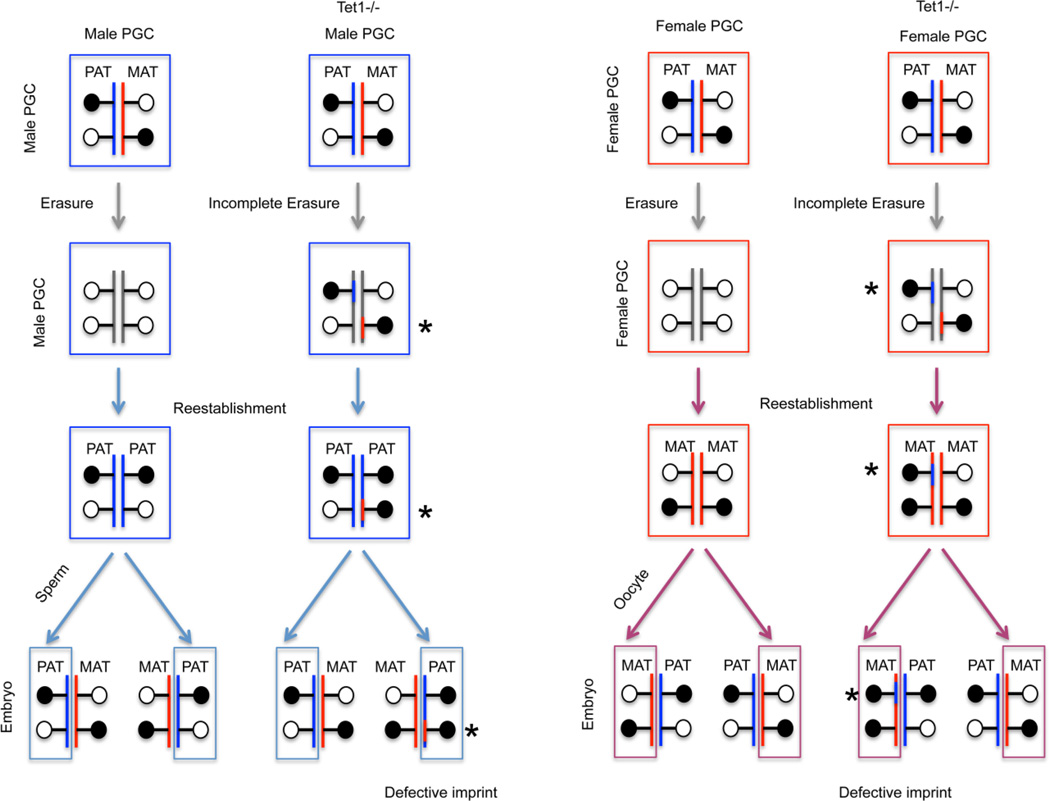
The conversion of 5mC to 5hmC at imprinted DMRs was suggested by the kinetics of these marks, measured by MeDIP and hMeDIP [88]. Genetic studies give us clues about the possible roles of TET1 and TET2 in the erasure of genomic imprints. Offspring of Tet1−/− [90] and Tet1−/−/Tet2−/− DKO mice [92] both exhibited variable phenotypes that are consistent with incomplete imprint erasure. There is controversy as to whether Tet1 KO alone results in failed PGC demethylation and imprint erasure, and the reason for this is unclear at this time. Heterozygous mice developing from Tet1−/− male germ cells exhibited variable phenotypes including early embryonic lethality due to placental defects and neonatal lethality or fetal or postnatal growth retardation. RNA-seq analysis and bisulfite sequencing experiments revealed that numerous imprinted genes were misregulated in the embryos and placentas. In addition, closely linked imprinted genes (e.g. Peg10 and Sgce) were coordinately misregulated and the pattern of misregulation often corresponded to hypermethylation of maternally methylated imprinted DMRs (e.g Peg10-Sgce DMR), consistent with failed demethylation of the maternal allele in male PGCs. Indeed, hypermethylation of maternal Peg10 DMRs was observed in Tet1−/− male PGCs at 13.5 dpc. Reduced representation bisulfite sequencing (RRBS) in 13.5 dpc Tet1−/− male germ cells and Tet1−/− sperm DNA showed that the hypermethylation was not affecting the entire genome [90], but rather a group of late-demethylating loci in the PGC genome, including 7 out of 12 known maternal DMRs. The imprinting phenotypes observed in paternally mutant Tet1+/− embryos can be traced back to Tet1−/− sperm and even further to DNA defects of methylation erasure in male Tet1−/− PGCs. Homozygous Tet1 mutation also had similar effect in the erasure of imprinting in the female germline. Variable phenotypes were observed in the offspring of Tet1−/− female mice, including embryonic lethality and increased growth. Hypermethylation of paternally methylated Dlk1/Gtl2 and Rasgrf1 DMRs were detected in the placentae of dead maternal mutant embryos consistent with misregulation of the respective imprinted genes. Another group reported that Tet1−/−/Tet2−/− DKO resulted in partially penetrant perinatal lethality [92]. Some DKO males that survived were fertile, but their Tet1+/−/Tet2+/− offspring exhibited partially penetrant lethality. Surviving DKO females had smaller ovaries, fewer mature oocytes and small litters, and only a small fraction of their Tet1−/+/Tet2−/−+ pups survived to adulthood. It was reported, based on genome-wide MeDIP assays, that the offspring of DKO male and female mice had increased 5mC levels across various imprinted loci. Bisulfite sequencing confirmed hypermethylation of maternal DMRs, Peg3 and Mest, in some offspring of DKO male corresponding to silenced transcription of these genes. Hypermethylation of paternal H19 DMR was also found in some offspring of female DKO. However, DKO sperm DNA methylation appeared normal at two maternally methylated DMRs. These authors interpreted the findings as “the establishment of imprinting is compromised in DKO gametes.” It is likely that erasure of methylation was defective in DKO PGCs. These two studies collectively suggest that imprint erasure in PGCs depends on TET-mediated oxidation. The variable phenotype is the results of incomplete erasure of DMRs in the mutant PGCs with variable level of remaining DNA methylation per DMR per chromosome and also from random distribution of the grandmaternally and grandpaternally inherited chromosomes from the male or female germ lines, respectively. In Figure 2 we depicted one pair of chromosomes out of 20 with a maternally and paternally methylated imprinted DMR.
Figure 2. Model for imprinting defects in Tet-deficient mice.
Imprint erasure and establishment are shown for the male (left) and female (right) germ lines. Methylated and unmethylated DMRs at imprinted genes are depicted as black and white circles, respectively. Paternally and maternally marked chromosomes are blue and red, respectively. Erased chromosomes are gray. Imprint erasure is compromised in Tet1-deficient mice as indicated by the asterisks. One chromosome is inherited from the germline via the sperm or oocyte. For each locus, the probability of inheriting the improperly remodeled allele is 50%. The level of demethylation failure may be also variable for each locus. These mice may exhibit developmental failure and/or lethality, depending on the role of the specific imprinted locus in development.
In addition to genetic studies, in vitro model systems provided further evidence for the role of TET1 and TET2 in PGC demethylation and imprint erasure. Bisulfite sequencing showed that demethylation of germline genes Dazl, Sycp3 and Mael, and repeat elements (IAP and LINE-1) was impaired and imprinted DMRs demethylation didn’t occur when Tet1 and Tet2 were knocked down in PGC-like cultured cells [88]. Embryonic germ cells (EGCs) and ES cells are capable of reprogramming the genome of fused somatic cells by activating pluripotency genes upon fusion. EGCs in addition have the capacity to erase DNA methylation imprints at DMRs of imprinted genes [93]. Bisulfite sequencing showed that upon fusing mouse B cells with EGCs, DNA methylation decreased at the H19/Igf2, Dlk1/Gtl2 (paternal) and Peg3 (maternal) DMRs. A Peg1 promoter-beta galactosidase transgene was demethylated and its transcription was reactivated by the fusion. 5hmC accumulated at the H19/IGF2, PEG3 and SNURF DMRs in human DNA 48 hrs after fusion with EGCs. ShRNA-mediated knockdown experiments suggested that Tet2 is required for the efficient reprogramming capacity of EGCs, whereas Tet1 was necessary to induce 5-methylcytosine oxidation-mediated erasure of genomic imprints at DMRs [93]. Another study investigated the role of Tet1 and Tet2 in the in vitro PGC (iPGCs) formation from ESCs. iPGCs recapitulate many aspects of in vivo PGC differentiation, including the expression pattern for each of the Tet1 genes [89]. WGBS showed that iPGC differentiation and global DNA demethylation doesn’t require Tet1 and Tet2, but a number of specific promoters and gene bodies (including germline specific genes e.g. Tdrd5 and Piwil4 and imprinted genes Snrpn and Peg3) exhibited DNA hypermethylation in Tet2−/− and Tet1 knockdown iPGCs. This again is consistent with the notion that 5hmC is an intermediate in locus-specific demethylation in PGCs that includes imprinted genes.
What is unclear at present is to what extent the 5mC oxidation pathway participates in the initial genome-wide wave of demethylation in PGCs before 9.5 dpc. Because the late-demethylating imprinted loci loose bisulfite-resistant moieties (5mC and 5hmC combined) with the rate expected from passive, replication-coupled demethylation, it is logical to expect that the early phase of fast global demethylation should involve some sort of active demethylation mechanism. Although genetic knockouts suggest that TET-mediated oxidation is not required for this global step, there still might be an important locus-specific role of 5mC oxidation to “kick-start” the demethylation process at certain genomic sites. It will be important to map 5hmC in PGCs at early demethylating loci. Interestingly, LINE1 elements and IAPs showed reduced 5hmC and elevated 5mC levels in Dppa3 null PGCs at 10.5 dpc, suggesting that DPPA3 may perhaps target 5mC oxidation to these repeats in migrating PGCs [94]. This is, indeed, the opposite of what role DPPA3 (PGC7) plays during zygotic demethylation [60] and it also occurs in the virtual absence of H3K9me2 in PGCs [73]. In addition, a partial role of DNA demethylation via deamination of 5mC by activation-induced cytidine deaminase (AID) was suggested based on bisulfite sequencing of the PGC genome at 13.5 dpc [95]. Because Aid is not expressed in the epiblast or in PGCs between 9.5 and 13.5 dpc [86, 87, 89], AID might exert its activity between 7.5–8.5 dpc in the early demethylation phase.
Immunostaining and mass spectrometry data suggest that a passive loss of 5hmC by replication-dependent dilution eliminates 5hmC after the Tet1-mediated oxidation step in PGCs [86, 96]. This data is in agreement with bisulfite sequencing data, because 5hmC is another bisulfite-sequencing-positive DNA base [97, 98]. TET proteins can oxidize 5hmC further to 5fC and 5caC, but this process has not been studied during PGC reprogramming [76, 96]. All of the experimental evidence suggests that 5hmC represents an intermediate (transient) stage during reprogramming of PGCs with one exception: 5hmC appears to be a stable mark at pericentric regions in oocytes up to the germinal vesicle stage, consistent with lack of replication in the female germ line after 14.5 dpc [96].
Whole genome TAB-sequencing (WGTABS) experiments in normal PGCs will reveal the precise dynamics of TET-mediated oxidation. WGBS and WGTABS in Tet1−/−, Tet2−/− and DKO PGCs will unequivocally determine the role of each TET proteins in PGC demethylation. It will be interesting to find out what protects the specific loci from global demethylation in the first phase and how the active second phase is orchestrated. What mechanism is behind the considerable heterogeneity with regard to demethylation of specific loci in the second phase? Transcription factors and histone covalent modifications are likely involved. Indeed, global reorganization of chromatin composition is an early event in the epigenetic reprogramming of PGCs [73, 99]. PRDM14 is an important factor for PGC specification and global reprogramming and for their migration into the genital ridges [100]. Based on somewhat artificial but intriguing evidence -- overexpressing of Prdm14 in ESCs --, it was suggested that PRDM14 might play a role in targeting TET1 and TET2 to the pluripotency genes, germ cell genes and imprinted DMRs in PGCs [101].
It is perhaps informative to compare the two major demethylation events. Specific loci are protected from global TET3-mediated demethylation in the zygote’s paternal pronucleus whereas specific loci survive the first wave of global demethylation in PGCs, and these late demethylated regions are oxidized by TET1 and perhaps TET2. What can be so different in the TET3 versus TET/TET2 enzymes to act more globally or at specific sites? Further experiments need to elucidate whether this depends on regulatory mechanisms affecting TET protein function and/or their accessibility to DNA.
5hmC in ES cells
ES cells contain 5hmC, which decreases upon differentiation [7]. Studies using knockdown strategies revealed that TET1, together with TET2, plays a central role in maintaining the pluripotent state since reduced TET activities caused ES cell differentiation, aberrant DNA methylation of promoters and repression of genes important for pluripotency including Nanog [10, 32]. Inconsistent with this data, Tet1/2 double knockout ES cells remained pluripotent, contributing to each germ layer in teratoma assays and contributed to chimeric embryos with the caveat that a skewed contribution to extraembryonic lineage was observed in teratomas and developmental defects occurred in chimeras, likely due to the group of developmental genes that became deregulated [92]. Further experiments with Tet1/2/3 triple-knockout (TKO) ES cells revealed that Tet-TKO embryoid bodies are characterized by aberrant methylation of promoters and gene bodies and repression of key genes important for development and cell differentiation thus compromising proper differentiation of ES cells [102].
Remarkably, TET1 and TET2 show different localizations in the genome of ES cells [44]. TET2 is present along with 5hmC along gene bodies whereas TET1 is responsible for 5hmC deposition at promoters and enhancers [44, 103]. Profiling of TET1 in the ES cell genome revealed that TET1 binding is specifically enhanced at active and bivalent promoters where TET1 presence is positively correlated with the H3K4me3 mark [103, 104]. 5hmC profiling in ES cells showed some general similarities in 5hmC patterns with brain tissues. For instance, in ES cells, 5hmC is accumulated at repressed promoters, marks gene bodies of actively transcribed genes where it reflects exon/intron structure and is associated with enhancers and p300 binding sites [40, 43, 103–105]. The specific genes marked by 5hmC along gene bodies, however, are different between ES cells, neuronal cells, or other cell types reflecting which genes are expressed in a particular type of tissue [106–108].
It is important to note that the TET1 binding pattern does not exactly reflect 5hmC occupancy. TET1 mostly binds to active promoters whereas, according to numerous profiling data, 5hmC is missing at active promoters but is enriched at sequences immediately surrounding these promoters. A similar situation is found at enhancers, which are associated with accumulation of 5hmC around p300 binding sites. This fact may suggest that enhancers and active promoters undergo enhanced TET-mediated surveillance for aberrantly introduced 5mC by extensive 5mC oxidation and possibly further oxidation and repair of 5hmC. This suggestion was confirmed by 5fC profiling in mouse ES cells with compromised TDG. Here TET1 binding sites were characterized by the highest rate of 5hmC oxidation [109]. In the absence of TDG, 5fC and 5CaC showed similar patterns to 5hmC in ES cells: both marks were found at bivalent and repressed promoters, at p300 sites, and in gene bodies of actively transcribed genes, particularly in exons [13, 109]. Thus 5hmC conversion may occur continuously, which may indicate that it is perhaps an unstable mark in ES cells at specific sites. One should bear in mind, however, that in these Tdg knockout or knockdown experiments, the levels of 5fC and 5caC were increased only ~2-fold to ~8-fold compared to the very low levels found in wildtype ES cells [13, 109]. If 5hmC were rapidly and continuously turned over to 5fC and 5caC, their levels should be much higher than those reported in ES cells lacking TDG.
On the other hand, TET activity and TDG are required for demethylation of key micro RNA genes that are required for the mesenchymal-to-epithelial transition in mouse embryo fibroblasts reprogrammed towards pluripotency [14]. TDG knockdown leads to an increase in 5fC and 5caC during neuronal and glial cell differentiation suggesting that active DNA demethylation may occur at cell type-specific promoters during lineage specification [110]. In these settings, 5hmC, as well as the higher oxidation products 5fC and 5caC, might function as transient intermediates. Notwithstanding these perhaps special situations, however, the balance of the evidence is currently in favor of 5hmC being a stable DNA modification.
Is 5hmC a DNA modification that has mostly methylation-inhibitory functions?
The question may be asked whether 5hmC is perhaps mostly a negative mark that repels DNMT proteins and many methyl-CpG binding proteins. Even before 5hmC has become known as an epigenetic and enzymatically produced DNA modification, earlier studies by the laboratory of Lawrence Sowers investigated the effect of this DNA modification on a DNA methyltransferase and a methyl-CpG recognizing protein domain [16, 111]. Methylation of CpG sites by DNMT1 is inhibited when 5hmC is present on one DNA strand [15, 16, 112]. This blockage of maintenance DNA methylation will lead to passive DNA demethylation and is though to represent an important biological function of 5hmC, being important for example during PGC DNA demethylation and during demethylation of the paternal genome after fertilization, as discussed above.
Earlier investigations showed that the methyl-binding domain of MeCP2 was unable to bind to 5hmC [111], which is in apparent contrast to data by Mellén et al. [26]. Furthermore, it was shown that several other proteins that contain an MBD domain, including MBD1, MBD2, and MBD4 were all incapable of binding to 5hmC [98]. One report suggested that MBD3, which displays poor binding to 5mC, indeed interacts with 5hmC [113]. However, other studies have come to a different conclusion and suggested poor binding of MBD3 to 5hmC [15, 114, 115]. One other protein, initially identified as a protein recognizing 5mC and promoting maintenance of DNA methylation is UHRF1 [116]. UHRF1 and a related protein, UHRF2, are capable of binding to 5hmC [27, 117, 118] and may help propagate methylation patterns regionally by recruiting DNMT1. While UHRF1 may have a better affinity to 5mC than to 5hmC [119], the binding of UHRF2 to 5hmC appears to be stronger than its binding to 5mC [27, 118]. Their respective role in distinguishing between 5mC and 5hmC and their functions in vivo are not completely understood at this time. Except perhaps for UHRF2, there is yet no well-characterized protein that specifically recognizes the hydroxymethylated form of cytosine. Interestingly, two studies based on mass spectrometric analysis showed that the number of proteins binding to 5fC and 5caC is in fact much greater than the number of those binding to 5hmC even though these modifications are much less abundant than 5hmC in tissues [27, 120].
Viewing all the data combined, it seems not unreasonable to propose that the major biological effects of 5hmC are mostly negative ones including the inhibition of DNA methyltransferases leading to passive DNA demethylation and the interference with methyl-CpG recognizing inhibitory factors such as most of the MBD proteins. Only MeCP2 and UHRF2 seem to have a reasonable or even preferential affinity towards 5hmC when compared to 5mC. Steric hindrance imposed by the hydroxymethyl groups may also negatively affect the affinity of other DNA binding proteins to their targets but this has not yet been investigated in much detail.
Conclusions
So, in summary, how stable is 5hmC in cells? In one extreme view, 5hmC can be considered as very labile, similar to reversible chromatin modifications, and much in contrast to 5mC, which has always been though to be exceptionally stable. The prevailing evidence based on currently available data would suggest, however that 5hmC is indeed rather stable in somatic tissues and serves specific biological functions that may include specific readers such as UHRF2 and MeCP2. Yet, its major functional role may in large part be based on blocking the interaction of 5mC-targeting proteins with DNA. During specific phases of germ cell and early embryonic development, 5hmC can be considered as a transient intermediate that promotes either passive, replication-dependent or active DNA demethylation. There is accumulating evidence that a TET-mediated active demethylation process also may occur at certain genomic sequences during cell differentiation and reprogramming. The available information suggests that there is a regulatory step that determines at what developmental stage and at what genomic loci oxidation of 5mC continues beyond the 5hmC base to produce 5caC and eventually complete DNA demethylation by converting 5caC to cytosine. In such gene-specific demethylation pathways, 5hmC would indeed be a transient intermediate of the oxidation-demethylation cycle but it would be difficult to detect as such, similar to 5fC and 5caC, which turn over rapidly due to TDG-initiated base excision repair. Further studies are necessary to define the role of 5hmC as both a stable and transient DNA modification in various biological contexts including developmental stages, cell differentiation and disease states.
Acknowledgements
The work of the authors was supported by NIH grant CA160965 to GPP and NIH grant GM064378 to PES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riggs AD. X inactivation differentiation, and DNA methylation. Cytogenet. Cell Genet. 1975;14:9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- 2.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- 3.Saluz HP, Jiricny J, Jost JP. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Zhang Y. Reversing DNA methylation: mechanisms genomics and biological functions. Cell. 2014;156:45–68. doi: 10.1016/j.cell.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorsbach RB, Moore J, Mathew S, Raimondi SC, Mukatira ST, Downing JR. TET1 a member of a novel protein family is fused to MLL in acute myeloid leukemia containing the t(10;11)(q22;q23) Leukemia. 2003;17:637–641. doi: 10.1038/sj.leu.2402834. [DOI] [PubMed] [Google Scholar]
- 9.Ono R, Taki T, Taketani T, Taniwaki M, Kobayashi H, Hayashi Y. LCX leukemia-associated protein with a CXXC domain is fused to MLL in acute myeloid leukemia with trilineage dysplasia having t(10;11)(q22;q23) Cancer Res. 2002;62:4075–4080. [PubMed] [Google Scholar]
- 10.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y, Role of Tet proteins in 5mC to 5hmC conversion. ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen L, Wu H, Diep D, Yamaguchi S, D'Alessio AC, Fung HL, Zhang K, Zhang Y. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153:692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Zhang L, Mao SQ, Li Z, Chen J, Zhang RR, Wu HP, Gao J, Guo F, Liu W, Xu GF, Dai HQ, Shi YG, Li X, Hu B, Tang F, Pei D, Xu GL. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell. 2014;14:512–522. doi: 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto H, Liu Y, Upadhyay AK, Chang Y, Howerton SB, Vertino PM, Zhang X, Cheng X. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res. 2012;40:4841–4849. doi: 10.1093/nar/gks155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valinluck V, Sowers LC. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–950. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 17.Jin SG, Jiang Y, Qiu R, Rauch TA, Wang Y, Schackert G, Krex D, Lu Q, Pfeifer GP. 5-Hydroxymethylcytosine is strongly depleted in human cancers but its levels do not correlate with IDH1 mutations. Cancer Res. 2011;71:7360–7365. doi: 10.1158/0008-5472.CAN-11-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeifer GP, Kadam S, Jin SG. 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin. 2013;6:10. doi: 10.1186/1756-8935-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeifer GP, Xiong W, Hahn MA, Jin SG. The role of 5-hydroxymethylcytosine in human cancer. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-1896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin SG, Wu X, Li AX, Pfeifer GP. Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res. 2011;39:5015–5024. doi: 10.1093/nar/gkr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Münzel M, Globisch D, Bruckl T, Wagner M, Welzmiller V, Michalakis S, Muller M, Biel M, Carell T. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew. Chem. Int. Ed. Engl. 2010;49:5375–5377. doi: 10.1002/anie.201002033. [DOI] [PubMed] [Google Scholar]
- 22.Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, Jui J, Jin SG, Jiang Y, Pfeifer GP, Lu Q. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013;3:291–300. doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotechnol. 2011;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, Vasanthakumar A, Godley LA, Chang Q, Cheng X, He C, Jin P. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, Munzel M, Wagner M, Muller M, Khan F, Eberl HC, Mensinga A, Brinkman AB, Lephikov K, Muller U, Walter J, Boelens R, van Ingen H, Leonhardt H, Carell T, Vermeulen M. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2 encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Kim M, Park YK, Kang TW, Lee SH, Rhee YH, Park JL, Kim HJ, Lee D, Lee D, Kim SY, Kim YS. Dynamic changes in DNA methylation and hydroxymethylation when hES cells undergo differentiation toward a neuronal lineage. Hum. Mol. Genet. 2014;23:657–667. doi: 10.1093/hmg/ddt453. [DOI] [PubMed] [Google Scholar]
- 32.Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 33.Colquitt BM, Allen WE, Barnea G, Lomvardas S. Alteration of genic 5-hydroxymethylcytosine patterning in olfactory neurons correlates with changes in gene expression and cell identity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14682–14687. doi: 10.1073/pnas.1302759110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, D'Alessio A, Zhang Y, Bredy TW. Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7120–7125. doi: 10.1073/pnas.1318906111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 2013;13:237–245. doi: 10.1016/j.stem.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013;79:1109–1122. doi: 10.1016/j.neuron.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauch TA, Wu X, Zhong X, Riggs AD, Pfeifer GP. A human B cell methylome at 100base pair resolution. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:671–678. doi: 10.1073/pnas.0812399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen L, Li X, Yan L, Tan Y, Li R, Zhao Y, Wang Y, Xie J, Zhang Y, Song C, Yu M, Liu X, Zhu P, Li X, Hou Y, Guo H, Wu X, He C, Li R, Tang F, Qiao J. Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain. Genome Biol. 2014;15:R49. doi: 10.1186/gb-2014-15-3-r49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Pan Q, Lin L, Szulwach KE, Song CX, He C, Wu H, Warren ST, Jin P, Duan R, Li X. Genome-wide DNA hydroxymethylation changes are associated with neurodevelopmental genes in the developing human cerebellum. Hum. Mol. Genet. 2012;21:5500–5510. doi: 10.1093/hmg/dds394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P, Tahiliani M, Daley GQ, Liu XS, Ecker JR, Milos PM, Agarwal S, Rao A. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473:394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maunakea AK, Chepelev I, Cui K, Zhao K. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013;23:1256–1269. doi: 10.1038/cr.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Z, Terragni J, Borgaro JG, Liu Y, Yu L, Guan S, Wang H, Sun D, Cheng X, Zhu Z, Pradhan S, Zheng Y. High-resolution enzymatic mapping of genomic 5-hydroxymethylcytosine in mouse embryonic stem cells. Cell Rep. 2013;3:567–576. doi: 10.1016/j.celrep.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, Min JH, Jin P, Ren B, He C. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Chavez L, Chang X, Wang X, Pastor WA, Kang J, Zepeda-Martinez JA, Pape UJ, Jacobsen SE, Peters B, Rao A. Distinct roles of the methylcytosine oxidases Tet1 and Tet2 in mouse embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1361–1366. doi: 10.1073/pnas.1322921111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J. Neurosci. Res. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- 46.Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PloS one. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE. Dnmt3a–dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li YQ, Zhou PZ, Zheng XD, Walsh CP, Xu GL. Association of Dnmt3a and thymine DNA glycosylase links DNA methylation with base-excision repair. Nucleic Acids Res. 2007;35:390–400. doi: 10.1093/nar/gkl1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu S, Wang J, Su Y, Guerrero C, Zeng Y, Mitra D, Brooks PJ, Fisher DE, Song H, Wang Y. Quantitative assessment of Tet-induced oxidation products of 5-methylcytosine in cellular and tissue DNA. Nucleic Acids Res. 2013;41:6421–6429. doi: 10.1093/nar/gkt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaffeneder T, Spada F, Wagner M, Brandmayr C, Laube SK, Eisen D, Truss M, Steinbacher J, Hackner B, Kotljarova O, Schuermann D, Michalakis S, Kosmatchev O, Schiesser S, Steigenberger B, Raddaoui N, Kashiwazaki G, Muller U, Spruijt CG, Vermeulen M, Leonhardt H, Schar P, Muller M, Carell T. Tet oxidizes thymine to 5-hydroxymethyluracil in mouse embryonic stem cell DNA. Nature chemical biology. 2014;10:574–581. doi: 10.1038/nchembio.1532. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds JJ, Stewart GS. A single strand that links multiple neuropathologies in human disease. Brain : a journal of neurology. 2013;136:14–27. doi: 10.1093/brain/aws310. [DOI] [PubMed] [Google Scholar]
- 52.Neil AJ, Belotserkovskii BP, Hanawalt PC. Transcription blockage by bulky end termini at single-strand breaks in the DNA template: differential effects of 5' and 3' adducts. Biochemistry. 2012;51:8964–8970. doi: 10.1021/bi301240y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kellinger MW, Song CX, Chong J, Lu XY, He C, Wang D. 5-formylcytosine and 5-carboxylcytosine reduce the rate and substrate specificity of RNA polymerase II transcription. Nat. Struct. Mol. Biol. 2012;19:831–833. doi: 10.1038/nsmb.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 55.Rougier N, Bourc'his D, Gomes DM, Niveleau A, Plachot M, Paldi A, Viegas-Pequignot E. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 1998;12:2108–2113. doi: 10.1101/gad.12.14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–502. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 57.Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabo PE, Pfeifer GP, Li J, Xu GL. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 59.Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature communications. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, Matoba S, Tachibana M, Ogura A, Shinkai Y, Nakano T. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012;486:415–419. doi: 10.1038/nature11093. [DOI] [PubMed] [Google Scholar]
- 61.Szabo PE, Pfeifer GP. H3K9me2 attracts PGC7 in the zygote to prevent Tet3-mediated oxidation of 5-methylcytosine. J. Mol. Cell. Biol. 2012;4:427–429. doi: 10.1093/jmcb/mjs038. [DOI] [PubMed] [Google Scholar]
- 62.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science. 2011;334:194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inoue A, Shen L, Dai Q, He C, Zhang Y. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res. 2011;21:1670–1676. doi: 10.1038/cr.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature. 2012;484:339–344. doi: 10.1038/nature10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, Yang L, Zhang J, Li G, Ci W, Li W, Zhou Q, Aluru N, Tang F, He C, Huang X, Liu J. Programming and inheritance of parental DNA methylomes in mammals. Cell. 2014;157:979–991. doi: 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smallwood SA, Tomizawa S, Krueger F, Ruf N, Carli N, Segonds-Pichon A, Sato S, Hata K, Andrews SR, Kelsey G. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat. Genet. 2011;43:811–814. doi: 10.1038/ng.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu W, Yin J, Kou X, Jiang Y, Gao H, Zhao Y, Huang B, He W, Wang H, Han Z, Gao S. Asymmetric reprogramming capacity of parental pronuclei in mouse zygotes. Cell Rep. 2014;6:1008–1016. doi: 10.1016/j.celrep.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Wossidlo M, Arand J, Sebastiano V, Lepikhov K, Boiani M, Reinhardt R, Scholer H, Walter J. Dynamic link of DNA demethylation DNA strand breaks and repair in mouse zygotes. EMBO J. 2010;29:1877–1888. doi: 10.1038/emboj.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J. Active demethylation of the paternal genome in the mouse zygote. Curr. Biol. 2000;10:475–478. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 70.Borgel J, Guibert S, Li Y, Chiba H, Schubeler D, Sasaki H, Forne T, Weber M. Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet. 2010;42:1093–1100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 71.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 72.Surani MA, Ancelin K, Hajkova P, Lange UC, Payer B, Western P, Saitou M. Mechanism of mouse germ cell specification: a genetic program regulating epigenetic reprogramming. Cold Spring Harb Symp Quant Biol. 2004;69:1–9. doi: 10.1101/sqb.2004.69.1. [DOI] [PubMed] [Google Scholar]
- 73.Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol. 2005;278:440–458. doi: 10.1016/j.ydbio.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 74.Guibert S, Forne T, Weber M. Global profiling of DNA methylation erasure in mouse primordial germ cells. Genome Res. 2012;22:633–641. doi: 10.1101/gr.130997.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell. 2012;48:849–862. doi: 10.1016/j.molcel.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hackett JA, Reddington JP, Nestor CE, Dunican DS, Branco MR, Reichmann J, Reik W, Surani MA, Adams IR, Meehan RR. Promoter DNA methylation couples genome-defence mechanisms to epigenetic reprogramming in the mouse germline. Development. 2012;139:3623–3632. doi: 10.1242/dev.081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelsey G, Feil R. New insights into establishment and maintenance of DNA methylation imprints in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20110336. doi: 10.1098/rstb.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lees-Murdock DJ, Walsh CP. DNA methylation reprogramming in the germ line. Adv. Exp. Med. Biol. 2008;626:1–15. doi: 10.1007/978-0-387-77576-0_1. [DOI] [PubMed] [Google Scholar]
- 79.Brandeis M, Kafri T, Ariel M, Chaillet JR, McCarrey J, Razin A, Cedar H. The ontogeny of allele-specific methylation associated with imprinted genes in the mouse. EMBO J. 1993;12:3669–3677. doi: 10.1002/j.1460-2075.1993.tb06041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 81.Lee J, Inoue K, Ono R, Ogonuki N, Kohda T, Kaneko-Ishino T, Ogura A, Ishino F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2002;129:1807–1817. doi: 10.1242/dev.129.8.1807. [DOI] [PubMed] [Google Scholar]
- 82.Sato S, Yoshimizu T, Sato E, Matsui Y. Erasure of methylation imprinting of Igf2r during mouse primordial germ-cell development. Mol Reprod Dev. 2003;65:41–50. doi: 10.1002/mrd.10264. [DOI] [PubMed] [Google Scholar]
- 83.Kawasaki Y, Lee J, Matsuzawa A, Kohda T, Kaneko-Ishino T, Ishino F. Active DNA demethylation is required for complete imprint erasure in primordial germ cells. Sci Rep. 2014;4:3658. doi: 10.1038/srep03658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Szabo PE, Mann JR. Biallelic expression of imprinted genes in the mouse germ line: implications for erasure establishment and mechanisms of genomic imprinting. Genes Dev. 1995;9:1857–1868. doi: 10.1101/gad.9.15.1857. [DOI] [PubMed] [Google Scholar]
- 85.Szabo PE, Hubner K, Scholer H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev. 2002;115:157–160. doi: 10.1016/s0925-4773(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 86.Kagiwada S, Kurimoto K, Hirota T, Yamaji M, Saitou M. Replication-coupled passive DNA demethylation for the erasure of genome imprints in mice. EMBO J. 2013;32:340–353. doi: 10.1038/emboj.2012.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hajkova P, Jeffries SJ, Lee C, Miller N, Jackson SP, Surani MA. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science. 2010;329:78–82. doi: 10.1126/science.1187945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–452. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vincent JJ, Huang Y, Chen PY, Feng S, Calvopina JH, Nee K, Lee SA, Le T, Yoon AJ, Faull K, Fan G, Rao A, Jacobsen SE, Pellegrini M, Clark AT. Stage-specific roles for tet1 and tet2 in DNA demethylation in primordial germ cells. Cell Stem Cell. 2013;12:470–478. doi: 10.1016/j.stem.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamaguchi S, Shen L, Liu Y, Sendler D, Zhang Y. Role of Tet1 in erasure of genomic imprinting. Nature. 2013;504:460–464. doi: 10.1038/nature12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamaguchi S, Hong K, Liu R, Shen L, Inoue A, Diep D, Zhang K, Zhang Y. Tet1 controls meiosis by regulating meiotic gene expression. Nature. 2012;492:443–447. doi: 10.1038/nature11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, Faull KF, Lyko F, Jaenisch R. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell. 2013;24:310–323. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piccolo FM, Bagci H, Brown KE, Landeira D, Soza-Ried J, Feytout A, Mooijman D, Hajkova P, Leitch HG, Tada T, Kriaucionis S, Dawlaty MM, Jaenisch R, Merkenschlager M, Fisher AG. Different roles for Tet1 and Tet2 proteins in reprogramming-mediated erasure of imprints induced by EGC fusion. Mol. Cell. 2013;49:1023–1033. doi: 10.1016/j.molcel.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakashima H, Kimura T, Kaga Y, Nakatani T, Seki Y, Nakamura T, Nakano T. Effects of dppa3 on DNA methylation dynamics during primordial germ cell development in mice. Biol Reprod. 2013;88:125. doi: 10.1095/biolreprod.112.105932. [DOI] [PubMed] [Google Scholar]
- 95.Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamaguchi S, Hong K, Liu R, Inoue A, Shen L, Zhang K, Zhang Y. Dynamics of 5-methylcytosine and 5-hydroxymethylcytosine during germ cell reprogramming. Cell Res. 2013;23:329–339. doi: 10.1038/cr.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PloS one. 2010;5:e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin SG, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res. 2010;38:e125. doi: 10.1093/nar/gkq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development. 2007;134:2627–2638. doi: 10.1242/dev.005611. [DOI] [PubMed] [Google Scholar]
- 100.Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet. 2008;40:1016–1022. doi: 10.1038/ng.186. [DOI] [PubMed] [Google Scholar]
- 101.Okashita N, Kumaki Y, Ebi K, Nishi M, Okamoto Y, Nakayama M, Hashimoto S, Nakamura T, Sugasawa K, Kojima N, Takada T, Okano M, Seki Y. PRDM14 promotes active DNA demethylation through the ten-eleven translocation (TET)-mediated base excision repair pathway in embryonic stem cells. Development. 2014;141:269–280. doi: 10.1242/dev.099622. [DOI] [PubMed] [Google Scholar]
- 102.Dawlaty MM, Breiling A, Le T, Barrasa MI, Raddatz G, Gao Q, Powell BE, Cheng AW, Faull KF, Lyko F, Jaenisch R. Loss of tet enzymes compromises proper differentiation of embryonic stem cells. Dev. Cell. 2014;29:102–111. doi: 10.1016/j.devcel.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu H, D'Alessio AC, Ito S, Xia K, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011;473:389–393. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol. Cell. 2011;42:451–464. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stroud H, Feng S, Morey Kinney S, Pradhan S, Jacobsen SE. 5-Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 2011;12:R54. doi: 10.1186/gb-2011-12-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ivanov M, Kals M, Kacevska M, Barragan I, Kasuga K, Rane A, Metspalu A, Milani L, Ingelman-Sundberg M. Ontogeny, distribution and potential roles of 5-hydroxymethylcytosine in human liver function. Genome Biol. 2013;14:R83. doi: 10.1186/gb-2013-14-8-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laird A, Thomson JP, Harrison DJ, Meehan RR. 5-hydroxymethylcytosine profiling as an indicator of cellular state. Epigenomics. 2013;5:655–669. doi: 10.2217/epi.13.69. [DOI] [PubMed] [Google Scholar]
- 108.Nestor CE, Ottaviano R, Reddington J, Sproul D, Reinhardt D, Dunican D, Katz E, Dixon JM, Harrison DJ, Meehan RR. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome Res. 2012;22:467–477. doi: 10.1101/gr.126417.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song CX, Szulwach KE, Dai Q, Fu Y, Mao SQ, Lin L, Street C, Li Y, Poidevin M, Wu H, Gao J, Liu P, Li L, Xu GL, Jin P, He C. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell. 2013;153:678–691. doi: 10.1016/j.cell.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wheldon LM, Abakir A, Ferjentsik Z, Dudnakova T, Strohbuecker S, Christie D, Dai N, Guan S, Foster JM, Correa IR, Jr, Loose L, Dixon JE, Sottile V, Johnson AD, Ruzov A. Transient accumulation of 5-carboxylcytosine indicates involvement of active demethylation in lineage specification of neural stem cells. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.05.003. in press. [DOI] [PubMed] [Google Scholar]
- 111.Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ji D, Lin K, Song J, Wang Y. Effects of Tet-induced oxidation products of 5-methylcytosine on Dnmt1- and DNMT3a–mediated cytosine methylation. Mol. Biosyst. 2014;10:1749–1752. doi: 10.1039/c4mb00150h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yildirim O, Li R, Hung JH, Chen PB, Dong X, Ee LS, Weng Z, Rando OJ, Fazzio TG. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147:1498–1510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baubec T, Ivanek R, Lienert F, Schubeler D. Methylation-dependent and -independent genomic targeting principles of the MBD protein family. Cell. 2013;153:480–492. doi: 10.1016/j.cell.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 115.Cramer JM, Scarsdale JN, Walavalkar NM, Buchwald WA, Ginder GD, Williams DC., Jr Probing the dynamic distribution of bound states for methylcytosine-binding domains on DNA. J. Biol. Chem. 2014;289:1294–1302. doi: 10.1074/jbc.M113.512236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 117.Frauer C, Hoffmann T, Bultmann S, Casa V, Cardoso MC, Antes I, Leonhardt H. Recognition of 5-hydroxymethylcytosine by the Uhrf1 SRA domain. PloS one. 2011;6:e21306. doi: 10.1371/journal.pone.0021306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou T, Xiong J, Wang M, Yang N, Wong J, Zhu B, Xu RM. Structural Basis for Hydroxymethylcytosine Recognition by the SRA Domain of UHRF2. Mol. Cell. 2014 doi: 10.1016/j.molcel.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Otani J, Kimura H, Sharif J, Endo TA, Mishima Y, Kawakami T, Koseki H, Shirakawa M, Suetake I, Tajima S. Cell cycle-dependent turnover of 5-hydroxymethyl cytosine in mouse embryonic stem cells. PloS one. 2013;8:e82961. doi: 10.1371/journal.pone.0082961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iurlaro M, Ficz G, Oxley D, Raiber EA, Bachman M, Booth MJ, Andrews S, Balasubramanian S, Reik W. A screen for hydroxymethylcytosine and formylcytosine binding proteins suggests functions in transcription and chromatin regulation. Genome Biol. 2013;14:R119. doi: 10.1186/gb-2013-14-10-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]