Abstract
To identify the most promising vaccine candidates for combinatorial strategies, we compared five SIV vaccine platforms including recombinant canary pox virus ALVAC, replication-competent adenovirus type 5 host range mutant RepAd, DNA, modified vaccinia Ankara (MVA), peptides and protein in distinct combinations. Three regimens used viral vectors (prime or boost) and two regimens used plasmid DNA. Analysis at necropsy showed that the DNA-based vaccine regimens elicited significantly higher cellular responses against Gag and Env than any of the other vaccine platforms. The T cell responses induced by most vaccine regimens disseminated systemically into secondary lymphoid tissues (lymph nodes, spleen) and effector anatomical sites (including liver, vaginal tissue), indicative of their role in viral containment at the portal of entry. The cellular and reported humoral immune response data suggest that combination of DNA and viral vectors elicits a balanced immunity with strong and durable responses able to disseminate into relevant mucosal sites.
Keywords: cellular immune response, prime-boost vaccination, DNA, protein, pox virus ALVAC MVA
1. Introduction
To date, four clinical efficacy trials against HIV have been conducted including: (i) gp120 protein vaccine (VaxGen) [1–4]; (ii) recombinant Ad5 (STEP) [5–7]; (iii) DNA prime-recombinant Ad5 boost (HVTN 505) [8]; (iv) combination of recombinant Canarypox ALVAC®-HIV (vCP1521; containing Gag, PR and Env) with gp120 Env protein (AIDSVAX® B/E) (referred to as RV144, conducted in Thailand) [9]. Only the RV144 trial showed modest statistically significant protection from infection [9]. This trial revealed a critical role of humoral responses in preventing infection [10–14]. The humoral immune response waned rapidly after vaccination, indicating the need for vaccine regimens provided longer-lasting immunity. In addition, no difference in the levels of viremia were found between infected vaccinees and unvaccinated controls, indicating suboptimal cellular immune responses induced by this vaccine protocol. Thus, there is a need to develop a vaccine regimen against HIV that is able to provide effective humoral responses to prevent virus acquisition as well as potent cytotoxic effector memory T cell responses able to contain infection. Importantly, it is critical that humoral and cellular responses disseminate efficiently to mucosal sites (rectum, vagina), since these are portals of entry for HIV infection.
The five sections of the National Cancer Institute’s Vaccine Branch have been studying distinct vaccine regimens, which have shown some degree of protection from virus acquisition and/or significant control of peak and/or chronic viremia such as: (i) ALVAC/Env vaccine using a recombinant canary pox virus (ALVAC) vector in combination with an Env protein boost delivered via the intramuscular route (IM) [15–19] (Vaccari M. et. al., manuscript in preparation); (ii) RepAd/Env vaccine consisting of mucosal priming by replication-competent adenovirus type 5 host range mutant recombinants (RepAd) followed by an IM-delivered Env protein boost [20–25]; (iii) DNA vaccine delivered via the IM route followed by electroporation (EP) [26–33]; (iv) DNA&Env vaccine consisting of DNA and Env protein co-immunization delivered as in (iii) [31, 32]; (v) IL-15-adjuvanted viral-specific peptides given together with a TLR agonist delivered intrarectally in combination with recombinant modified vaccinia Ankara (MVA) vectors and Env protein [34–37]. In a comparative study, we tested these five vaccine regimens side-by-side in rhesus macaques and we have recently reported on our comparison and characterization of the humoral responses induced by these platforms [38]. We found that the ALVAC/Env, RepAd/Env and DNA&Env regimens induced robust systemic binding antibodies with neutralizing activity and able to mediate antibody dependent cellular cytotoxicity (ADCC) and opsonization. Mucosal IgA and IgG responses were readily detected in animals vaccinated with ALVAC/Env, RepAd/Env, DNA&Env and DNA at necropsy, but the RepAd/Env regimen induced the earliest mucosal SIV-specific IgA responses.
Several lines of evidence support the importance of cellular responses for the control of viral propagation in HIV-infected individuals. Some studies reported an association between CTL responses against HIV proteins and control of viremia [39–46]; other studies demonstrated that high avidity CTLs targeting strictly conserved viral regions are preferentially found in HIV-infected controllers and long-term non-progressors [47, 48]. Similarly, a correlation between vaccine-induced cellular responses and improved control of viremia has also been described using the SIV/rhesus macaque model [22, 27, 32, 49–64]. Among the vaccine platforms studied in our branch, a correlation between vaccine-induced cell mediated responses and reduction of viremia was found in DNA immunized animals challenged with SIVmac251 [27, 62], in DNA and DNA&Env immunized macaques challenged with SIVsmE660 [32], in DNA-ALVAC immunized animals challenged with SIVmac251 [19], in RepAd/Env vaccinated animals challenged with SIVmac251 [22, 63, 64] and upon intrarectal peptide and MVA vaccine vaccination challenged with SIVmac251 or SHIVKu2 [34–37]. The referred vaccination regimens also induced humoral responses against Env, therefore it was unclear whether vaccine-induced T cell responses only, in the absence of humoral responses, were sufficient to mediate control of viremia. However, several studies in macaques unequivocally demonstrated the efficacy of T cell responses in controlling highly pathogenic SIVmac: (i) animals vaccinated with recombinant CMV expressing SIV antigens controlled viremia to undetectable level in the presence of vaccine-induced CTL responses and absolute absence of anti-SIV humoral responses [65–67]; (ii) macaques vaccinated with immunogens lacking an Env component were able to significantly control viremia [68–72]. In the present study, we report a comparison of systemic cellular immune responses induced by the different vaccine platforms being explored in our Vaccine Branch, which may provide suggestions for combinations that further optimize vaccine regimens.
2. Methods
2.1. Vaccination regimens
The Indian rhesus macaques included in the study were housed and maintained at Advanced BioScience Laboratories, Inc. (ABL, Rockville, MD) following the standards of the American Association for Accreditation of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals of the NIH. The ABL Animal Care and Use Committee approved the protocols prior to implementation. All macaques enrolled in the study (N=22) were positive for the MamuA*01 MHC class I allele. The animals were negative for infection by SIV, simian T-cell leukemia virus-type 1 and simian type D retrovirus.
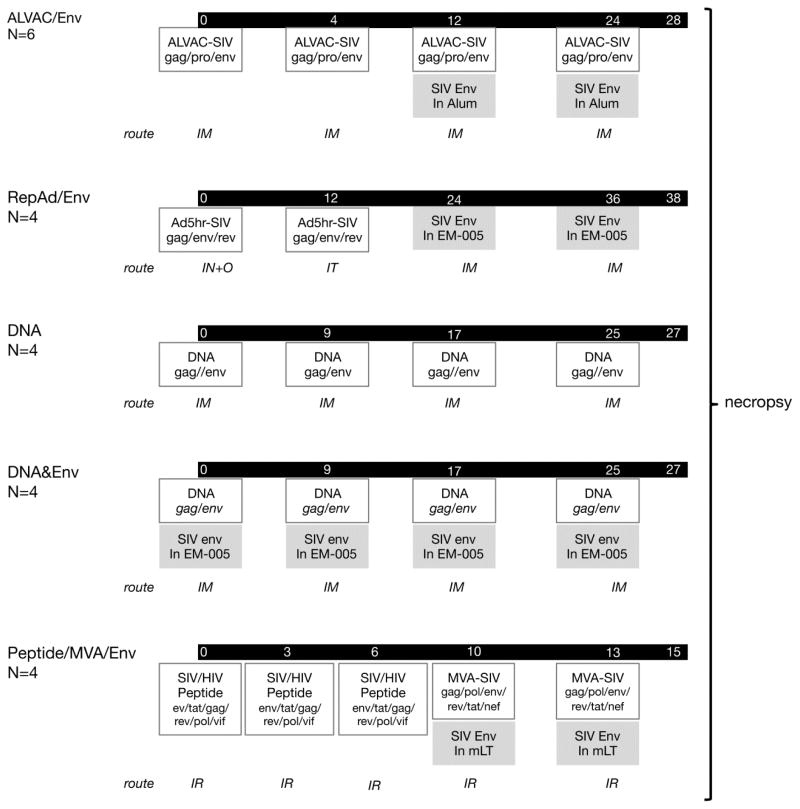
The animals were immunized with five different vaccine regimens (ALVAC/Env, RepAd/Env, DNA&Env, DNA, and Peptide/MVA/Env) as previously described [38]; the details for each protocol are summarized in Figure 1. Briefly, the ALVAC/Env protocol (N=6) consisted of 4 vaccinations (weeks 0, 4, 12, 24) with SIV gag/pro/env ALVAC vector (1×108 pfu VCP2432) via the intramuscular (IM) route including 2 vaccinations (weeks 12, 24) with 400 μg of SIV gp120 protein adjuvanted in Alum (200 μg of SIVM766.4 gp120 and 200 μg of SIVCG7V gp120).
Figure 1.
Schematic representation of the vaccination regimens used in the study, an adaptation from Vargas-Inchaustegui et al [38]. The five immunization regimens are detailed and the times of vaccination are given in weeks. The number of animals per group and the route of vaccine administration (IM: intramuscular; IN+O: intranasal and orally; IT: intratracheal; IR: intrarectal) are shown to the left. The key vaccine components are shown in boxes, and the proteins and adjuvants are highlighted in grey.
The RepAd/Env protocol (N=4) consisted of Ad5hr-SIVsmH4env/rev and Ad5hr-SIV239gag (5 × 108 pfu) delivered intranasally (IN) and orally (O) at week 0, and intratracheally (IT) at week 12, followed by two protein boosts (100 μg of M766 gp120 adjuvanted in 10 μg of EM-005; Infectious Disease Research Institute, Seattle, WA) at weeks 24 and 36.
The DNA (N=4) and DNA&Env (N=4) protocols consisted of the same plasmid DNA mixture (3 mg of Env DNA, 1 mg of Gag DNA and 0.2 mg of macaque IL-12 DNA) administered 4 times (weeks 0, 9, 17 and 25) via the IM route followed by in vivo electroporation (IM/EP; Inovio Pharmaceuticals, Inc., Blue Bell, PA). The DNA&Env co-immunization regimen included administration of 100 μg Env protein (M766-like gp140 and CG7V gp140; adjuvanted in 10 μg EM-005) delivered by IM route into the same muscle following the DNA electroporation.
The Peptide/MVA/Env protocol (N=4) consisted of a mixture of SIV/HIV peptides (13 peptides including epitopes of HIV Env and Tat, and SIV Gag, Pol, Rev, Tat, and Vif at 0.5 mg/peptide) delivered intrarectally (IR) at weeks 0, 3 and 6 together with a cocktail containing IL-15 (300 μg), the TLR agonists MALP2 (10 μg), polyI:C (1 mg) and CpG (500 μg) per dose as adjuvant. The boost consisted of recombinant MVA vectors (dose of 5 × 108 pfu MVA-SIVmac239 env, gag, and pol, and MVA-SIVmac239 tat, nef, and rev) together with the above described adjuvant cocktail, and 100 μg M766 gp120 adjuvanted with mutant E. coli labile toxin R192G (mLT, 50 μg/dose, a kind gift of J. Clements, Tulane University, New Orleans, LA) administered IR at weeks 10 and 13. This vaccine was designed to elicit mostly colorectal mucosal immunity.
The proteins used in these vaccine regimens included HEK293 cell produced M766 gp120 (RepAd/Env; Peptide/MVA/Env) and the trimeric gp140 proteins (DNA&Env) purified from cells grown in serum-free media in a Hollow Fiber bioreactor; CHO cell produced gD-tagged M766 and CG7V proteins (ALVAC/Env).
2.2. Sample collection and tissue processing
Tissues collected at necropsy (axillary and inguinal lymph nodes, spleen, liver and vagina and rectum) were placed in RPMI 1640 medium and kept on ice until processing. PBMC were isolated from blood samples drawn in EDTA-tubes by Ficoll-Histopaque (Histopaque, Sigma, St. Louis, MO) gradient centrifugation. For spleen and lymph nodes lymphocyte purification, the tissues were gently squeezed through a 100-μm cell strainer (Thomas Scientific) and washed in PBS supplemented with 0.2% heat-inactivated human AB+ serum. The cells were resuspended in RPMI 1640 containing 10% FCS and counted using Acridine Orange (Molecular Probes) and ethidium bromide (Fisher Scientific) dye to assess cell viability. To isolate lymphocytes from liver and vaginal biopsies, the tissues were minced and incubated in RPMI 1640 with 200 U/ml collagenase (Sigma-Aldrich) and 30 U/ml DNase (Roche) for 1.5 h at 37°C under continuous shaking. Clumps and tissue debris were removed by centrifugation at 800 rpm for 1 minute and the fluids containing single cells were collected, transferred into a new tube and washed with PBS supplemented with 0.2% human serum.
2.3. Antigen-specific cell mediated responses
Analysis of vaccine-induced cellular responses upon peptide stimulation was performed in cryopreserved PBMC. After thawing, macaque PBMCs were cultured in RPMI medium supplemented with 10% fetal bovine serum at a concentration of 2×106 cells/ml. PBMCs were stimulated overnight with peptide pools (final concentration of 1 μg/ml for each peptide) in the presence of monensin (BD Pharmingen, San Diego, CA). The peptide pools consisted of 15-mers overlapping by 11 AA covering p39gag and gp160 Env of SIVmac239. Antigen-specific T cells were monitored by a protocol that combines cell surface phenotyping and intracellular cytokine staining followed by flow cytometry. The cells were stained with the following cocktail of cell surface antibodies: CD3-APCCy7 (clone SP34-2), CD4-V500 (clone L200), CD95-FITC (clone DX2) (BD Pharmingen), CD8-Alexa Fluor-405 (clone 3B5, Invitrogen, Carlsbad, CA), and CD28-PerCP Cy5.5 (clone CD28.2, BioLegend, San Diego, CA). After cell permeabilization with Cytofix/Cytoperm (BD Biosciences), intracellular staining was performed using IFN-γ-PE Cy7 (clone B27, BD Pharmingen), TNF-α-AF700 (clone Mab11, BD Pharmingen) and Granzyme B-PE antibodies (clone GB12, Invitrogen). PBMC cultured in medium without peptide pools or stimulated with phorbol myristate acetate (PMA) and calcium ionophore (Sigma, St. Louis, MO) were used as negative and positive control, respectively. At least 105 T cells from each sample were acquired on an LSR II flow cytometer (BD Biosciences, San Jose, CA) and the data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). Samples were considered positive if the frequency of the cytokine positive T cells in the peptide stimulated samples was more than 2-fold higher than the frequency obtained in the unstimulated medium only control sample. Statistical analysis was performed using ANOVA (Graphpad Prism version 6).
2.4. Tetramer staining
Lymphocytes recovered from the different tissues collected at necropsy were washed with PBS supplemented with 0.2% heat-inactivated human serum and centrifuged at 1200 rpm for 10 min. The cells were resuspended in 5 μl of CM9-PE tetramer (Mamu-A*01-CTPYDINQM, Beckman Coulter) and, after 5 minutes, a cocktail containing CD3-APCCy7 (clone SP34-2; BD Pharmingen), CD4-V500 (clone L200; BD Pharmingen), CD95-FITC (clone DX2; BD Pharmingen) CD8-Alexa Fluor-405 (clone MHCD0826, Invitrogen) CD28-PerCP Cy5.5 (clone CD28.2, BioLegend, San Diego, CA), CD45RA-AF700 (clone F8-11-13, ABD Serotec, UK) and CCR7-APC (clone 150503, R&D) antibodies was added to the samples and further incubated for 30 minutes at room temperature. After the incubation, the cells were washed with PBS and acquired in a LSR II flow cytometer (BD Biosciences, San Jose, CA). Tetramer staining in PBMC was performed using cryopreserved samples collected at necropsy. After thawing, the cells were counted and 106 PBMC were stained with the Aqua Live/Dead (Invitrogen) viability dye. After washing, the cells were exposed to the CM9-PE tetramer, and after incubation a cocktail containing CD3-APC, CD4-PerCP Cy5.5, CD8-APCCy7, CD28-FITC, CD95-PE Cy7 (BD Pharmingen) was added to the cells. After 20 minutes of incubation, the cells were washed, fixed in 1% Paraformaldehyde and acquired in the flow cytometer. For all the tetramer stained samples, at least 5×104 CD8+ T cells were acquired from each tube and the data were analyzed using FlowJo software (Tree Star, Inc.). Samples were considered positive if the frequency of the Gag CM9 tetramer positive CD8+ T cells was more than 2-fold higher than the frequency obtained in samples collected before vaccination or in MamuA*01 negative samples.
3. RESULTS
3.1 Comparison of the five vaccine platforms tested in macaques
Macaques were vaccinated with regimens expressing SIV antigen as outlined in Figure 1. The ALVAC/Env regimen consisted of four vaccinations with recombinant ALVAC expressing SIV gag/pol and env, including two SIV Env protein boosts. The RepAd/Env regimen included two vaccinations with recombinant replicating Adenovirus expressing gag, env and rev followed by two boosts with SIV Env protein. The DNA-based protocols included four vaccinations with a mixture of DNAs expressing gag and env, whereas the DNA&Env co-immunization regimen included codelivery of Env protein in the same muscle following the DNA electroporation. The Peptide/MVA/Env regimen consisted of three vaccinations with a mixture of HIV and SIV peptides covering helper and cytotoxic T cell epitopes, followed by two boosts with recombinant MVA expressing different SIV genes together with SIV Env protein, all delivered intrarectally to induce colorectal mucosal immunity [34–36]. Of note the vaccines were delivered via different routes such as intramuscular (IM) for ALVAC/Env, DNA&Env and DNA vaccines, intrarectal route (IR) for the Peptide/MVA/Env vaccine and several mucosal routes including oral (O), intranasal (IN) and intratracheal (IT) for the RepAd regimen. The Env protein was formulated with different adjuvants including Alum (ALVAC/Env), EM-005 (RepAd/Env; DNA&Env) and mLT (Peptide/Env). We previously reported on the humoral responses in these macaques [38]. This report focuses primarily on the cellular immune responses monitored in peripheral blood and in different tissues at necropsy at 2 to 4 weeks after the last vaccination.
3.2. Peptide-specific cellular immune responses in blood at necropsy
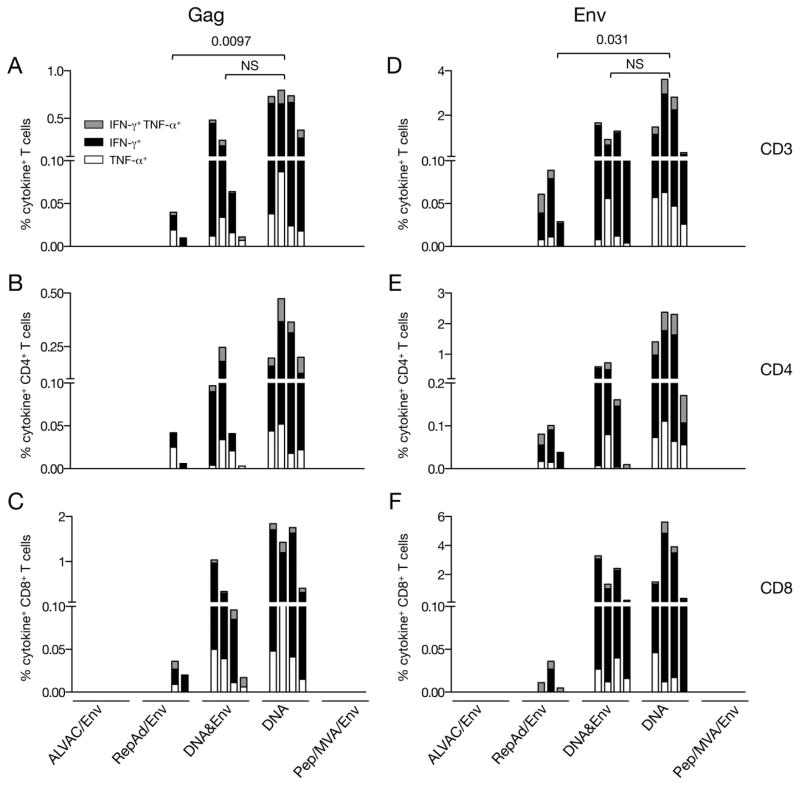
For the measurement of SIV-specific cellular immune responses at the time of necropsy, peptide-stimulated PBMC were analyzed by intracellular staining with antibodies against IFN-γ and TNF-α and the frequency of both Gag- and Env-specific cytokine+ T cells was determined by flow cytometry (Figure 2). All animals immunized with plasmid DNA (DNA&Env and DNA only groups) as well as 2 of the 4 animals from the RepAd/Env group showed Gag-specific cytokine+ T cell responses (Figure 2A). In contrast, Gag-specific T cells were absent in the animals immunized with either the ALVAC/Env or Pep/Env regimens. The highest level of Gag responses was found in the DNA only group (range 0.4–0.7% of the total T cells), followed by the DNA&Env group (range 0.01–0.5%) and the RepAd/Env group (range 0.01 and 0.04% of the 2 responders). Comparison of these three groups (Figure 2A) showed significantly higher level of responses in the DNA only group compared to RepAd/Env and no difference between DNA and DNA&Env group using ANOVA. All positive animals, except one in the RepAd/Env group, developed anti-Gag responses characterized by the production of both IFN-γ+ and TNF-α+, although we noted that the responses were clearly dominated by the production of IFN-γ. The Gag-specific responses were mediated by both CD4+ (Figure 2B) and CD8+ T lymphocytes (Figure 2C), although the CD8+ T responses were higher for the majority of the animals (Figure 2C, note different scale).
Figure 2.
SIV-specific cellular immune responses in PBMC at the time of necropsy. PBMC from blood collected 2 weeks after the last immunization (4 weeks for macaques of the ALVAC/Env group) were stimulated with peptides covering (A–C) Gag or (D–E) Env. The frequency of SIV-specific cells producing IFN-γ, TNF-α or both (cytokine+) were measured by flow cytometry. Total CD3+ T cell responses (A, D), CD4+ T cell responses (B, E) and CD8+ T cell responses (C, F) are shown. The order of the animals within each vaccine group were ALVAC/Env: P464; P836; P841; P851; P862; P863; RepAd/Env: P445, P450, P451, P576; DNA&Env: P181; P447; P515; P520; DNA: P516; P517; P518; P519; Pep/MVA/Env: R216; R217; R452; R451.
Next, PBMC were analyzed for the presence of Env-specific cytokine+ T cell responses. Only the vaccine regimens including plasmid DNA (DNA, DNA&Env) and RepAd/Env (one of the animals in this group could not be evaluated due to the very low number of cells in the sample) showed Env-specific cellular responses at necropsy (Figure 2D). Animals enrolled in the DNA-only vaccine group had the highest anti-Env cellular responses (range between 0.3–3.6% of the total T cells), followed by the DNA&Env regimen (range 0.13–1.7%), and the RepAd/Env protocol (range 0.03–0.09%) (Figure 2D), which is similar to the responses observed for Gag (see above, Figure 2A). Similar to the observation of the Gag responses, we found significantly higher level of Env-specific responses (Figure 2D) in the DNA only group compared to RepAd/Env and no difference between DNA and DNA&Env group using ANOVA. The responses in the two groups that included plasmid DNA were preferentially mediated by CD8+ T lymphocytes (Figure 2F), whereas CD4+ T cells dominated the anti-Env cellular responses elicited by the RepAd/Env regimen (Figure 2E, note the different scale). The Env-specific T cells produced primarily IFN-γ, although one animal from the DNA-only regimen showed a higher frequency of TNF-α secreting cells CD4+ T cells (Figure 2E). Together, we found distinct efficacy and magnitude by the different vaccine regimens in inducing SIV-specific cellular T cell responses. We cannot rule out that the time point selected for the ALVAC/Env group may have been suboptimal, since this group showed positive responses at 2 weeks after the 3rd vaccination (see below Figure 7). Thus, vaccine platforms such as plasmid DNA, DNA&Env co-immunization and RepAd/Env were the most potent in eliciting Gag- and Env-specific cellular immune responses in the blood at necropsy.
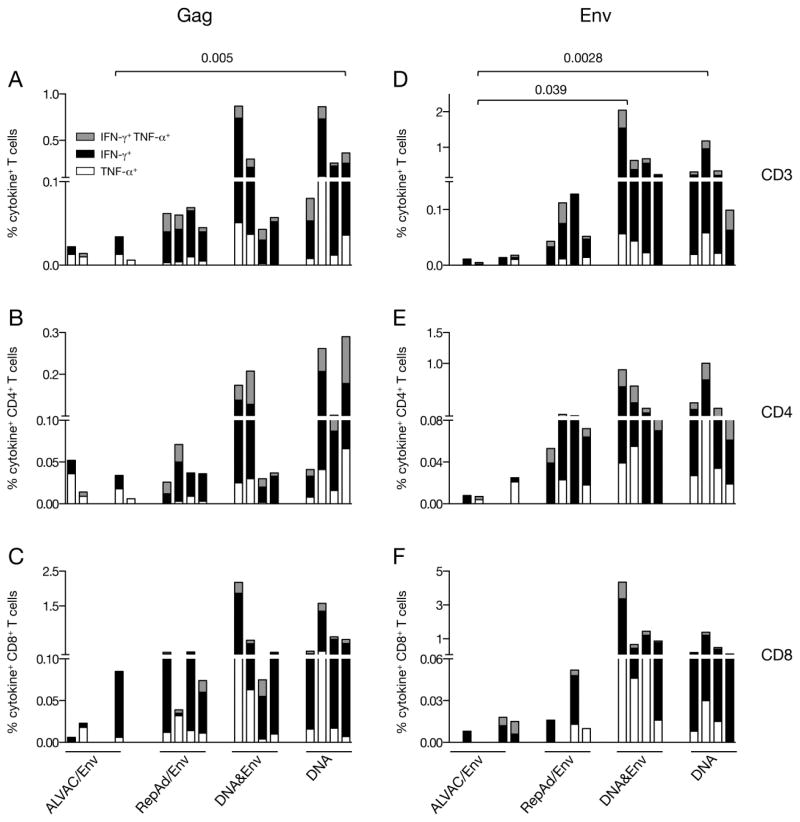
Figure 7.
Antigen-specific T cells in PBMC measured during the course of vaccination. PBMC from blood collected 2 weeks after the 3rd immunization for all vaccine groups except at 2 weeks after the 2nd vaccination for the ALVAC/Env group were stimulated with peptides covering (A–C) Gag and (D–F) Env. The frequency of SIV-specific cells producing IFN-γ, TNF-α or both (cytokine+) were measured by flow cytometry. Total (A, D), CD4+ (B, E) and CD8+ (C, F) T cell responses are shown. Samples from the Pep/Env group were not included in the analysis. The order of the animals within the vaccine groups were kept the same as described in Figure 2.
3.3. Gag CM9 tetramer responses in blood at necropsy
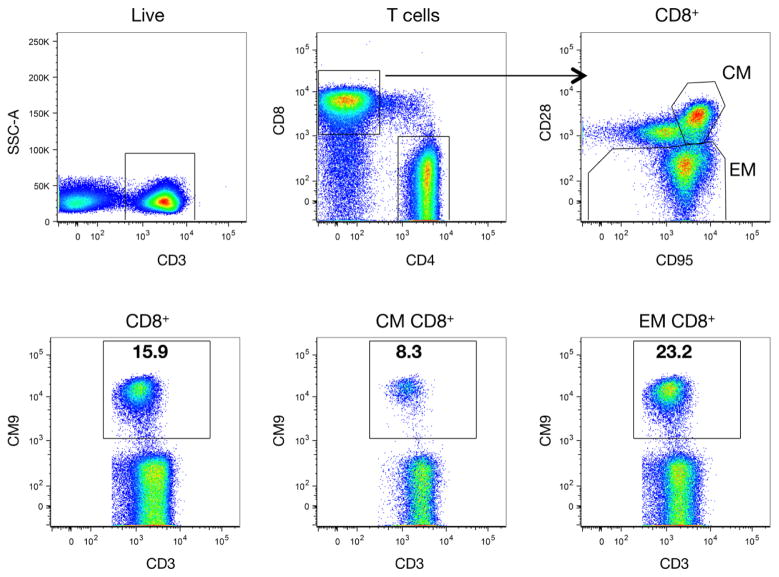
Since all macaques enrolled in this study expressed the mamuA*01 MHC class I allele, we also analyzed the Gag181-189 (CM9) tetramer responses in PBMC, because this epitope was present in all the vaccine platforms. The sequential gating strategy used for the analysis of tetramer responses in PBMC is shown in Figure 3. Briefly, the main lymphocyte population was identified by the scatter properties within single cells. After excluding dead cells, T lymphocytes were gated based on CD3 expression, and CD4+ and CD8+ T cells were identified within CD3+ lymphocytes. Central memory (CM) and effector memory (EM) T cells were defined based on the expression of CD95 and CD28, and, finally, the percentage of Gag CM9 tetramer positive cells was determined within these lymphocyte populations were shown for a representative DNA vaccinated animal.
Figure 3.
Gating strategy for the flow cytometric analysis of Gag CM9-specific tetramer+ CD8+ T cell responses. Single CD4+ and CD8+ T lymphocyte subsets were determined from the live CD3+ T cell population. Central memory (CM) and effector memory (EM) subsets were defined within the CD8+ T cells by staining with CD95 and CD28. Gag CM9 Tetramer positive cells are shown from the CD8+ CM and EM T cell populations. Numbers within the gates represent the percentage of tetramer positive cells.
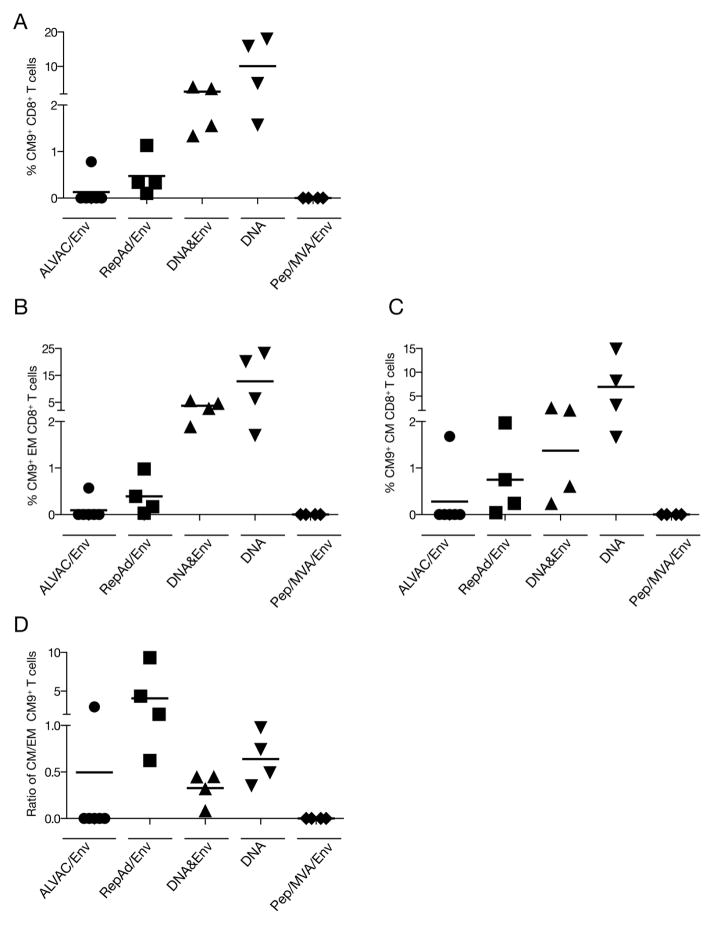
Analysis of all the animals showed (Figure 4A) that vaccination protocols including DNA, especially the DNA only group, had the highest CM9 tetramer responses among the CD8+ T cells (range 1.3% to 4% for the DNA/Protein and 1.6% to 18% for the DNA only group), Interestingly, although two of the animals from the RepAd/Env group were negative for peptide-induced Gag responses (Figure 2A), all the animals within this group showed positive CM9 tetramer responses (range from 0.1 to 1.1% of total CD8+ T cells) (Figure 4A). Similarly, one animal in the ALVAC/Env group was also found positive for CM9-specific CD8+ T cell responses by tetramer staining (0.8% of total CD8 T cells) (Figure 4A), although this animal was negative upon peptide stimulation (Figure 2A). No tetramer positive CD8+ T cells were found in PBMC of any of the animals vaccinated with the Pep/Env regimen.
Figure 4.
Gag CM9 tetramer responses in CD8+ T lymphocytes at necropsy. The frequency of Gag-specific CM9+ CD8+ T lymphocytes elicited by the different vaccine regimens was measured in PBMC by combined tetramer staining and cell surface phenotyping for memory T cells subsets followed by flow cytometry. The plots show the percentage of CM9+ CD8+ T lymphocytes among (A) total CD8+ T cells, (B) effector memory (CD95+CD28−) and (C) central memory (CD95+CD28+) CD8+ T lymphocytes. (D) The ratio of CM/EM among the tetramer+ CD8+ T cells is shown.
Since the CM9 tetramer staining was performed in combination with antibodies against CD28 and CD95, the memory phenotype of this CD8+ T cell population was further analyzed (Figures 4B and 4C). The DNA-based vaccines induced preferentially effector memory EM (CD95+ CD28−) cells (Figure 4B). The responses showed ranges of 1.9% to 5.7% for the DNA&Env; 1.7% to 23.2% for the DNA only, and 0.17% to 1% in the RepAd/Env). A substantial fraction of the CM9+ CD8+ T cells was also found among the CM (CD95+CD28+) subpopulation (0.2% to 2.5% in the DNA&Env; 1.6% to 14.9% in the DNA only, and 0.2% to 2% in the RepAd/Env group) (Figure 4C). Similarly, the only macaque with detectable CM9 responses in the ALVAC/Env group had both EM and CM CM9+ CD8+ T cells (0.57% and 1.7%, respectively), with a higher fraction of CM memory CD8+ T lymphocytes. Together, these data showed that inclusion of DNA in the vaccine resulted in the highest CM9 tetramer responses, including both EM and CM CD8+ T cells. Interestingly, we noted that although animals in the RepAd/Env group had overall lower responses, they showed the most balanced distribution of CM9 tetramer positive cells as judged by the higher CM/EM ratio found among the tetramer positive CD8+ T lymphocytes (Figure 4D). In contrast, the DNA-based vaccines induced responses that favored EM phenotype. These data demonstrate the vaccine regimens compared in this study induce cellular responses with distinct efficacy, magnitude, and characteristics.
3.4. Tissue distribution of antigen-specific CD8+ T cells
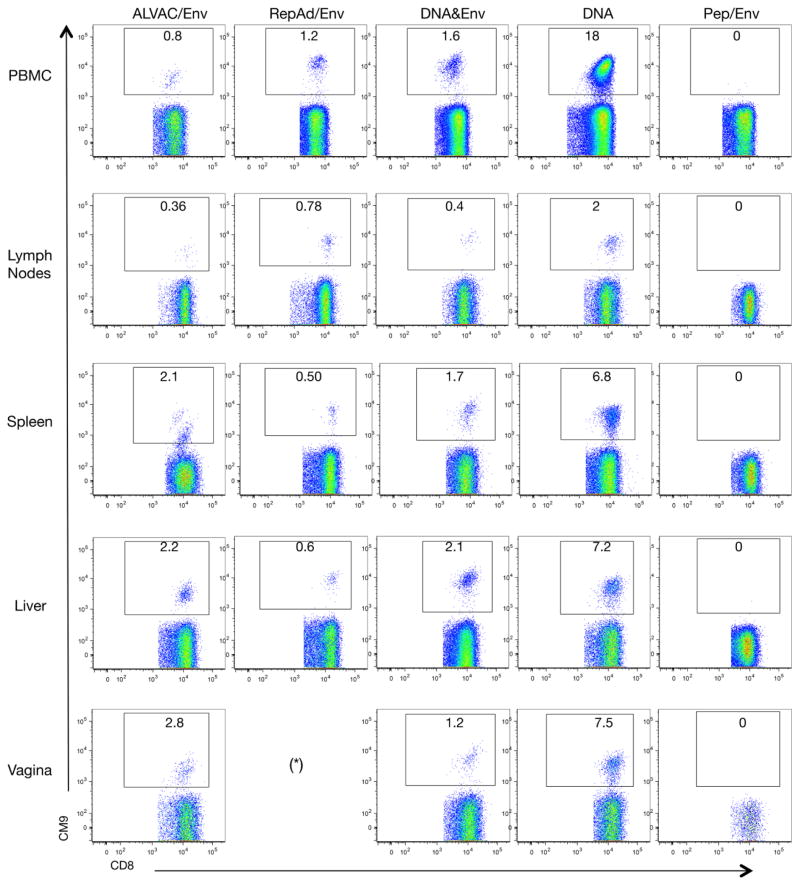
Samples from different organs were collected at necropsy and the systemic dissemination of vaccine-induced cellular responses was analyzed by Gag CM9 tetramer staining. Primary flow plot data from one representative animal from each vaccination group are shown in Figure 5, including all the tissues that were analyzed by Gag CM9 tetramer staining: PBMC (A, see also Figure 4), lymphoid tissue (lymph nodes; B), spleen (C), liver (D) and mucosal sites (vagina) (E). Note that all the animals included in the RepAd/Env regimen were males and, therefore, dissemination of cellular responses into the genital tract could not be addressed. No rectal samples were available due to a technical error, making it impossible to assess responses induced in this mucosal site by the different vaccine protocols.
Figure 5.
Flow cytometric analysis of Gag CM9-spexific responses in different tissues at necropsy of an exemplary animal from each group. Flow plots show the frequency (numbers given within the gate) of CM9 tetramer+ CD8+ T lymphocytes among PBMC, lymph nodes (axillary, inguinal), spleen, liver, and vaginal samples from a representative macaque from each vaccine regimen. Asterisk denotes the absence of samples from the vagina because all animals in the RepAd/Env group were all males.
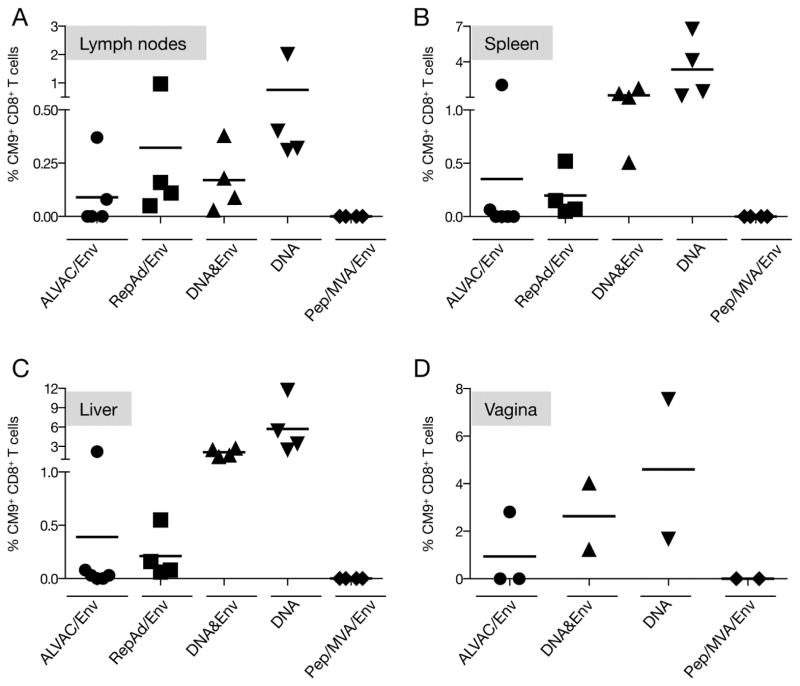
The analysis of the different tissues from all the animals is shown in Figure 6. Table 1 compares the numbers of positive responders among the different groups from the data shown in Figures 4 and 6. All vaccine platforms tested, except the Pep/Env regimen, showed the presence of Gag CM9-specific responses in different tissues, albeit we noted a great difference in the efficacy of inducing cellular immune responses among the groups. CM9-specific T cells present in lymph nodes reflect the dissemination of the cellular responses into secondary lymphoid organs (Figure 6A). Compared to the other tissues (Figure 6B–D, note different scale), the frequency of CM9-specific CD8+ T cells was lowest in lymph nodes. This finding is expected because typically effector memory T cells do not accumulate in lymph nodes. The frequency of CM9-specific CD8+ T cells ranged from 0.03–0.38% for the DNA&Env group; 0.31% to 2% for the DNA only group; 0.05% to 0.97% for the RepAd/Env, and 0.08% to 0.37% for the two positive animals in the ALVAC/Env group. Interestingly, one animal from the ALVAC/Env group lacking both detectable Gag peptide-induced responses and Gag CM9-specific CD8+ T cells in PBMC (Figures 2A and 4A) showed Gag CM9-specific responses in the lymph nodes (Figure 6A).
Figure 6.
Gag CM9 tetramer responses analyzed in different tissues from all vaccine groups. The presence of Gag-specific CM9+ CD8+ T lymphocytes was detected by flow cytometry after tetramer staining of lymphocytes recovered from (A) lymph nodes, (B) spleen, (C) liver, and (D) vagina. The plots show the frequency of CM9+ CD8+ T lymphocytes as a percentage of the parental total CD8+ T lymphocyte population.
Table 1.
Macaques with positive cellular immune responses at necropsy.
| Tissue | Assay | Number of positive animals per vaccine group | ||||
|---|---|---|---|---|---|---|
| ALVAC/Env (N=6) | RepAd/Env (N=4) | DNA&Env (N=4) | DNA (N=4) | Peptide/MVA/Envb (N=4) | ||
| PBMC | Gag peptides | 0 | 2 | 4 | 4 | 0 |
| Env peptides | 0 | 3 | 4 | 4 | 0 | |
| CM9 | 1 | 4 | 4 | 4 | 0 | |
| LN | CM9 | 2 | 4 | 4 | 4 | 0 |
| Spleen | CM9 | 2 | 4 | 4 | 4 | 0 |
| Liver | CM9 | 4 | 4 | 4 | 4 | 0 |
| Vagina | CM9 | 1 of 3 | NDa | 2 of 2 | 2 of 2 | 0 of 2 |
ND, Not done, only male macaques in this group
Designed to induce primarily colorectal immunity
Lymphocytes recovered from the spleen represent a mixture of secondary lymphoid tissue and peripheral blood, and therefore the percentage of Gag CM9-specific CD8+ T cells is expected to be higher than in lymph nodes (Figure 6B). The range of CM9-specific CD8+ T cells measured in these samples were 0.5% to 1.8% for DNA&Env group; 1.1 % to 6.7% for DNA only; 0.05% to 0.5% for RepAd/Env, and 0.06% to 2% for ALVAC/Env group. Similar to the results obtained in lymph nodes, all macaques in the DNA, DNA&Env, and RepAd/Env groups and two of the animals in the ALVAC/Env group showed positive tetramer responses in the spleen.
Lymphocytes recovered from liver were analyzed to monitor dissemination of cellular responses into a non-lymphoid effector site (Figure 6C). The vaccine regimens including DNA induced the highest CM9 tetramer responses (range 1.4% to 2.7% for DNA&Env group and 2.4% to 11.7% for the DNA only group). All the animals in the RepAd/Env group had Gag CM9+ T cells in this effector site (range 0.06–0.55%), while four of the six macaques from the ALVAC/Env group showed tetramer responses (0.03% to 2.2%), albeit the responses in three of the four responders were very low.
Vaginal samples were collected to address the dissemination of vaccine-induced cellular responses to mucosal sites (Figure 6D). This site is highly relevant because HIV infection is mainly transmitted at mucosal sites including the genital tract. With the exception of RepAd/Env group, half of the animals (2–3 animals) in each group were females. Four of the macaques, which received a DNA-based vaccine, showed CM9-specific CD8+ T cells with a frequency of 1.24% and 4% in the DNA&Env group, and 1.6% and 7.5% in the DNA only group. Only one of three females from the ALVAC/Env group was showed a positive response (2.8% CM9-specific CD8+ T cells). This animal had the highest tetramer responses in all the analyzed tissues and, therefore, was clearly different from the other macaques included in the group. Finally, no Gag CM9-specific T cells were found in any tissue for the macaques vaccinated with the Pep/Env regimen. Taken together (Table 1), the analysis of the Gag CM9-specific T cells showed that the vaccine-induced cellular responses were able to disseminate systemically, including genital tract mucosa, which is a desirable feature for an effective anti-HIV vaccine.
3.5 Detection of early cellular responses in blood
The results obtained of CM9 tetramer responses for the ALVAC/Env and RepAd/Env groups suggested that the time of necropsy could have been suboptimal for the evaluation of vaccine induced cellular immunity in some vaccine groups. Therefore, we also analyzed the peptide-induced T cell responses for both Gag and Env in blood samples collected earlier during the vaccination schedule. The time point selected was two weeks after the 3rd vaccination except for the RepAd/Env group, which was analyzed two weeks after the 2nd immunizations. Comparison of the responses for RepAd/Env, DNA&Env and DNA groups did not show statistical differences between this time point (Figure 7) and after the 4th vaccination (Figure 2). Because animals in the Pep/Env group were found negative for both peptide and CM9 tetramer responses performed in the necropsy samples, they were excluded from this analysis.
Cellular immune responses were examined upon stimulation of the PBMC with peptide pools covering Gag (Figure 7A) and the complete Env (Figure 7D) followed by flow cytometry and the responses were analyzed as described for Figure 2. In contrast to the results obtained in blood samples collected at necropsy (Figure 2), all four macaques in the RepAd/Env group induced positive responses (IFN-γ, TNF-α production) upon stimulation with both Gag and Env antigens (range of 0.04–0.07% and 0.04–0.13% of T cells for Gag (Figures 7A) and Env (Figure 7D), respectively. Interestingly, although negative at the time of necropsy, five of the six macaques in the ALVAC/Env group had peptide-induced responses at this time point: three animals were positive for both Gag and Env, while one animal each was positive for either of the two antigens (ranges of 0.006% to 0.034% for Gag and 0.005% to 0.018% for Env). As expected, all the macaques in the two DNA groups were positive also at this time point. The responses for the animals in the DNA&Env group had a range for the antigen-specific T cells of 0.04% to 0.85% (Gag) and of 0.23% to 2.1% (Env), and in the DNA only group of 0.08% to 0.85% (Gag) and 0.1% to 1.17% (Env). Comparison of these four groups (Figure 7A and 7B) showed significantly higher levels of Gag- as well as Env-specific responses in the DNA group compared to ALVAC/Env using ANOVA. Similarly, analysis of total (Gag and Env) SIV-specific responses also showed significant difference between the ALVAC/Env and DNA group (p=0.013) and DNA&Env group (p=0.005). At this time point, we did not find a significant difference between RepAd/Env and DNA groups as we found at necropsy (Figure 2), although we noted a trend of the higher responses in the groups that received DNA. We further observed that all the macaques with measurable vaccine-induced cellular responses elicited antigen-specific cytotoxic T lymphocytes armed with Granzyme B (data not shown), indicating that these cells are capable of killing SIV-infected cells.
Similar to the results obtained at necropsy (Figure 2), the antigen-specific responses against both Gag (Figures 7B–C) and Env (Figures 7E–F) were mediated by CD4+ (Figures 7B and 7E) and CD8+ (Figures 7C and 7F) T cells producing IFN-γ, TNF-α and both cytokines. All vaccine regimens induced preferentially CD8+ T cell responses with the exception of the Env responses in the RepAd/Env group that showed a skewing of the responses towards CD4+ T cells, similar to the data obtained at necropsy (Figure 2).
In summary, analysis of PBMC collected at earlier time points during the vaccination schedule demonstrate that for some vaccine regimens, especially ALVAC/Env and RepAd/Env, the peak of cellular responses were elicited prior to the final immunization. With regard to RepAd/Env, a similar decline in cellular immune responses following Env immunization was seen in a recent study in which the Env boost was administered in the same EM-005 adjuvant. The effect was attributed to complex innate immune signaling arising from persistent RepAd replication and the adjuvant in the booster immunization, leading to a re-orientation of induced adaptive responses (Thomas et al., submitted). An alternate adjuvant pairing might be more appropriate for this vaccine regimen.
4. DISCUSSION
In this report, we examined and compared the immune responses induced by five different SIV vaccine regimens in macaques to develop improved combinatorial vaccine strategies aiming to improve the partially protective responses we had previously reported. The main focus of this work was to provide an analysis of the induced cellular immunity, while the induced humoral immune responses have already been reported elsewhere [38]. A summary of the key findings of the induced cellular (this work) and humoral responses [38] found at necropsy including binding antibody (bAb), neutralizing antibody (Nab), antibody-dependent cell mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibition (ADCVI) are presented in Table 2.
Table 2.
Comparison of cellular and humoral immune responses at necropsy.
| Tissue | Assay | Immune responses in different vaccine groups | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ALVAC/Env (N=6) | RepAd/Env (N=4) | DNA&Env (N=4) | DNA (N=4) | |||
|
| ||||||
| PBMC | Gag CM9 cellular responsesa | − | + | +++ | +++ | |
|
|
|
|||||
| Vagina | 1 of 3 | N/A | +++ | +++ | ||
|
| ||||||
| plasma | Humoral responsesb | bAbc | +++ | ++ | +++ | ++ |
| Nabc | ++ | +++ | +++ | + | ||
| ADCCc | + | +++ | ++ | + | ||
| ADCPc | ++ | +++ | ++ | + | ||
|
|
|
|||||
| mucosa | IgA | 3 of 6 | 2 of 4 | 3 of 4 | 1 of 4 | |
| IgG | 5 of 6 | 4 of 4 | 4 of 4 | 3 of 4 | ||
data from this report (Figures 4A and 6D)
humoral immune response data are from Vargas-Inchaustegui et al (38)
reflects relative magnitude
At necropsy, the highest frequency of Gag CM9-specific T cells in PBMC were found in animals that receive vaccine regimens including DNA and positive responses were also found in all animals of the RepAd/Env group. The antigen-specific T cells induced by the DNA vaccine were preferentially differentiated effector memory cells, while the RepAd/Env induced more CM-like CD8+ T cells. Whether the different phenotype of the Gag CM9-specific T cells will translate into extended longevity or different effector function against infected cells could not be addressed in this study since immunogenicity of the different vaccines was analyzed immediately after the last vaccination. However, we have previously shown that DNA-based vaccines elicit potent cytotoxic T cells and, importantly, we reported long-lasting cellular immune responses, persisting for more the 5 years in vaccinated macaques [32, 33, 73]. Similarly, persistent elite control of viremia in macaques for more than 6.5 years following SIVmac251 challenges was attributed in part to cellular immunity elicited by RepAd/Env immunization [64].
We also compared SIV peptide-specific responses at a selected time point during vaccination and at necropsy. The DNA based vaccines induced robust responses detectable at both time points as expected since we previously showed elicitation of high antigen-specific responses even after 2 vaccinations using the efficient intramuscular delivery followed by electroporation [27, 28, 30, 32, 33, 73]. In contrast, we noted a significant difference for the ALVAC/Env and the RepAd/Env regimens with more responders and higher responses at the earlier time point. As discussed above, this may have involved a reorientation of immune responses in the RepAd/Env group (Thomas et al., submitted). In any case, depending on the vaccine regimen selection of optimal time points for vaccine evaluation is critical to assess the potency of the cellular immune responses. Importantly, all vaccines (RepAd/Env, ALVAC/Env and DNA) were able to induce cytotoxic T cell responses, an important characteristic to evaluate the potency of the cellular immune response. In addition, since we analyzed the Gag peptide and Gag CM9 tetramer responses at necropsy, this allowed us to directly compare the results. In fact, we found that some of the immunized macaques that failed to respond to Gag peptide stimulation had indeed circulating Gag-specific cells in peripheral blood (two animals from the RepAd/Env and one from the ALVAC/Env group). It is possible that Gag-specific T cells in these cases produce cytokines different than IFN-γ and TNF-α measured in our assays. These data showed the importance of employing both assays to get a comprehensive evaluation of the vaccine induced cellular immunity.
The presence of SIV-specific cellular responses at effector sites, especially mucosal surfaces such as the genital tract, is critical for the containment of the virus. Using live-attenuated SIV as a vaccine model, others have demonstrated the potent role of SIV-specific CD8+ T cells in the genital tract including vagina and protection from vaginal SIV challenge [74, 75]. Therefore, induction of immunity that readily disseminates into these areas is a desirable feature of anti-HIV candidate vaccines. We examined the dissemination of vaccine-induced cell-mediated immunity into the genital tract using vaginal samples taken at necropsy and observed that the highest frequency of Gag CM9-specific T cells was consistently found in the animals immunized with a vaccine regimens that included DNA. Interestingly, we noted that the DNA vaccine regimen administered by the IM route induced robust Gag CM9-specific responses reaching up to 7% of the CD8+ T cells in the vaginal samples. In contrast, the ALVAC/Env vaccine that was also administered via the IM route showed only one of the three females with Gag CM9-specific T cells in the genital tract. Unfortunately, we could not assess these responses in RepAd/Env group, which received the vaccine via mucosal routes but did not have any female vaccinees. A few other reports demonstrated the successful induction of cellular responses in vaginal tissues of macaques using a vaccine regimen consisting of DNAs, rMVA, and inactivated SIVmac239 particles administered via the oral route [76] or intraperitoneal vaccination with a gp96-Ig chaperoning SIV antigens [77]. Of note, none of these vaccines were administered via the IM route.
The analysis of the humoral responses at necropsy by the different vaccine regimens revealed robust responses in the plasma (binding and neutralizing antibodies) when the vaccine included a protein component [38] (Table 2). Similarly, DNA/Env, RepAd/Env and ALVAC/Env regimens showed dissemination of SIV-specific IgG and IgA to mucosal surfaces [38]. The DNA only vaccine regimen was clearly less potent in eliciting SIV-specific mucosal IgG. The peptide/MVA/Env regimen also had one of four animals with a strong mucosal IgA response [38].
Thus, different vaccine platforms induce responses with different characteristics. It is possible that the presence of Env protein shifted the immune responses towards antibody development at the expense of cellular immunity, since the only vaccine regimen lacking a protein component (DNA only) had the highest and more consistent cell mediated responses in the analyzed tissues. On the other hand the RepAd/Env and ALVAC/Env showed good SIV-specific IgG and IgA levels in rectal secretions, albeit RepAd/Env showed relative low gag CM9 responses while ALVAC/Env did not show detectable cellular responses at this time point. The DNA&Env regimen, combining the robust cellular responses associated with DNA and the higher antibody responses that typically are induced by the protein component, induced the more balanced immunity (Table 2). The immunity induced by this regimen was characterized by high cellular responses against Gag and Env and high levels of antibodies that were shown to have several functional properties (neutralizing activity, ADCC) [38]. The responses induced by the DNA&Env protocol, both humoral and cellular, efficiently disseminated into mucosal surfaces as demonstrated by the presence of antigen-specific T cells in the genital tract and, similar to animals vaccinated by the RepAd/Env protocol, the presence of SIV-specific IgG and IgA in rectal secretions. Interestingly, in addition to providing an excellent mucosal prime for antibody responses, the RepAd/Env regimen induced cellular immunity that had clearly distinct features: (i) the anti-Env cellular responses were dominated by CD4+ T cells, and (ii) the Gag CM9-specific CD8+ T cells induced by this regimen were skewed towards a CM memory phenotype, showing the highest CM/EM ratio among all the vaccine regimens. Consistent with these findings, the frequency of Gag CM9-specific CD8+ T cells in lymph nodes, a site where differentiated effector cells are typically excluded, were higher in the RepAd/Env group.
Taken together, these results suggest that the RepAd vaccine vector in combination with DNA or ALVAC including Env protein represent promising combinatorial vaccination strategies that may induce potent long-lasting cellular immunity with early dissemination of both cellular and humoral responses into mucosal sites. The efficacy of such combination should be explored in the rhesus macaque model.
5. Conclusion
We compared the cellular immune responses induced by five vaccine regimens previously shown to confer protection in macaques. We found potent dissemination of T cell responses into secondary lymphoid tissues and effector anatomical sites, including the genital tract, even when the vaccine regimen was administered by the intramuscular route. Combination of different presented vaccine regimens may induce a more balanced, durable and protective immune responses.
HIGHLIGHTS.
Cellular immune responses induced by five SIV vaccine regimens in macaques
Identification of promising vaccine candidates for combination strategies
Systemic cellular immune responses capable of disseminating to mucosal sites
Presence of vaccine-induced T cells in vaginal mucosa
Likely contribution to containment of HIV/SIV at the portal of entry.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH) (B.K.F., G.N.P., J.A.B., G.F., M.R.G.) and NIH/NIAID/DAIDS under HVDDT Contract HHSN272200800063C (NYS). We gratefully acknowledge N. Miller (NIAID, NIH) for help with gD-env protein expression, J. Tartaglia (Sanofi Pasteur) for the ALVAC vectors, J. Clements (Tulane University) for reagents, the animal caretakers and research team at Advanced BioScience Laboratories, and B. Chowdhury for technical assistance.
Abbreviations
- PBMCs
peripheral blood mononuclear cells
- TNF-α
tumor necrosis factor α
- IFN-γ
interferon γ
- IM
intramuscular
- IN
intranasal
- O
oral
- IT
intratracheal
- IR
intrarectal
- ADCC
antibody-dependent cell mediated cytotoxicity
- ADCVI
antibody-dependent cell-mediated viral inhibition
Footnotes
Conflict of interest statement
G.N.P. and B.K.F. are inventors on US Government-owned patents and patent applications related to DNA vaccines and gene expression optimization. G.F. is an inventor on a US Government patent filed jointly with Sanofi Pasteur on the use of the ALVAC vector as a platform for an HIV vaccine. N.Y.S. is a full time employee of Inovio Pharmaceuticals and as such receives compensation in the form of salary and stock options. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berman PW. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14(Suppl 3):S277–289. [PubMed] [Google Scholar]
- 2.Francis DP, Gregory T, McElrath MJ, Belshe RB, Gorse GJ, Migasena S, Kitayaporn D, Pitisuttitham P, Matthews T, Schwartz DH, Berman PW. Advancing AIDSVAX to phase 3. Safety, immunogenicity, and plans for phase 3. AIDS Res Hum Retroviruses. 1998;14(Suppl 3):S325–331. [PubMed] [Google Scholar]
- 3.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K, Bangkok G. vaccine evaluation, randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok. Thailand, J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 4.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF H.I.V.V.S.G. rgp. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 5.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, Heath L, Magaret CA, Bose M, Bradfield A, O’Sullivan A, Crossler J, Jones T, Nau M, Wong K, Zhao H, Raugi DN, Sorensen S, Stoddard JN, Maust BS, Deng W, Hural J, Dubey S, Michael NL, Shiver J, Corey L, Li F, Self SG, Kim J, Buchbinder S, Casimiro DR, Robertson MN, Duerr A, McElrath MJ, McCutchan FE, Mullins JI. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17:366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB, Team HS. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 10.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O’Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O’Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, Berman P, Shen X, Andrews C, O’Connell RJ, Ngauy V, Nitayaphan S, de Souza M, Korber B, Koup R, Bailer RT, Mascola JR, Pinter A, Montefiori D, Haynes BF, Robb ML, Rerks-Ngarm S, Michael NL, Gilbert PB, Kim JH. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One. 2013;8:e53629. doi: 10.1371/journal.pone.0053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2012;28:1444–1457. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, Tryniszewska E, Lewis MG, VanCott TC, Hirsch V, Woodward R, Gibson A, Grace M, Dobratz E, Markham PD, Hel Z, Nacsa J, Klein M, Tartaglia J, Franchini G. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, Hudacik L, Rose N, Nacsa J, Edghill-Smith Y, Moniuszko M, Hel Z, Belyakov IM, Berzofsky JA, Parks RW, Markham PD, Letvin NL, Tartaglia J, Franchini G. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J Virol. 2006;80:3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hel Z, Nacsa J, Tsai WP, Thornton A, Giuliani L, Tartaglia J, Franchini G. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SIVmac251-infected macaques. Virology. 2002;304:125–134. doi: 10.1006/viro.2002.1722. [DOI] [PubMed] [Google Scholar]
- 18.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccari M, Keele BF, Bosinger SE, Doster MN, Ma ZM, Pollara J, Hryniewicz A, Ferrari G, Guan Y, Forthal DN, Venzon D, Fenizia C, Morgan T, Montefiori D, Lifson JD, Miller CJ, Silvestri G, Rosati M, Felber BK, Pavlakis GN, Tartaglia J, Franchini G. Protection afforded by an HIV vaccine candidate in macaques depends on the dose of SIVmac251 at challenge exposure. J Virol. 2013;87:3538–3548. doi: 10.1128/JVI.02863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson LJ, Robert-Guroff M. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin Biol Ther. 2008;8:1347–1363. doi: 10.1517/14712598.8.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy SC, Chanda PK, Nigida SM, Jr, Markham PD, Zolla-Pazner S, Steimer K, Wade M, Reitz MS, Jr, Arthur LO, Mizutani S, Davis A, Hung PP, Gallo RC, Eichberg J, Robert-Guroff M. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 22.Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH, Kalyanaraman VS, Pal R, Lee EM, Zhao J, Cristillo A, Robert-Guroff M. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology. 2006;353:83–98. doi: 10.1016/j.virol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Demberg T, Florese RH, Heath MJ, Larsen K, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Venzon D, Grant R, Patterson LJ, Korioth-Schmitz B, Buzby A, Dombagoda D, Montefiori DC, Letvin NL, Cafaro A, Ensoli B, Robert-Guroff M. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2007;81:3414–3427. doi: 10.1128/JVI.02453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogers WM, Davis D, Baak I, Kan E, Hofman S, Sun Y, Mortier D, Lian Y, Oostermeijer H, Fagrouch Z, Dubbes R, van der Maas M, Mooij P, Koopman G, Verschoor E, Langedijk JP, Zhao J, Brocca-Cofano E, Robert-Guroff M, Srivastava I, Barnett S, Heeney JL. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology. 2008;382:217–225. doi: 10.1016/j.virol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, von Gegerfelt A, Huang W, Guan Y, Broderick KE, Sardesai NY, Labranche C, Montefiori DC, Pavlakis GN, Felber BK. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother. 2012;8:1620–1629. doi: 10.4161/hv.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Patel V, von Gegerfelt AS, Montefiori DC, Venzon D, Khan AS, Draghia-Akli R, Van Rompay KKA, Felber BK, Pavlakis GN. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A. 2009;06:15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, Markham PD, Marques ET, August JT, Khan A, Draghia-Akli R, Felber BK, Pavlakis GN. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni V, Jalah R, Ganneru B, Bergamaschi C, Alicea C, von Gegerfelt A, Patel V, Zhang GM, Chowdhury B, Broderick KE, Sardesai NY, Valentin A, Rosati M, Felber BK, Pavlakis GN. Comparison of immune responses generated by optimized DNA vaccination against SIV antigens in mice and macaques. Vaccine. 2011;29:6742–6754. doi: 10.1016/j.vaccine.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni V, Valentin A, Rosati M, Alicea C, Singh AK, Jalah R, Broderick KE, Sardesai NY, Le Gall S, Mothe B, Brander C, Rolland M, Mullins JI, Pavlakis GN, Felber BK. Altered Immunodominance Hierarchy and Increased T-cell Breadth upon HIV-1 Conserved Element DNA Vaccination in Macaques. PLoS One. 2014;9:e86254. doi: 10.1371/journal.pone.0086254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, Alicea C, Bear J, Hannaman D, Reed SG, Felber BK, Pavlakis GN. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine. 2013;31:3747–3755. doi: 10.1016/j.vaccine.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele B, Bess J, Jr, Piatak M, Jr, Lifson JD, Willliams WT, Shen X, Tomaras GD, Amara RR, Robinson HL, Johnson W, Broderick KE, Sardesai NY, Venzon D, Hirsch VM, Felber BK, Pavlakis GN. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous SIV challenge. Proc Natl Acad Sci U S A. 2013;110:2975–2980. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, Alicea C, Minang JT, Trivett MT, Ohlen C, Zhao J, Robert-Guroff M, Khan AS, Draghia-Akli R, Felber BK, Pavlakis GN. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine. 2010;28:4827–4836. doi: 10.1016/j.vaccine.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal R, Clements JD, Lewis MG, Strober W, Franchini G, Berzofsky JA. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, Woodward R, Markham PD, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 36.Sui Y, Zhu Q, Gagnon S, Dzutsev A, Terabe M, Vaccari M, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Belyakov IM, Berzofsky JA. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc Natl Acad Sci U S A. 2010;107:9843–9848. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sui Y, Gagnon S, Dzutsev A, Zhu Q, Yu H, Hogg A, Wang Y, Xia Z, Belyakov IM, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Berzofsky JA. TLR agonists and/or IL-15 adjuvanted mucosal SIV vaccine reduced gut CD4(+) memory T cell loss in SIVmac251-challenged rhesus macaques. Vaccine. 2011;30:59–68. doi: 10.1016/j.vaccine.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas-Inchaustegui DA, Tuero I, Mohanram V, Musich T, Pegu P, Valentin A, Sui Y, Rosati M, Bear J, Kulkarni V, Alicea C, Pilkington GR, Venzon V, Liyanage NP, TDT, Gordon SN, Wang Y, Hogg AE, Frey B, Patterson LJ, DiPasquale J, Montefiori DC, Sardesai NY, Reed SG, Tartaglia J, Berzofsky JA, Franchini G, Felber BK, Pavlakis GN, Robert-Guroff M. Humoral immunity induced by mucosal and/or systemic SIV-specific vaccine platforms suggest novel combinatorial approaches for enhancing responses. Clin Immunol. 2014;153:308–322. doi: 10.1016/j.clim.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 40.Honeyborne I, Prendergast A, Pereyra F, Leslie A, Crawford H, Payne R, Reddy S, Bishop K, Moodley E, Nair K, van der Stok M, McCarthy N, Rousseau CM, Addo M, Mullins JI, Brander C, Kiepiela P, Walker BD, Goulder PJ. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J Virol. 2007;81:3667–3672. doi: 10.1128/JVI.02689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneidewind A, Brockman MA, Yang R, Adam RI, Li B, Le Gall S, Rinaldo CR, Craggs SL, Allgaier RL, Power KA, Kuntzen T, Tung CS, LaBute MX, Mueller SM, Harrer T, McMichael AJ, Goulder PJ, Aiken C, Brander C, Kelleher AD, Allen TM. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007;81:12382–12393. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ngumbela KC, Day CL, Mncube Z, Nair K, Ramduth D, Thobakgale C, Moodley E, Reddy S, de Pierres C, Mkhwanazi N, Bishop K, van der Stok M, Ismail N, Honeyborne I, Crawford H, Kavanagh DG, Rousseau C, Nickle D, Mullins J, Heckerman D, Korber B, Coovadia H, Kiepiela P, Goulder PJ, Walker BD. Targeting of a CD8 T cell env epitope presented by HLA-B*5802 is associated with markers of HIV disease progression and lack of selection pressure. AIDS Res Hum Retroviruses. 2008;24:72–82. doi: 10.1089/aid.2007.0124. [DOI] [PubMed] [Google Scholar]
- 43.Rolland M, Heckerman D, Deng W, Rousseau CM, Coovadia H, Bishop K, Goulder PJ, Walker BD, Brander C, Mullins JI. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One. 2008;3:e1424. doi: 10.1371/journal.pone.0001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, Allen S, Kumwenda N, Taha T, Gray G, McIntyre J, Karim SA, Sheppard HW, Gray CM. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J Virol. 2004;78:3233–3243. doi: 10.1128/JVI.78.7.3233-3243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mothe B, Ibarrondo J, Llano A, Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers. 2009;27:105–120. doi: 10.3233/DMA-2009-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuniga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, Hildebrand W, Altfeld M, Allen TM, Walker BD, Korber BT, Leitner T, Sanchez J, Brander C. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–3125. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mothe B, Llano A, Ibarrondo J, Zamarreno J, Schiaulini M, Miranda C, Ruiz-Riol M, Berger CT, Herrero MJ, Palou E, Plana M, Rolland M, Khatri A, Heckerman D, Pereyra F, Walker BD, Weiner D, Paredes R, Clotet B, Felber BK, Pavlakis GN, Mullins JI, Brander C. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One. 2012;7:e29717. doi: 10.1371/journal.pone.0029717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mothe B, Llano A, Ibarrondo J, Daniels M, Miranda C, Zamarreno J, Bach V, Zuniga R, Perez-Alvarez S, Berger CT, Puertas MC, Martinez-Picado J, Rolland M, Farfan M, Szinger JJ, Hildebrand WH, Yang OO, Sanchez-Merino V, Brumme CJ, Brumme ZL, Heckerman D, Allen TM, Mullins JI, Gomez G, Goulder PJ, Walker BD, Gatell JM, Clotet B, Korber BT, Sanchez J, Brander C. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med. 2011;9:208. doi: 10.1186/1479-5876-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manrique M, Kozlowski PA, Cobo-Molinos A, Wang SW, Wilson RL, del Martinez-Viedma PM, Montefiori DC, Carville A, Aldovini A. Resistance to infection, early and persistent suppression of simian immunodeficiency virus SIVmac251 viremia, and significant reduction of tissue viral burden after mucosal vaccination in female rhesus macaques. J Virol. 2014;88:212–224. doi: 10.1128/JVI.02523-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manrique M, Kozlowski PA, Cobo-Molinos A, Wang SW, Wilson RL, Montefiori DC, Mansfield KG, Carville A, Aldovini A. Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara. J Immunol. 2011;186:3581–3593. doi: 10.4049/jimmunol.1002594. [DOI] [PubMed] [Google Scholar]
- 53.Pahar B, Gray WL, Phelps K, Didier ES, deHaro E, Marx PA, Traina-Dorge VL. Increased cellular immune responses and CD4+ T-cell proliferation correlate with reduced plasma viral load in SIV challenged recombinant simian varicella virus - simian immunodeficiency virus (rSVV-SIV) vaccinated rhesus macaques. Virology. 2012;9:160. doi: 10.1186/1743-422X-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultheiss T, Schulte R, Sauermann U, Ibing W, Stahl-Hennig C. Strong mucosal immune responses in SIV infected macaques contribute to viral control and preserved CD4+ T-cell levels in blood and mucosal tissues. Retrovirology. 2011;8:24. doi: 10.1186/1742-4690-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawada M, Tsukamoto T, Yamamoto H, Takeda A, Igarashi H, Watkins DI, Matano T. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J Virol. 2007;81:5202–5211. doi: 10.1128/JVI.02881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattapallil JJ, Hill B, Douek DC, Roederer M. Systemic vaccination prevents the total destruction of mucosal CD4 T cells during acute SIV challenge. J Med Primatol. 2006;35:217–224. doi: 10.1111/j.1600-0684.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 57.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O’Connor DH, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto T, Johnson MJ, Price DA, Wolinsky DI, Almeida JR, Petrovas C, Nason M, Yeh WW, Shen L, Roederer M, Rao SS, McDermott AB, Lefebvre F, Nabel GJ, Haddad EK, Letvin NL, Douek DC, Koup RA. Virus inhibition activity of effector memory CD8(+) T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J Virol. 2012;86:5877–5884. doi: 10.1128/JVI.00315-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boyer JD, Maciag PC, Parkinson R, Wu L, Lewis MG, Weiner DB, Paterson Y. Rhesus macaques with high levels of vaccine induced IFN-gamma producing cells better control viral set-point following challenge with SIV239. Vaccine. 2006;24:4498–4502. doi: 10.1016/j.vaccine.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 61.Vaccari M, Mattapallil J, Song K, Tsai WP, Hryniewicz A, Venzon D, Zanetti M, Reimann KA, Roederer M, Franchini G. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J Virol. 2008;82:9629–9638. doi: 10.1128/JVI.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori DC, Markham P, Felber BK, Pavlakis GN. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol. 2005;79:8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, Kalyanaraman VS, Markham PD, Robey FA, Robert-Guroff M. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol. 2004;78:2212–2221. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson LJ, Daltabuit-Test M, Xiao P, Zhao J, Hu W, Wille-Reece U, Brocca-Cofano E, Kalyanaraman VS, Kalisz I, Whitney S, Lee EM, Pal R, Montefiori DC, Dandekar S, Seder R, Roederer M, Wiseman RW, Hirsch V, Robert-Guroff M. Rapid SIV Env-specific mucosal and serum antibody induction augments cellular immunity in protecting immunized, elite-controller macaques against high dose heterologous SIV challenge. Virology. 2011;411:87–102. doi: 10.1016/j.virol.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, Bett AJ, Liang X, Wang F, Thoryk E, Heidecker GJ, Citron MP, Huang L, Lin J, Vitelli S, Ahn CD, Kaizu M, Maness NJ, Reynolds MR, Friedrich TC, Loffredo JT, Rakasz EG, Erickson S, Allison DB, Piatak M, Jr, Lifson JD, Shiver JW, Casimiro DR, Shaw GM, Hahn BH, Watkins DI. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009;83:6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martins MA, Wilson NA, Piaskowski SM, Weisgrau KL, Furlott JR, Bonaldo MC, Veloso de Santana MG, Rudersdorf RA, Rakasz EG, Keating KD, Chiuchiolo MJ, Piatak M, Jr, Allison DB, Parks CL, Galler R, Lifson JD, Watkins DI. Vaccination with gene fragments of gag, vif, and nef affords partial control of viral replication after mucosal challenge with SIVmac239. J Virol. 2014 doi: 10.1128/JVI.00601-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giraldo-Vela JP, Bean AT, Rudersdorf R, Wallace LT, Loffredo JT, Erickson P, Wilson NA, Watkins DI. Simian immunodeficiency virus-specific CD4+ T cells from successful vaccinees target the SIV Gag capsid. Immunogenetics. 2010;62:701–707. doi: 10.1007/s00251-010-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawada M, Tsukamoto T, Yamamoto H, Iwamoto N, Kurihara K, Takeda A, Moriya C, Takeuchi H, Akari H, Matano T. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol. 2008;82:10199–10206. doi: 10.1128/JVI.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jalah R, Kulkarni V, Patel V, Rosati M, Alicea C, Bear J, Yu L, Guan Y, Shen X, Tomaras GD, LaBranche C, Montefiori DC, Bess J, Jr, Lifson JD, Reed S, Sardesai NY, Venzon D, Valentin A, Pavlakis GN, Felber BK. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in rhesus macaques. PLoS One. 2014;9:e91550. doi: 10.1371/journal.pone.0091550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Genesca M, Skinner PJ, Bost KM, Lu D, Wang Y, Rourke TL, Haase AT, McChesney MB, Miller CJ. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 2008;1:219–228. doi: 10.1038/mi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genesca M, McChesney MB, Miller CJ. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J Intern Med. 2009;265:67–77. doi: 10.1111/j.1365-2796.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manrique M, Kozlowski PA, Cobo-Molinos A, Wang SW, Wilson RL, Montefiori DC, Carville A, Aldovini A. Immunogenicity of a vaccine regimen composed of simian immunodeficiency virus DNA, rMVA, and viral particles administered to female rhesus macaques via four different mucosal routes. J Virol. 2013;87:4738–4750. doi: 10.1128/JVI.03531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strbo N, Vaccari M, Pahwa S, Kolber MA, Fisher E, Gonzalez L, Doster MN, Hryniewicz A, Felber BK, Pavlakis GN, Franchini G, Podack ER. Gp96 SIV Ig immunization induces potent polyepitope specific, multifunctional memory responses in rectal and vaginal mucosa. Vaccine. 2011;29:2619–2625. doi: 10.1016/j.vaccine.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]