Abstract
Study objective
To determine if day 3 FSH and E2 levels at the upper limits of normal affect live birth rates and treatment trajectory in a conventional vs. ‘fast track’ treatment program for IVF.
Design
Secondary analysis of two randomized controlled trials, FASTT and FORT-T.
Setting
Multicenter study in a state with mandated insurance coverage.
Patients
Infertile women ages 21 to 42 years randomized to conventional or accelerated treatment with controlled ovarian hyperstimulation (COH)-IUI and/or IVF (n=603 patients contributing 2,717 total cycles).
Interventions
Patients were stratified according to basal FSH and E2: FSH <10mIU/mL and E2 <40pg/mL (Group 1A), FSH <10mIU/mL and E2 ≥40 pg/mL (Group 1B), FSH 10–15 mIU/mL and E2 <40pg/mL (Group 2A) and FSH 10–15 mIU/mL and E2 ≥40 pg/mL (Group 2B).
Main outcome measures
Number of cancelled cycles, disenrollment for poor response and cumulative live birth rates per couple.
Results
Women in Groups 2A and 2B were more likely to have cancelled cycles and be disenrolled for poor response. While no live births occurred in Group 2B during COH-IUI (0/19 couples, 0/58 cycles), IVF still afforded these patients a reasonable chance of success (6/18 couples, 6/40 cycles, 33.3% live birth rate per couple). The specificity and positive predictive value of basal FSH 10–15 mIU/mL and E2 ≥40 pg/mL for no live birth during COH-IUI treatment were both 100%.
Conclusions
Women who initiated infertility treatment with FSH 10–15 mIU/mL and E2 ≥ 40 pg/mL on day 3 testing were unlikely to achieve live birth after COH-IUI treatment.
Keywords: day 3 testing, fast track, FSH cut-off, IVF, ovarian reserve
Introduction
Fecundity rates decrease with age due to lower quantity and quality of available oocytes. Basal testing with cycle day 3 follicle stimulating hormone (FSH) and estradiol (E2) are commonly used to screen women for poor response to ovulation induction prior to undergoing in vitro fertilization (IVF) (1–7). The utility of these endocrine markers in predicting ongoing pregnancy from intrauterine insemination (IUI) is less well established. Two prospective observational trials of controlled ovarian hyperstimulation (COH)-IUI are available to guide treatment recommendations. The first study demonstrated that women ≥35 years of age do not achieve live birth if the day 3 FSH is ≥24 mIU/mL (8). Notably, this study was performed with an FSH assay using the human menopausal gonadotropin (hMG) standard, which has since been replaced with the World Health Organization Second International standard (WHO 2nd IRP 78/549). Accordingly, the corresponding FSH threshold reported by Magarelli et al. using the current assay is 16.1 mIU/mL. A second study in older women ≥38 years demonstrated an even lower FSH threshold: no live births from COH-IUI occurred among women with FSH >13mIU/mL or E2 >80 pg/mL (9).
The objective of the current study was to determine if FSH and/or E2 levels at the upper limits of normal, but below these previously published thresholds, affect cancellation rates per cycle start, clinical pregnancy and live birth rates per couple, and mean time to live birth in patients enrolled in a standardized infertility treatment program.
Materials and Methods
Experimental design
A secondary analysis of data from the Fast Track and Standard Treatment Trial (FASTT) and Forty and Over Treatment Trial (FORT-T) was performed to assess the effect of FSH and/or E2 levels considered to be at the upper limits of normal on pregnancy rates in a standardized infertility treatment program. FASTT (n=503) randomized women ages 21 to 39 with 12 months of unexplained infertility to conventional treatment (three cycles of clomiphene citrate IUI [CC-IUI], three cycles of gonadotropin IUI [FSH-IUI], then up to six cycles of IVF) or accelerated treatment (three cycles of CC-IUI then up to six cycles of IVF) (10). FORT-T (n=154) randomized women ages 38 to 42 (through their 43rd birthday) with six months of unexplained infertility to COH-IUI (two cycles of CC or FSH) then up to six cycles of IVF, or directly to IVF (11). Both FASTT and FORT-T were approved by the institutional review boards at participating institutions. All patients provided written informed consent. An independent Data and Safety Monitoring Board met annually.
Due to mandated insurance coverage and study design, patients were treated until they no longer demonstrated a reasonable chance for success (i.e., were disenrolled) according to predetermined stopping criteria. In the current analysis, four groups of patients were identified according to day 3 FSH and E2 concentrations: Group 1A (FSH <10 mIU/mL and E2 <40 pg/mL), Group 1B (FSH <10 mIU/mL and E2 ≥40 pg/mL), Group 2A (FSH 10–15 mIU/mL and E2 <40 pg/mL), and Group 2B (FSH 10–15 mIU/mL and E2 ≥40 pg/mL). The basal FSH and E2 for patients at study initiation (and not necessarily the worst recorded value for each patient) were used to stratify the groups. The day 3 FSH threshold of <10 or 10–15 mIU/mL was chosen a priori as it is a well established cut-off used clinically to diagnose a patient with diminished ovarian reserve and is less than the previously published thresholds of 16.1 and 13 mIU/mL, respectively (8,9). The day 3 E2 value of < or ≥40 pg/mL was chosen because the median E2 among all patients included in our analysis was 38.1 pg/mL. Accordingly, we were interested in treatment trajectory and outcomes for those patients with moderately abnormal day 3 E2, defined as ≥ the 50th percentile in our population, but less than the previously published threshold of 80 pg/mL (9).
Study population
Patient characteristics were as described elsewhere (10, 11). Briefly, participants in FASTT were ages 21 to 39 with a minimum of 12 months of attempted conception, while patients in FORT-T were ages 38 to 42 with a minimum of six months of attempted conception at the time of study enrollment. All patients had at least one ovary with ipsilateral patent tube confirmed by hysterosalpingogram or chromopertubation and adequate ovarian reserve, indicated by day 3 FSH <15 mIU/mL and E2 <100 pg/mL. A total motile sperm count of ≥15 million, or ≥5 million total motile sperm upon IUI preparation, was required for participation. Patients were excluded for the following reasons: previous infertility treatment (beyond three cycles of CC without IUI), prior ectopic pregnancy affecting tubal patency or history of two ectopic pregnancies, hydrosalpinges, stage 3 or 4 endometriosis, body mass index (BMI) > 38 kg/m2, use of donor gametes, preimplantation genetic diagnosis or other biopsied embryos, abnormal thyroid stimulating hormone or prolactin, or use of zygote or gamete intrafallopian transfer (GIFT). Our final sample size was restricted to 603 couples (2,717 total cycles); 54 couples were excluded because they were randomized but did not initiate treatment, or were missing a baseline E2 level.
Clinical protocols
All patients were treated by standardized protocol. FASTT participants underwent up to three cycles of CC-IUI (100 mg orally on cycle days 3 through 7 and a single IUI the day following a positive ovulation predictor kit result, or when the lead follicle was ≥ 18mm in the absence of LH surge by cycle day 15, ovulation was triggered with hCG 10,000 IU SC). Patients in the conventional arm who did not conceive an ongoing pregnancy proceeded to three cycles of FSH-IUI, while those in the accelerated arm omitted the FSH-IUI and moved directly to IVF. The starting dose of FSH was 150 IU and was titrated as necessary to achieve a lead follicle of ≥17mm and two to three additional follicles of ≥15mm size. Approximately 36 hours after hCG administration, a single IUI was performed. Patients in the conventional arm who still had not conceived initiated up to six cycles of IVF therapy, with a maximum of two cryopreserved embryo transfers allowed. FORT-T patients were randomized to two cycles of CC-IUI then IVF, two cycles of FSH-IUI then IVF, or immediate IVF.
A standardized stimulation protocol was used for all IVF cycles as previously reported (10, 11). Briefly, a long luteal or poor responder (microdose leuprolide flare) protocol was prescribed based on patient age, day 3 testing and prior ovarian response. FSH dose adjustments were made according to standard monitoring criteria. Transvaginal oocyte retrieval was performed approximately 36 hours after hCG trigger with 10,000 IU SC.
Laboratory protocols
All gametes and embryos were incubated at 37 °C in humidified air with 5% CO2 content in media microdrops under oil. Motile sperm were isolated using a discontinuous gradient consisting of an upper and lower layer. Oocytes were inseminated with 50,000 sperm in 30 microliter drops 4–6 hours after retrieval. Intracytoplasmic sperm injection (ICSI) of mature metaphase II (MII) oocytes was only allowed for cases of failed fertilization, total motile sperm counts <5 million on several occasions during previous attempts, or when there were <10 million total motile sperm available for IVF. ICSI gradient-isolated sperm pellets were re-suspended and used for microinjection 4–6 hours post-retrieval. At 17–20 hours post-insemination the oocytes were assessed for fertilization. The presence of two pronuclei (PN) and two polar bodies was considered normal. A re-check was performed 4–6 hours later to identify any delayed fertilization. Embryo morphology was assessed on days 2 and 3 by analyzing blastomere number and fragmentation. Standard practice was for day 3 embryo transfer. Embryo culture was extended to day 5 when ≥ 6 embryos had reached the 6–8 cell stage with <20% fragmentation on day 3. Embryo transfer was performed under ultrasound guidance. The number of embryos transferred was determined by institutional algorithm in accordance with guidelines from the American Society for Reproductive Medicine (ASRM); parameters considered were the number and morphology of available embryos, patient age and clinical history. Luteal phase support with vaginal progesterone was used until clinical pregnancy was documented at approximately 7 weeks gestation or negative hCG.
Outcome variables
Female patient demographics, including patient age, ethnicity, BMI, day 3 FSH and E2 levels at study enrollment, gravidity, parity and smoking history were compared between the four FSH and E2 groups. Sperm concentration, sperm motility, and total motile sperm count were compared among the male partners. Other variables considered for stimulated cycles (FSH-IUI and IVF) were total gonadotropin dose and peak E2. Variables specific to the IVF cohort were number of mature and immature oocytes, 1PN and 2PN zygotes, failed fertilizations, percent of cycles with ICSI, and day 3 or day 5 transfer. Numbers of embryos transferred and cryopreserved were also recorded, along with blastomere number on day 3 and fragmentation scores (<20%, 20–50%, or >50%). A good quality embryo was defined as having ≥8 cells with <20% fragmentation on day 3.
The cycle cancellation rate and disenrollment rate for poor ovarian response were calculated according to the predetermined stopping criteria. Clinical pregnancy, spontaneous abortion (SAB) and live birth rates per couple were also calculated. Clinical pregnancy rate was defined as the first clinically recognized pregnancy with at least one intrauterine gestational sac on ultrasound per initiated couple. Loss of a clinical pregnancy prior to 20 weeks gestation was defined as a SAB. Live birth rate was defined as the proportion of couples delivering a viable infant. Cumulative live birth rates and the mean time to pregnancy resulting in a live birth were determined for each FSH and E2 group and treatment modality (CC-IUI, FSH-IUI and IVF).
FSH and E2 Assays
Baseline serum FSH and E2 levels at study enrollment were analyzed by solid phase, competitive chemiluminescent enzyme immunoassay in the program’s own endocrinology laboratory. The Immulite 2000 (Siemens Medical Solutions Diagnostics, Flanders, NJ) system was used for all assays. The system was calibrated to detect a range of FSH values from 1 to 170 mIU/mL using the WHO 2nd IRP 78/549 standard with an analytic sensitivity of 0.1 mIU/mL. The calibration range for E2 was 20 to 2000 pg/mL with an analytic sensitivity of 15 pg/mL. The intra-assay and inter-assay coefficients of variation for FSH ranged from 2.9–4.2% and 4.1–7.9%, respectively. For E2, the intra-assay and inter-assay coefficients of variation ranged from 4.3–9.9% and 6.7–16%, respectively.
Statistical analyses
Analysis of variance and Fisher’s exact test were used to compare the FSH/E2 groups for continuous and categorical variables, respectively. Proportions were compared using Fisher’s exact tests. Paired nominal data (comparing Group 2B to itself according to treatment modality) were analyzed using binomial probability models, and the null hypothesis was rejected if the point estimate for one treatment modality fell outside the 95th confidence interval (CI) of the point estimate for the other. P-values for the binomial model were calculated according to the one-sample binomial test-exact method. In order to account for multiple cycles contributed by the same couple, discrete-event survival analysis was performed, using the Kaplan-Meier (or product-limit estimator) method of estimating one minus the survival function. Cycle data were stratified by treatment modality to generate separate survival functions for COH-IUI and IVF within a particular FSH/E2 group, and log-log transformation was used to calculate 95% CI bands. Differences in the cumulative incidence of live births were determined by the log-rank test. Performance tests including sensitivity, specificity, and positive and negative predictive values were carried out to examine the test characteristics of FSH and E2 levels at the upper limits of normal on live birth during COH/IUI treatment. In these calculations the ‘diseased state’ was defined as not achieving live birth. Statistical significance was defined as P <.05 (two-sided). Data analyses were performed using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC).
Results
Patient demographics and cycle characteristics
Six hundred and fifty seven couples were enrolled in FASTT and FORT-T during the study period; 54 couples were excluded from this analysis because they were randomized but did not initiate treatment or were missing a baseline E2 level. The distribution of subjects in the pre-defined FSH and E2 groups was as follows: Group 1A (291 subjects, 1271 total CC-IUI, FSH-IUI and IVF cycles), Group 1B (256 subjects, 1205 total cycles), Group 2A (35 subjects, 143 total cycles) and Group 2B (21 subjects, 98 total cycles). The mean FSH and E2 levels for all groups (1A to 2B) were statistically significantly different, and subjects with FSH 10–15 mIU/mL were slightly older than those with FSH <10 mIU/mL (P=.01) (Table 1). The remaining demographic and reproductive characteristics, including prior gravidity, prior live birth, BMI, smoking history and total motile sperm count, were not statistically significantly different.
Table 1.
Demographic and reproductive characteristics by day 3 FSH and E2 groups
| Characteristic | Mean ± SD or N (%) | ||||
|---|---|---|---|---|---|
| Group 1A FSH <10 E2 <40 (n=291) |
Group 1B FSH <10 E2 ≥40 (n=256) |
Group 2A FSH 10–15 E2 <40 (n=35) |
Group 2B FSH 10–15 E2 ≥40 (n=21) |
P-value | |
| Age (y) | 34.5 ± 4.2 | 34.6 ± 4.2 | 36.4 ± 4.4 | 36.6 ± 4.1 | .01 |
| BMI a (kg/m2) | 24.1 ± 4.4 | 24.0 ± 4.5 | 23.3 ± 5.3 | 23.6 ± 2.8 | NS b |
| Current or past smoker | 80 (27.9) | 74 (29.4) | 8 (23.5) | 6 (28.6) | NS |
| Day 3 FSH (mIU/mL) | 6.5 ± 1.6 | 6.4 ± 1.9 | 11.7 ± 1.5 | 11.7 ± 1.4 | <.0001 |
| Day 3 E2 (pg/mL) | 29.9 ± 5.8 | 55.6 ± 13.7 | 30.9 ± 5.4 | 52.5 ± 13.9 | <.0001 |
| Prior gravidity | 128 (44.0) | 111 (43.4) | 13 (37.1) | 10 (47.6) | NS |
| Prior live birth | 57 (19.6) | 52 (20.3) | 8 (22.9) | 5 (23.8) | NS |
| Total motile sperm count (million) mean ± SD 25th-50th-75th percentiles |
161.4 ± 236.5 53.8 – 99.4 – 176.8 |
146.4 ± 163.0 51.8 – 103.3 – 185.8 |
132.1± 108.6 66.2 – 90.2 – 166.4 |
93.2 ± 81.8 21.6 – 52.8 – 184.4 |
NS |
BMI=body mass index
NS=not statistically significant
While fewer patients in Groups 2A and 2B were treated with CC-IUI (P=.02), there was no difference in the percentage of subjects initiating FSH-IUI cycles according to FSH and E2 group (Table 2). Subjects in Groups 1A and 1B required significantly lower total gonadotropin doses per FSH-IUI cycle (P<.0001) and achieved significantly higher peak E2 levels, compared to subjects in Group 2A and 2B who required more aggressive stimulation (P=.04; Table 2).
Table 2.
Treatment cycle and embryology parameters by day 3 FSH and E2 groups
| Mean ± SD or N (%) | |||||
|---|---|---|---|---|---|
| Treatment Cycle | Group 1A FSH <10 E2 <40 (n=291) |
Group 1B FSH <10 E2 ≥40 (n=256) |
Group 2A FSH 10–15 E2 <40 (n=35) |
Group 2B FSH 10–15 E2 ≥40 (n=21) |
P-value |
| CC-IUI Cycles | |||||
| No. subjects initiating CC-IUI a | 243 (83.5) | 215 (84.0) | 23 (65.7) | 14 (66.7) | .02 |
| FSH-IUI Cycles | |||||
| No. subjects initiating FSH-IUI b | 90 (30.9) | 94 (36.7) | 14 (40.0) | 8 (38.1) | NS c |
| Total gonadotropin dose (IU) in FSH-IUI cycle | 1264.6 ± 787.9 | 1195.9 ± 575.1 | 1905.2 ± 779.4 | 1923.7 ± 987.5 | <.0001 |
| Peak E2 (pg/mL) in FSH-IUI cycle | 756.8 ± 507.0 | 772.2 ± 567.5 | 558.9 ± 269.3 | 511.2 ± 332.1 | .04 |
| IVF cycles | |||||
| No. subjects initiating IVF | 178 (61.2) | 171 (66.8) | 22 (62.9) | 18 (85.7) | NS |
| Total gonadotropin dose (IU) in IVF cycle | 2891.0 ± 1466.2 | 2761.1 ± 1459.8 | 4290.0 ± 1787.8 | 3974.6 ± 1923.4 | <.0001 |
| Peak E2 (pg/mL) in IVF cycle | 1968 ± 1188.8 | 1953.5 ± 1333.0 | 1293.5 ± 751.7 | 1236.4 ± 984.4 | <.0001 |
| No. oocytes retrieved | 11.6 ± 6.0 | 11.0 ± 6.8 | 7.2 ± 5.0 | 5.5 ± 2.8 | <.0001 |
| No. MII oocytes d | 9.7 ± 5.4 | 9.1 ± 5.8 | 6.0 ± 4.8 | 5.0 ± 2.8 | <.0001 |
| ICSI | 12 (6.9) | 21 (13.0) | 4 (19.1) | 0 (0) | NS |
| No. 2PN zygotes e | 7.4 ± 4.9 | 6.4 ± 4.4 | 4.2 ± 4.4 | 3.7 ± 2.3 | <.0001 |
| No. embryos transferred | 2.6 ± 1.5 | 2.5 ± 1.5 | 2.2 ± 1.4 | 2.0 ± 0.9 | NS |
| No. cycles with ≥1 good quality embryo transferred f | 224 (63.6) | 206 (63.2) | 23 (46.9) | 17 (56.7) | NS |
| Day of ETg Day 3 Day 5 |
307 (94.5) 18 (5.5) |
291 (97.4) 6 (2.0) |
44 (100.0) 0 (0) |
28 (100.0) 0 (0) |
NS |
| Elective single embryo transfer | 33 (10.1) | 27 (9.0) | 10 (22.7) | 4 (14.3) | NS |
| Cycles with no supernumerary embryos available for cryopreservation | 223 (64.1) | 229 (70.5) | 43 (87.8) | 25 (86.2) | <.001 |
CC-IUI=intrauterine insemination following treatment with clomiphene citrate
FSH-IUI=intrauterine insemination following stimulation with exogenous gonadotropins
Not statistically significant
MII=metaphase II (mature) oocytes
2PN=two pronuclei observed after fertilization
Good quality embryo was defined as having ≥8 cells on day 3, with <20% fragmentation
ET=embryo transfer
There was no statistically significant difference in the percentage of subjects from each group who initiated IVF. Total gonadotropin doses required for stimulation were significantly lower in Groups 1A and 1B (P<.0001), and peak E2 levels were significantly higher (P<.0001). Despite more aggressive stimulation, fewer total oocytes and MII oocytes were retrieved in Groups 2A and 2B (P<.0001). There were no differences in the rates of ICSI among all groups (Table 2).
Embryo morphology, cryopreservation and transfer characteristics
Fewer 2PN zygotes were observed among patients in Group 2A and 2B (P<.0001; Table 2). There were no differences in the day of embryo transfer (day 3 vs. day 5), the mean number of embryos transferred, the number of cycles with at least one good quality embryo transferred or the percentage of cycles with elective single embryo transfer (Table 2). Likewise, the percentage of cycles containing embryos with minimal (<20%), moderate (20–50%), and severe (>50%) fragmentation were not different between groups (data not shown). Subjects in Groups 2A and 2B were statistically significantly more likely to have no remaining supernumerary embryos available for cryopreservation (P<.001; Table 2).
Clinical outcomes
Cycle completion outcomes varied according to basal FSH and E2 group; patients in Groups 2B were more likely to be disenrolled or have at least one cancelled cycle due to poor ovarian response according to predetermined stopping criteria (P<.0001; Table 3).
Table 3.
Cycle completion outcomes per couple by day 3 FSH and E2
| Cycle completion outcomes | FSH (mIU/mL) and E2 (pg/mL) groups N couples (%) | P-value a | |||
|---|---|---|---|---|---|
| Group 1A FSH <10 E2 <40 (n=291) |
Group 1B FSH <10 E2 ≥40 (n=256) |
Group 2A FSH 10–15 E2 <40 (n=35) |
Group 2B FSH 10–15 E2 ≥40 (n=21) |
||
| All cycles completed | 248 (85.2) | 207 (80.9) | 24 (68.6) | 9 (42.9) | <.0001 |
| ≥1 cancelled cycle(s) | 39 (13.4) | 43 (16.8) | 6 (17.1) | 58 (38.1) | <.0001 |
| Disenrolled for poor ovarian responseb | 4 (1.4) | 6 (2.3) | 5 (14.3) | 4 (19.0) | <.0001 |
Fisher’s Exact test for comparison of cycle completion outcomes by FSH/E2 group
Predetermined stopping criteria for disenrollment: In FASTT patients undergoing CC-IUI with two cycles lacking both an LH surge and mature follicles then proceeded to FSH-IUI, and were subsequently disenrolled for absent follicular development (dominant follicle <15mm) and E2 <100 pg/ml after five days of gonadotropin stimulation. IVF patients were disenrolled after one previous cancelled IUI cycle due to poor ovarian response in conjunction with one cancelled IVF cycle. IVF cancellation occurred due to absent follicular development, E2<100 pg/ml after six days of gonadotropin therapy, and less than two lead follicles on the day of hCG trigger. In FORT-T, CC-IUI patients were disenrolled according to the same criteria as FASTT. FSH-IUI patients stopped treatment if two cycles were characterized by fewer than three lead follicles and E2 <500 pg/mL. IVF patients were disenrolled after one poor ovarian response in a prior IUI cycle plus one cancelled IVF cycle, or two cancelled IVF cycles (either due to poor response or insufficient quality or number of embryos, defined as three or more embryos with six to eight cells and < 50% fragmentation or two four-cell mitotically active embryos if accompanied by at least one embryo with six or more cells).
Clinical pregnancy rates per couple were lowest for Group 2B among those treated with CC-IUI and/or FSH-IUI (henceforth referred to as COH-IUI): Group 1A (93/268, 34.7%), Group 1B (59/233, 25.3%), Group 2A (10/30, 33.3%) and Group 2B (1/19, 5.3%; P=.008). Similarly, subjects treated with IVF had lower clinical pregnancy rates per couple if FSH was 10–15 mIU/mL: Group 1A 131/178, 73.6%, Group 1B 117/171, 68.4%, Group 2A 9/22, 40.9% and Group 2B 8/18, 44.4% (P=.003).
Spontaneous abortion rates per couple were as follows: for COH-IUI (Group 1A: 17/268, 6.3%; Group 1B: 7/233, 3.0%; Group 2A: 0/30, 0%; Group 2B: 1/19, 5.3%) and for IVF (Group 1A: 24/178, 13.5%; Group 1B: 32/171, 18.7%; Group 2A: 3/22, 13.6%; Group 2B: 1/18, 5.6%).
Live birth rates per couple following COH-IUI were significantly different according to FSH and E2 group (P=.01): the live birth rates ranged from 20.6% to 27.6% for Groups 1A, 1B and 2A (Supplementary Table 1). There were no live births from COH-IUI when FSH was 10–15 mIU/mL and E2 was ≥40 pg/mL, regardless of age (0/19 couples, 0/58 cycles; P=.01). Among women over age 40, there were no live births from COH-IUI when FSH was 10–15 mIU/mL, regardless of E2 level (0/14 couples, 0/28 cycles). Patients in Groups 2A and 2B were significantly less likely to achieve a live birth following IVF (Group 1A:98/178, 55.1%; Group 1B: 86/171, 50.3%; Group 2A: 5/22, 22.7%; Group 2B: 6/18, 33.3%; P=.02). IVF live birth rates according to patient age, FSH and E2 may be found in Supplementary Table 1.
When the live birth rates per couple in Group 2B were restricted to those patients who were treated with both COH-IUI and IVF (n=16 couples, 51 COH-IUI cycles and 37 IVF cycles), the point estimate for the probability of live birth following COH-IUI was 0 (95% CI 0–0.21); the point estimate for IVF was 0.31 (well outside the CI for COH-IUI), indicating a statistically significant difference in the probability of live births between the two treatment modalities (P<.0001). If a more conservative estimate for the probability of live birth in Group 2B following COH-IUI were adopted (e.g., the midpoint of the CI—10.5%, rather than 0%), this difference remained statistically significant (P=.02). Discrete-event survival analysis of Group 2B stratified by COH-IUI vs. IVF is shown in Figure 1. Differences in the cumulative incidence of live birth for these patients according to treatment modality were statistically significant by log-rank test (P=.005).
Figure 1.
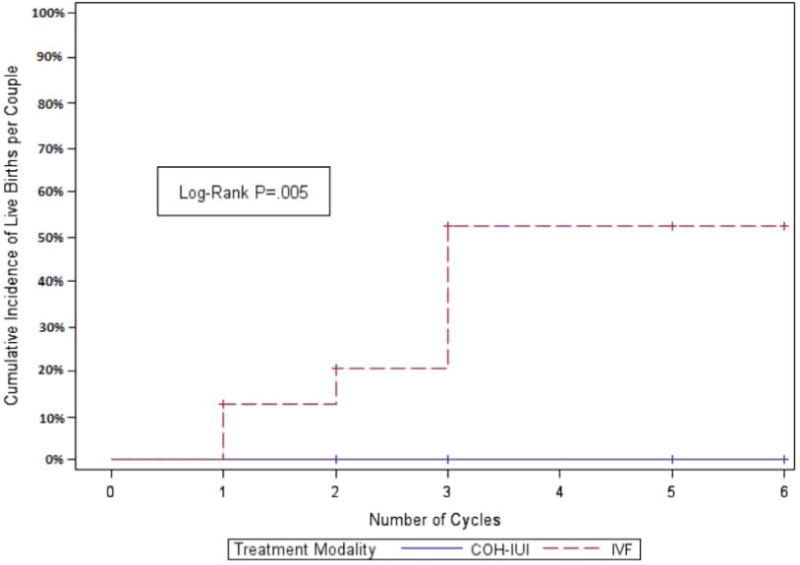
Kaplan-Meier estimates of cumulative incidence of live births following controlled ovarian hyperstimulation (COH)-IUI vs. IVF for patients in Group 2B (FSH 10–15 mIU/mL and E2 ≥40 pg/mL). Analysis used a discrete-event survival function; P=.005 by log-rank test. + indicates censoring.
Cumulative live birth rates per couple across all treatment modalities were 59.1% (172/291), 52.3% (134/256), 37.1% (13/35) and 28.6% (6/21) for Groups 1A to 2B, respectively (P=.005). Statistical significance remained after controlling for female age at baseline and number of treatment cycles initiated (P=.02).
There were no statistically significant differences in the following parameters among women who delivered: multiple live births (Group 1A: 37/172, 21.5%; Group 1B: 31/134, 23.1%; Group 2A: 2/13, 15.4%; Group 2B: 2/5, 40.0%); mean time to conception leading to a live birth (Group 1A 13.5 ± 0.7; Group 1B 13.4 ± 0.6; Group 2A 7.9 ± 0.7; Group 2B 8.8 ± 0.6 months); or mean time from randomization to initiation of IVF (Group 1A 6.0 ± 3.4; Group1B 5.7 ± 3.4; Group 2A 4.4 ± 2.8; Group 2B 4.3 ± 1.5 months).
Performance characteristics of day 3 FSH and E2 at the upper limits of normal
The sensitivity and specificity of FSH 10–15 mIU/mL and E2 ≥40 pg/mL for not achieving a live birth during COH-IUI treatment were 4.5% (19/420) and 100% (130/130), respectively. The corresponding negative and positive predictive values of the parameters were 24.5% (130/531) and 100% (19/19), respectively.
Discussion
This secondary analysis of two prospective randomized studies of conventional vs. accelerated infertility treatment was undertaken to determine if ovarian reserve testing at the initial evaluation could predict patient trajectory through a standardized treatment algorithm. The principal finding of the current study was that women at all ages studied who initiated infertility treatment with FSH 10–15 mIU/mL and E2 ≥40 pg/mL on day 3 testing were unlikely to achieve live birth following COH-IUI treatment. The specificity and positive predictive value of these parameters for no live birth during COH-IUI treatment were both 100%. Despite more aggressive stimulation regimens, a higher likelihood of cycle cancellation or disenrollment due to poor ovarian response, lower peak E2, and fewer MII oocytes, 2PN zygotes, and cycles with supernumerary embryos available for cryopreservation, these same women had a reasonable live birth rate (>33%) when treated with IVF (P=.005 by log-rank test; Figure 1).
ASRM defines fertility treatment as ‘futile’ when the prognosis for the desired end, that is, live birth is ≤1%. According to the Ethics Committee Opinion on poor prognosis patients, “the provision of futile therapies is not ethically justifiable” in most cases, based on the non-existent or extremely remote chance of success, along with the potential for physiologic and psychological harm to the patient (12). Some ethicists go even further, suggesting that physicians have a professional duty to withhold treatment that may be potentially harmful and unlikely to achieve the desired result (13, 14). The observation that no live births occurred among women with FSH 10–15 mIU/mL and E2 ≥40 pg/mL treated with IUI challenges the current paradigm of stepwise treatment for these patients in mandated states, where several cycles of CC-IUI may still be required by some insurers prior to the initiation of IVF.
While the association of abnormal day 3 testing and poor response to stimulation in IVF is well established (1–7), surprisingly few studies address the rates of success from COH-IUI according to basal FSH and E2 values. There is one externally validated prediction model for natural cycle and COH-IUI, but the authors did not evaluate day 3 testing as a prognostic factor in their stepwise regression (15). The two prospective studies that have addressed this question (both using the outdated hMG standard), report a cut-off value for FSH between 13 and 16.1 mIU/mL, or an E2 >80 pg/mL, above which no live births occurred following IUI treatment (8, 9). Our study effectively lowers both of the thresholds for FSH and estradiol when they are interpreted together to FSH 10–15 mIU/mL and E2 ≥40 pg/mL.
In the FORT-T trial we determined that immediate IVF was most effective for older couples (11). In the current study, there were no live births from COH-IUI when FSH 10–15 mIU/mL and E2 ≥40 pg/mL, regardless of patient age (0/19 couples, 0/58 cycles). Despite small numbers, the difference in the cumulative incidence of live births between patients in Group 2B who were treated with both COH-IUI and IVF was statistically significant. This observation may prove helpful when counseling women with day 3 testing at the upper limits of normal who prefer not to go to immediate IVF: COH-IUI does not appear to be effective, while the live birth rate after IVF exceeds 33%.
There are several limitations of this study. First, there can be considerable fluctuation in the measurement of basal FSH values between cycles. The intercycle variability of day 3 FSH may be as high as 1.7 ± 0.1 for patients with FSH <10 mIU/mL and 4.9 ± 0.5 for patients with FSH ≥10 mIU/mL (16). As we stratified patients according to their initial day 3 testing, some patients may have been misclassified. The immunoassay used in our study, however, employs the WHO 2nd IRP 78/549 standard with an analytic sensitivity of 0.1 mIU/mL and intra-assay and inter-assay coefficients of variation of 2.9–4.2% and 4.1–7.9%, respectively; thus, intercycle variability might not be so pronounced. Second, we assessed outcomes according to patient age, day 3 FSH and E2 only and did not examine the predictive value of antral follicle count on baseline ultrasound or antimullerian hormone (AMH) levels. We recognize the limitations of this approach, but also its parsimony—many patients in our practice will not have had an antral follicle count or AMH level performed prior to initiating an IUI cycle. Third, there were relatively few patients at the extremes of ovarian reserve testing. Previous reports on the success rates of COH-IUI among patients with FSH ≥10 mIU/mL indicate a clinical pregnancy rate of 3.9–4.8% (8,17). The clinical pregnancy rate in Group 2B was indeed 1/19 (5.3%), consistent with prior reports, suggesting adequate power to detect this outcome. To our knowledge there are no studies of poor prognosis patients stratified by FSH and E2 treated with IUI that report live birth data, and as such, we were unable to determine whether our reported live birth rate of zero was due to type 1 error. A prospective study aimed to detect a 33% difference in live birth between patients with moderately abnormal testing randomized to COH-IUI or IVF would only require 19 subjects per arm to achieve a power of 80% with an α=.05.
Notable strengths of the study include the standardized treatment algorithm that still allowed flexibility in gonadotropin dosing, predetermined stopping criteria for cycle cancellation and disenrollment, and the application of widely available, and vetted, endocrine markers to stratify patients at initial consultation. This study also provides clinically useful cut-offs for day 3 testing for patients considering COH-IUI. Currently in states with mandated coverage such as Massachusetts, there are predefined upper limits of FSH and E2 to determine eligibility for infertility treatment, but no threshold values to help triage eligible patients to the most effective treatment option.
In summary, this study supports a lower threshold value for basal FSH and E2 among patients undergoing COH-IUI and provides further evidence for beginning treatment with immediate IVF among infertile women of advanced reproductive age. It is our hope that consideration of this threshold in designing a couple’s treatment plan, along with forthright counseling about relative success rates, will allow patients to more efficiently realize their goals of building a family.
Supplementary Material
Acknowledgments
Financial support: Grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (R01 HD38561 and R01 HD44547). The National Institute of Child Health and Human Development had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: D.J. Kaser: none, M.B. Goldman: none, J.L. Fung: none, M.M. Alper: none, R.H. Reindollar: none
References
- 1.Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu HC, et al. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298–307. doi: 10.1016/s0015-0282(16)60077-8. [DOI] [PubMed] [Google Scholar]
- 2.Toner JP, Philput CB, Jones GS, Muasher SJ. Basal follicle-stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril. 1991;55:784–91. doi: 10.1016/s0015-0282(16)54249-6. [DOI] [PubMed] [Google Scholar]
- 3.Scott RT, Toner JP, Muasher SJ. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1988;51:651–4. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 4.Henne MB, Stegmann BJ, Neithardt AB, Catherino WH, Armstrong AY, Kao TC, et al. The combined effect of age and basal follicle-stimulating hormone on the cost of a live birth at assisted reproductive technology. Fertil Steril. 2008;89:104–10. doi: 10.1016/j.fertnstert.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Licciardi FL, Liu HC, Rosenwaks Z. Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing IVF. Fertil Steril. 1995;64:991–4. doi: 10.1016/s0015-0282(16)57916-3. [DOI] [PubMed] [Google Scholar]
- 6.Evers JLH, Slaats P, Land JA, Dumoulin JC, Dunselman GA. Elevated levels of basal estradiol-17B predict poor response in patients with normal basal levels of FSH undergoing IVF. Fertil Steril. 1998;69:1010–14. doi: 10.1016/s0015-0282(98)00080-6. [DOI] [PubMed] [Google Scholar]
- 7.Smotrich DB, Widra EA, Gindoff PR, Levy MJ, Hall JL, Stillman RJ. Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil Steril. 1995;64:1136–40. [PubMed] [Google Scholar]
- 8.Magarelli PC, Pearlstone AC, Buyalos RP. Discrimination between chronological and ovarian age in infertile women aged 35 years and older: predicting pregnancy using basal follicle stimulating hormone, age and number of ovulation induction/intra-uterine insemination cycles. Hum Reprod. 1996;11:1214–9. doi: 10.1093/oxfordjournals.humrep.a019358. [DOI] [PubMed] [Google Scholar]
- 9.Buyalos RP, Daneshmand S, Brzechffa PR. Basal estradiol and follicle stimulating hormone predict fecundity in women of advanced reproductive age undergoing ovulation induction intrauterine insemination. Fertil Steril. 1997;68:272–7. doi: 10.1016/s0015-0282(97)81514-2. [DOI] [PubMed] [Google Scholar]
- 10.Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, et al. A randomized clinical trial to evaluate optimal treatment for unexplained fertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94:888–99. doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Goldman MB, Thornton KL, Ryley D, Alper MM, Fung JL, Hornstein MD, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T) Fertil Steril. 2014;101:1574–81. doi: 10.1016/j.fertnstert.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ethics Committee, American Society for Reproductive Medicine. Fertility treatment when the prognosis is very poor or futile: a committee opinion. Fertil Steril. 2012;98:e6–9. doi: 10.1016/j.fertnstert.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Jecker NS, Schneiderman LJ. Medical futility: the duty not to treat. Camb Q Healthc Ethics. 1993;2:151–9. doi: 10.1017/s0963180100000852. [DOI] [PubMed] [Google Scholar]
- 14.Greiner GG. The physician’s authority to withhold futile treatment. J Med Philos. 1995;20:207–24. doi: 10.1093/jmp/20.2.207. [DOI] [PubMed] [Google Scholar]
- 15.Steures P, van der Steeg JW. Prediction of an ongoing pregnancy after intrauterine insemination. Fertil Steril. 2004;82:45–51. doi: 10.1016/j.fertnstert.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297–302. doi: 10.1016/s0015-0282(16)53707-8. [DOI] [PubMed] [Google Scholar]
- 17.Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706–10. doi: 10.1093/oxfordjournals.humrep.a136159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


