Abstract
Streptococcus mutans is the primary agent of dental caries, which is often detected in transient bacteremia. Lactoferrin is a multifunctional glycoprotein showing antibacterial activities against several Streptococcus species. We reported here the prophylactic effect of human lactoferrin (hLF) in a lactoferrin knockout mouse (LFKO−/−) bacteremic model. The hLF treatment significantly cleared S. mutans from the blood and organs of bacteremic mice when compared to the non-hLF treated mice. Further, analysis of serum cytokines, spleen and liver cytokine mRNA levels revealed that hLF prophylaxis modulates their release differently when compared to the non-hLF treated group. C-reactive protein level (P=0.003) also decreased following hLF prophylaxis in S. mutans induced bacteremic mice. Additional quantitative RT-PCR analysis revealed that hLF prophylaxis significantly decreased the expression level of IFN-γ, TNF-α, IL-1β, IL-6, MPO and iNOS in spleen and liver. These results suggested that the hLF protects the host against S. mutans-induced experimental bacteremia.
Keywords: human lactoferrin, prophylaxis, Streptococcus mutans, bacteremia
1. Introduction
Streptococcus mutans, causative agent of dental caries, has also been isolated from the blood of patients with bacteremia [1, 2], infective endocarditis [3–5] and sepsis following tooth extraction [4]. Studies suggest that periodontal probing, tooth brushing and poor oral hygiene are the most common ways of introducing oral pathogens into the bloodstream, which increases the risk of bacteremia and systemic diseases [6, 7]. Lactoferrin (LF) constitutes an important component of the innate immune system and has a significant immunomodulatory effect on humans. The major ability of LF is to induce the innate immune mediator cells that subsequently impact the adaptive immune cell functions. Also, the affinity of LF towards iron is considered as a main component of non-specific host defense system against numerous pathogens [8–10]. High level of plasma LF concentration has been suggested to be a predictive indicator of sepsis-related morbidity and mortality [11]. LF also modulates the differentiation of leukocytes of the innate immune system, by increasing natural killer (NK) cell activity [12, 13]. The degranulation of neutrophils in response to an inflammatory signal introduces LF into an environment that is populated with a mix of both innate leukocytes and adaptive immune cells (T-cells and B-cells). The LF receptors for different immune cells confirm the potential for LF to function as a modulator of both the innate and adaptive immune system [14–16]. LF exhibit bacteriostatic and bactericidal effects in addition to controlling systemic inflammation [8]. Studies have shown that LF has direct bactericidal effect against S. mutans [17, 18]. However, none of these studies looked at the bactericidal effect of LF in blood. We have recently demonstrated the importance of the host LF against the Gram-negative periodontal pathogen, Aggregatibacter actinomycetemcomitans [19]. Subsequently, we have also reported the effect of human lactoferrin (hLF) against A. actinomycetemcomitans-induced experimental bacteremia [10]. Prophylactic treatment of hLF against Gram-positive bacteria S. mutans-induced bacteremia however, has not been studied to date. Therefore knowledge about the significance of hLF is a prerequisite. Furthermore, this study will provide information on the importance of hLF prophylaxis on antibacterial and immunomodulatory effects during such bacteremic conditions.
2. Materials and Methods
2.1. Mice
Experimental groups comprised of 7–8 week-old male LFKO−/− mice, a generous gift from Dr. Orla Conneely, (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX, USA) [20]. Mice colonies were bred and maintained in the transgenic animal facility of Rutgers School of Dental Medicine, Newark, New Jersey, USA. The experimental protocol was approved by the campus Institutional Animal Care and Use Committee (IACUC) of Rutgers School of Dental Medicine, Newark, NJ, USA.
2.2. Bacterial strain and tail vein injections
S. mutans-VSK1, a spontaneous streptomycin resistant serotype c strain was isolated from a plaque sample routinely grown in brain heart infusion broth (BHI) or on mitis salivarius agar (MSA) plates (BD & company, Sparks, MD, USA) as reported earlier [21]. PCR amplification of a 727 bp with the following primers SCF 5′-CGGAGTGCTTTTTACAAGTGCTGG-3′, and SCR 5′-AACCACGGCCAGCAAACCCTTTAT-3′ confirmed serotype c specific strain as reported earlier [22]. Cells that were grown in BHI broth for 12 h was centrifuged and resuspended to 1×107 colony forming unit (CFU) per 0.1 ml of phosphate-buffered saline (PBS) and it was intravenously (i.v) injected into the tail vein as reported earlier [23]. The recovered S. mutans after i.v. injection from blood and organ samples was plated on MSA agar plates supplemented with streptomycin (50 μg/ml). The recovered streptomycin resistant colonies were confirmed by PCR [5]. The hLF (100 μg/g body wt) for the prophylactic treatment was prepared as reported earlier [10].
2.3. Experimental design
Experimental groups included; 1) sham infected control mice i.v. injected with PBS (LFKO−/−C), 2) mice i.v. injected with hLF (LFKO−/−+hLF), 3) mice i.v. injected with S. mutans (LFKO−/−I) and 4) mice i.v. injected with hLF followed by S. mutans i.v. injection 2 h later (LFKO−/−I+hLF). All injection volumes were adjusted to 100 μl. Mice (n=5–8) were euthanized at 6, 12, 48 and 96 h following injection of either S. mutans or hLF or both. The recovered S. mutans were expressed in CFU/ml or CFU/g tissue in three independent experiments and the data were statistically analyzed using one-way ANOVA as described earlier [10].
2.4. Determination of the viable bacterial levels
To determine the S. mutans clearance upon hLF prophylaxis, samples of blood, brain, heart, kidney, liver, lungs, and spleen were aseptically harvested and processed after each time point. Blood samples were serially diluted and plated on the MSA plates supplemented with streptomycin (50 μg/ml). The organs were placed in 1 ml of PBS in 50-ml Kendall tissue homogenizer (Tyco Healthcare Group, Mansfield, MA). Following homogenization of the tissues, serially diluted tissue samples were plated on MSA plates. Colonies enumerated after 48 h were expressed as CFU/ml (blood) and CFU/mg (tissue weight). In total, three independent experiments representing three biological replicates were performed.
2.5. Blood sample analysis
The serum inflammatory cytokines and C-reactive protein (CRP) levels were quantified using multiplex ELISA kit (Millipore Corporation, Billerica, MA, USA). The differential leukocyte blood cell count and enzyme-linked immunosorbent assay (ELISA) were performed as reported earlier [10].
2.6. Quantitative RT-PCR
The Total RNA was extracted from spleen and liver tissue using an RNeasy Mini Kit. DNA contamination in the samples was removed by on-column DNase I digestion (Qiagen Inc, Valencia, CA, USA). The relative gene expression of proinflammatory cytokines, inducible nitric oxide synthase (iNOS) and myeloperoxidase (MPO) was performed as reported earlier [10]. Results are presented as the mean±SEM of the duplicate experiments of three independent samples. The data was analyzed by one-way analysis of variance (ANOVA).
2. 7. Effect of hLF treatment in spleen during bacteremic conditions
Mice (n=5–8) were euthanized at different time points and the intact spleen was collected from each group and weighed. The spleen size variation between the groups was photographed using a dissection microscope (Olympus SZ61, Tokyo, Japan). For histological analysis, aseptically collected spleen from 96 h time point was fixed with 4 % paraformaldehyde for 48 h. The tissue samples were then processed and stained with hematoxylin and eosin as described earlier [10]. Tissue sections were observed under Olympus light microscope at 1000× magnification.
2. 8. Statistical analysis
Significance of differences between the groups was calculated using Student’s t test, ANOVA and post hoc Tukey’ HST test between the groups with the JMP software SAS 9.1. P values of less than 0.05 were considered statistically significant. Continuous variables were compared by pairwise t test for two independent samples. Bonferroni correction was used to protect against multiple comparison. Correlation analysis of two variables was carried out with the JMP software SAS 9.1 (SAS, Cary, NC).
3. Results
3.1. In vivo effect of hLF against S. mutans bacteremia
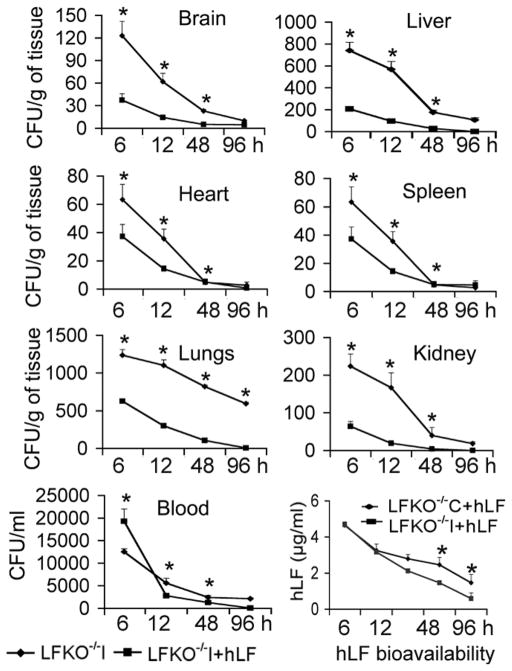
To demonstrate the prophylactic effect of hLF against S. mutans-induced experimental bacteremia, blood and various organs were collected from experimental groups at different time points. The results revealed that hLF treated mice exhibited low levels of S. mutans CFU compared to the non-hLF treated infected group of mice. Analysis of the bacterial clearance data revealed that the LFKO−/−I+hLF mice showed complete clearance at 48 h (P=0.04) while a significantly higher bacterial burden was observed in the LFKO−/−I mice. There was also a significant clearance of S. mutans found at 48 h in LFKO−/−I+hLF mice’ different organ tissues including brain (P=0.03), heart (P=0.003), liver (P=0.02), spleen (P=0.004) and kidney (P=0.006) when compared to LFKO−/−I mice (Fig. 1).
Fig. 1. The prophylactic antibacterial activity of hLF against S. mutans-induced bacteremia in LFKO−/− mice.
Experimental groups (n=5–8) were i.v. injected hLF and S. mutans to induce bacteremia. The number of bacteria from blood and different organs are expressed as ±SEM mean CFU/ml blood or CFU/g of tissue-wet weight, respectively. The hLF bioavailability was assessed by ELISA in duplicate experiments for each mouse. Asterisks indicate statistical significance (P<0.05) of differences between the groups (◆-S. mutans alone i.v. injected; ■-hLF prophylactic treatment), as calculated by the ANOVA. The data shown are from the mean of triplicate experiments (±SEM).
3.2. Bioavailability of serum hLF concentration
To analyze the residual hLF in serum, samples were subjected to ELISA. Estimation of hLF in the serum samples indicated that there was a significant decrease (0.58 μg/ml) (P=0.01) in hLF concentration observed at 96 h in LFKO−/−I+hLF group compared to LFKO−/−C+hLF mice (4.47 μg/ml; P=0.01) (Fig. 1).
3.3. Immunomodulatory effect of hLF during S. mutans bacteremia
To evaluate the immunomodulatory effect of hLF during prophylactic treatment against S. mutans-induced experimental bacteremia, the serum samples collected at 48 h post infection were subjected to multiplex ELISA analysis. S. mutans bacteremia in LFKO−/−I mice significantly increased the levels of serum IFN-γ (P=0.007), TNF-α (P=0.008), IL-1β (P=0.03) and IL-6 (P=0.001) but there was no increase in the levels of IL-10 and IL-12p70 at 48 h when compared to LFKO−/−C (Table 1). Assessment of cytokine levels in hLF prophylactic treatment group (LFKO−/−I+hLF) revealed that the levels of IFN-γ (P=0.002) and TNF-α (P=0.03) were significantly decreased when compared to those in the non-hLF treated infected group (LFKO−/−I). There was a significant increase in IL-6 (P=0.01) level in the LFKO−/−I+hLF mice compared to the LFKO−/−I mice. Evaluation of serum CRP release also shows that the levels were significantly decreased (P=0.007) in LFKO−/−I+hLF mice compared to LFKO−/−I mice (Table 1).
Table 1.
Assessment of effect of hLF on S. mutans induced proinflammatory cytokine and CRP release in the serum samples.
| Mice group | Serum inflammatory mediators following treatments (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-1β | IL-6 | IL-10 | IL12p70 | CRP | |
| LFKO−/−C | 30.0±2.1 | 16.5±2. | 115.0±1.4 | 27.5±2.1 | 47.5±11 | 21.0±1.4 | 0.5±0.09 |
| LFKO−/−C+hLF | 36.0±1.4 | 22.5±0.7 | 18.0±0.08 | 2.0±2.8* | 49.5±4.9 | 26.0±1.4 | 0.9±0.00 |
| LFKO−/−I | 813.0±29.3# | 42.5±2.4# | 17.5±0.0# | 96.0±4.9# | 46.0±2.1# | 19.0±1.4# | 1.8±0.04# |
| LFKO−/−I+hLF | 81.0±11.6¶§ | 24.5±4.7¶§ | 20.0±1.7¶§ | 216.0±4.9$§ | 59.0±7.1$§ | 23.5±3.8¶§ | 1.0±0.04¶§ |
Serum samples were collected at 48 h post-infection with or without hLF prophylaxis. The inflammatory cytokines were quantified after S. mutans and or hLF i.v. injections from serum samples. Significance in the levels was analyzed in triplicate experiments using Student’s t test and ANOVA and post hoc Tukey’ HST test between the groups.
p<0.05 between LFKO−/−C vs. LFKO−/−C+hLF groups,
p<0.05 significant difference between LFKO−/−C vs. LFKO−/−I groups,
p<0.05 significant difference between LFKO−/−I vs. LFKO−/−I+hLF groups,
p<0.05 significant difference between LFKO−/−C+hLF vs. LFKO−/−I+hLF groups,
p<0.05 significant difference between LFKO−/−C vs. LFKO−/−I+hLF groups.
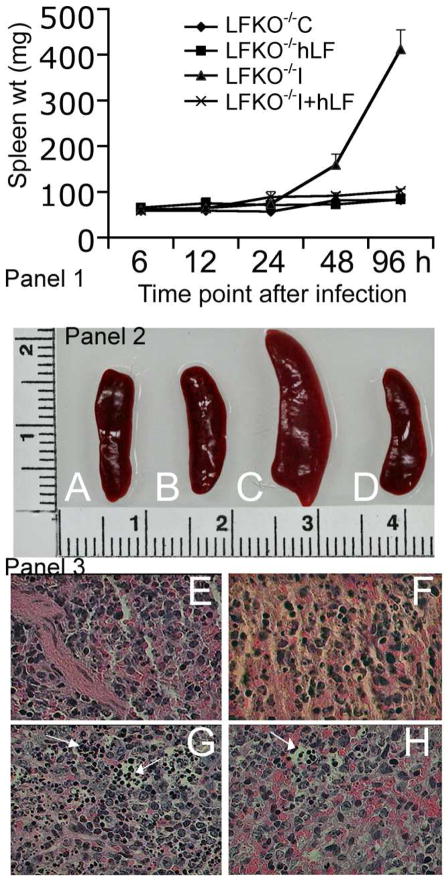
As a result of S. mutans inflammation, there was a significant increase in splenic weight from 6 h to 96 h. Maximum weight increase was observed at 96 h in LFKO−/−I mice (412.7±42.6 mg) compared to LFKO−/−C mice (82.4±1.21 mg) (Fig. 2 panel 1). At this time point, in LFKO−/−I+hLF mice, the splenic weight was significantly decreased to 101.4±5.1 mg (P=0.001) (Fig. 2A–D). The histopathological analysis also suggested an increased in inflammatory cells migration in LFKO−/−I mice, whereas it was decreased in LFKO−/−I+hLF mice (Fig. 2E–H). This was also supported by a significant reduction in the mRNA levels of IFN-γ (P=0.002), TNF-α (P=0.03), IL-1β (P=0.001) and IL-6 (P=0.001) when compared to non-hLF treated infected mice (LFKO−/−I) (Table 2). In liver, the expression levels of proinflammatory cytokine expression levels of IFN-γ (P=0.01), TNF-α (P=0.02), IL-1β (P=0.01) and IL-6 (P=0.001) were also significantly decreased (Table 2). Analysis of mRNA expression levels of acute phase reactions such as iNOS (P=0.01) and MPO (P=0.002) in spleen tissue revealed that S. mutans infection caused a significant increase in these levels. Concomitantly, there was a 2.5 and a 4.9-fold decrease in iNOS and MPO expression levels respectively in hLF treated infected mice (LFKO−/−I+hLF) when compared to non-hLF treated infected mice (LFKO−/−I). The liver iNOS (P=0.004) and MPO (P=0.01) expression levels also showed a significant decrease compared to non-hLF treated mice (Table 2). There was a significant increase in the neutrophil count (P=0.03) in hLF treated and infected mice (LFKO−/−I+hLF) compared to sham-infected mice (LFKO−/−C). However, there was no difference seen in the cell counts of monocytes, eosinophils and basophils among the groups (Table 3).
Fig. 2. Prophylactic effect of hLF against S. mutans bacteremia in the spleen samples of LFKO−/− mice.
Panel 1. Changes in the splenic weight during S. mutans bacteremia and hLF prophylaxis. The spleen of different treatment groups were harvested from 6–96 h post infection and weighed. Data shown are the ±SEM of 5–8 mice spleen weight.
Panel 2. Differences in the splenic size during S. mutans bacteremia and hLF treatment.
Panel 3. Histopathological analysis of hematoxylin and eosin stained spleen section during S. mutans bacteremic conditions treated with hLF. The infiltrations of the increased inflammatory cells are shown in the arrow mark. A–E) LFKO−/−C, B–F) LFKO−/−+hLF, C–G) LFKO−/−I and D–H) LFKO−/−I+hLF mice.
Table 2.
Quantitative RT-PCR analysis of splenic and hepatic inflammatory cytokines, MPO and iNOS mRNA levels.
| organs | Mice group | Relative mRNA expression levels of genes | |||||
|---|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-1β | IL-6 | MPO | iNOS | ||
| Spleen | LFKO−/−C | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| LFKO−/−I | 8.0±0.9 | 16.2±1.8 | 12.8±1.4 | 11.2±1.3 | 78.2±9.3 | 124.2±27.0 | |
| LFKO−/−I+hLF | 3.2±0.3* | 7.0±0.6* | 3.0±0.3* | 4.0±0.4* | 41.2±6.1* | 56.5±18.0* | |
|
| |||||||
| Liver | LFKO−/−C | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| LFKO−/−I | 15.8±4.9 | 8.1±1.1 | 4.4±0.8 | 3.4±0.9 | 54.0±1.7 | 189.1±33.7 | |
| LFKO−/−I+hLF | 3.1±0.2* | 4.6±0.3* | 0.7±0.04* | 1.5±0.3* | 11.0±1.0* | 73.8±23.9* | |
Data shown are mean±SEM) relative levels of expression of mRNA. Asterisks indicate statistically significant difference compared to uninfected controls (P<0.05) as calculated by the ANOVA.
Table 3.
Peripheral leukocytes during S. mutans bacteremia treatment in LFKO−/− mice
| Mice group | Percentage of leukocytes in the blood | ||||
|---|---|---|---|---|---|
| Lymphocyte | Neutrophils | Monocytes | Eosinophils | Basophiles | |
| LFKO−/−C | 70.6±3.1 | 12.0±1.1 | 2.1±0.5 | 6.4±2.3 | 1.0±0.6 |
| LFKO−/−C+hLF | 82.5±2.7 | 11.5±0.8 | 3.4±0.4 | 2.5±0.2 | 1.5±0.2 |
| LFKO−/−I | 78.8±2.7 | 17.3±1.1* | 2.3±0.3 | 2.1±0.8 | 1.1±0.3 |
| LFKO−/−I+hLF | 75.0±0.5 | 18.0±0.6* | 4.0±1.4 | 2.3±1.2 | 1.0±0.1 |
The blood samples were analyzed for the differential cell count during hLF and/or S. mutans i.v. injection at 48 h.
Indicates significant difference between the groups.
4. Discussion
The antibacterial activity of LF has been widely documented both in vitro and in vivo on Gram-positive and Gram-negative bacteria [24]. LF’s bacteriostatic function is due to its ability to take up the Fe3+ ion, limiting the use of this nutrient by bacteria at the infection site and inhibiting the growth of these microorganisms as well as the expression of their virulence factors. LF’s bactericidal function has been attributed to its direct interaction with bacterial surfaces [24]. The mechanism of action against Gram-positive bacteria is based on binding due to its net positive charge that binds to anionic molecules on the bacterial surface, resulting in a reduction of negative charge on the cell wall and thus favoring contact between lysozyme and the underlying peptidoglycans over which it exerts an enzymatic effect [25, 26]. It has been reported that the virulence factors of S. mutans such as glucan-binding protein C and cell surface protein antigen PAc contributes to systemic infection [27, 28]. Streptococci are the most abundant inhabitants of the human mouth and they gain frequent access to the bloodstream through periodontal lesions [29]. It has also been reported that the dental extraction without prophylaxis leads to S. mutans bacteremia and IE [30]. Clinical studies have also shown that the S. mutans DNA is present in blood samples [31]. Due to this invasive nature of intruding pathogen into the bloodstream, a rapid increase in serum LF levels and reduced the circulating iron levels has been reported to protect the host system [32]. The present study shows that the antibacterial effects achieved by injecting a single dose of hLF as prophylaxis greatly reduced bacteremia as evidenced by reduced S. mutans CFUs in blood and all other organs. There have been many reports on antibacterial effect of LF in support of the present results [33–36]. We also found a corroboration of the presence of residual hLF in blood and bacterial clearance.
In addition to the primary killing mechanism of hLF against S. mutans, it plays a critical role in suppressing bacteremia induced cytokine levels. It has been reported that TNF-α, IL-1β and keratinocyte chemoattractant (a functional homologue of human IL-8) cytokines are the distinctive biomarkers for fatal bacteremic conditions [33]. It has also been reported that S. mutans is capable of inducing TNF-α and IL-1β pathways [37]. The LFKO−/− mice with hLF supplementation decreased the expression of such cytokines suggestive of immunomodulatory role of LF. This could be due to multiple components involved in the differential regulation of cellular immune responses in this study. It could be also due to a generalized deactivation of the monocytes and macrophages as manifested by the significant suppression of both pro- and anti-inflammatory mediators of inflammation. This was supported by an in vitro study that LF can down-regulate TNF-α production [38]. Studies have shown that S. mutans induces high titers of inflammatory cytokines and chemokines [39–41]. Cell-free supernatant fluids of Streptococci have also shown to induce proinflammatory cytokines [42, 43]. Alternatively, in the present study, the decrease in S. mutans colony counts may simply result from the direct interaction of hLF leading to dissociation of bacterial membrane integrity [38]. This is also supported by a time dependent decrease in the residual hLF in the blood of LFKO−/−C+hLF or LFKO−/−I+hLF mice. Therefore, we hypothesize that the clearance of S. mutans from the blood and organs might be the cumulative effect of hLF by inducing host defense system as well as by direct killing effect. Thus, the anti-inflammatory capacity of hLF in our model could be due to the down regulation of inflammatory cytokines. The LFKO−/− mouse model is an appropriate model for studying the effect of hLF, which is not yet completely explored. This could explain the essential role of endogenous LF in the system for host protection as well as immunomodulatory properties.
Acknowledgments
The corresponding author would like to thanks Dr. Orla M. Conneely for providing us the LFKO−/− mice. This research was supported by the NIH NIDCR grant R21 DE019548 and New Jersey Health Foundation for providing partial financial support in the form of Grant #PC123-13 to V.K. Authors also thank the confocal imaging core facility and Molecular Resource Facility of Rutgers New Jersey Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nomura R, Hamada M, Nakano K, Nemoto H, Fujimoto K, Ooshima T. Repeated bacteraemia caused by Streptococcus mutans in a patient with Sjogren’s syndrome. J Med Microbiol. 2007;56:988–992. doi: 10.1099/jmm.0.47186-0. [DOI] [PubMed] [Google Scholar]
- 2.Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun. 2011;2:485–491. doi: 10.1038/ncomms1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vose JM, Smith PW, Henry M, Colan D. Recurrent Streptococcus mutans endocarditis. Am J Med. 1987;82:630–632. doi: 10.1016/0002-9343(87)90111-2. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara T, Nakano K, Kawaguchi M, Ooshima T, Sobue S, Kawabata S, et al. Biochemical and genetic characterization of serologically untypable Streptococcus mutans strains isolated from patients with bacteremia. Eur J Oral Sci. 2001;109:330–334. doi: 10.1034/j.1600-0722.2001.00119.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakano K, Nomura R, Nakagawa I, Hamada S, Ooshima T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J Clin Microbiol. 2004;42:198–202. doi: 10.1128/JCM.42.1.198-202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238–1244. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clare DA, Catignani GL, Swaisgood HE. Biodefense properties of milk: the role of antimicrobial proteins and peptides. Curr Pharm Des. 2003;9:1239–1255. doi: 10.2174/1381612033454874. [DOI] [PubMed] [Google Scholar]
- 10.Velusamy SK, Poojary R, Ardeshna R, Alabdulmohsen W, Fine DH, Velliyagounder K. Protective effects of human lactoferrin during Aggregatibacter actinomycetemcomitans-induced bacteremia in lactoferrin-deficient mice. Antimicrob Agents Chemother. 2014;58:397–404. doi: 10.1128/AAC.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baynes RD, Bezwoda WR. Lactoferrin and the inflammatory response. Adv Exp Med Biol. 1994;357:133–141. doi: 10.1007/978-1-4615-2548-6_13. [DOI] [PubMed] [Google Scholar]
- 12.Damiens E, El Yazidi I, Mazurier J, Elass-Rochard E, Duthille I, Spik G, et al. Role of heparan sulphate proteoglycans in the regulation of human lactoferrin binding and activity in the MDA-MB-231 breast cancer cell line. Eur J Cell Biol. 1998;77:344–351. doi: 10.1016/S0171-9335(98)80093-9. [DOI] [PubMed] [Google Scholar]
- 13.Shau H, Kim A, Golub SH. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J Leukoc Biol. 1992;51:343–349. [PubMed] [Google Scholar]
- 14.Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci. 2005;62:2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mincheva-Nilsson L, Hammarstrom S, Hammarstrom ML. Activated human gamma delta T lymphocytes express functional lactoferrin receptors. Scand J Immunol. 1997;46:609–618. doi: 10.1046/j.1365-3083.1997.d01-165.x. [DOI] [PubMed] [Google Scholar]
- 16.Iyer S, Lonnerdal B. Lactoferrin, lactoferrin receptors and iron metabolism. Eur J Clin Nutr. 1993;47:232–241. [PubMed] [Google Scholar]
- 17.Arnold RR, Russell JE, Champion WJ, Gauthier JJ. Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of the target microorganism. Infect Immun. 1981;32:655–660. doi: 10.1128/iai.32.2.655-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velliyagounder K, Kaplan JB, Furgang D, Legarda D, Diamond G, Parkin RE, et al. One of two human lactoferrin variants exhibits increased antibacterial and transcriptional activation activities and is associated with localized juvenile periodontitis. Infect Immun. 2003;71:6141–6147. doi: 10.1128/IAI.71.11.6141-6147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velusamy SK, Ganeshnarayan K, Markowitz K, Schreiner H, Furgang D, Fine DH, et al. Lactoferrin knockout mice demonstrates greater susceptibility to Aggregatibacter actinomycetemcomitans-induced periodontal disease. J Periodontol. 2013;84:1690–1701. doi: 10.1902/jop.2013.120587. [DOI] [PubMed] [Google Scholar]
- 20.Ward PP, Conneely OM. Lactoferrin: role in iron homeostasis and host defense against microbial infection. Biometals. 2004;17:203–208. doi: 10.1023/b:biom.0000027693.60932.26. [DOI] [PubMed] [Google Scholar]
- 21.Stinson MW, Alder S, Kumar S. Invasion and killing of human endothelial cells by viridans group Streptococci. Infect Immun. 2003;71:2365–2372. doi: 10.1128/IAI.71.5.2365-2372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata Y, Ozaki K, Seki M, Kawato T, Tanaka H, Nakano Y, et al. Analysis of loci required for determination of serotype antigenicity in Streptococcus mutans and its clinical utilization. J Clin Microbiol. 2003;41:4107–4112. doi: 10.1128/JCM.41.9.4107-4112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima A, Nakano K, Wada K, Takahashi H, Katayama K, Yoneda M, et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep. 2012;2:1–12. doi: 10.1038/srep00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Chavez SA, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33:e301–308. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Lassiter MO, Newsome AL, Sams LD, Arnold RR. Characterization of lactoferrin interaction with Streptococcus mutans. J Dent Res. 1987;66:480–485. doi: 10.1177/00220345870660021601. [DOI] [PubMed] [Google Scholar]
- 26.Soukka T, Lumikari M, Tenovuo J. Combined bactericidal effect of human lactoferrin and lysozyme against Streptococcus mutans serotype c. Microb Ecol Health Dis. 1991;4:259–264. [Google Scholar]
- 27.Nomura R, Nakano K, Ooshima T. Contribution of glucan-binding protein C of Streptococcus mutans to bacteremia occurrence. Arch Oral Biol. 2004;49:783–788. doi: 10.1016/j.archoralbio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Nakano K, Ooshima T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 2009;4:891–902. doi: 10.2217/fmb.09.64. [DOI] [PubMed] [Google Scholar]
- 29.Kitten T, Munro CL, Zollar NQ, Lee SP, Patel RD. Oral streptococcal bacteremia in hospitalized patients: taxonomic identification and clinical characterization. Clin Microbiol. 2012;50:J 1039–1042. doi: 10.1128/JCM.06438-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas S, Bowler IC, Bunch C, Prendergast B, Webster DP. Streptococcus mutans infective endocarditis complicated by vertebral discitis following dental treatment without antibiotic prophylaxis. J Med Microbiol. 2010;59:1257–1259. doi: 10.1099/jmm.0.020974-0. [DOI] [PubMed] [Google Scholar]
- 31.Gauduchon V, Chalabreysse L, Etienne J, Celard M, Benito Y, Lepidi H, et al. Molecular diagnosis of infective endocarditis by PCR amplification and direct sequencing of DNA from valve tissue. J Clin Microbiol. 2003;41:763–766. doi: 10.1128/JCM.41.2.763-766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutteberg TJ, Rokke O, Andersen O, Jorgensen T. Early fall of circulating iron and rapid rise of lactoferrin in septicemia and endotoxemia: an early defence mechanism. Scand J Infect Dis. 1989;21:709–715. doi: 10.3109/00365548909021701. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berg S, Laman JD, Boon L, Ten Kate MT, De Knegt GJ, Verdijk RM, et al. Distinctive cytokines as biomarkers predicting fatal outcome of severe Staphylococcus aureus bacteremia in mice. PLoS One. 2013;8:e59107. doi: 10.1371/journal.pone.0059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsh KJ, Hwang SA, Boyd S, Kruzel ML, Hunter RL, Actor JK. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis (Edinb) 2011;91:S105–113. doi: 10.1016/j.tube.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zagulski T, Jarzabek Z, Zagulska A, Jaszczak M, Kochanowska IE, Zimecki M. Lactoferrin stimulates killing and clearance of bacteria but does not prevent mortality of diabetic mice. Arch Immunol Ther Exp (Warsz) 2001;49:431–438. [PubMed] [Google Scholar]
- 36.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74:183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 37.Kim JS, Kim KD, Na HS, Jeong SY, Park HR, Kim S, Chung J. Tumor necrosis factor-alpha and interleukin-1beta expression pathway induced by Streptococcus mutans in macrophage cell line RAW 264.7. Mol Oral Microbiol. 2012;27:149–159. doi: 10.1111/j.2041-1014.2012.00639.x. [DOI] [PubMed] [Google Scholar]
- 38.Kruzel ML, Zimecki M. Lactoferrin and immunologic dissonance: clinical implications. Arch Immunol Ther Exp (Warsz) 2002;50:399–410. [PubMed] [Google Scholar]
- 39.Hahn CL, Best AM, Tew JG. Cytokine induction by Streptococcus mutans and pulpal pathogenesis. Infect Immun. 2000;68:6785–6789. doi: 10.1128/iai.68.12.6785-6789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soell M, Holveck F, Scholler M, Wachsmann RD, Klein JP. Binding of Streptococcus mutans SR protein to human monocytes: production of tumor necrosis factor, interleukin 1, and interleukin 6. Infect Immun. 1994;62:1805–1812. doi: 10.1128/iai.62.5.1805-1812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y, Russell TR, Schilder H, Graves DT. Endodontic pathogens stimulate monocyte chemoattractant protein-1 and interleukin-8 in mononuclear cells. J Endod. 1998;24:86–90. doi: 10.1016/S0099-2399(98)80083-6. [DOI] [PubMed] [Google Scholar]
- 42.Takada H, Kawabata Y, Tamura M, Matsushita K, Igarashi H, Ohkuni H, et al. Cytokine induction by extracellular products of oral viridans group Streptococci. Infect Immun. 1993;61:5252–5260. doi: 10.1128/iai.61.12.5252-5260.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto A, McWhinney PH, Kibbler CC, Cohen J. Cytokine release and mitogenic activity in the viridans streptococcal shock syndrome. Cytokine. 1998;10:370–376. doi: 10.1006/cyto.1997.0300. [DOI] [PubMed] [Google Scholar]