Abstract
Background
The use of stable isotope tracer techniques to measure muscle protein fractional synthesis rate (FSR) has been well established and widely used. The most common method that has been utilized so far is a primed constant infusion (CI) method, which requires 3–4 h of tracer infusion. However, recently our group has developed a bolus injection (BI) method, which requires an injection of bolus of tracer and can be completed within 1 h. In this study, we compared calf (gastrocnemius) muscle protein FSR measured using these two different methods — CI and BI.
Method
FSRs were measured in eight people (5 men and 3 women; age: 62.3 ± 6.9 years (mean ± SD); body weight: 75.4 ± 21.5 kg) at basal, postabsorptive state using L-[ring-2H5]-phenylalanine. In the CI protocol, a primed continuous infusion was given for 4 h, and muscle biopsies were taken at 120 and 240 min; in the BI, a bolus injection of the tracer was given at 0 min and biopsies were taken at 5 and 60 min. Tracer enrichments in blood and muscle tissue were determined by gas chromatography–mass spectrometry. Data are expressed as mean ± SE; t-test, linear regression and Levene Median equal variance test analyses were performed.
Results
CI FSR was 0.066 ± 0.006%/h, whereas BI FSR was 0.058 ± 0.008%/h, p = NS. The linear regression analysis showed a significant relationship between BI and CI, p = 0.038. The intra-class correlation coefficient was 0.83. The standard deviation of the differences in the measurements was 0.015%/h. The Levene Median equal variance test demonstrated no difference in variance between the CI and BI measurements (p = 0.722).
Conclusion
No difference could be detected in calf muscle protein FSR measured by CI and BI methods; the BI method can be used for the measurement of muscle protein FSR in humans.
Keywords: Stable isotope tracer techniques, Constant infusion method, Bolus injection method, Muscle, Protein, Fractional synthesis rate
1. Introduction
In vivo measurement of muscle protein synthesis is an essential element in metabolic studies evaluating lean body mass metabolism in health and pathology. Primed constant infusion (CI) and flooding dose (FD) methods have been introduced to measure muscle protein fractional synthesis rate (FSR) [1]. The CI method is well established and a proven approach in this area. However, it requires isotopic steady-state conditions, which take at least an hour to achieve depending on the tracer, and then at least another two or three hours to achieve adequate change in protein-bound enrichment over time for accurate measurements. Therefore, if the goal of the experiment is to determine the basal postabsorptive muscle protein FSR, the length of the infusion protocol may take up to four hours [1]. The FD method eliminates this issue; however, the total amount of amino acid given with the flooding dose exceeds the endogenous free amino acid level several-fold [2]. This flooding dose can stimulate protein synthesis by itself when an essential amino acid is used [3–5]. The usage of a non-essential amino acid has not shown any effect on FSR; however, intracellular physiological production of non-essential amino acid (from breakdown or de novo synthesis) will dilute the pool [4,5].
Recently, a new bolus injection (BI) method was introduced that uses a much smaller dose than the flooding dose method [6]. FSR calculation by the BI method is based on the precursor–product principle similar to both CI and FD approaches, using muscle intracellular free and bound tracer enrichments as precursor and product, respectively. The design of the BI method includes a bolus injection of a tracer with subsequent blood draws and muscle biopsies at five and sixty minutes. Zhang et al. [6] showed that this method does not affect muscle protein kinetics in rabbits, is suitable to be used with essential amino acid tracer (e.g., 2H5-phenylalanine), gives reliable results and can be completed within one hour. In humans, to our best knowledge only one study using the BI method has been reported [7], and it has not been validated in comparison with the CI method in humans. Therefore, the purpose of this study was to use the BI method to measure in vivo muscle protein FSR in humans and compare the results with FSR measured by the CI method.
2. Materials and methods
2.1. Subjects
Eight elderly adults (5 men and 3 women; age: 62.3 ± 6.9 years old (mean ± SD); body weight: 75.4 ± 21.5 kg) participated in the study. Two subjects were healthy elderly; the other six had been diagnosed with peripheral arterial disease (PAD). All subjects underwent measurements of their calf muscle protein FSR by the CI and BI methods at two different occasions. This study is a part of a larger clinical study on muscle protein metabolism in patients with PAD and a part of the study has been already reported [8]. However, none of the subjects reported here underwent any interventions between the study trials. Written informed consent was obtained from all subjects before the participation in the study. The protocol was approved by the Institutional Review Board (IRB) and the General Advisory Committee of the General Clinical Research Center (GCRC) at the University of Texas Medical Branch (UTMB).
2.2. Tracer infusion/injection protocol
Subjects were admitted to the GCRC at UTMB the day before the study. After an overnight fast, a polyethylene catheter was inserted into a forearm vein for infusion or injection of the tracer L-[ring2H5]-phenylalanine (Cambridge Isotope Laboratories, Andover, MA, USA). Another catheter was inserted in retrograde fashion into the hand vein of the other arm for arterialized blood sampling, as described previously [8,9]. Blood samples were obtained for measurement of blood tracer enrichment and pO2.
2.3. Constant infusion protocol
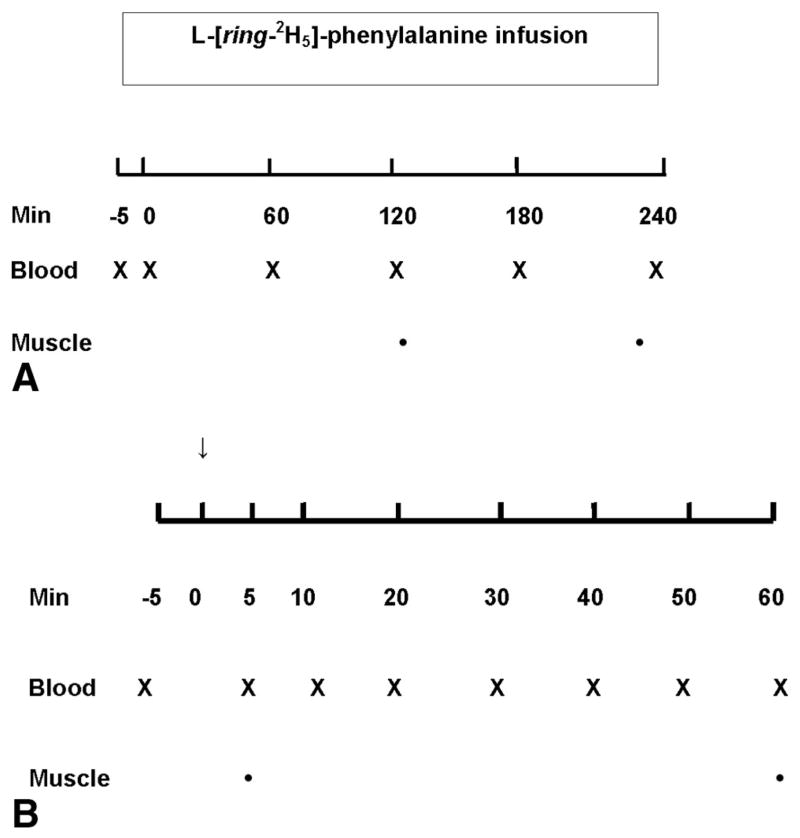
A background blood sample was obtained before the start of the tracer infusion, after which a primed (2 μmol/kg) constant infusion (0.05 μmol/kg/min) of labeled phenylalanine was started and maintained throughout the study [8]. The study design is illustrated in Fig. 1A. Arterialized blood samples from the retrograde dorsal vein catheter were collected hourly. Muscle biopsies were obtained from gastrocnemius muscle of one leg of each participant under sterile conditions and local anesthesia using a 5-mm Bergstrom biopsy needle, as previously described [8]. Biopsies were performed at 120 and 240 min after start of the tracer infusion (Fig. 1A).
Fig. 1.
Experimental protocol. (A) Constant infusion protocol; (B) Bolus injection protocol. Min, minutes of study; Blood, blood sampling from arterialized dorsal hand vein; Muscle, gastrocnemius muscle biopsy. ↓, Bolus injection of stable isotope.
2.4. Bolus injection protocol
After the background blood draw a bolus injection of 15 μmol/kg of labeled phenylalanine was given at time 0 min [6]. The study design is illustrated in Fig. 1B. Blood samples were drawn at 5, 10, 20, 30, 40, 50 and 60 min after the tracer injection and muscle biopsies were taken at 5 and 60 min from gastrocnemius muscle in the same leg as for the CI study.
2.5. Analytical methods
2.5.1. Blood
pO2 was measured by a Rapidpoint 405 System (Bayer HealthCare, Clayton, NC) in the Department of Pathology, UTMB. Blood for amino acid (AA) enrichment was immediately deproteinized by mixing with 15 % sulfosalicylic acid solution. Samples were centrifuged, and the subsequent supernatant was removed and frozen at −20 °C. Upon thawing, AAs were extracted with cation-exchange chromatography, and enrichment was determined after derivatization to tert-butyldimethylsilyl by gas chromatography–mass spectrometry (GC-MS HP 5989; Hewlett-Packard, Palo Alto, CA) with electron impact ionization. Ions 234 and 239 were monitored [1,10].
2.5.2. Muscle
20–25 mg of muscle was homogenized twice in 800 μL of 10% perchloric acid. The free intracellular enrichment of phenylalanine was measured on the supernatant obtained after tissue homogenization and centrifugation. The bound enrichment was measured from the pellet obtained after centrifugation. The pellet was washed and dried, and the proteins were hydrolyzed in 6 N HCl at 110 °C for 24 h. The hydrolysate was processed in the same fashion as the blood samples, and the phenylalanine enrichment was measured by GC-MS [1].
2.6. Calculations
For both methods FSR was calculated by the following equation [1]:
where, t2 and t1 are the times of muscle biopsies, EM(t2) and EM(t1) are the muscle protein enrichments at t2 and t1, AUC is area under curve of intracellular free amino acid enrichment; T is time between t2 and t1. AUC was calculated by the following equations [1]:
where, EF(t1) and EF(t2) are muscle intracellular free enrichments at t1 and t2 expressed as tracer to tracee ratio; and ln is the natural log. The factor of (1 + AUC/(t2 − t1)) in the above formula accounts for the fact that the amount of tracer in the precursor pool is relatively high during the measurement period. Without this factor, fractional synthesis rate of tracee is computed by the above formula. However, if total synthesis remains constant then tracee incorporation should decrease to the extent that tracer is incorporated into bound muscle protein. Therefore to calculate the total synthesis rate, the tracee incorporation rate needs to be multiplied by the average muscle intracellular free (tracer + tracee) to tracee ratio. (AUC/(t2 − t1)) gives average muscle intracellular free tracer to tracee ratio, so (1 + AUC/(t2 − t1)) gives average muscle intracellular free (tracer + tracee) to tracee ratio, which is the desired correction factor. The calculation of AUC for the bolus injection assumes that the decay of enrichment is exponential, which is reasonable [11].
2.6.1. Statistical analysis
Values are presented as mean ± SE unless otherwise noted. Differences in demographic parameters and FSR between the studies were evaluated by paired t-test. Relationships in FSR between BI and CI were evaluated by linear regression analyses, calculation of the intra-class correlation coefficient, and presentation of the standard deviation of the differences. The Levene Median equal variance test was performed to test the difference in variance between the measurements. A p-value ≤ 0.05 was considered statistically significant.
3. Results
Individual characteristics of the subjects and results are presented in Table 1. There were no differences in mean age and weight between two studies (CI vs BI (mean ± SD); age: 62.0 ± 7.3 vs 62.6 ± 6.9 years; weight: 75.7 ± 21.3 vs 75.0 ± 21.6 kg, p = 0.26). However, in the subgroup of PAD patients, body weight was significantly lower during BI study (CI vs BI (mean ± SD): 69.9 ± 13.3 vs 68.6 ± 13.6 kg, p = 0.03). However, there was no statistically significant difference in FSR values between the two studies for this group (p = 0.17). There was no correlation between changes in body weight and FSR (r = 0.03).
Table 1.
Demographics and individual values.
| Subject | Age, y a | Gender | Weight, kg a | FSR (%/h)
|
Interval between studies | |
|---|---|---|---|---|---|---|
| CI | BI | |||||
| 8 | 54 (56) | F | 51 (49) | 0.059 | 0.054 | 25 months |
| 15 | 60 (61) | M | 81.6 (79.3) | 0.079 | 0.056 | 18 months |
| 21 b | 67 (68) | M | 66.2 (68.6) | 0.090 | 0.061 | 15 months |
| 30 | 57 (58) | F | 86.2 (84.9) | 0.066 | 0.078 | 6 months |
| 32 | 76 (76) | F | 58.7 (56.5) | 0.044 | 0.029 | 5 months |
| 35 b | 56 (56) | M | 120.4 (120.4) | 0.081 | 0.096 | 2 months |
| 40 | 66 (66) | M | 70.7 (70.7) | 0.071 | 0.063 | 1 week |
| 42 | 60 (60) | M | 71 (71) | 0.040 | 0.029 | 1 week |
| Average | 62 ± 7.3 (62.6 ± 6.9) | 3/5 | 75.7 ± 21.3 (75 ± 21.6) | 0.066 ± 0.006 | 0.058 ± 0.008 | 8.9 ± 9.2 months |
Demographic averages are given in mean ± SD.
Age and weight are given for constant infusion (CI); in parenthesis are the values for the bolus injection (BI) study.
Healthy subjects.
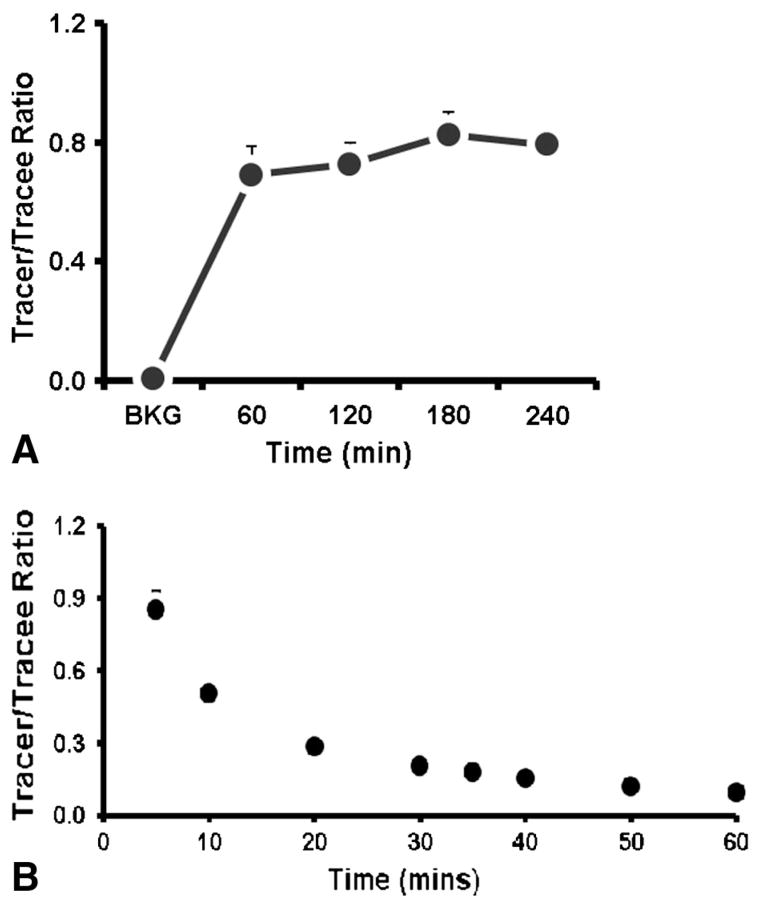
In the CI group, the enrichment of L-[2H5]-Phenylalanine in the arterialized blood had reached a steady state after 60 min of primed-constant infusion (Fig. 2A) and remained constant until the end of the study. The plasma enrichment decay curve for the BI study is presented in Fig. 2B.
Fig. 2.
Plasma tracer enrichment. (A) Constant infusion enrichment; (B) Bolus injection decay curve.
Muscle intracellular free phenylalanine enrichment increased by 0.011 ± 0.002 tracer/tracee ratio (t/T) in the CI protocol, whereas it decreased in the BI experiments by 0.086 ± 0.02 t/T ratio, p = 0.004. Muscle bound protein enrichment increased by 0.0056 ± 0.004 t/T ratio in CI versus an increase of 0.007 ± 0.003 t/T ratio in BI, p = 0.039.
The individual and mean values of muscle protein FSR, calculated from the CI and BI methods, are shown in Table 1 and Fig. 3, and there was no significant difference in mean FSR between the methods (p = 0.18). The linear regression analysis showed a significant relationship between CI and BI, p = 0.038 (Fig. 4). The intercept was 0.0037 with 95% confidence interval of −0.0627 (lower 95%) and 0.0553 (higher 95%), which includes zero point. The slope was 0.935 with 95% confidence interval of 0.069 (lower 95%) and 1.800 (higher 95%), which includes 1. The intra-class correlation coefficient was 0.83. The standard deviation of the differences in the measurements was 0.015%/h. The Levene Median equal variance test demonstrated no difference in variance between the CI and BI measurements (p = 0.722).
Fig. 3.
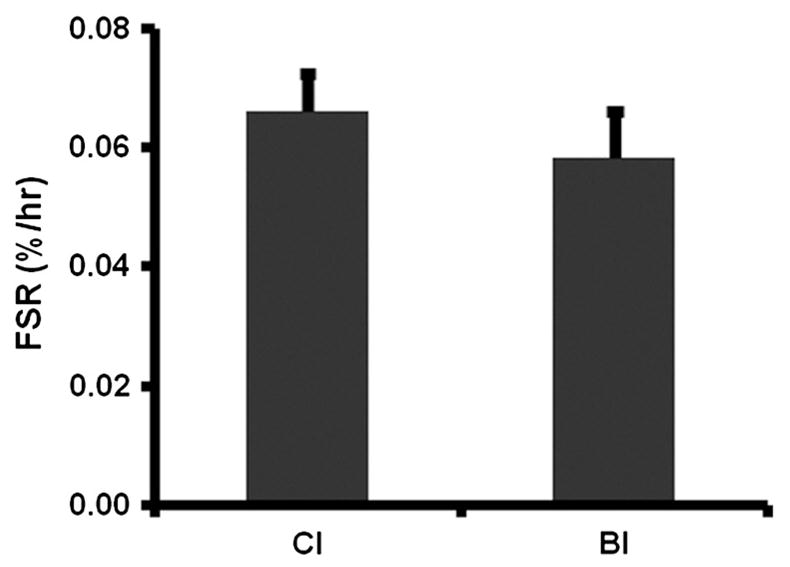
Calf muscle (gastrocnemius) protein fractional synthesis rate (FSR) measured by Constant Infusion (CI) and Bolus Injection (BI) methods. Means ± SE.
Fig. 4.
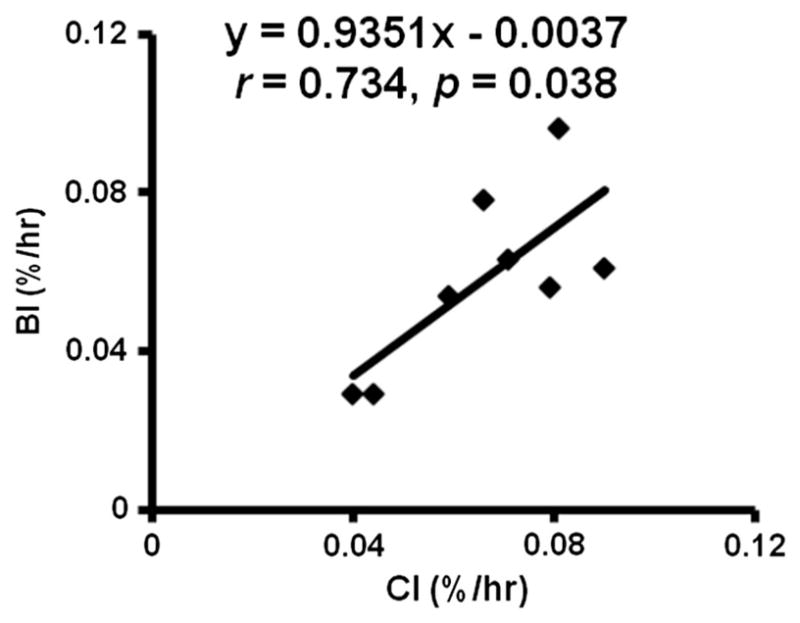
Linear regression analysis of Fractional synthesis rates measured by Constant Infusion (CI) and Bolus Injection (BI) methods.
4. Discussion
The purpose of this study was to compare the constant infusion and bolus injection methods to measure human muscle protein FSR. The results demonstrated that in our study patients, the measurement of FSR was not significantly different using the two methods. An acceptable intra-class correlation coefficient and standard deviation of the differences were achieved. These findings indicate that the bolus tracer injection method can be used to measure human muscle protein synthesis rate in vivo, and that it gives comparable results to the constant infusion method.
The flooding dose technique [12] became popular because it enabled determination of muscle FSR in a short period of time and it eliminated uncertainty about the precursor enrichment. However, the method became controversial because it was shown that certain amino acids (mostly the essential amino acids) stimulate muscle FSR when given as a flooding dose [4,5]. However, a typical flooding dose is on the order of 50 mg/kg [12], which is much larger than the dose of 2.5 mg/kg (or 15 μmol/kg) of phenylalanine given in this study. A flooding dose produces comparable intracellular and plasma enrichments [12]. In our study intracellular enrichment was markedly lower than the plasma enrichment at 5 min after the bolus of tracer was given (0.24 ± 0.08 vs 0.85 ± 0.21 t/T, mean ± SD), which suggests that this dosage is below that necessary to affect the measured FSR. The lack of an effect of the bolus of tracer on the FSR is supported by our results when the two methods (BIvsCI) were compared. The FSR computed with the bolus dose was similar to the value obtained at a different time with the constant infusion method (Table 1); in fact the mean with the BI method was actually lower than the CI value, although not statistically lower (Fig. 3).
Although the FSR results (Fig. 3) are within the reported ranges [13–15] there is a three-fold range in FSR values between subjects measured by either method (Table 1). In six out of eight study subjects the FSR results were lower when measured by BI method. One explanation for these variations is that the studies were not conducted within a limited period of time (one or two months) but rather over an average of 8 months (Table 1). However, trends in ranges of FSR values were consistent between two methods within the subjects (Table 1), which suggest that the discrepancy is due to individual pathophysiological specificities of the study participants. Since we did not conduct body composition or muscle strength measurements during these studies we can only speculate regarding this point. However, the fact that in the subgroup of PAD patients, body weight was significantly lower during BI study (p = 0.03) supports our theory.
In the CI method, muscle intracellular free enrichment tended to increase with time (0.035 ± 0.015 vs 0.045 ± 0.015 t/T, 120 vs 240 min, mean ± SD) probably at least in part because of a small increase in plasma phenylalanine enrichment over that time (0.73 ± 0.14 vs 0.79 ± 0.08 t/T, 120 vs 240 min, mean ± SD). However, the CI method is still valid under these conditions because steady state precursor enrichment (intracellular free enrichment) is not required for the calculations, provided some account is taken of the change in precursor enrichment over time.
In the BI method, there was a larger change in protein bound enrichment as compared to the CI method. This is expected, since the increase in the precursor enrichment with the BI method is higher than the CI method to offset the shorter time for tracer incorporation. A greater amount of tracer incorporation should yield more reliable measurement of enrichment, although the current study was not designed to specifically test this point.
One limitation of this study is the relatively large time period between some of the paired studies, with the longest up to 25 months (Table 1); this may explain the difference in the mean age and weight. However, we believe that this did not affect the study conclusions. Although, the results of the later study (BI) are lower than the earlier study (CI), the fact that the difference between the studies did not reach statistical significance suggests that the factor “time” did not affect current results.
Villareal et al., [16] reported muscle protein FSR measured using CI method in individuals, who underwent weight loss intervention (6 months) and maintained their weight for another 6 months. Thus there was 12-month gap between the measurements of FSR in these individuals. From their data it seems that there may have been in average about 10 % difference between the measurements. Smith et al. [17] conducted a comprehensive review of published literature and concluded that variability in FSR is partly due to a considerable within-subject variability. These data suggest that even with CI method some sort of variability may exist.
Our results are comparable to previously published FSR values [13–15]. Most measurements of muscle FSR have been conducted in vastus lateralis, whereas our current results are from gastrocnemius muscle. However, it has been reported that, at least in humans, neither anatomical location nor fiber-type composition influences muscle protein FSR [15]. Or this may in part simply reflect the lack of precision of the method.
We have conducted several statistical analyses comparing these 2 methods. Firstly, the linear regression analysis demonstrated a significant association between the methods with slope and intercept ranges (95 percentile) including 1 and zero, respectively. This demonstrates that even with intra-subject variability the BI method does not over/underestimate the “true” value. Furthermore, the equal variance test demonstrated that the difference in variance was far from being statistically significant (p = 0.722) suggesting that we will need a large cohort to demonstrate the difference between the 2 methods.
There are three major differences between the BI and CI methods. Muscle protein FSR can be measured within one hour using the BI technique because enrichment in the protein-bound pool increases rapidly. In contrast, the CI method requires a minimum of about 3–4 h of tracer incorporation to achieve sufficient enrichment in protein-bound enrichment to reliably measure. The shorter time requirement for the BI method may be an advantage in some circumstances, such as when a value at a particular time is desired. On the other hand, the CI technique allows the determination of an FSR value that integrates over a long period of time, such as 24 h or longer, which is not feasible with the BI. Logistically, the BI method is simpler than the CI method because a tracer infusion is not necessary. This could potentially facilitate field studies in which a constant tracer infusion is not feasible. A third difference between the two methods is that with the BI method protein fractional breakdown rate (FBR) can be measured simultaneously with the measurement of FSR [11]. Simultaneous measurement of the FSR and FBR enables the important calculation of net protein balance. The current methods for measuring FBR rely on the rate of decay of the intracellular enrichment, either after a bolus injection or cessation of a constant infusion [1]. This approach can only be performed over about an hour or less because of the rapid half life of the intracellular free amino acid pool [6,11]. As a result of this fast turnover, the tracer enrichment in the intracellular pool will have essentially disappeared three hours after giving a labeled bolus or stopping a continuos infusion, so that the decay cannot be accurately quantified over this long of a time period. Consequently, with the CI method measurement of FBR cannot be done in a concordant time frame as FSR, even if a different labeled tracer amino acid is used at the same time at which the FSR is measured. Furthermore, measurement of FBR using a constant tracer infusion requires that a steady state in enrichment be achieved, and then the tracer infusion stopped and the FBR determined from the rate of decay of tracer enrichment [1]. This protocol makes the CI logistically more challenging than the bolus technique. Further, it is harder to measure the decay enrichment accurately after a constant infusion is stopped because the enrichment is much higher in the first hour after a bolus injection of tracer.
In conclusion, the bolus injection method gives comparable results to the constant infusion method, and has potential advantages, depending on the specific experimental protocol.
Acknowledgments
We wish to thank the study volunteers for their patience and dedication. We thank Sue Minello R.N., Roxana Hirst M.S., and Nancy Poore at the Pepper Center for help in recruiting the volunteers, and the nurses and the personnel of the General Clinical Research Center (GCRC), University of Texas Medical Branch for their help with conducting the clinical portion of this study. We thank Guarang Jariwala and Guy Jones for performing GCMS analysis.
This study was supported by grants 5K08-AG020115 and P30AG028718 from the National Institute of Aging/NIH and M01-RR-00073 from the National Center for Research Resources/NIH to GCRC, and Shriners Grant8490 to Metabolism Unit, Shriners Hospitals for Children.
Abbreviations
- BI
bolus method
- CI
constant infusion method
- FD
flooding dose
- FBR
fractional breakdown rate
- FSR
fractional synthesis rate
- GC-MS
gas chromatography–mass spectrometry
- PAD
peripheral arterial disease
Footnotes
Contribution of authors
- Study conduction: DT, JB, MSM, LAK.
- Sample analyses: DT.
- Data analyses: DT, DLC, XJZ, LAK, MSM, RRW.
- Manuscript preparation, edition, approval: DT, DLC, JB, XJZ, MSM, LAK, RRW.
- This manuscript has no potential conflict of interest for all authors.
References
- 1.Wolfe RR, Chinkes DL. Isotopic tracers in metabolic research: principles and practice of kinetic analysis. New Jersey: Wiley-Liss; 2005. [Google Scholar]
- 2.Garlick PJ, McNurlan MA. Measurement of protein synthesis in human tissues by the flooding method. Curr Opin Clin Nutr Metab Care. 1998;1(5):455–60. doi: 10.1097/00075197-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Chinkes DL, Rosenblatt J, Wolfe RR. Assessment of the mathematical issues involved in measuring the fractional synthesis rate of protein using the flooding dose technique. Clin Sci. 1993;84:177–83. doi: 10.1042/cs0840177. [DOI] [PubMed] [Google Scholar]
- 4.Jahoor F, Zhang XJ, Baba H, Sakurai Y, Wolfe RR. Comparison of constant infusion and flooding dose techniques to measure muscle protein synthesis rate in dogs. J Nutr. 1992;122:878–87. doi: 10.1093/jn/122.4.878. [DOI] [PubMed] [Google Scholar]
- 5.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–8. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol. 2002;283:E753–64. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]
- 7.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–8. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]
- 8.Killewich LA, Tuvdendorj D, Bahadorani J, Hunter GC, Wolfe RR. Amino acids stimulate leg muscle protein synthesis in peripheral arterial disease. J Vasc Surg. 2007;45:554–60. doi: 10.1016/j.jvs.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30:936–40. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt J, Chinkes DL, Wolfe MH, Wolfe RR. Stable isotope tracer analysis by GC-MS, including quantification of isotopomer effects. Am J Physiol. 1992;263:E584–96. doi: 10.1152/ajpendo.1992.263.3.E584. [DOI] [PubMed] [Google Scholar]
- 11.Chinkes DL. Methods for measuring tissue protein breakdown rate in vivo. Curr Opin Clin Nutr Metab Care. 2005;8:534–7. doi: 10.1097/01.mco.0000170754.25372.37. [DOI] [PubMed] [Google Scholar]
- 12.Garlick PJ, Wernerman J, McNurlan MA, Essen P, Lobley GE, Milne E, et al. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a “flooding dose” of [1-13C]leucine. Clin Sci (Lond) 1989;77(3):329–36. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- 13.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol. 1997;82(3):807–10. doi: 10.1152/jappl.1997.82.3.807. [DOI] [PubMed] [Google Scholar]
- 15.Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, et al. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563(1):203–11. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villareal DT, Smith GI, Shah K, Mittendorfer B. Effect of weight loss on the rate of muscle protein synthesis during fasted and fed conditions in obese older adults. Obesity. 2012;20(9):1780–6. doi: 10.1038/oby.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover—why is it so variable? J Appl Physiol. 2011;110(2):480–91. doi: 10.1152/japplphysiol.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]