Abstract
Lysine residues are subject to a multitude of reversible post-translational modifications, including acetylation and SUMOylation. In the heart, enhancement of lysine acetylation or SUMOylation using histone deacetylase (HDAC) inhibitors or SUMO-1 gene transfer, respectively, has been shown to be cardioprotective. Here, we addressed whether there is crosstalk between lysine acetylation and SUMOylation in the heart. Treatment of cardiomyocytes and cardiac fibroblasts with pharmacological inhibitors of HDAC catalytic activity robustly increased conjugation of SUMO-1, but not SUMO-2/3, to several high molecular weight proteins in both cell types. Use of a battery of selective HDAC inhibitors and short hairpin RNAs demonstrated that HDAC2, which is a class I HDAC, is the primary HDAC isoform that controls cardiac protein SUMOylation. HDAC inhibitors stimulated protein SUMOylation in the absence of de novo gene transcription or protein synthesis, revealing a post-translational mechanism of HDAC inhibitor action. HDAC inhibition did not suppress the activity of de-SUMOylating enzymes, suggesting that increased protein SUMOylation in HDAC inhibitor-treated cells is due to stimulation of SUMO-1 conjugation rather than blockade of SUMO-1 cleavage. Consistent with this, multiple components of the SUMO conjugation machinery were capable of being acetylated in vitro. These findings reveal a novel role for reversible lysine acetylation in the control of SUMOylation in the heart, and suggest that cardioprotective actions of HDAC inhibitors are in part due to stimulation of protein SUMO-1-ylation in myocytes and fibroblasts.
Keywords: SUMO, acetylation, HDAC
1. Introduction
Lysine residues in a variety of proteins are subject to diverse modifications, including acetylation, methylation, ubiquitlyation and SUMOylation. The most well characterized post-translational modification of lysine is acetylation (1). In the context of chromatin, acetylation of histone tails impacts gene transcription by altering electrostatic interactions between DNA and nucleosomes, and by creating docking sites (a histone code) for multi-subunit regulatory complexes (2). Additionally, proteomic studies have revealed reversible acetylation of thousands of non-histone proteins that regulate a multitude of biological processes (3–5).
Acetyl groups are conjugated to lysine by histone acetyltransferases (HATs) and removed from lysine by histone deacetylases (HDACs). With regard to the heart, several studies have shown that small molecule HDAC inhibitors have beneficial effects in pre-clinical models of heart failure (6,7). HDACs are grouped into four classes and are encoded by 18 different genes. Class I, II, and IV HDACs require cellular zinc as a cofactor, whereas class III HDACs, better known as sirtuins, require nicotinamide adenine dinucleotide as a cofactor for catalytic activity (8). Inhibitors of zinc-dependent HDACs have been shown to block pathological cardiac hypertrophy and cardiac fibrosis, and improve cardiac contractile performance. Nonetheless, the molecular mechanisms for the beneficial effects of HDAC inhibitors in the setting of heart failure remain poorly characterized.
Lysine SUMOylation provides another mechanism for controlling protein function (9,10). The four SUMO family members (SUMO-1, -2, -3 and -4) belong to the ubiquitin-like protein modifying family and have a characteristic β-grasp fold (11). SUMO-1/2/3 are covalently ligated to their targets in a manner analogous to ubiquitin that involves an E1 activating heterodimer (SAE1/SAE2), a single E2 conjugating enzyme (Ubc9), and, in many cases, an E3 sumo ligase, which is thought to confer substrate specificity (9). Sentrin specific proteases (SENPs) are de-SUMOylases that are responsible for cleaving pro-SUMO into mature-SUMO, and also remove SUMO from target proteins, contributing to the dynamic nature of this lysine modification (12).
It is well established that SUMOylation plays a role in cardiac development (13–17). Recently, dysregulation of the SUMO pathway was also shown to contribute to the pathogenesis of adult heart failure. Myocardial expression of SUMO-1 protein was found to be dramatically reduced in failing human hearts and in animal models of heart failure (18). Remarkably, in rodent and pig models, adeno-associated virus (AAV)-mediated overexpression of SUMO-1 in the heart was demonstrated suppress cardiac hypertrophy and fibrosis and improve contractile function, at least in part, through SUMO-1-ylation of sarcoplasmic reticulum Ca2+ ATPase SERCA2a (18–21).
Given the critical roles of lysine acetylation and SUMOylation in the control of cardiac structure and function, we assessed the potential for crosstalk between these post-translational modifications in the heart. Here, we define a novel role for class I HDACs as negative regulators of protein SUMOylation in the heart. The data suggest that beneficial effects of HDAC inhibitors in models of heart failure may in part be due to the ability of these compounds to stimulate SUMO-1 conjugation to proteins in cardiac myocytes and fibroblasts.
2. Materials and methods
2.1 Inhibitors and agonists
Reagents were purchased or synthesized in-house and used at the indicated final concentrations. HDAC inhibitors: trichostatin A (TSA) (Sigma; 100 nM), MGCD0103 (Selleck; 1 μM), diphenylacetohydroxamic acid (DPAH) (Sigma [D6071]; 10 μM), tubastatin A (Selleck; 1μM), MS-275 (Selleck; 1μM). BA-60 (1 μM) was synthesized in-house, and its purity was confirmed to be greater than 95%. Phenylephrine (PE), phorbol 12-myristate 13-acetate (PMA), actinomycin D and cycloheximide were purchased from Sigma and used at final concentrations of 10 μM, 50 nM, 1 μg/mL and 100 μM, respectively.
2.2 Cell Isolation and culture
Neonatal rat ventricular myocytes (NRVMs) and neonatal rat ventricular fibroblasts (NRVFs) were prepared from hearts of 1- to 3- day old Sprague–Dawley rats, as previously described (22). Cells were cultured overnight on 10-cm plates coated with gelatin (0.2%; Sigma) in Dulbecco’s Modified Eagle’s Medium (DMEM) containing calf serum (10%), L-glutamine (2 mM), and penicillin–streptomycin. After overnight culture, cells were washed with serum-free medium and maintained in DMEM supplemented with L-glutamine, penicillin–streptomycin, and Neutridoma-SP (0.1%; Roche Applied Science), which contains albumin, insulin, transferrin, and other defined organic and inorganic compounds. Adult rat ventricular fibroblasts (ARVFs) were collected by the Langendorff-perfusion method from Sprague Dawley rats (23). NRVFs and ARVFs were cultured in DMEM with 20% FBS containing penicillin (100 U/ml), streptomycin (100 U/ml) and L-glutamine (29.2 μg/ml) (PSG); cells were used at passage one for all experiments. All cell culture supplies were purchased from Cellgro (Mediatech, Inc.), unless otherwise noted.
2.3 Immunoblotting and immunoprecipitation
Cells were washed twice in ice-cold PBS (pH 7.4) containing 20 mM n-ethylmaleimide (NEM) (Sigma, E3876) prior to lysis. Cells were lysed in radioimmunoprecipitation assay buffer (RIPA) containing 50 mM Tris (pH 8), 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 20 mM NEM, and HALT™ protease/phosphatase inhibitor mixture (Thermo Fisher). Cellular lysates were sonicated before clarification by centrifugation. Proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes (BioRad) and probed with antibodies for SUMO-1 (Cell Signaling Technology, 4930; Santa Cruz Biotechnology, sc-5308; University of Iowa Hybridoma Bank, 21C7); SUMO-2/3 (Cell Signaling Technology, 4971); ubiquitin (Santa Cruz Biotechnology, sc-8017); cFos (Cell Signaling Technology, 4384); HDAC1 (Cell Signaling Technology, 5356); HDAC2 (Cell Signaling Technology, 5113); HDAC3 (Santa Cruz Biotechnology, sc-136290); α Tubulin (Abcam, ab7291); RanGAP1 antibody #1 was kindly provided by Dr. Frauke Melchior; RanGAP1 antibody #2 (Santa Cruz Biotechnology, sc-25630); acetylated-lysine antibodies were used as a cocktail (Cell Signaling Technology, 9441 and 9681). SUMO-1 conjugates were immunoprecipitated using antibody-agarose conjugated beads (Santa Cruz Biotechnology, sc-5308 AC). Briefly, cellular lysates were pre-cleared with protein A agarose at 4°C for one hour, a 10% input sample was removed, and the remaining lysate was incubated with anti-SUMO-1 beads at 4°C overnight. Beads were pelleted and a supernatant sample was taken prior to washing beads three times with ice-cold lysis buffer and two times with ice-cold PBS. Immunoprecipitated SUMO-1 conjugates were resolved by SDS-PAGE and detected by immunoblotting.
2.4 Lentiviral production and infection
Lentiviruses were produced with pLKO.1 short-hairpin RNA plasmids (Sigma) provided by the University of Colorado Boulder Functional Genomics Facility. On Day 1, 2 x 106 HEK L293 cells were plated on 10-cm plates in DMEM containing calf serum (10%), L-glutamine (2 mM), and penicillin–streptomycin. On Day 2, each plate was transfected with 9 μg of packaging plasmid (psPAX2), 0.9 μg envelope plasmid (pMD2.G), and 9 μg pLKO.1 containing shRNA sequence of interest. Plasmids were combined with a 3μL:1μg polyethyleneimine (PEI):DNA mixture; PEI (Polysciences, Inc., cat# 23966) was used at a concentration was 1 mg/mL. On Day 3, media was replaced with fresh DMEM containing calf serum (10%), L-glutamine (2 mM), and penicillin–streptomycin. On Day 5, culture medium containing viral particles was passed through a 0.45μm syringe filter and stored at −80°C until use. For infection of NRVMs on 60-mm plates, 3 mL of lentivirus-containing medium was added, and 3μL polybrene (10 mg/mL) was included to enhance infection efficiency. Cells were cultured for four days in serum-free DMEM containing Neutridoma-SP supplement and PE (10 μM). Cell homogenates were prepared in RIPA buffer supplemented with 20 mM NEM and HALT™ protease/phosphatase inhibitor mixture (Thermo Fisher). pLKO.1 plasmids: shControl, SHC016 non-mammalian targeting; shHDAC1, TRCN0000039402; shHDAC2, TRCN0000039395; shHDAC3, TRCN0000318152.
2.5 SENP activity assay
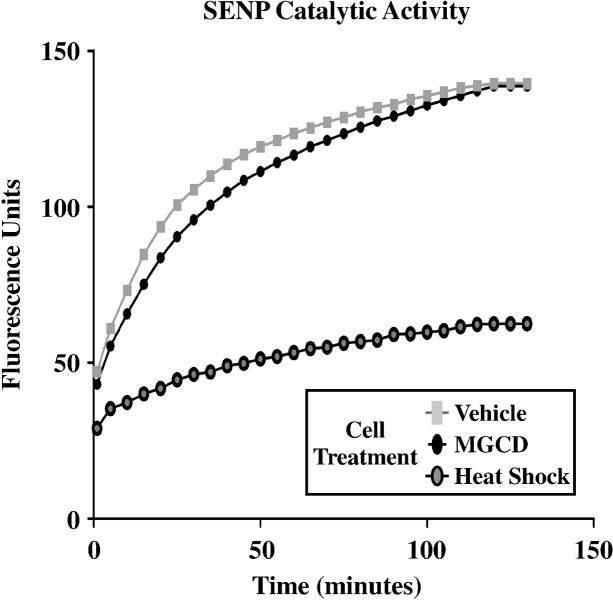
Sentrin specific protease (SENP) activity was assessed as described previously (24). Cardiac fibroblasts were treated with vehicle (DMSO) or MGCD0103 for 48 hours. Some cells received vehicle and were heat shocked at 43°C for 30 minutes prior to lysis. Cells were lysed in PBS pH 7.4 supplemented with 300 mM NaCl and 0.5% Triton X-100. Protein homogenates were incubated with 500 nM SUMO-1-7-amidomethylcoumarin (AMC) (Enzo Life Sciences) in 110 μL of assay buffer (50 mM Tris HCl, pH 7.5, 0.1 mg/mL bovine serum albumin, and 10 mM DTT). Hydrolysis of SUMO-1 AMC was measured by determining the increase in fluorescence (λex=340 nm and λem=440nm) every 30 seconds for 2 hours using a BioTek Synergy 2 plate reader.
2.6 Purification of recombinant proteins
Recombinant protein components of the SUMO conjugation machinery were produced in E. coli using pET28a-Aos1 (SAE1), pET28b-Uba2 (SAE2), pET23a-Ubc9, pET-11a-SUMO-1, and pET11-hRanGAP1, and were purified as previously described (25).
2.7 Chemical acetylation followed by in vitro SUMOylation
Chemical acetylation was adapted from a previously described method (26). Recombinant forms of each component of the SUMO conjugation machinery (1 ug each) were chemically acetylated by 0.1 mM acetic anhydride (Sigma, 320102) in PBS for 1 hour at room temperature. After chemical acetylation, proteins were resolved by SDS-PAGE and detected by immunoblotting with anti-acetyl-lysine antibodies. Protein levels were confirmed by Coomassie Brilliant Blue staining. Pre-acetylated proteins were incorporated into in vitro SUMOylation assays with RanGAP1 substrate based on prior optimization studies; SAE1/SAE2 (140 ng), Ubc9 (220 ng), SUMO-1 (2 μg), RanGAP1 (2 μg, unacetylated) (27). Reaction buffer consisted of 40 mM HEPES pH 7.3, 220 mM KOAc, 4 mM Mg(OAc)2, 4 mM DTT, and protease/phosphatase inhibitor cocktail (Thermo Fisher). Reactions were carried out at 30°C for 30 minutes in the absence or presence of ATP (5 mM) and terminated by boiling in SDS-PAGE sample buffer.
2.8 In vitro acetylation by p300 followed by in vitro SUMOylation
Recombinant p300 histone acetyltransferase was produced as previously described (28). Reactions were performed with recombinant SUMO-1, SAE1/2 and Ubc9 (1 μg each) in reaction buffer containing 25 mM Tris HCl pH 7.9, 50 mM KCl, 6.25 mM MgCl2, 10% glycerol, 1 mM DTT and p300 for 1 hour at 30°C. Assessment of protein acetylation and in vitro SUMOylation activity of pre-acetylated proteins was performed as described above for acetic anhydride.
3. Results
3.1 Selective inhibition of HDAC1 and HDAC2 stimulates protein SUMOylation in cardiac myocytes and fibroblasts
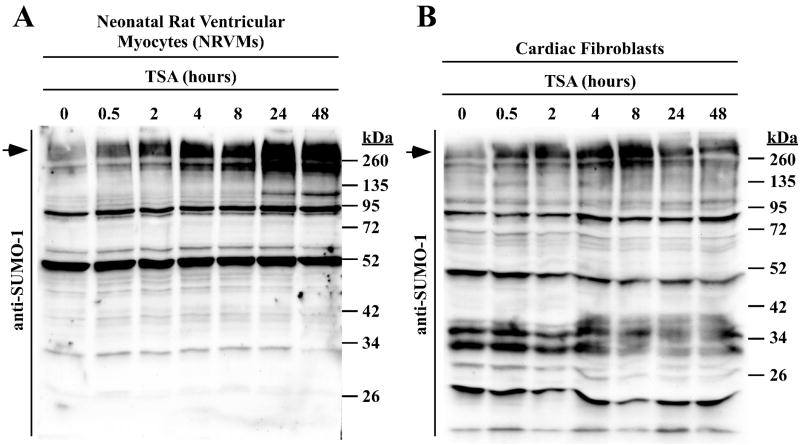
Since acetylation or SUMOylation of a given lysine residue occurs in a mutually exclusive manner (1), we hypothesized that globally increasing protein acetylation through the use of an HDAC inhibitor would result in suppression of SUMO-1 conjugation. To address this hypothesis, primary neonatal rat ventricular myocytes (NRVMs) were treated with the HDAC inhibitor trichostatin A (TSA) over a time course of 48 hours, and lysates were immunoblotted with a SUMO-1-specific antibody. Surprisingly, rather than inhibiting SUMOylation, TSA treatment resulted in a robust, time-dependent accumulation of high molecular weight SUMO-1 conjugated proteins. A similar increase in protein SUMOylation was observed in primary rat cardiac fibroblasts treated with TSA, although the stimulatory effect in these cells appeared to be transient compared to cardiac myocytes (Fig. 1B).
Fig. 1.
HDAC inhibition stimulates SUMOylation in cardiac cells. (A) Neonatal rat ventricular myocytes (NRVMs) were treated with the pan-HDAC inhibitor trichostatin A (TSA) for the indicated amounts of time. SUMO-1 conjugates were examined by immunoblotting. (B) Primary adult rat cardiac fibroblasts were serum starved for 24 hours prior to treatment with TSA. Arrows indicate the high molecular weight SUMO conjugates that are referenced throughout the text.
TSA is a pan-HDAC inhibitor that efficiently inhibits the catalytic activity of at least nine Zn2+-dependent HDACs (HDACs 1–9) (29). The recent discovery of isoform-selective HDAC inhibitors provides an opportunity to use a chemical biological approach to more precisely address the role of specific HDACs in the control of a given process. For example, MGCD0103 and MS-275 are benzamide-containing compounds that are highly specific inhibitors of class I HDACs (HDACs -1, -2 and -3), while biaryl benzamide derivatives such as biaryl-60 (BA-60) selectively inhibits HDACs -1 and -2 (30,31). Diphenylacetohydroxamic acid (DPAH) blocks the activity of class IIa HDACs (HDACs -4, -5, -7 and -9) (32), while Tubastatin A is highly specific for HDAC6, which is a class IIb HDAC (33). Selectivity profiles of these compounds are summarized in Fig. 2A.
Fig. 2.
Selective inhibition of HDAC1 and HDAC2 is sufficient to stimulate SUMOylation in cardiac myocytes and cardiac fibroblasts. (A) Selectivity profiles for the indicated HDAC inhibitors are shown; X = inhibited. (B) NRVMs were stimulated with phenylephrine (PE) in the absence or presence of the indicated HDAC inhibitors for 48 hours; DMSO vehicle (Veh.; 0.1% final concentration) was used as a negative control. SUMO-1 conjugates were examined by immunoblotting. (C) Unstimulated NRVMs were treated for 48 hours with the indicated HDAC inhibitors prior to immunoblotting for SUMO-1 conjugates. (D) Neonatal rat ventricular fibroblasts were serum-starved for 24 hours prior to treatment with the indicated HDAC inhibitors for 48 hours. (E) Independent plates of adult rat ventricular fibroblasts were serum starved for 24 hours prior to treatment with MGCD. SUMO-1 conjugates were resolved on a 7.5% polyacrylamide gel as opposed to (A – D), which employed 10% polyacrylamide gels.
As shown in Fig. 2B, MGCD0103 and MS-275 induced SUMOylation in NRVMs as efficiently as TSA, suggesting that a class I HDAC(s) regulates this post-translational modification. Class I HDAC inhibition was equally effective at promoting SUMOylation in hypertrophic cardiomyocytes and unstimulated cardiomyocytes (Fig. 2B and 2C). Class IIa and IIb inhibition failed to stimulate SUMO-1 conjugation. Remarkably, selective inhibition of only HDAC1 and HDAC2 with BA60 was sufficient to promote SUMO-1 conjugation in both cardiomyocytes and cardiac fibroblasts (Fig. 2C and 2D). These findings define a novel role for HDAC1 and/or HDAC2 as negative regulators of protein SUMOylation in the two dominant cell types in the heart, myocytes and fibroblasts.
HDAC inhibitor-mediated SUMOylation appeared to occur primarily on high molecular weight proteins. To more accurately characterize SUMO-1 targets that were enhanced by class I HDAC inhibition, cardiac fibroblast lysates were resolved through a lower percentage polyacrylamide gel and subjected to anti-SUMO-1 immunoblotting. Two prominent basally SUMOylated proteins were detected near the 100 kDa marker, and MGCD0103 treatment enhanced SUMO-1 conjugation to both of these targets (Fig. 2E). Based on molecular weight, it is predicted that the more rapidly migrating protein is RanGAP1, which is the most stably SUMOylated protein described to date (34). Interestingly, class I HDAC inhibition stimulated SUMOylation of many additional proteins ranging from 100 kDa to over 170 kDa, suggesting a general role of HDAC1/2 in the control of SUMO-1 conjugation and/or cleavage.
3.2 HDAC2 suppresses cardiac protein SUMO-1-ylation
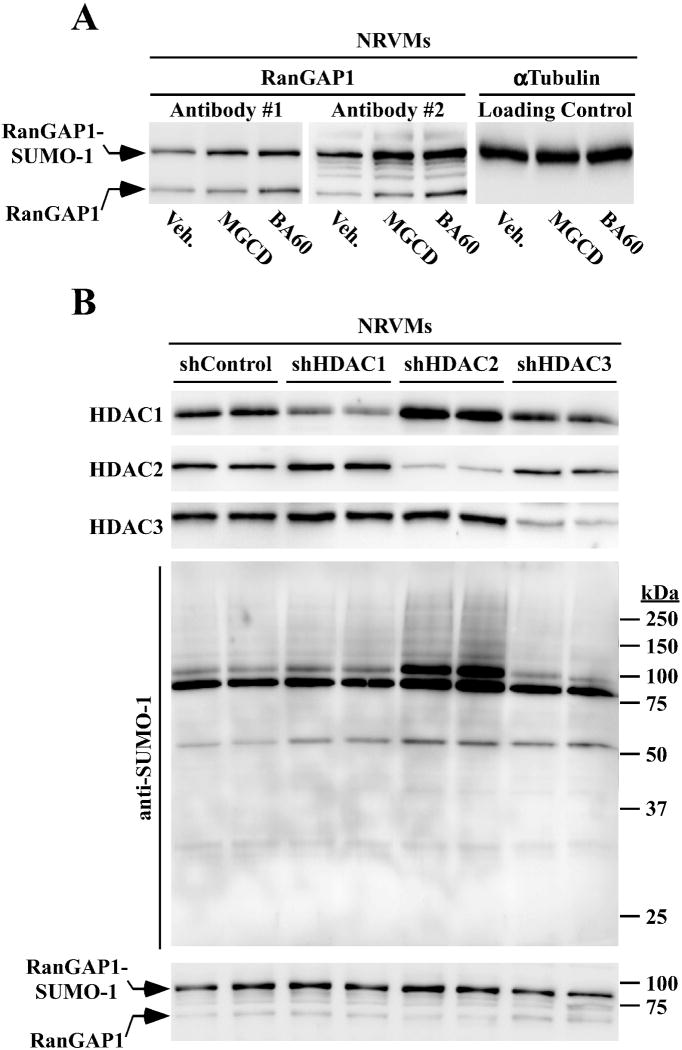
To directly assess whether class I HDAC inhibition stimulates RanGAP1 SUMOylation, NRVMs were treated with MGCD0103 or BA60 for 48 hours and levels of free RanGAP1 and SUMO-1-conjugated RanGAP1 were assessed by immunoblotting. Two distinct antibodies revealed elevated levels of SUMO-1-ylated RanGAP1 in NRVMs treated with the HDAC inhibitors (upper bands).
Since BA60 selectively inhibits HDAC1 and HDAC2, experiments were next performed to determine the relative contribution of these HDAC isoforms to the control of cardiac protein SUMOylation. Lentiviral expression of short hairpin RNAs (shRNAs) effectively and selectively inhibited expression of endogenous HDAC1, HDAC2 or HDAC3 protein in NRVMs (Fig. 3B, top panel). Remarkably, knockdown of HDAC2 alone dramatically increased the abundance of multiple SUMO-1-conjugated proteins in NRVMs, while knockdown of HDAC1 or HDAC3 failed to stimulate SUMOylation (Fig. 3B, middle panel). Subsequent immunoblotting revealed a modest increase in RanGAP1 SUMOylation in cells in which HDAC2 expression was suppressed (Fig. 3B, bottom panel). These findings define a novel role for HDAC2 in the control of protein SUMOylation.
Fig. 3.
HDAC2 regulates cardiac protein SUMOylation. (A) NRVMs were treated with MGCD0103 or BA60 for 48 hours, and protein homogenates were immunoblotted with two distinct antibodies that detect free RanGAP1 (lower band) and SUMO-1-ylated RanGAP1 (upper band). (B) NRVMs were infected with lentiviruses encoding shRNAs directed toward HDAC1, HDAC2 or HDAC3. shControl is an shRNA that is predicted to fail to target any mammalian mRNA transcript. After 96 hours of infection in the presence of PE, cells were lysed and HDAC1, HDAC2, HDAC3, SUMO-1, and RanGAP1 were detected by immunoblotting, as indicated.
3.3 Class I HDAC inhibition enriches for SUMO-1 conjugates rather than SUMO-2/3 conjugates in cardiac myocytes and fibroblasts
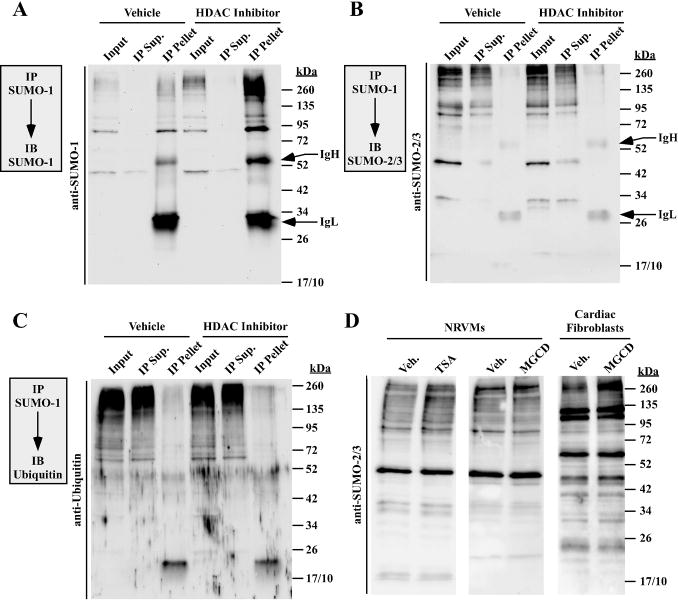
SUMO-1 and its paralogs, SUMO-2/3, are conjugated to target proteins via the same core enzymatic machinery (9,35). However, while SUMO-1 is conjugated to lysine as a monomer, SUMO-2/3 can form chains analogous to ubiquitin (36). Proteins that are modified by SUMO-2/3 appear as a stepwise ladder when resolved through polyacrylamide gels. Given the periodicity of the SUMO-1-conjugated proteins derived from HDAC inhibitor treated cells (Fig. 2E), sequential immunoprecipitation (IP) and immunoblotting was performed to determine if the target proteins were also conjugated to SUMO-2/3 and/or ubiquitin. High molecular weight SUMO-1 conjugated proteins were quantitatively immunoprecipitated with a SUMO-1-specific antibody (Fig. 4A). Remarkably, neither SUMO-2/3 nor ubiquitin conjugates were detected in SUMO-1 immunoprecipitates from DMSO or HDAC inhibitor-treated cells (Fig. 4B and C). Consistent with these findings, analysis of whole cell lysates revealed that, relative to SUMO-1, pan- and class I HDAC inhibition only modestly elevated SUMO-2/3 conjugates in cardiomyocytes and cardiac fibroblasts (Fig. 4D). These findings suggest that class I HDAC inhibition preferentially stimulates SUMO-1-ylation.
Fig. 4.
Inhibition of class I HDACs preferentially enhances SUMO-1 conjugation. SUMO-1 conjugates from DMSO vehicle or HDAC inhibitor-treated cardiac fibroblasts (A) or NRVMs (B and C) were immunoprecipitated (IP) with anti-SUMO-1 antibody-conjugated beads and immunoblotted (IB) with either anti-SUMO-1 antibody (A) anti-SUMO-2/3 antibody (B) or anti-ubiquitin antibody (C). Input = 10% of pre-IP volume; IP supernatant (Sup) = 10% of post-IP volume; pellet = immunoprecipitate. Immunoglobulin heavy (IgH) and light (IgL) chains from the IP antibody are indicated. (D) NRVMs or 24 hour serum-starved adult rat ventricular fibroblasts were treated with DMSO, TSA or MGCD for 48 hours, and SUMO-2/3 conjugates were detected by immunoblotting.
3.4 HDAC inhibitor-mediated SUMOylation occurs independently of de novo protein synthesis
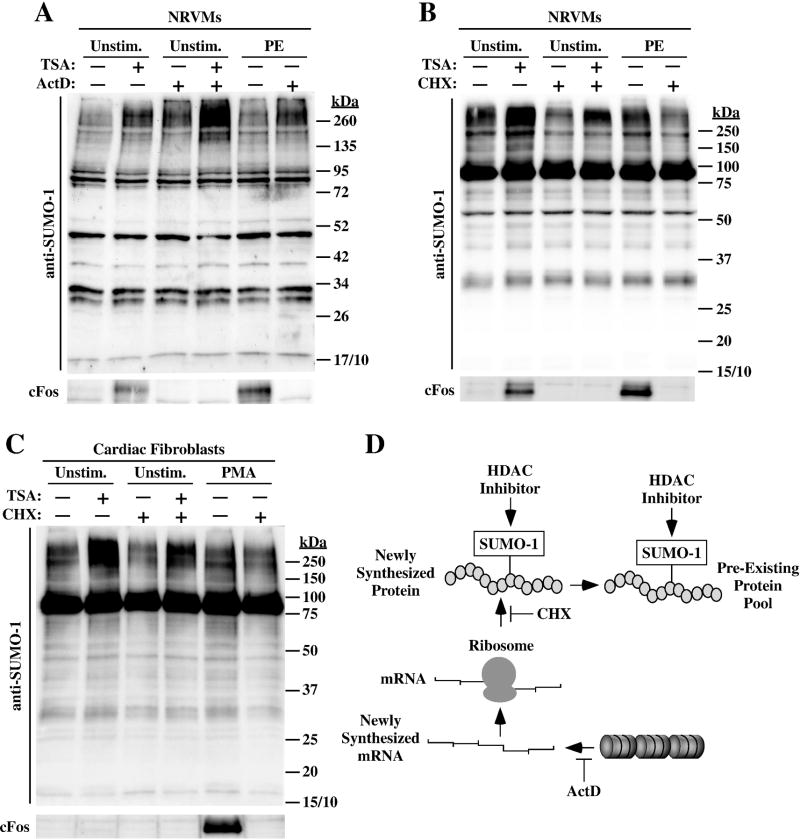
HDAC inhibitors have profound effects on gene expression. To begin to address the mechanism by which HDAC inhibition leads to accumulation of SUMOylated proteins, we determined whether de novo gene expression is required for the HDAC inhibitor-mediated effect on SUMO-1. NRVMs were treated with TSA in the absence or presence of actinomycin D (ActD), which blocks gene transcription. In order to minimize the duration that cells were exposed to ActD, which is cytotoxic, these studies were performed with TSA; TSA rapidly inhibits HDAC catalytic activity while benzamide class I HDAC inhibitors such as MGCD0103 have a slow on-rate (37,38). ActD treatment alone led to modest SUMOylation in NRVMs (Fig. 5A, lane 3). Importantly, TSA efficiently promoted SUMOylation in NRVMs co-treated with ActD (Fig. 5A, compare lanes 2 and 4). The integrity of the ActD preparation was confirmed by its ability to block PE-mediated induction of the immediate early gene, cFos (lower panel). HDAC inhibition also induced cFos protein expression, which was blocked by ActD treatment (lane 2).
Fig. 5.
Class I HDAC inhibitor-mediated SUMOylation does not require de novo gene transcription or protein synthesis. (A) NRVMs were pre-treated with actinomycin D (ActD) for two hours to block gene transcription, and were subsequently exposed to either vehicle (DMSO; -) or TSA for 4 hours. As controls, some cells received PE for two hours in the absence or presence of ActD. Protein homogenates were immunoblotted with anti-SUMO-1 antibody. In parallel, cFos protein was assessed to confirm that ActD efficiently inhibited gene expression. (B) NRVMs were pre-treated with the protein translation inhibitor cycloheximide (CHX) for 30 minutes, and were subsequently exposed to vehicle (DMSO; -) or TSA for 4 hours. Immunoblotting was performed with anti-SUMO-1 antibody or cFos antibody to confirm CHX efficacy. (C) Adult rat ventricular fibroblasts were serum-starved for 24 hours, pretreated with CHX for 30 minutes, and exposed to DMSO or TSA for 4 hours. As controls, some cells received PMA for 2 hours in the absence or presence of CHX. ActD did not block TSA-mediated SUMO-1-ylation (A), and CHX only partially reduced SUMO-1 conjugation in cardiac myocytes (B) and fibroblasts (C), suggesting that HDAC inhibition stimulates SUMOylation of a pre-existing protein pool (D).
To rule out the possibility that induction of SUMO-1 conjugates by ActD alone complicated interpretation of the data, experiments were also performed with cycloheximide (CHX) to block de novo protein synthesis. Consistent with the findings made with ActD, TSA was still capable of enhancing protein SUMOylation in NRVMs and cardiac fibroblasts treated with CHX (Fig. 5B and C). However, unlike what was observed with ActD, CHX treatment did result in a modest reduction in the degree of induction of SUMO-1 conjugates by TSA (Fig. 5B and C, lanes 2 and 4). The data suggest that HDAC inhibition leads to accumulation of SUMO-1-conjugated proteins through a post-translational mechanism. Based on the findings with CHX, we propose that HDAC inhibition enhances SUMOylation of a pre-existing pool of protein that is activity synthesized in cardiac myocytes and fibroblasts (Fig. 5D).
3.5 Class I HDAC inhibition does not suppress SENP catalytic activity
The increase in protein SUMOylation in HDAC inhibitor-treated cells could be due to stimulation of SUMO conjugation or suppression of de-SUMOylation. To address the latter possibility, we assessed the impact of class I HDAC inhibition on the catalytic activity of sentrin-specific proteases (SENPs), which are a family of enzymes that cleave SUMO conjugates from proteins. Cardiac fibroblasts were treated with MGCD0103 or vehicle control. As a positive control, a third group of cells was subjected to heat shock, which has previously been shown to inhibit SENP activity (24). After cell lysis, SENP activity was quantified by incubation of protein homogenates with a SUMO-1-7-amidomethylcoumarin (AMC) substrate; de-SUMOylation of the substrate results in enhanced fluorescence. As shown in Fig. 6, SENP activity was similar in extracts from MGCD0103-treated NRVMs and untreated controls, while heat shock led to a profound decrease in SENP catalytic activity. These data suggest that the post-translational mechanism by which HDAC inhibition enhances SUMOylation does not involve suppression of SUMO proteases.
Fig. 6.
Global SENP catalytic activity is not suppressed by a class I HDAC inhibitor. Adult rat ventricular fibroblasts were serum-starved for 24 hours and treated with DMSO vehicle or the class I HDAC inhibitor MGCD for 48 hours. As controls, some cells were subjected to 43°C for 30 minutes prior lysis. Protein homogenates were incubated with a SUMO-1-AMC probe and de-SUMOylase activity (as measured by increased fluorescence) was monitored over 2 hours. Heat shock inactivates SENPs and served as a positive control. For each condition, homogenates from three independent plates of cells were pooled for assessment of SENP activity.
3.6 Multiple components of the SUMO conjugation machinery are acetylated on lysine residues in vitro
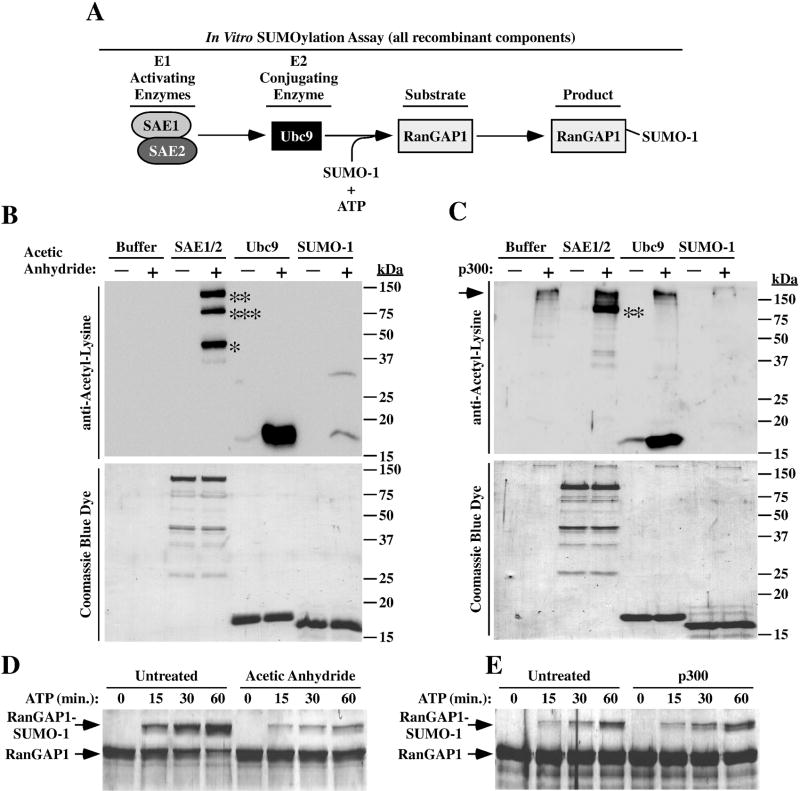
Additional experiments were performed to address the possibility that acetylation regulates proteins that control SUMO conjugation. Components of the in vitro SUMOylation assay that was employed for these studies are shown in Fig. 7A. Recombinant forms of the E1 activating enzyme complex (SAE1/SAE2), the E2 conjugating enzyme (Ubc9), SUMO-1, and the canonical SUMO-1 substrate, RanGAP1, were produced in E. coli. Immunoblotting with an anti-acetyl-lysine antibody revealed that SAE1, SAE2, Ubc9 and SUMO-1 were all capable of being chemically acetylated with acetic anhydride in vitro, albeit to different extents (Fib. 6B). Additionally, SAE2 and Ubc9 were efficiently acetylated by recombinant p300, which possesses intrinsic histone acetyltransferase (HAT) activity (Fig. 7C). However, p300 was unable to acetylate SAE1 and SUMO-1 in vitro (Fig. 7C, lanes 4 and 8).
Fig. 7.
Acetylation of SUMO conjugation machinery. (A) Schematic depiction of the in vitro SUMOylation assay. (B) The indicated recombinant proteins were incubated for one hour with acetic anhydride to assess non-enzymatic acetylation of the proteins. Proteins were resolved by SDS-PAGE and either immunoblotted with anti-acetyl-lysine antibody (top panel) or stained with Coomassie blue dye (bottom panel); *SAE1, **SAE2, ***presumed degradation product of SAE2. (C) Recombinant proteins were incubated for one hour with p300 to assess their capacity to be enzymatically acetylated. Samples were analyzed as in (B); **SAE2. Note that p300 (the upper-most acetylated protein) undergoes auto-acetylation (arrow). (D) In vitro SUMOylation assays were performed as outlined in (A) after acetic anhydride treatment (D) or exposure to p300 (E). Reactions were terminated at the indicated times. Proteins were resolved by SDS-PAGE and stained with Coomassie blue dye. The reduced mobility of RanGAP1 is indicative of SUMOylation.
To assess whether acetylation affects SUMO conjugation, SAE1/2, Ubc9 and SUMO-1 were pre-acetylated with acetic anhydride or p300 and incorporated into in vitro enzymatic assays with ATP and recombinant RanGAP1 substrate. Neither mode of pre-acetylation resulted in enhanced conjugation of SUMO-1 to RanGAP1 (Fig. 7D and E). Thus, under these experimental conditions, acetylation of core SUMO conjugation machinery fails to stimulate enzymatic activity.
4. Discussion
The findings of this study establish a novel role for class I HDACs, specifically HDAC2, in the control of protein SUMOylation in cardiac myocytes and fibroblasts. Since combined inhibition of HDAC1/2 catalytic activity (Fig. 2), or selective knockdown of HDAC2 expression (Fig. 3), leads to the accumulation of SUMO-1-conjugated proteins in cardiac cells, the results suggest that HDAC2 functions as an endogenous inhibitor of SUMOylation in the heart. The work extends the ever-expanding list of non-canonical functions of HDACs, and reversible lysine acetylation, beyond the control of epigenetics and gene transcription.
Crosstalk between acetylation and SUMOylation at the level of individual substrates has previously been demonstrated. For example, acetylation of a conserved lysine residue in the myocyte enhancer factor 2 (MEF2) transcription factor stimulates MEF2-dependent transcription of downstream target genes (39). Conversely, when this same lysine residue is SUMOylated, MEF2 transcriptional activity is repressed. Similar to MEF2, acetylation or SUMOylation of lysine-386 in the p53 transcription factor has opposing effects on p53 target gene activation (40).
More recently, acetylation has emerged as a mechanism for influencing the activity of components of the SUMO conjugation machinery. Consistent with our findings (Fig. 7), the E2 conjugating enzyme Ubc9 was shown to be acetylated at lysine-65, resulting in differential SUMOylation of downstream substrates (41). In addition, two studies demonstrated that SUMO itself is acetylated. When conjugated to p53, SUMO-1 is either unacetylated or acetylated on lysine-37, and the acetylation state of SUMO-1 dictates which downstream p53-target genes are activated (42). Acetylation of SUMO-1 or SUMO-2/3 was also shown to negatively regulate interactions between SUMO and proteins harboring SUMO-interaction motifs (SIMs) (43). It is noteworthy that we failed to detect significant levels of SUMO-1 acetylation in our in vitro assays (Fig. 7). This discrepancy could be due to the antibodies that were used for immunoblotting. We employed pan-anti-acetyl-lysine antibodies, while the aforementioned studies used an antibody specific for acetylated lysine-37 of SUMO-1; it is possible that the pan-anti-acetyl-antibodies fail to recognize acetyl-SUMO-1.
Enhancement of global SUMOylation has been observed in cells exposed to osmotic and oxidative stress, heat shock, ethanol and the lipid peroxidation product 4-hydroxy-nonenal (4-HNE) (44–46). There is also a rich body of literature showing that ischemia enhances the formation of SUMO-1/2/3 conjugates in organs such as brain and kidney (47). Much of this elegant work was performed using a ground squirrel model of hibernation torpor, where oxygen and glucose deprivation during torpor was demonstrated to result in a dramatic increase in SUMO-1 and SUMO-2/3 conjugation to proteins in a variety of organs (48). SUMOylation appears to provide a mechanism for ischemic tolerance (49). Interestingly, HDAC inhibitors have been shown to be protective in pre-clinical models of ischemic brain injury and in models of ischemic cardiomyopathy (50–53). Based on our findings, it is possible that at least a portion of the observed protective effects of HDAC inhibitors in the setting of ischemia are due to stimulation of SUMOylation.
The molecular basis for the enhancement of cardiac protein SUMOylation by HDAC inhibitors remains unknown. Given that de novo protein synthesis is not required for HDAC inhibitor-mediated SUMOylation, we hypothesized that the observed effect was due to stimulation of SUMO conjugation or suppression of de-SUMOylation. Concerning the latter possibility, class I HDAC inhibition did not reduce global SENP activity in cardiac fibroblasts (Fig. 6). Although it remains possible that HDACs have as-yet-unidentified effects on de-SUMOylation via one or more of the seven SENP family members, these data suggest that HDAC inhibition targets components of the SUMO conjugation machinery to promote SUMOylation of proteins. Surprisingly, acetylation of the E1 activating enzyme complex (SAE1/2) or Ubc9 failed to enhance SUMO-1 conjugation to RanGAP1 in our in vitro assay (Fig. 7). While it is possible that E1 or E2 acetylation stimulates SUMOylation of substrates other than RanGAP1, we favor a model in which acetylation of an E3 SUMO ligase(s) promotes SUMO-1 conjugation to select targets. Although SUMOylation can proceed in the absence of an E3 ligase, these enzymes enhance conjugation and confer target specificity to the reaction (9). There are seven bona fide E3 ligases in mammals and, intriguingly, interrogation of databases associated with proteomic studies reveals that several of these enzymes are acetylated on lysine residues (4,5). Elucidation of the identity of proteins within the pool of high molecular weight factors that are SUMOylated in an HDAC inhibitor-dependent manner should facilitate efforts to define the possible role of reversible lysine acetylation in the control of E3 SUMO ligases.
5. Conclusions
Inhibition of class I HDAC catalytic activity stimulates SUMO-1-ylation in the two most abundant cell types in the heart, cardiomyocytes and cardiac fibroblasts. HDAC inhibition elicits cardiac protein SUMOylation through a post-translational mechanism that is likely mediated by acetylation of a component(s) of the SUMO conjugation machinery. Independently, HDAC inhibition and SUMO-1 overexpression suppress pathological cardiac remodeling in response to stresses such as pressure overload and myocardial infarction in pre-clinical models. Thus, our findings raise the intriguing possibility that concomitant HDAC inhibitor therapy and SUMO-1 gene transfer will provide synergistic efficacy in the setting of heart failure.
Highlights.
HDAC inhibition promotes cardiac protein SUMOylation
Class I HDACs suppress cardiac protein SUMOylation
SUMO conjugating proteins are acetylated
Acknowledgments
Grants
W.W.B. was funded by the University of Colorado Denver Pharmacology Program NIH T32 Training Grant (GM007635). This work was supported by NIH grants to T.A.M. (HL116848 and AG043822).
We thank J. Mahaffey, B. Ferguson and M. Stratton for preparation of primary cardiac cells, and M. Wempe for medicinal chemistry. We are grateful to F. Melchior and A. Werner (U. Heidelberg) for reagents and invaluable advice.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang XJ, Seto E. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. Nat Struct Mol Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster DB, Liu T, Rucker J, O’Meally RN, Devine LR, Cole RN, O’Rourke B. PLoS One. 2013;8:e67513. doi: 10.1371/journal.pone.0067513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 5.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinsey TA. Annu Rev Pharmacol Toxicol. 2012;52:303–319. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- 7.Xie M, Hill JA. Trends Cardiovasc Med. 2013;23:229–235. doi: 10.1016/j.tcm.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregoretti IV, Lee YM, Goodson HV. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Flotho A, Melchior F. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 10.Hay RT. Biochem Soc Trans. 2013;41:463–473. doi: 10.1042/BST20130015. [DOI] [PubMed] [Google Scholar]
- 11.van der Veen AG, Ploegh HL. Annu Rev Biochem. 2012;81:323–357. doi: 10.1146/annurev-biochem-093010-153308. [DOI] [PubMed] [Google Scholar]
- 12.Hickey CM, Wilson NR, Hochstrasser M. Nat Rev Mol Cell Biol. 2012;13:755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Feng XH, Schwartz RJ. J Biol Chem. 2004;279:49091–49098. doi: 10.1074/jbc.M407494200. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Li A, Wang Z, Feng X, Olson EN, Schwartz RJ. Mol Cell Biol. 2007;27:622–632. doi: 10.1128/MCB.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa MW, Lee S, Furtado MB, Xin L, Sparrow DB, Martinez CG, Dunwoodie SL, Kurtenbach E, Mohun T, Rosenthal N, Harvey RP. PLoS One. 2011;6:e24812. doi: 10.1371/journal.pone.0024812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EY, Chen L, Ma Y, Yu W, Chang J, Moskowitz IP, Wang J. PLoS One. 2011;6:e20803. doi: 10.1371/journal.pone.0020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Schwartz RJ. Circ Res. 2010;107:19–29. doi: 10.1161/CIRCRESAHA.110.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilemann L, Lee A, Ishikawa K, Aguero J, Rapti K, Santos-Gallego C, Kohlbrenner E, Fish KM, Kho C, Hajjar RJ. Sci Transl Med. 2013;5:211ra159. doi: 10.1126/scitranslmed.3006487. [DOI] [PubMed] [Google Scholar]
- 20.Vejpongsa P, Yeh ET. Circ Res. 2014;114:1561–1563. doi: 10.1161/CIRCRESAHA.114.304125. [DOI] [PubMed] [Google Scholar]
- 21.Lee A, Jeong D, Mitsuyama S, Oh JG, Liang L, Ikeda Y, Sadoshima J, Hajjar R, Kho C. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2014.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer JN, Hartogensis WE, Patten M, Fortuin FD, Long CS. J Clin Invest. 1995;95:2555–2564. doi: 10.1172/JCI117956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemon DD, Horn TR, Cavasin MA, Jeong MY, Haubold KW, Long CS, Irwin DC, McCune SA, Chung E, Leinwand LA, McKinsey TA. J Mol Cell Cardiol. 2011;51:41–50. doi: 10.1016/j.yjmcc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto MP, Carvalho AF, Grou CP, Rodriguez-Borges JE, Sa-Miranda C, Azevedo JE. Biochim Biophys Acta. 2012;1823:1958–1966. doi: 10.1016/j.bbamcr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Flotho A, Werner A, Winter T, Frank AS, Ehret H, Melchior F. Methods Mol Biol. 2012;832:93–110. doi: 10.1007/978-1-61779-474-2_5. [DOI] [PubMed] [Google Scholar]
- 26.Fritz KS. Methods Mol Biol. 2013;1077:191–201. doi: 10.1007/978-1-62703-637-5_13. [DOI] [PubMed] [Google Scholar]
- 27.Flotho A, Werner A, Winter T, Frank AS, Ehret H, Melchior F. Methods Mol Biol. 2012;832:93–110. doi: 10.1007/978-1-61779-474-2_5. [DOI] [PubMed] [Google Scholar]
- 28.Georges SA, Kraus WL, Luger K, Nyborg JK, Laybourn PJ. Mol Cell Biol. 2002;22:127–137. doi: 10.1128/MCB.22.1.127-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, Mazitschek R. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, Harsch A, Heidebrecht R, Hughes B, Jung J, Kenific CM, Kral AM, Meinke PT, Middleton RE, Ozerova N, Sloman DL, Stanton MG, Szewczak AA, Tyagarajan S, Witter DJ, Secrist JP, Miller TA. Bioorg Med Chem Lett. 2008;18:973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 31.Moradei OM, Mallais TC, Frechette S, Paquin I, Tessier PE, Leit SM, Fournel M, Bonfils C, Trachy-Bourget MC, Liu J, Yan TP, Lu AH, Rahil J, Wang J, Lefebvre S, Li Z, Vaisburg AF, Besterman JM. J Med Chem. 2007;50:5543–5546. doi: 10.1021/jm701079h. [DOI] [PubMed] [Google Scholar]
- 32.Tessier P, Smil DV, Wahhab A, Leit S, Rahil J, Li Z, Deziel R, Besterman JM. Bioorg Med Chem Lett. 2009;19:5684–5688. doi: 10.1016/j.bmcl.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 35.Hay RT. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. J Biol Chem. 2001;276:35368–35374. doi: 10.1074/jbc.M104214200. [DOI] [PubMed] [Google Scholar]
- 37.Chou CJ, Herman D, Gottesfeld JM. J Biol Chem. 2008;283:35402–35409. doi: 10.1074/jbc.M807045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauffer BE, Mintzer R, Fong R, Mukund S, Tam C, Zilberleyb I, Flicke B, Ritscher A, Fedorowicz G, Vallero R, Ortwine DF, Gunzner J, Modrusan Z, Neumann L, Koth CM, Lupardus PJ, Kaminker JS, Heise CE, Steiner P. J Biol Chem. 2013;288:26926–26943. doi: 10.1074/jbc.M113.490706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP. Mol Cell Biol. 2005;25:8456–8464. doi: 10.1128/MCB.25.19.8456-8464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu SY, Chiang CM. EMBO J. 2009;28:1246–1259. doi: 10.1038/emboj.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh YL, Kuo HY, Chang CC, Naik MT, Liao PH, Ho CC, Huang TC, Jeng JC, Hsu PH, Tsai MD, Huang TH, Shih HM. EMBO J. 2013;32:791–804. doi: 10.1038/emboj.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheema A, Knights CD, Rao M, Catania J, Perez R, Simons B, Dakshanamurthy S, Kolukula VK, Tilli M, Furth PA, Albanese C, Avantaggiati ML. J Cell Physiol. 2010;225:371–384. doi: 10.1002/jcp.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullmann R, Chien CD, Avantaggiati ML, Muller S. Mol Cell. 2012;46:759–770. doi: 10.1016/j.molcel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitoh H, Hinchey J. J Biol Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- 45.Shrivastava V, Pekar M, Grosser E, Im J, Vigodner M. Reproduction. 2010;139:999–1010. doi: 10.1530/REP-09-0492. [DOI] [PubMed] [Google Scholar]
- 46.Manza LL, Codreanu SG, Stamer SL, Smith DL, Wells KS, Roberts RL, Liebler DC. Chem Res Toxicol. 2004;17:1706–1715. doi: 10.1021/tx049767l. [DOI] [PubMed] [Google Scholar]
- 47.Lee YJ, Hallenbeck JM. Neuromolecular Med. 2013;15:771–781. doi: 10.1007/s12017-013-8239-9. [DOI] [PubMed] [Google Scholar]
- 48.Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. J Cereb Blood Flow Metab. 2007;27:950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YJ, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM. PLoS One. 2011;6:e25852. doi: 10.1371/journal.pone.0025852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fessler EB, Chibane FL, Wang Z, Chuang DM. Curr Pharm Des. 2013;19:5105–5120. doi: 10.2174/1381612811319280009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aune SE, Herr DJ, Mani SK, Menick DR. J Mol Cell Cardiol. 2014;72:138–145. doi: 10.1016/j.yjmcc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Qin X, Zhao Y, Fast L, Zhuang S, Liu P, Cheng G, Zhao TC. J Pharmacol Exp Ther. 2012;341:285–293. doi: 10.1124/jpet.111.189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Circulation. 2014;129:1139–1151. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]