Abstract
A variety of both RNA and DNA viruses envelop their capsids in a lipid bilayer. One of the more recently appreciated benefits this envelope is incorporation of phosphatidylserine (PtdSer). Surface exposure of PtdSer disguises viruses as apoptotic bodies; tricking cells into engulfing virions. This mechanism is termed apoptotic mimicry. Several PtdSer receptors have been identified to enhance virus entry and we have termed this group of proteins PtdSer-mediated virus entry enhancing receptors or PVEERs. These receptors enhance entry of a range of enveloped viruses. Internalization of virions by PVEERs provides a broad mechanism of entry with little investment by the virus itself. PVEERs may allow some viruses to attach to cells, thereby making viral glycoprotein/cellular receptor interactions more probable. Alternatively, other viruses may rely entirely on PVEERs for internalization into endosomes. This review provides an overview of PtdSer receptors that serve as PVEERs and the biology behind virion/PVEER interaction.
Keywords: Enveloped virus, Virus entry, Phosphatidylserine, Phosphatidylserine receptor, Filovirus, Flavivirus, Alphavirus, Baculovirus, PVEER, TIM-1, TIM-4, TAM, Tyro3, Axl, Mer, MFG-E8, Integrin αvβ3, Integrin αvβ5, Receptors
Highlights
-
•
Phosphatidylserine (PtdSer) receptors can mediate entry of enveloped viruses.
-
•
PtdSer is present on the outer leaflet of the virion envelope.
-
•
PtdSer receptors are expressed on a variety of primary cells and cell lines.
-
•
Characteristics of PtdSer receptors that mediate virus entry are defined.
Introduction
A variety of both RNA and DNA viruses envelop their capsids in a lipid bilayer. This outer membrane is obtained during virus budding from either plasma or organelle membranes. While reliance on an envelope sensitizes viruses to desiccation, detergents, and heat, these envelopes provide a number of benefits for the virus, including protection of viral structural proteins from immune recognition and neutralizing antibodies, a platform for displaying viral proteins, a barrier to enclose viral and cellular proteins necessary for early steps during infection, and a mechanism for virus egress without lysing infected cells. In addition, a more recently appreciated benefit is the incorporation of phospholipids into viral envelopes. Presentation of phosphatidylserine (PtdSer) on the outer leaflet of these membranes disguises viruses as apoptotic bodies, thereby conning cells into engulfing virions through cell clearance mechanisms. This mechanism of enhanced virus entry is termed apoptotic mimicry.
Over the past three years, receptors and receptor complexes have recently been identified that enhance entry of a diverse range of enveloped viruses. This group of viral receptors shares the ability to bind to PtdSer present on the viral envelope and, consequently, we have termed them PtdSer-mediated virus entry enhancing receptors or PVEERs. The broad expression of these receptors and their ability to interact with PtdSer on a wide array of enveloped viruses has huge potential implications for virus infection. Most importantly, PVEERs enhance virus binding to cells and facilitate internalization. These receptors also contribute to immune evasion through both anti-inflammatory signaling and a mechanism of entry that does not require extracellular exposure of key receptor-binding domain and fusion epitopes. In this review we summarize what is known about the identified PVEERs and their role in virus entry and identify important gaps in current knowledge.
History of PVEER discovery
Apoptotic mimicry was first hypothesized to be used by hepatitis B virus (Vanlandschoot and Leroux-Roels, 2003), but was experimentally confirmed with vaccinia virus (Mercer and Helenius, 2008). Inactivation of vaccinia virus by NP-40-mediated lipid depletion could be rescued by incubation with PtdSer liposomes (Oie, 1985). However, the contribution of PtdSer to infection was not understood. Mercer et al. furthered these studies and determined that not only PtdSer is present on the surface of some vaccinia infectious particles, but Annexin V (AnxV), a PtdSer-binding protein, can bind to PtdSer on viral envelopes and inhibit vaccinia infection (Mercer and Helenius, 2008). These results were confirmed by another study that found substitution with a non-biologically relevant isomer of PtdSer restored infectivity (Laliberte and Moss, 2009). In addition, a role for viral envelope PtdSer during infection was demonstrated for Pichinde virus and HIV-1 (Soares et al., 2008, Callahan et al., 2003).
A protein complex composed of growth-arrest-specific 6 (Gas6) and the tyrosine kinase receptor, Axl, was the first set of cellular proteins to be implicated in PtdSer-binding enhancement of viral entry (Morizono et al., 2011). It was shown that the soluble protein Gas6 binds to PtdSer on the virion surface and bridges virus to the cell surface via interaction with the tyrosine kinase receptor, Axl and formation of this complex is necessary for enhancement of virus entry. This study found in addition to vaccinia virus, the Gas6/Axl complex enhances binding and entry of lentiviruses pseudotyped with Ross River GP, baculovirus GP64, or Sindbis env, demonstrating for the first time that PtdSer binding can enhance entry of viral particles bearing a variety of different viral glycoproteins. More recently, a variety of additional PVEERs were identified, including T-cell immunoglobulin and mucin domain 1 and 4 (TIM-1 and 4) proteins and MFG-E8/integrin αvβ3 or αvβ5 complexes (Meertens et al., 2012, Jemielity et al., 2013, Moller-Tank et al., 2013, Morizono and Chen, 2014). The details of virion interactions with different PVEERs are highlighted below.
Several recent studies have expanded our understanding of PVEERs. The group of viruses whose entry is enhanced by PVEERs now include members of the flavivirus, filovirus, New World arenavirus, baculovirus, and alphavirus families (Meertens et al., 2012, Jemielity et al., 2013, Moller-Tank et al., 2013, Morizono and Chen, 2014). Between viruses within these families, the extent to which PVEERs enhance virus entry varies, but generally viruses do not appear to utilize one PVEER effectively and not another. This suggests that virion uptake mediated by the different PVEERs is mechanistically similar. Despite the strong evidence that PVEERs interact with virion associated PtdSer, the utilization of PVEER-dependent virus entry is influenced by the viral glycoprotein presence on the virion. For instance, entry of viruses bearing the envelope proteins of Old World arenaviruses, coronaviruses, influenza A virus, vesicular stomatitis virus, or herpes simplex virus 1 is not enhanced by PVEER expression (Morizono et al., 2011, Meertens et al., 2012, Jemielity et al., 2013, Moller-Tank et al., 2013, Kondratowicz et al., 2011). Thus, while PVEERs do not enhance entry all enveloped viruses, they do represent a potentially important class of receptors for a large and diverse collection of important human pathogenic viruses.
Characterization of identified PVEERs
Six PVEERs have been identified to date: TIM-1, TIM-4, Gas6 or Protein S/Axl, Mer, and Tyro3, and MFG-E8/integrin αvβ3 and αvβ5. A summary of characteristics of these PVEERs is shown in Table 1. The key property that these proteins or complexes utilize to enhance virus entry is their ability to bind PtdSer and a native function of all of these receptors is to bind and clear apoptotic bodies (Ravichandran, 2011). Mutation of residues involved in PtdSer binding or complex formation results in inhibition of PVEER function (Morizono et al., 2011, Meertens et al., 2012, Moller-Tank et al., 2013, Morizono and Chen, 2014). Further, competition with PtdSer liposomes, but not phosphatidylcholine liposomes, inhibits entry enhancement by PVEERs (Jemielity et al., 2013, Moller-Tank et al., 2013). Phosphatidylethanolamine liposomes are also somewhat inhibitory, likely due to sharing similar structure to PtdSer (Jemielity et al., 2013). In some cases, prebinding virus with the PtdSer-binding protein, Annexin V (AnxV), has been shown to inhibit PVEER enhancement of entry (Mercer and Helenius, 2008, Callahan et al., 2003, Meertens et al., 2012), but not in others (Morizono and Chen, 2014). Consistent with the ability of an array of PtdSer receptors to serve as PVEERS, an artificially generated PVEER containing the PtdSer-binding domain from AnxV was highly effective at mediating uptake of vesicular stomatitis virus (VSV) pseudovirions bearing a filovirus, alphavirus or baculovirus glycoprotein (Moller-Tank et al., 2014).
Table 1.
PtdSer receptors: distribution and efficacy as a viral receptor.
| Protein name (s) | PtdSer binding domain | Tissue/cell type expression | Pseudoviruses for which entry is enhanced (>2 fold) | Pseudoviruses for which entry is unaffected (<2 fold) | Cell lines reported to express PtdSer receptor |
|---|---|---|---|---|---|
| TIM-1 | IgV(Kobayashi et al., 2007) | Immune cells: | Filovirus: | Old World Arenavirus: | Human: |
| B cells (Sizing et al., 2007), mast cells (Nakae et al., 2007), TH2 CD4+ T cells (Meyers et al., 2005, Umetsu et al., 2005, Khademi et al., 2004), and NKT cells (Lee et al., 2010, Kim et al., 2013) | Ebola● and Marburg (Jemielity et al., 2013, Moller-Tank et al., 2013, Kondratowicz et al., 2011) | Lassa Virus, LCMV (Jemielity et al., 2013, Moller-Tank et al., 2013) | Huh-7, ACHN, A498, 786-O, Caco-2, CAKI-1, TK-10, UO-3, Vero, A549, Cos-7 (Meertens et al., 2012, Kondratowicz et al., 2011) | ||
| Epithelial cells: | Alphavirus: | New World Arenavirus: | |||
| Kidney (Kondratowicz et al., 2011, Ichimura et al., 1998) and airway and eye mucosa (Kondratowicz et al., 2011) | Ross River●, Chikungunya, Sindbis●, Eastern Equine Encephalitis (Jemielity et al., 2013, Moller-Tank et al., 2013, Morizono and Chen, 2014) | Oliveros (Jemielity et al., 2013) | |||
| Baculovirus: | Influenza A virus: | ||||
| Autographa californica multicapsid nucleopolyhedrovirus● (Moller-Tank et al., 2013) | H7N1, H1N1● (Jemielity et al., 2013) | ||||
| Rhabdovirus: | Coronavirus: | ||||
| Vesicular stomatitis virus (Morizono and Chen, 2014) | SARS (Jemielity et al., 2013) | ||||
| New World Arenavirus: | Rhabdovirus: | ||||
| Amapari, Tacaribe●, Junín, and Machupo virus (Jemielity et al., 2013) | Vesicular stomatitis virus (Jemielity et al., 2013) | ||||
| Flavivirus: | Herpes simplex virus: | ||||
| West Nile Virus●, Dengue●, Yellow Fever virus● (Meertens et al., 2012, Jemielity et al., 2013) | HSV-1● (Meertens et al., 2012) | ||||
| TIM-3 | IgV(DeKruyff et al., 2010) | Immune cells: | Flavivirus: | Alphavirus: | Mouse: |
| TH1 and TH17 CD4+ T cells (Khademi et al., 2004, Hastings et al., 2009, Monney et al., 2002, Nakae et al., 2007), mast cells (Nakae et al., 2007), DCs (DeKruyff et al., 2010, Anderson et al., 2007), and monocytes (Anderson et al., 2007) | West Nile Virus, Dengue Virus● (Meertens et al., 2012, Jemielity et al., 2013) | Sindbis (Morizono and Chen, 2014) | Raw264.7 (DeKruyff et al., 2010) | ||
| Epithelial cells: | New World Arenavirus: | Filovirus: | Human: | ||
| Bronchial (DeKruyff et al., 2010) | Tacaribe (Jemielity et al., 2013) | Ebola, Marburg (Jemielity et al., 2013) | PMA stimulated THP-1 cells (DeKruyff et al., 2010) | ||
| Old World Arenavirus: | |||||
| LASV, LCMV (Jemielity et al., 2013) | |||||
| New World Arenavirus: | |||||
| Tacaribe, Junín, Machupo, Oliveros (Jemielity et al., 2013) | |||||
| Influenza A virus: | |||||
| H7N1 (Jemielity et al., 2013) | |||||
| Rhabdovirus: | |||||
| Vesicular stomatitis virus (Jemielity et al., 2013) | |||||
| TIM-4 | IgV(Kobayashi et al., 2007; Santiago et al., 2007; Miyanishi et al., 2007) | Immune cells: | Filovirus: | New World Arenavirus: | |
| Macrophages and mature DCs (Kobayashi et al., 2007, DeKruyff et al., 2010, Miyanishi et al., 2007, Mizui et al., 2008, Meyers et al., 2005), and B-1 cells (Rodriguez-Manzanet et al., 2010) | Ebola, Marburg (Jemielity et al., 2013, Moller-Tank et al., 2013) | Oliveros (Jemielity et al., 2013) | |||
| Tissues: | Alphavirus: | Old World Arenavirus: | |||
| Spleen, lymph node, and peritoneum (Miyanishi et al., 2007, Meyers et al., 2005) | Sindbis, Ross River (Morizono and Chen, 2014), Eastern Equine Encephalitis (Jemielity et al., 2013) | LASV, LCMV (Jemielity et al., 2013) | |||
| New World Arenavirus: | Influenza A virus: | ||||
| Tacaribe, Junín, Machupo (Jemielity et al., 2013) | H7N1 (Jemielity et al., 2013) | ||||
| Baculovirus: | Herpes simplex virus: | ||||
| Autographa californica multicapsid nucleopolyhedrovirus (Morizono and Chen, 2014) | HSV-1● (Meertens et al., 2012) | ||||
| Rhabdovirus: | |||||
| Vesicular stomatitis virus (Jemielity et al., 2013) | |||||
| Flavivirus: | |||||
| West Nile Virus●, Dengue●, Yellow Fever virus● (Meertens et al., 2012, Jemielity et al., 2013) | |||||
| TAM ligand (Protein S or Gas6) / TAM receptor kinases (Tyro3, Mer, or Axl) | Gla(Huang et al., 2003; Rajotte et al., 2008) domain of TAM ligand | Reviewed here: (Lemke and Rothlin, 2008) | Filovirus: | Old World Arenavirus: | Human: |
| Blood cells: | Ebola, Marburg (Jemielity et al., 2013, Shimojima et al., 2006) | LASV, LCMV (Jemielity et al., 2013) | A549, Vero, Cos-7, HeLa, SNB19, and SN12C (Meertens et al., 2012, Shimojima et al., 2006, Brindley et al., 2011) | ||
| Platelets (Angelillo-Scherrer et al., 2001) | Rhabdovirus: | New World Arenavirus: | |||
| Immune cells: | Vesicular stomatitis virus (Morizono and Chen, 2014) | Machupo (Jemielity et al., 2013), | |||
| Macrophages, NK cells, NKT cells, and DCs (Seitz et al., 2007, Caraux et al., 2006, Behrens et al., 2003) | Baculovirus: | Oliveros (Jemielity et al., 2013) | |||
| Connective tissue: | Autographa californica multicapsid nucleopolyhedrovirus (Morizono et al., 2011, Morizono and Chen, 2014) | Rhabdovirus: | |||
| Bone marrow stromal cells (Caraux et al., 2006) | Alphavirus: | Vesicular stomatitis virus (Jemielity et al., 2013) | |||
| Tissues: | Sindbis, Ross River (Morizono et al., 2011, Morizono and Chen, 2014) | Influenza A virus: | |||
| Testes (Lu et al., 1999, Wang et al., 2005), CNS (Stitt et al., 1995, Prieto et al., 2000), retina (Prasad et al., 2006), and foreskin fibroblasts (Brindley et al., 2011) | Pox virus: | H7N1 (Jemielity et al., 2013) | |||
| Vaccinia Virus● (Morizono et al., 2011) | Herpes simplex virus: | ||||
| New World Arenavirus: | HSV-1● (Meertens et al., 2012) | ||||
| Amapari, Tacaribe, Junín (Jemielity et al., 2013) | |||||
| Alphavirus: | |||||
| Chikingunya, Eastern Equine Encephalitis (Jemielity et al., 2013) | |||||
| Flavivirus: | |||||
| Dengue● (Meertens et al., 2012) | |||||
| MFG-E8/ αvβ3-5 integrin | C2 domain (Andersen et al., 2000, Ye et al., 2013) | Immune cells: | Alphavirus: | Rhabdovirus: | Human: |
| Macrophages (Hanayama et al., 2002, Hanayama et al., 2004, Finnemann and Rodriguez-Boulan, 1999, Antonov et al., 2004), Immature DCs (Miyasaka et al., 2004, Albert et al., 1998) | Ross River and Sindbis (Morizono and Chen, 2014) | Vesicular stomatitis virus (Morizono and Chen, 2014) | HEC-1A, Ishikawa, HEEC, K562, HL60 (Bocca et al., 2012, Kruger et al., 2000); | ||
| Tissues: | Baculovirus: | Mouse: | |||
| Mammary glands (Oshima et al., 1999, Stubbs et al., 1990), spleen, lymph node, brain (Hanayama et al., 2004, Garmy‐Susini et al., 2007, Uehara and Uehara, 2014), and vascular system (Silvestre et al., 2005, Brooks et al., 1994) | Autographa californica multicapsid nucleopolyhedrovirus (Morizono and Chen, 2014) | COMMA-1D, D1, P388D1(Hanayama et al., 2004; Thery et al., 1999; Oshima et al., 2002) | |||
| CD300a | IgV (Simhadri et al., 2012) | Immune cells: | Alphavirus: | Alphavirus: | Human: |
| CD8+ T cells (Xu et al., 2012), CD4+ T cells (Clark et al., 2007, Simhadri et al., 2011, Narayanan et al., 2010), B cells (Silva et al., 2011), and NK cells (Lankry et al., 2010, Cantoni et al., 1999) | Sindbis (Morizono and Chen, 2014)●● | Ross River and Sindbis (Morizono and Chen, 2014) | THP1, U937, REC-1, SUDHL5, 721.221 (Silva et al., 2011, Kim et al., 2012) | ||
| Baculovirus: | |||||
| Autographa californica multicapsid nucleopolyhedrovirus (Morizono and Chen, 2014) | |||||
| Rhabdovirus: | |||||
| Vesicular stomatitis virus (Morizono and Chen, 2014) | |||||
| BAI1 | Type 1 thrombospondin repeats (TSRs) (Park et al., 2007) | Tissues: | Alphavirus: | Human: | |
| Brain (Shiratsuchi et al., 1997, Sokolowski et al., 2011), muscle(Hochreiter-Hufford et al., 2013), bone marrow, and spleen (Park et al., 2007) | Ross River and Sindbis (Morizono and Chen, 2014) | J774 and RAW264.7 (Park et al., 2007) | |||
| Immune cells: | Baculovirus: | ||||
| Macrophages (Park et al., 2007) | Autographa californica multicapsid nucleopolyhedrovirus (Morizono and Chen, 2014) | ||||
| Rhabdovirus: | |||||
| Vesicular stomatitis virus (Morizono and Chen, 2014) | |||||
| Flavivirus: | |||||
| Dengue● (Meertens et al., 2012) | |||||
| RAGE | IgV (Friggeri et al., 2011)●●● | Immune cells: | Old World Arenavirus: | Human: | |
| CD8+ and CD4+ T cells (Akirav et al., 2012, Moser et al., 2007), DCs (Dumitriu et al., 2005), macrophages (Zhang et al., 2009, Sunahori et al., 2006), and monocytes (Ohashi et al., 2010) | Lassa (Moller-Tank et al., 2013) | HaCaT (Zhu et al., 2012), A549 (Nakano et al., 2006) | |||
| Tissues: | Filovirus: | ||||
| Smooth muscle (Zhang et al., 2009, Kamioka et al., 2011), | Ebola virus (Moller-Tank et al., 2013) | ||||
| cartilidge chondrocytes (Loeser et al., 2005), skin keratinocytes (Zhu et al., 2012), and vascular system (Pollreisz et al., 2010, Liu et al., 2010) | |||||
| Stabilin-1/-2 | Epidermal growth factor-like domain (Park et al., 2009, Park et al., 2008, Kim et al., 2010) | Immune cells: | Alphavirus: | Human: | |
| Macrophages (Park et al., 2008, Park et al., 2009, Martens et al., 2006) | Ross River and Sindbis (Morizono and Chen, 2014) | PMA stimulated THP1(Park et al., 2008) | |||
| Tissues: | Baculovirus: | Mouse: | |||
| Sinusoidal endothelial cells (Martens et al., 2006, Falkowski et al., 2003), spleen, lymph node, liver, bone marrow, cornea, brain, heart, and kidney (Falkowski et al., 2003, Goerdt et al., 1991) | Autographa californica multicapsid nucleopolyhedrovirus (Morizono and Chen, 2014) | PMA stimulated P388D1 cells (Park et al., 2008) | |||
| Rhabdovirus: | |||||
| Vesicular stomatitis virus (Morizono and Chen, 2014) | |||||
●Confirmed using infectious virus.
●●Enhances binding only, but not infection.
●●●Hypothesized but not shown
Recent studies have demonstrated that virus internalization into HEK 293T cells can occur entirely independently of the presence of a viral glycoprotein. This virion internalization is significantly enhanced by PVEER overexpression and is inhibited by competition with PtdSer liposomes (Jemielity et al., 2013, Moller-Tank et al., 2013), providing compelling evidence that virion associated PtdSer/PVEER interactions are responsible for virion uptake, not just virus binding. While the cellular compartment(s) into which PVEERs deliver virions has yet to be identified, indirect evidence indicates that uptake of cargo is into endosomes (Kobayashi et al., 2007, Albacker et al., 2010). Once the virion is internalized, the presence of a viral glycoprotein on the virion is necessary for fusion events with the endosomal membrane. An overview of each group of PVEERs is given below.
TIM-1 and TIM-4
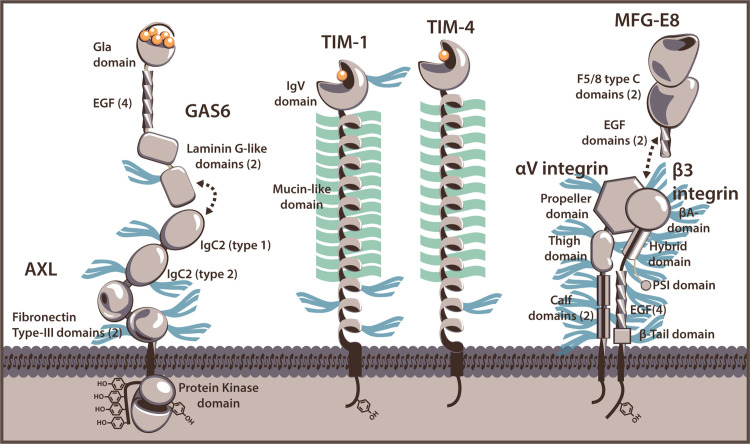
TIM-1 was first implicated in enveloped virus entry as a specific receptor for filoviruses (Kondratowicz et al., 2011). However, it was later discovered that both TIM-1 and family member, TIM-4, enhance virus entry as PVEERs through binding of PtdSer (Meertens et al., 2012, Jemielity et al., 2013, Moller-Tank et al., 2013). The human TIM family members are type I, cell-surface glycoproteins that along with their murine counterparts, share a common structure. Their amino terminal immunoglobulin variable (IgV)-like domain extends from the plasma membrane by a heavily O-linked-glycosylated mucin-like domain (MLD), which is attached to the cell surface by a transmembrane domain followed by a cytoplasmic tail ( Fig. 1) (McIntire et al., 2004). TIM-1 signals through phosphorylation of cytoplasmic tail tyrosines (Binne et al., 2007, de Souza et al., 2005, de Souza et al., 2008); however, none of the TIM family members require their cytoplasmic domains for PVEER function (Meertens et al., 2012, Moller-Tank et al., 2014).
Fig. 1.
PtdSer-binding receptors that function as PVEERs. Cartoon representations of Gas 6/Axl (representatives of TAM ligand and receptor kinases), TIM-1, TIM-4, and MFG-E8/ αvβ3 integrin are displayed. Estimations of N-linked glycan sites are represented as blue tridents and O-link glycoslyations (TIM-1 and TIM-4 only) are shown as green lines. Domains for which calcium-binding is necessary for interaction with PtdSer are indicated with calcium ions (orange spheres). Gas6 and Axl interact through their respective laminin G-like and IgC2 domains. MFG-E8 contains an Arg-Gly-Asp (RGD) motif in the second EGF domain that likely binds at the interface of the αvβ3/5 integrin complex (Xiong et al., 2002).
TIM-1 and TIM-4 are unique among PVEERs as their PtdSer-binding domains are a portion of a transmembrane protein rather than an independent, small protein that binds to a plasma membrane receptor. The PtdSer-binding pocket is located between two loops of the IgV domain ( Fig. 2) (Kobayashi et al., 2007, Santiago et al., 2007, DeKruyff et al., 2010, Miyanishi et al., 2007). Conserved aspartate and asparagine residues within the upper loop are involved in coordination of a cation that, in conjunction with residues of the lower loop, form hydrogen bonds with the phosphate and serine groups of PtdSer (Santiago et al., 2007). This PtdSer binding is necessary for the native functions of TIM-1 and TIM-4: apoptotic body clearance and immune cell regulation (Albacker et al., 2010, Miyanishi et al., 2007, Lee et al., 2010, Kim et al., 2013, Ichimura et al., 2008, Rodriguez-Manzanet et al., 2010, Mizui et al., 2008, Wong et al., 2010). Mutation of aspartate and/or asparagine or chelation of free cations with EGTA results in significant loss in PtdSer binding and subsequent PVEER efficacy (Meertens et al., 2012, Moller-Tank et al., 2013). A recent study suggests TIM-4 is more sensitive than TIM-1 or -3 to differences in membrane-PtdSer concentrations due to additional PtdSer-binding residues outside of the pocket (Tietjen et al., 2014). This altered sensitivity may have implications for virus binding.
Fig. 2.
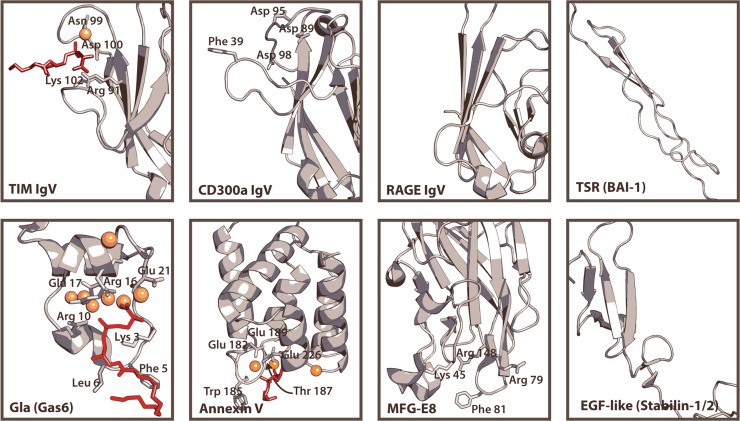
Structures of PtdSer-binding domains. Representative renders of the PtdSer-binding domains of TIM-1/-3/-4, CD300a, RAGE, BAI-1, Gas6/Protein S, Annexin V, MFG-E8, and Stabilin-1/2 are shown. PtdSer-like ligands (red) and calcium ions (orange) are also displayed for structures with which they were solved. For domains that do not have solved structures, equivalent domains from other proteins are shown. The IgV domain of TIM-1/-3/-4 is represented by the mTIM-4 IgV domain (2OR8) (Santiago et al., 2007). The human CD300a IgV domain crystal structure (Dimasi et al., 2007) is shown with residues hypothesized for interaction (Simhadri et al., 2012). The region of the RAGE IgV domain (3O3U) that corresponds to the binding pockets of CD300a and TIM-1 IgV domains is shown for comparison (Park et al., 2010); however, this domain is only hypothesized to interact with PtdSer and no residues have been implicated (Friggeri et al., 2011). The thrombospondin type-1 repeats (TSRs) of BAI1 are represented by TSR domain 3 of human thrombospondin-1 (3R6B) (Klenotic et al., 2011). Gas6 binds PtdSer via a Gla domain, represented by that of bovine prothrombin (1NL2) with residues identified that interact with PtdSer (red) (Huang et al., 2003). For AnxV, the structure of one annexin repeat domain from rat Anx V is shown (1A8A) with residues identified that interact with PtdSer (red) (Swairjo et al., 1995). The C2 domain of bovine MFG-E8 binds to PtdSer (3BN6) (Shao et al., 2008) and while the structure was not crystallized with PtdSer, key residues involved in interaction have been determined experimentally (Ye et al., 2013). An EGF-like domain of stabilin-1 or -2 is represented by that from human heregulin alpha (1HRE) (Nagata et al., 1994).
While all human TIM family members can bind PtdSer and enhance the uptake of apoptotic bodies (Kobayashi et al., 2007, DeKruyff et al., 2010), TIM-3 does not effectively enhance virus entry (<2 fold) (Meertens et al., 2012, Jemielity et al., 2013, Kondratowicz et al., 2011). Though the IgV domain of TIM-3 has reduced affinity for PtdSer (DeKruyff et al., 2010), replacing the TIM-1 or TIM-4 IgV domain with that of TIM-3 results in a functional PVEER (Moller-Tank et al., 2014). Thus, the reduced efficacy is not due to the TIM-3 IgV domain. Rather, the inability of TIM-3 to function effectively as a PVEER is likely due to the short length of the TIM-3 MLD which is ~2.5-fold shorter than that of TIM-1 and -4 (Fig. 1). This is supported by studies showing that deletions within the MLD of TIM-1 reduce PVEER efficacy (Jemielity et al., 2013, Moller-Tank et al., 2014).
Protein S and Gas6/Tyro3, Axl, and Mer
Tyro3, Axl, and Mer (TAM) are members of the TAM family of receptor tyrosine kinases. These highly related proteins contain two N-terminal immunoglobulin-like domains, followed by two fibronectin type III domains, a single transmembrane domain, and a cytoplasmic protein tyrosine kinase (PTK) domain (Fig. 2). TAM N-terminal interaction with Gas6 leads to activation of the TAM receptors and autophosphorylation of tyrosines within the PTK domain (Stitt et al., 1995). Tyro3 and Mer, but not Axl, are similarly activated by binding Protein S (Stitt et al., 1995, Prasad et al., 2006). Both Gas6 and Protein S consist of an N-terminal domain rich in γ-carboxyglutamic acid residues (Gla) that binds to PtdSer (Fig. 1) (Huang et al., 2003, Ishimoto et al., 2000, Rajotte et al., 2008), a loop region, four epidermal growth factor-like repeats, and two C- terminal laminin G-like domains forming a sex hormone-binding globulin-like structure that binds to the Ig-like domains of the TAM receptors (Sasaki et al., 2006, Sasaki et al., 2002). Dimerization of TAM receptors occurs after binding of their ligands, resulting in a 2:2 complex, and is necessary for signaling (Sasaki et al., 2006).
All TAM receptors in combination with their Gas6 or Protein S ligand have been shown to effectively enhance virus entry, although studies suggest that the relative effectiveness of the three TAM members as PVEERs varies (Morizono et al., 2011, Meertens et al., 2012, Jemielity et al., 2013, Bhattacharyya et al., 2013). This may be due to differences in binding affinities between the TAM receptors and their ligands (Nagata et al., 1996) or relative expression. As the PtdSer-binding activity of PVEERs is essential for efficacy, in the case of the TAM receptors, this occurs through the Gla domain of Gas6/Protein S and removal of this domain eliminates PVEER function of the TAMs (Morizono et al., 2011, Meertens et al., 2012). Similarly mutation of the Gas6 binding residues of Axl inhibits virus uptake by the complex (Meertens et al., 2012). Thus, the PVEER efficacy of the TAM ligand/receptor complexes requires both efficient binding of PtdSer by ligand and binding of ligand by receptor.
Contrary to the TIM family PVEERs where cytoplasmic tail signaling is not required for enhancing virus entry into cells (Moller-Tank et al., 2014), signaling through the PTK domain of TAM receptors is essential for enhancement of viral infection (Meertens et al., 2012, Bhattacharyya et al., 2013). While the Gas6/Axl complex is still able to enhance WNV binding and internalization without kinase activity, subsequent infection is significantly impaired compared to infection of cells expressing WT Axl. This kinase activity is important in vivo for TAM-receptor-inhibition of DC activation and inflammation (Rothlin et al., 2007, Tibrewal et al., 2008). Ligand binding by the receptors and assocation with the type I interferon receptor leads to activation of STAT1 and induction of suppressor of cytokine signaling (SOCS) proteins (Rothlin et al., 2007). Viruses appear to utilize this anti-inflammatory signaling pathway to dampen the immune response and promote replication (Bhattacharyya et al., 2013). While TAM signaling is triggered by interaction with ligand alone, signaling is significantly enhanced by the presence of virus which helps to facilitate the interaction of TAM ligands with their receptors (Bhattacharyya et al., 2013). Thus, the TAM receptors enhance virus infection through PVEER functions by enhancing virus binding and internalization and non-PVEER functions by inhibiting innate immunity, both of which require binding to PtdSer on the virion surface and likely contribute to enhancing in vivo virus loads.
MFG-E8/integrin αvβ3 and αvβ5
Milk fat globule-EGF factor 8 (MFG-E8), the most recently identified PVEER, is a secreted protein that contains two N-terminal EGF repeats followed by a C1 and C2 domain, of which the latter binds to PtdSer (Andersen et al., 2000, Ye et al., 2013). Several key positively charged residues within the C2 domain are responsible for interacting with the phosphate and serine groups, while hydrophobic residues of the domain stabilize the positioning of PtdSer (Fig. 1) (Ye et al., 2013). Like the Gas6, MFG-E8 bridges PtdSer containing membranes to cells through interaction with a membrane bound protein, either integrin αvβ3 or αvβ5 (Andersen et al., 2000, Hanayama et al., 2002). This occurs through an Arg-Gly-Asp (RGD) motif present in the second EGF repeat of MFGE-8 and mutation of this motif inhibits PVEER efficacy (Morizono and Chen, 2014).
Several aspects of MFG-E8 biology may contribute to infection. Similar to the Gas6/Axl complex, MFG-E8/integrin complexes have been shown to induce production of anti-inflammatory cytokines such as IL-10 (Aziz et al., 2009, Jinushi et al., 2009), some of which are initiated by activation of STAT-3 (Brissette et al., 2012, Jinushi et al., 2011). Interestingly, STAT-3 has also been identified as an upstream regulator of TIM-1 expression (Ajay et al., 2014). However, currently MFG-E8 induced signaling activity has not yet been associated with enhancement of virus entry.
Expression of PVEERs and implications for tropism
The presence of PVEERs in various tissues permissive to infection may contribute to the tropism of some viruses, particularly for flaviviruses and filoviruses, whose entries are most enhanced by PVEER expression. PVEERs are expressed in a variety of tissues and cell types (summarized in Table 1) that are key targets of infection by enveloped viruses. TIM-1, TIM-4, and Axl (Li et al., 2013, Dorfman et al., 2010, Bauer et al., 2012) may enhance uptake of Dengue virus into Langerhans dendritic cells (DCs), one of the first targets during flavivirus infection (Wu et al., 2000, Marovich et al., 2001). TIM-1 is also present on the mucosal epithelia of the airway and eye (Kondratowicz et al., 2011), both of which may be routes of infection for filoviruses (Jaax et al., 1996). Early during infection, flaviviruses and filoviruses replicate in antigen-presenting cell (APC) populations: DCs and macrophages (Geisbert et al., 2003a, Geisbert et al., 2003b, Jessie et al., 2004). Similarly, alphaviruses infect DCs (Labadie et al., 2010, Gardner et al., 2000, Nishimoto et al., 2007, Shabman et al., 2007) and establish persistent infections in macrophages (Labadie et al., 2010, Linn et al., 1996). Interestingly, these APCs express a variety of PVEERs including TIM-4 (Kobayashi et al., 2007, Rodriguez-Manzanet et al., 2010, Mizui et al., 2008, Meyers et al., 2005, Baghdadi et al., 2013), TAM receptors (Seitz et al., 2007, Caraux et al., 2006, Behrens et al., 2003), and MFG-E8/integrin αvβ3 or αvβ5 (Hanayama et al., 2004, Miyasaka et al., 2004, Albert et al., 1998, Finnemann and Rodriguez-Boulan, 1999, Antonov et al., 2004). However, little is known about whether these PVEERs play critical roles in entry of virus into APC populations. Knock out of TAM receptors from bone marrow-derived DCs significantly reduces infection by West Nile virus or lentiviruses pseudotyped with Ebola, Marburg, vesicular stomatitis, or murine leukemia virus entry proteins (Bhattacharyya et al., 2013). In addition, PtdSer liposomes compete for uptake of EBOV virus-like particles (VLPs) in mouse peritoneal macrophages (Jemielity et al., 2013). These data suggest these receptors are relevant for uptake into early infection targets.
PVEERs are also expressed in tissues that are later targets of virus spread. After infection by flaviviruses, Langerhans cells traffic to the lymph nodes (Johnston et al., 2000). Once there, flaviviruses infect lymph node DCs (Balsitis et al., 2009, Kyle et al., 2007), which have been shown to express TIM-4 (Kobayashi et al., 2007, Meyers et al., 2005). The alphavirus, Chikungunya virus, and filoviruses also replicate in lymph nodes (Geisbert et al., 2003a, Geisbert et al., 2003b, Labadie et al., 2010), where TIM-4, in conjunction with MFG-E8/integrin αvβ3 and αvβ5 (Hanayama et al., 2004, Garmy‐Susini et al., 2007), may mediate infection. Infected APCs also traffic flaviviruses and filoviruses to the liver and spleen, two major sites of replication (Geisbert et al., 2003a, Jessie et al., 2004, Balsitis et al., 2009, Baskerville et al., 1985, Bhoopat et al., 1996). Expression of MFG-E8/integrin αvβ5 and TIM-4 has been detected in spleen (Meyers et al., 2005, Hanayama et al., 2004, Uehara and Uehara, 2014). In particular, TIM-4 is expressed on splenic macrophages (Kobayashi et al., 2007, Wong et al., 2010), a target of flaviviruses (Balsitis et al., 2009). TIM-1 and integrin αvβ5 have been detected on hepatocellular, Huh7-derived cell lines (Meertens et al., 2012, Kondratowicz et al., 2011) and Kupffer cells (Wheeler et al., 2001), respectively, and may contribute to flavivirus and filovirus infection of the liver (Geisbert et al., 2003b, Balsitis et al., 2009, Couvelard et al., 1999, Hall et al., 1991, Xiao et al., 2001, Ryabchikova et al., 1999). Flaviviruses also infect bone marrow myeloid cells (Jessie et al., 2004, Balsitis et al., 2009), which were recently shown to express TIM-4 after stimulation (Baghdadi et al., 2013).
PVEER expression does not appear to affect the tropism of every virus for which they enhance entry. For instance, Ross River, Chikungunya, and Sindbis virus infect muscle cells, which do not express any currently identified PVEERs (Morrison et al., 2006, Johnson, 1965, Ozden et al., 2007), suggesting that either other unidentified PVEERs exist or virus entry into muscle cells is independent of PVEER expression. Interestingly, the PtdSer-binding receptor BAI1 has been shown to be important for myoblast fusion (Hochreiter-Hufford et al., 2013). Although BAI1 does not effectively enhance virus entry in vitro ( Meertens et al., 2012 ), it may contribute to virus entry in vivo in its native cell type and environment. This may also be the case for other PtdSer receptors that do not function as PVEERs in cell culture as the efficacy of these receptors was assessed after exogenous expression in cell types in which they are not endogenously expressed. Interestingly, PVEERs may also not function effectively in every cell population in which they are endogenously expressed as TIM-1 is present on some populations of T-cells (Meyers et al., 2005, Umetsu et al., 2005, Khademi et al., 2004), but, T cells are refractory to EBOV infection (Wool-Lewis and Bates, 1998). However, this may also be due to defects in virion internalization machinery or subsequent steps in the viral life cycle and not PVEER activity. Vaccinia virus does infect activated T-cells (Chahroudi et al., 2005) and in this case TIM-1 may contribute to entry. Nonetheless, the effect of PtdSer binding protein expression on virus tropism has not been thoroughly explored in tissue culture and, as discussed below, has yet to be assessed in vivo.
PtdSer-binding proteins without PVEER function
While some PtdSer-binding receptors function effectively as PVEERs, this is not true for all. Receptor for advanced glycation end-products (RAGE), brain-specific angiogensis inhibitor 1 (BAI1), CD300a, Stabilin-1 and -2, and TIM-3 have all been shown to bind to phosphatidylserine and enhance engulfment of apoptotic cells ( Fig. 3) (DeKruyff et al., 2010, Hochreiter-Hufford et al., 2013, Park et al., 2007, He et al., 2011, Friggeri et al., 2011, Nakahashi-Oda et al., 2012, Simhadri et al., 2012, Park et al., 2008, Park et al., 2009). However, expression of these proteins does not enhance virus entry (Meertens et al., 2012, Moller-Tank et al., 2013, Morizono and Chen, 2014). Of these proteins, only the mechanism responsible for reduced PVEER efficacy of TIM-3 has been studied. The inability of TIM-3 to serve as a PVEER is due to the absence of a MLD stalk of sufficient length (Moller-Tank et al., 2014). However, this may also explain the reduced efficacy of CD300a that, like TIM-3, binds PtdSer using an N-terminal IgV-like domain (Simhadri et al., 2012) and has a short stalk region between its IgV domain and the transmembrane domain.
Fig. 3.
PtdSer-binding receptors that do not function as PVEERs. Cartoon representations of Stabilin-1, BAI1, CD300a, RAGE, and TIM-3 are shown. Estimations of N-linked glycan sites are indicated with blue tridents and O-link glycosylation sites (TIM-3 only) are indicated with a green line. The binding pocket of the RAGE IgV domain is only hypothesized, as indicated by the presence of a dotted line. Domains for which calcium-binding is necessary for interaction with PtdSer are shown with calcium ions (orange spheres).
Stabilin-1/-2 and BAI1 bind PtdSer through epidermal growth factor-like (EGF) domain repeats (Park et al., 2009, Kim et al., 2010, Park et al., 2008) and type 1 thrombospondin repeats (TSRs), respectively (Park et al., 2007) (Fig. 2), although the residues within these domains mediating PtdSer binding have yet to be identified. While it is unknown why these motifs are able to bind apoptotic cells, but not virions, none of the PtdSer binding receptors that function as PVEERs bind PtdSer using repeat regions. One explanation may be that the repeats require a larger or flatter surface area to bind PtdSer than a virion envelope allows. This biological conundrum requires further examination and may provide insight for identification of other PVEERs.
RAGE is the only PtdSer receptor listed above for which the PtdSer-binding-domain is unidentified. The extracellular portion of RAGE consists of an N terminal IgV domain followed by two IgC2 domains (Fig. 3) (Park et al., 2010). The IgV domain of RAGE has been suggested to be responsible for PtdSer binding although this has not yet been experimentally shown (Fig. 2) (Friggeri et al., 2011). The lack of a similar PtdSer-binding pocket to that of the CD300a and TIM IgVs and inability to functionally replace the IgV domain of TIM-1 (SMT, unpublished data) would suggest at least the IgV domain of RAGE cannot bind to PtdSer on viral envelopes. Additionally, the condensed structure of the RAGE stalk provided by the IgC2 domains likely also further reduces PVEER efficacy by reducing overall length (Moller-Tank et al., 2014). Thus, both the absence of a clear PtdSer-binding pocket in the IgV domain and the short stalk likely explain the absent PVEER activity of RAGE.
Mechanism of virus entry enhancement
The key factors required for proteins to function as PVEERs are PtdSer-binding activity and attachment to the plasma membrane (Morizono et al., 2011, Meertens et al., 2012, Jemielity et al., 2013, Moller-Tank et al., 2013). While TIM-1 and TIM-4 individually perform both functions, the TAM receptors and αvβ3 or αvβ5 integrins provide membrane attachment and their ligands bind to PtdSer. In the case of the TIM family PVEERs, the spacer domain between the PtdSer-binding pocket and plasma membrane attachment is a MLD. MLDs from other molecules can substitute for the TIM MLDs, provided the MLD is of sufficient length, while the more compact structure of RAGE IgC2 domains is unable to substitute (Moller-Tank et al., 2014). These studies led us to propose that an extended structure was needed in the spacer region, perhaps to extend the PtdSer binding-pocket above the extracellular matrix that surrounds the cell (Moller-Tank et al., 2014). Thus, sufficient distance is necessary between the two essential functions of PVEERs for virus binding.
While we understand how PVEERs bind virus, much less is currently known about the pathways and/or mechanisms for PVEER mediated internalization of virus. There is no evidence for direct internalization. Ebola, baculo-, and vaccinia virus enter predominantly or at least partially through macropinocytosis (Mercer and Helenius, 2008, Aleksandrowicz et al., 2011, Nanbo et al., 2010, Kataoka et al., 2012, Saeed et al., 2010, Mulherkar et al., 2011) and these viruses are amongst those whose entry is most significantly enhanced by PVEER expression. However, entry of flaviviruses and alphaviruses is enhanced by PVEER expression, but primarily occurs through clathrin-mediated endocytosis (Chu and Ng, 2004, Peng et al., 2009, Acosta et al., 2009, Sourisseau et al., 2007, DeTulleo and Kirchhausen, 1998, Marsh et al., 1984). Thus, if PVEERs directly mediate virus internalization, there are currently no data to support entry via a single pathway. It is certainly possible that different PVEERs mediate uptake through different pathways. Alternatively, in some cases, PVEERs may function only as attachment factors to facilitate interaction of viral proteins with other cell surface receptors that trigger internalization. This may be true for New World arenaviruses that are known to enter cells using transferrin receptor 1 (Radoshitzky et al., 2007) and whose entry is only moderately enhanced by PVEER expression (Jemielity et al., 2013). However, this mechanism does not account for the ability of PVEERs to stimulate rapid uptake of viruses lacking a glycoprotein (Moller-Tank et al., 2013, Jemielity et al., 2013).
Incorporation of PtdSer into viral envelopes
Several viruses have been shown to expose PtdSer on the outer leaflet of their membrane (Soares et al., 2008, Morizono et al., 2011, Meertens et al., 2012, Moller-Tank et al., 2013). However, it remains to be determined how PtdSer, which is normally present within the inner leaflet of the cell membrane, is exposed on the outer leaflet of viral membranes. On cellular membranes the asymmetrical distribution of PtdSer is maintained by the activity of flippases and floppases that transfer phospholipids unidirectionally from either the extracellular side to the cytosolic side or the reverse, respectively (Leventis and Grinstein, 2010). Meanwhile, scramblases disrupt asymmetry by mediating random bidirectional transfer of phospholipids. In healthy cells, exposure of PtdSer can be induced by several mechanisms, including apoptosis and elevated levels of intracellular calcium (Leventis and Grinstein, 2010, Boon and Smith, 2002). Some viruses encode proteins that increase cytosolic calcium levels such as Nef of HIV-1 (Manninen and Saksela, 2002) and p7 of hepatitis C virus (Griffin et al., 2003), reviewed in (Zhou et al., 2009), which may contribute to PtdSer exposure. Entry of flaviviruses into cells also increases intracellular calcium levels (Nour et al., 2013). It is also possible that cellular stresses associated with virus infection trigger apoptosis as is seen with influenza A (Shiratsuchi et al., 2000) and flaviviruses (Su et al., 2002, Liu et al., 2014, Desprès et al., 1996).There is evidence that West Nile, Sindbis, and Chikungunya virus actively activate apoptosis to their advantage (Yang et al., 2008, Krejbich-Trotot et al., 2011, Levine et al., 1993). In contrast, however, apoptosis is not induced in cells that are infected by Ebola viruses (Geisbert et al., 2000, Olejnik et al., 2013), although internalization of Ebola VLPs and infection of Ebola virus is still enhanced by TIM-1 expression (Jemielity et al., 2013, Moller-Tank et al., 2013, Kondratowicz et al., 2011). Further, some viruses for which entry is enhanced by PVEERs also encode anti-apoptotic genes, such as NS1 of vaccinia virus (Maluquer de Motes et al., 2011, Cooray et al., 2007) and P35 of baculovirus (Bertin et al., 1996). Thus, there is not a clear correlation between induction of apoptosis and incorporation of PtdSer on viral envelopes.
Viruses may not utilize cellular functions to induce PtdSer flipping, but rather, concentrate the limited PtdSer present on the outer leaflet. PtdSer may be incorporated into the outer leaflet of viral envelopes during budding for promotion of favorable membrane curvature (Graham and Kozlov, 2010). Alternatively, viruses may associate with PtdSer in the inner leaflet due to its anionic charge as has been suggested for the matrix proteins of Ebola virus (Adu-Gyamfi et al., 2013) and human immunodeficiency virus-1 (Chukkapalli et al., 2013, Vlach and Saad, 2013). The VSV matrix has also been shown to associate with PtdSer enriched domains (Luan et al., 1995). Thus, the inner leaflet would be enriched with PtdSer and, after budding, the absence of flippases that normally regulate PtdSer asymmetry may lead to equilibration of PtdSer between the outer and inner leaflets. Nonetheless, the expression of the matrix protein alone results in sufficient PtdSer accumulation on the outer leaflet of Ebola virus VLPs to allow for TIM-1 enhancement of internalization (Jemielity et al., 2013, Moller-Tank et al., 2013). These data suggest that regardless of the mechanism, activity of the matrix protein is sufficient for PtdSer incorporation into viral envelopes.
Uptake of apoptotic bodies by PVEERs and implications for virus entry
Assuming PVEER enhancement of virus entry occurs through misrecognition of viruses as apoptotic bodies, it stands to reason that the pathways of PVEER-mediated virus internalization overlap with those involved in apoptotic body uptake. Initially it was believed that apoptotic bodies were internalized through macropinocytosis (Hoffmann et al., 2001, Ogden et al., 2001) which involves the ruffling of membrane to form large cups that engulf fluid (Swanson, 1989). Macropinocytosis can be stimulated by receptor signaling induced by a variety of ligands including growth factors (Racoosin and Swanson, 1989) or PMA (Swanson, 1989). While spontaneous ruffling occurs (Sallusto et al., 1995), only signaling-induced macropinocytosis leads to complete closure of the macropinosomes (Li et al., 1997). Contrary to what was previously thought, studies using SEM revealed that while necrotic cells are taken up through macropinocytosis, apoptotic bodies are phagocytosed (Krysko et al., 2006). Unlike macropinocystosis, which does not specifically engulf cargo, phagocytosis involves direct interaction between ligands and receptors. Targets to be engulfed must be entirely covered in ligand and become enclosed as binding between receptor and ligand facilitates the binding of adjacent receptors to ligand (Griffin et al., 1976). This mechanism is appropriately compared to a zipper (Griffin et al., 1975).
In theory, phagocytosis would best explain the mechanism of PVEER-mediated internalization with PVEERs sequentially binding PtdSer on the virion surface. This is particularly fitting for TIM-1- and TIM-4-mediated internalization of apoptotic bodies, as they have been shown to form phagocytic cups around cargo (Ichimura et al., 2008, Wong et al., 2010). However, both PtdSer and VLPs have been shown to induce macropinocytosis (Aleksandrowicz et al., 2011, Hoffmann et al., 2001). Additionally, amiloride and its derivative 5-(N-Ethyl-N-isopropyl) amiloride (EIPA), considered specific inhibitors of macropinocytosis (West et al., 1989) and not phagocytosis (Fukushima et al., 1996), inhibit Ebola virus uptake into and infection of Vero cells or SNB19 cells that express TIM-1 and Axl respectively (Kondratowicz et al., 2013, Saeed et al., 2010, Mulherkar et al., 2011, Hunt et al., 2011). Thus, there exists disparity between mechanisms of apoptotic body and virus uptake. Some of this confusion may be attributed to many components being shared between phagocytosis and macropinocytosis. Both require phosphoinositide 3-kinase (PI3K) signaling (Araki et al., 1996), phospholipase Cγ (Amyere et al., 2000, Cheeseman et al., 2006), Rac1 (Caron and Hall, 1998, Ridley et al., 1992), and dynamin (Liu et al., 2008, Cao et al., 2007, Gold et al., 1999, Tse et al., 2003), making differentiation between the two processes by targeting components difficult. The only macropinocytosis specific inhibitor, EIPA, functions by deregulating intracellular pH and thus may disrupt additional aspects of the viral life cycle that contribute to inhibition of virus infection (Koivusalo et al., 2010).
Neither phagocytosis nor macropinocytosis have been studied in the context of PVEERs. Phagocytosis is associated with phagocytic cells such as macrophages and dendritic cells (Rabinovitch, 1995), while all cells can initiate macropinocytosis. It is possible that both mechanisms are being used and are cell-type dependent. For example Ebola virus initially infects macrophages and dendritic cells, which may occur through phagocytosis, and subsequently spreads and enters a broad variety of tissues and cell types, perhaps by macropinocytosis (Feldmann and Geisbert, 2011). Interestingly, receptors that induce either phagocytosis or macropinocytosis require phosphorylation and recruitment of kinases and adapter proteins (Sobota et al., 2005, Swanson, 2008), but several PVEERs have been shown not to require signaling through cytoplasmic tails for internalization of virus (Meertens et al., 2012, Moller-Tank et al., 2014, Bhattacharyya et al., 2013).
Neither phagocytosis nor macropinocytosis of have been shown to be directly elicited by PVEERs. While Axl has been associated with macropinocytosis in some cells (Hunt et al., 2011), it is unknown whether Axl triggers macropinocytosis. There are, however, several studies that show PtdSer receptors coordinate to induce uptake signaling. In macrophages there is evidence that Mer induces internalization of apoptotic bodies, but requires TIM-4 for initial attachment due to a higher binding affinity for PtdSer (Nishi et al., 2014). Mer has also been shown to work synergistically with integrin αvβ5 (Wu et al., 2005). Similarly, TIM-4 and MFG-E8 have been implicated as partners for uptake of apoptotic bodies in which MFG-E8/integrin signaling triggers uptake (Toda et al., 2012). Several details of these mechanisms remain to be elucidated, such as whether or not TIM-4 actively associates with signaling partners. TIM-4 has also been shown to interact with adenosine monophosphate activating kinase (AMPK) (Baghdadi et al., 2013), a protein important for macropinocytosis of Ebola and vaccinia virus (Kondratowicz et al., 2013, Moser et al., 2010). However, this interaction is believed to occur after phagocytosis and does not explain how initial internalization events are triggered. This is also complicated by evidence that the cytoplasmic tail of TIM-4, the only domain accessible to AMPK, is unnecessary for internalization (Park et al., 2009). Further, these mechanisms do not explain why entry of viruses that utilize clathrin-coated pits is enhanced by PVEER expression. Thus, additional studies are required to determine if and how PVEERs contribute directly to internalization.
Viruses that do not utilize PVEERs
As mentioned above, PVEER expression does not enhance entry of all enveloped viruses into every cell type. In the case of arenaviruses, there exists an interesting dichotomy between the ability of PVEERs to enhance entry of New World but not Old World arenaviruses. Unlike for filo-, baculo-, alpha-, vaccinia, and flaviviruses, definitive cellular receptors have been identified for both Old World and New World arenaviruses: α-dystroglycan (Cao et al., 1998) and transferrin receptor 1 (Radoshitzky et al., 2007, Abraham et al., 2009), respectively. These viruses also use different entry pathways as New World arenaviruses such as Junín virus enter using clathrin coated pits (Martinez et al., 2007) while Old World arenaviruses such as Lassa and lymphocytic choriomeningitis virus use an unknown pathway that is clathrin, caveolin, and dynamin independent (Rojek et al., 2008a, Rojek et al., 2008b). PVEER-mediated enhancement of some New World arenaviruses entry into TIM-1 expressing HEK 293T cells is more modest than that found with filoviruses and flaviviruses, but still significant (Jemielity et al., 2013, Moller-Tank et al., 2013). These New World arenaviruses can bind to host cells and mediate efficient entry through interaction with transferrin receptor 1, and this can clearly be supplemented by PVEER expression. For Old World arenaviruses, expression of and/or affinity to α-dystroglycan may be sufficiently robust that PVEER expression does not notably enhance entry further. Indeed, in cells lacking α-dystroglycan, other attachment factors, including Axl, can enhance Old World arenavirus entry (Shimojima et al., 2012). However, PVEERs are likely not biologically relevant for Old World arenavirus entry as α-dystroglycan expression is ubiquitous (Cao et al., 1998, Sullivan et al., 2013).
When carrying its native glycoprotein, entry of VSV is in most cases, like the Old World arenaviruses, not enhanced by PVEER expression (Table 1). However, in a few cases we (unpublished data) and others (Jemielity et al., 2013) have observed a slight increase in VSV G-mediated entry into cells expressing PVEERs. The modest effect of PVEER expression on VSV entry is not due to an absence of PtdSer on the viral envelope as PtdSer can be readily detected on VSV pseudovirions (Moller-Tank et al., 2013). Instead, as we propose above for LASV, the utilization by VSV of PVEERs would be anticipated to be dependent upon the relative availability of its native receptor. If the native glycoprotein of VSV is sufficient for optimal virus binding to and entry into cells, PVEER expression would make little to no contribution to VSV entry.
Entry of SARS corona-, influenza A, and herpes simplex 1 viruses is not enhanced by PVEER expression (Meertens et al., 2012, Jemielity et al., 2013). Influenza A and SARS-CoV have viral envelope proteins that bind effectively to sialic acid (Weis et al., 1988) and ACE2 (Li et al., 2003) respectively. Expression of TIM-1 on HEK 293T cells enhances internalization of pseudovirions bearing either envelope protein, but does not result in enhancement of transduction (Jemielity et al., 2013). These results indicate that PVEER-mediated or -enhanced internalization results in unproductive infection by these viruses. This could occur due to virions being delivered to compartments with incompatible conditions for fusion (i.e. lacking correct pH or fusion triggers). An alternative possibility is that PVEERs enhance internalization of defective SARS or influenza virions with low or negligible levels of glycoprotein on the viral envelope. These particles would not effectively bind cellular receptors or be able to fuse, and thus their enhanced internalization would not contribute to infection. Internalization into endosomes may similarly inhibit entry of herpes simplex virus 1, which fuses at the cell surface (Akhtar and Shukla, 2009). However, as the effects of PVEER expression on herpes simplex virus 1 internalization have not been tested, it is possible that, in a manner similar to that proposed for Old World arenaviruses and VSV, strong association with cellular receptors masks any enhancement. Interestingly in all cases, PVEER overexpression on permissive cells does not appear to inhibit overall virus entry, suggesting that either a majority of these viruses either escapes these compartments or enters through natural productive routes.
In vivo relevance of PVEERs
A major question remaining regarding PVEER-mediated enhancement of virus entry is its relevancy in vivo. The efficacy of PVEERs on wild-type infectious virus has been demonstrated in vitro with virus harvested from both cell culture and mice (Jemielity et al., 2013, Moller-Tank et al., 2013). Additionally, entry of Ebola virus VLPs into mouse peritoneal macrophages is inhibited by PtdSer liposomes, providing evidence of PVEER importance for filovirus entry into relevant primary cell populations (Jemielity et al., 2013). However, to date only limited studies have been done in mice to determine the effect of PtdSer-binding inhibitors and none using PVEER knock down or knock out. Difficulty of testing in vivo relevancy of PVEERs using knockout mice arises from the potential for compensation by other PVEERs. Additionally, many PVEERs play critical roles in regulating adaptive immunity and single or combination knockout of genes could make interpretation of results difficult and/or lead to development of autoimmunity (Rodriguez-Manzanet et al., 2010, Hanayama et al., 2004, Miyanishi et al., 2012, Xiao et al., 2012, Li et al., 2013). Nonetheless, in a single study, a chimeric antibody that recognizes PtdSer was shown to inhibit infection by the New World arenavirus, Pichinde (Soares et al., 2008). Future studies will benefit from the development of a broad PtdSer-binding inhibitor that can target multiple PVEERs.
Conclusions
Utilization of PtdSer-binding proteins by viruses to enhance internalization provides a broad mechanism of viral entry with little investment by the virus itself. This mechanism may allow some viruses to attach to cells, thereby making viral glycoprotein/cellular receptor interactions more probable. Alternatively, other viruses may rely entirely on PVEERs for internalization into endosomes. This latter mechanism would eliminate the need of viruses to expose sensitive viral epitopes extracellularly, thereby protecting critical receptor binding or membrane fusion motifs from neutralizing antibodies. Once virions are internalized into endosomes, glycoprotein structural alterations that lead to membrane fusion can occur unhindered by antibodies. Additionally, the broad expression of PVEERs might contribute to the extensive tropism of viruses such as flaviviruses and alphaviruses that infect a broad array of insect and mammalian hosts.
Many questions still remain regarding PVEER-mediated entry. Although there is preliminary evidence as discussed above, the most important question is what is the in vivo significance of PVEERs and their relative contribution to infection? If PVEER utilization by viruses in vivo proves to be important, a model for testing inhibitors of PtdSer binding by PVEERs would be valuable and could lead to development of a single antiviral or cocktail capable of inhibiting multiple human viruses. However, as PVEER expression does not enhance entry of all enveloped virus families, targeting of PVEERs as an antiviral therapeutic may be limited to those such as the filoviruses and flaviviruses. Nonetheless, these viral families include highly pathogenic viruses for which limited antivirals are available. Second, how is PtdSer being incorporated into viral envelopes and does it matter which membrane the envelope is derived from? Third, what is the mechanism mediating PVEER-dependent entry? Is there a mechanism of direct internalization that results in productive infection? Is any cell signaling involved in these events? Finally, are there additional unidentified PVEERs that contribute to this newly appreciated means of viral infection? This may include potential PtdSer receptors that have not been tested as PVEERs. CD36, for example, has been shown to bind oxidized PtdSer for uptake of apoptotic cells into macrophages (Driscoll et al., 2013, Greenberg et al., 2006). HEK 293 T cells do not express a detectable amount of the identified PVEERs on their surface by surface staining, yet support low but detectable levels of transduction of filoviruses, flaviviruses, and alphaviruses. Certainly one possibility is that there are additional undiscovered PVEERs mediating entry. Alternatively, while studies detecting expression of Tyro3 and Mer on HEK 293T cells have been contradictory, low levels of expression may allow for entry (Morizono et al., 2011, Bhattacharyya et al., 2013, Shimojima et al., 2006). Further research on these receptors will elucidate these gaps in knowledge and determine feasibility of broad-spectrum antivirals to target them.
References
- Abraham J, Kwong JA, Albarino CG, Lu JG, Radoshitzky SR, Salazar-Bravo J, Farzan M, Spiropoulou CF, Choe H. Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic new world clade B arenaviruses. PLoS Pathog. 2009;5:e1000358. doi: 10.1371/journal.ppat.1000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta EG, Castilla V, Damonte EB. Alternative infectious entry pathways for dengue virus serotypes into mammalian cells. Cell. Microbiol. 2009;11:1533–1549. doi: 10.1111/j.1462-5822.2009.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu-Gyamfi E, Soni SP, Xue Y, Digman MA, Gratton E, Stahelin RV. The Ebola virus matrix protein penetrates into the plasma membrane: a key step in viral protein 40 (VP40) oligomerization and viral egress. J. Biol. Chem. 2013;288:5779–5789. doi: 10.1074/jbc.M112.443960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajay AK, Kim T-M, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS. A bioinformatics approach identifies signal transducer and activator of ranscription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J. Am. Soc.Nephrol. 2014;25:105–118. doi: 10.1681/ASN.2013020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav EM, Preston-Hurlburt P, Garyu J, Henegariu O, Clynes R, Schmidt AM, Herold KC. RAGE expression in human T cells: a link between environmental factors and adaptive immune responses. PLoS One. 2012;7:e34698. doi: 10.1371/journal.pone.0034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albacker LA, Karisola P, Chang YJ, Umetsu SE, Zhou M, Akbari O, Kobayashi N, Baumgarth N, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J. Immunol. 2010;185:6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrowicz P, Marzi A, Biedenkopf N, Beimforde N, Becker S, Hoenen T, Feldmann H, Schnittler HJ. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J. Infect. Dis. 2011;204(Suppl 3):S957–S967. doi: 10.1093/infdis/jir326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyere M, Payrastre B, Krause U, Smissen PVD, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of Phosphoinositide 3-Kinase and Phospholipase C. Mol. Biol. Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT. Functional analyses of two cellular binding domains of Bovine Lactadherin†. Biochemistry. 2000;39:6200–6206. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, Collen D, Dahlback B, Carmeliet P. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat. Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- Antonov AS, Kolodgie FD, Munn DH, Gerrity RG. Regulation of macrophage foam cell formation by αvβ3 integrin: potential role in human atherosclerosis. Am. J. Pathol. 2004;165:247–258. doi: 10.1016/s0002-9440(10)63293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MM, Ishihara S, Mishima Y, Oshima N, Moriyama I, Yuki T, Kadowaki Y, Rumi MAK, Amano Y, Kinoshita Y. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent αvβ3 Integrin signaling. J.. Immunol. 2009;182:7222–7232. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Takeya M, Viollet B, Yagita H, Jinushi M. TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity. 2013;39:1070–1081. doi: 10.1016/j.immuni.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, Beatty PR, Harris E. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J. Trop. Med Hyg. 2009;80:416–424. [PubMed] [Google Scholar]
- Baskerville A, Fisher-Hoch SP, Neild GH, Dowsett AB. Ultrastructural pathology of experimental ebola haemorrhagic fever virus infection. J. Pathol. 1985;147:199–209. doi: 10.1002/path.1711470308. [DOI] [PubMed] [Google Scholar]
- Bauer T, Zagorska A, Jurkin J, Yasmin N, Koffel R, Richter S, Gesslbauer B, Lemke G, Strobl H. Identification of Axl as a downstream effector of TGF-beta1 during Langerhans cell differentiation and epidermal homeostasis. J. Exp. Med. 2012;209:2033–2047. doi: 10.1084/jem.20120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens EM, Gadue P, Gong SY, Garrett S, Stein PL, Cohen PL. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur. J. Immunol. 2003;33:2160–2167. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- Bertin J, Mendrysa SM, LaCount DJ, Gaur S, Krebs JF, Armstrong RC, Tomaselli KJ, Friesen PD. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Zagórska A, Lew Erin D, Shrestha B, Rothlin Carla V, Naughton J, Diamond Michael S, Lemke G, Young John AT. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoopat L, Bhamarapravati N, Attasiri C, Yoksarn S, Chaiwun B, Khunamornpong S, Sirisanthana V. Immunohistochemical characterization of a new monoclonal antibody reactive with dengue virus-infected cells in frozen tissue using immunoperoxidase technique. Asian Pac. J Allergy Immunol. 1996;14:107–113. [PubMed] [Google Scholar]
- Binne LL, Scott ML, Rennert PD. Human TIM-1 associates with the TCR complex and up-regulates T cell activation signals. J. Immunol. 2007;178:4342–4350. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- Bocca SM, Anderson S, Amaker B, Swanson RJ, Franchi A, Lattanzio F, Oehninger S. Milk fat globule epidermal growth factor 8 (MFG-E8): A novel protein in the mammalian endometrium with putative roles in implantation and placentation. Placenta. 2012;33:795–802. doi: 10.1016/j.placenta.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Boon JM, Smith BD. Chemical control of phospholipid distribution across bilayer membranes. Med. Res. Rev. 2002;22:251–281. doi: 10.1002/med.10009. [DOI] [PubMed] [Google Scholar]
- Brindley MA, Hunt CL, Kondratowicz AS, Bowman J, Sinn PL, McCray PB, Jr., Quinn K, Weller ML, Chiorini JA, Maury W. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology. 2011;415:83–94. doi: 10.1016/j.virol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette M-J, Lepage S, Lamonde A-S, Sirois I, Groleau J, Laurin L-P, Cailhier J-F. MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming. PLoS One. 2012;7:e36368. doi: 10.1371/journal.pone.0036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Popernack PM, Tsutsui S, Truong L, Schlegel RA, Henderson AJ. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J. Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, Biassoni R, Moretta L, Moretta A. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J. Immunol. 1999;29:3148–3159. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cao H, Chen J, Awoniyi M, Henley JR, McNiven MA. Dynamin 2 mediates fluid-phase micropinocytosis in epithelial cells. J. Cell Sci. 2007;120:4167–4177. doi: 10.1242/jcs.010686. [DOI] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, Lemke G, Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat. Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J. Virol. 2005;79:10397–10407. doi: 10.1128/JVI.79.16.10397-10407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman KL, Ueyama T, Michaud TM, Kashiwagi K, Wang D, Flax LA, Shirai Y, Loegering DJ, Saito N, Lennartz MR. Targeting of protein kinase C-∈ during Fcγ receptor-dependent phagocytosis requires the ϵC1B domain and phospholipase C-γ1. Mol. Biol. Cell. 2006;17:799–813. doi: 10.1091/mbc.E04-12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli V, Inlora J, Todd GC, Ono A. Evidence in support of rna-mediated inhibition of phosphatidylserine-dependent HIV-1 Gag membrane binding in cells. J. Virol. 2013;87:7155–7159. doi: 10.1128/JVI.00075-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GJ, Rao M, Ju X, Hart DNJ. Novel human CD4+ T lymphocyte subpopulations defined by CD300a/c molecule expression. J. Leukoc. Biol. 2007;82:1126–1135. doi: 10.1189/jlb.0107035. [DOI] [PubMed] [Google Scholar]
- Cooray S, Bahar MW, Abrescia NGA, McVey CE, Bartlett NW, RA-J Chen, Stuart DI, Grimes JM, Smith GL. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J.Gen. Virol. 2007;88:1656–1666. doi: 10.1099/vir.0.82772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvelard A, Marianneau P, Bedel C, Drouet MT, Vachon F, Henin D, Deubel V. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum. Pathol. 1999;30:1106–1110. doi: 10.1016/s0046-8177(99)90230-7. [DOI] [PubMed] [Google Scholar]
- de Souza AJ, Oriss TB, O’Malley K J, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc. Natl. Acad. Sci. USA. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza AJ, Oak JS, Jordanhazy R, DeKruyff RH, Fruman DA, Kane LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J. Immunol. 2008;180:6518–6526. doi: 10.4049/jimmunol.180.10.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J. Immunol. 2010;184:1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. EMBO J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprès P, Flamand M, Ceccaldi PE, Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J. Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimasi N, Roessle M, Moran O, Candiano G, Svergun DI, Biassoni R. Molecular analysis and solution structure from small-angle X-ray scattering of the human natural killer inhibitory receptor IRp60 (CD300a) Int.J. Biol. Macromol. 2007;40:193–200. doi: 10.1016/j.ijbiomac.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Hornick JL, Shahsafaei A, Freeman GJ. The phosphatidylserine receptors, T cell immunoglobulin mucin proteins 3 and 4, are markers of histiocytic sarcoma and other histiocytic and dendritic cell neoplasms. Hum. Pathol. 2010;41:1486–1494. doi: 10.1016/j.humpath.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll WS, Vaisar T, Tang J, Wilson CL, Raines EW. Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circ. Res. 2013;113:52–61. doi: 10.1161/CIRCRESAHA.112.300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls t cell activation via the receptor for advanced glycation end products. J. Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- Falkowski M, Schledzewski K, Hansen B, Goerdt S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 2003;120:361–369. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J. Exp. Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, Abraham E. Participation of the receptor for advanced glycation end products in efferocytosis. J. Immunol. 2011;186:6191–6198. doi: 10.4049/jimmunol.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Waddell TK, Grinstein S, Goss GG, Orlowski J, Downey GP. Na+/H+ exchange activity during phagocytosis in human neutrophils: role of Fcgamma receptors and tyrosine kinases. J. Cell Biol. 1996;132:1037–1052. doi: 10.1083/jcb.132.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JP, Frolov I, Perri S, Ji Y, MacKichan ML, zur Megede J, Chen M, Belli BA, Driver DA, Sherrill S, Greer CE, Otten GR, Barnett SW, Liu MA, Dubensky TW, Polo JM. Infection of human dendritic cells by a sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 2000;74:11849–11857. doi: 10.1128/jvi.74.24.11849-11857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy‐Susini B, Makale M, Fuster M, Varner JA. Methods to study lymphatic vessel integrins. In: David AC, editor. Volume 426. Academic Press; 2007. pp. 415–438. (Methods .Enzymol.). [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Investig.; J Tech. Methods Pathol. 2000;80:171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 2003;163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. Pathogenesis of Ebola Hemorrhagic fever in Cynomolgus Macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerdt S, Walsh LJ, Murphy GF, Pober JS. Identification of a novel high molecular weight protein preferentially expressed by sinusoidal endothelial cells in normal human tissues. J. Cell Biol. 1991;113:1425–1437. doi: 10.1083/jcb.113.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 1999;190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Kozlov MM. Interplay of proteins and lipids in generating membrane curvature. Curr. Opin. Cell Biol. 2010;22:430–436. doi: 10.1016/j.ceb.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine–CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin FM, Griffin JA, Leider JE, Silverstein SC. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin FM, Griffin JA, Silverstein SC. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J. Exp. Med. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SDC, Beales LP, Clarke DS, Worsfold O, Evans SD, Jaeger J, Harris MPG, Rowlands DJ. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, amantadine. FEBS Lett. 2003;535:34–38. doi: 10.1016/s0014-5793(02)03851-6. [DOI] [PubMed] [Google Scholar]
- Hall WC, Crowell TP, Watts DM, Barros VLR, Kruger H, Pinheiro F, Peters CJ. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J. Trop. Med. Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in mfg-e8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J. Immunol. 2004;172:3876–3882. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, Yamada M, Yamaya M, Maekawa T, Yamamoto Y, Yamamoto H. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, Ravichandran KS. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor–mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001;155:649–660. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Rigby AC, Morelli X, Grant MA, Huang G, Furie B, Seaton B, Furie BC. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nature structural biology. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- Hunt CL, Kolokoltsov AA, Davey RA, Maury W. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. J. Virol. 2011;85:334–347. doi: 10.1128/JVI.01278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J. Biochem. 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- Jaax NK, Davis KJ, Geisbert TJ, Vogel P, Jaax GP, Topper M, Jahrling PB. Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure. Arch. Patho. Lab. Med. 1996;120:140–155. [PubMed] [Google Scholar]
- Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. TIM-family proteins promote infection of multiple enveloped viruses through Virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, Dranoff G, Tahara H. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J. Exp. Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT. Virus invasion of the central nervous system: a study of sindbis virus infection in the mouse using fluorescent antibody. Am. J. Pathol. 1965;46:929–943. [PMC free article] [PubMed] [Google Scholar]
- Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J.Investig. Dermatol. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- Kamioka M, Ishibashi T, Ohkawara H, Nagai R, Sugimoto K, Uekita H, Matsui T, Yamagishi S-I, Ando K, Sakamoto T, Sakamoto N, Takuwa Y, Wada I, Shiomi M, Maruyama Y, Takeishi Y. Involvement of membrane type 1-matrix metalloproteinase (MT1-MMP) in RAGE activation signaling pathways. J. Cell. Physiol. 2011;226:1554–1563. doi: 10.1002/jcp.22492. [DOI] [PubMed] [Google Scholar]
- Kataoka C, Kaname Y, Taguwa S, Abe T, Fukuhara T, Tani H, Moriishi K, Matsuura Y. Baculovirus GP64-mediated entry into mammalian cells. J. Virol. 2012;86:2610–2620. doi: 10.1128/JVI.06704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi M, Illes Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallstrom E. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J. Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Lee SM, Suk K, Lee WH. CD300a and CD300f differentially regulate the MyD88 and TRIF-mediated TLR signalling pathways through activation of SHP-1 and/or SHP-2 in human monocytic cell lines. Immunology. 2012;135:226–235. doi: 10.1111/j.1365-2567.2011.03528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Chang Y-J, Chuang Y-T, Lee H-H, Kasahara DI, Martin T, Hsu JT, Savage PB, Shore SA, Freeman GJ, DeKruyff RH, Umetsu DT. T-cell immunoglobulin and mucin domain 1 deficiency eliminates airway hyperreactivity triggered by the recognition of airway cell death. J.. Allergy . Clin. Immunol. 2013;132 doi: 10.1016/j.jaci.2013.03.025. (414–425.e416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bae D-J, Hong M, Park S-Y, Kim I-S. The conserved histidine in epidermal growth factor-like domains of stabilin-2 modulates pH-dependent recognition of phosphatidylserine in apoptotic cells. Int.. J.. Biochem.. Cell Biol. 2010;42:1154–1163. doi: 10.1016/j.biocel.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Page RC, Misra S, Silverstein RL. Expression, purification and structural characterization of functionally replete thrombospondin-1 type 1 repeats in a bacterial expression system. Protein Expr. Purif. 2011;80:253–259. doi: 10.1016/j.pep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010;188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB, Jr., Chiorini J, Maury W. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. USA. 2011;108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratowicz AS, Hunt CL, Davey RA, Cherry S, Maury WJ. AMP-activated protein kinase is required for the macropinocytic internalization of ebolavirus. J. Virol. 2013;87:746–755. doi: 10.1128/JVI.01634-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejbich-Trotot P, Denizot M, Hoarau JJ, Jaffar-Bandjee MC, Das T, Gasque P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2011;25:314–325. doi: 10.1096/fj.10-164178. [DOI] [PubMed] [Google Scholar]
- Kruger W, Lohner R, Jung R, Kroger N, Zander AR. Expression of human milk fat globulin proteins in cells of haemopoietic origin. Br. J. Cancer. 2000;83:874–879. doi: 10.1054/bjoc.2000.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D’Herde K, Vandenabeele P. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J. Infect. Dis. 2007;195:1808–1817. doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberte JP, Moss B. Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc. Natl. Acad. Sci. USA. 2009;106:17517–17521. doi: 10.1073/pnas.0909376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankry D, Simic H, Klieger Y, Levi-Schaffer F, Jonjic S, Mandelboim O. Expression and function of CD300 in NK cells. J. Immunol. 2010;185:2877–2886. doi: 10.4049/jimmunol.0903347. [DOI] [PubMed] [Google Scholar]
- Lee HH, Meyer EH, Goya S, Pichavant M, Kim HY, Bu X, Umetsu SE, Jones JC, Savage PB, Iwakura Y, Casasnovas JM, Kaplan G, Freeman GJ, DeKruyff RH, Umetsu DT. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. J. Immunol. 2010;185:5225–5235. doi: 10.4049/jimmunol.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Li G, D’Souza-Schorey C, Barbieri MA, Cooper JA, Stahl PD. Uncoupling of membrane ruffling and pinocytosis during ras signal transduction. J. Biol. Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- Li J, Cao D, Guo G, Wu Y, Chen Y. Expression and anatomical distribution of TIM-containing molecules in Langerhans cell sarcoma. J. Mol. Histol. 2013;44:213–220. doi: 10.1007/s10735-012-9475-2. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Lu H, Tian S, Lu Q. Systemic autoimmunity in TAM triple knockout mice causes inflammatory brain damage and cell death. PLoS One. 2013;8:e64812. doi: 10.1371/journal.pone.0064812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn ML, Aaskov JG, Suhrbier A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J. Gen. Virol. 1996;77(Pt 3):407–411. doi: 10.1099/0022-1317-77-3-407. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang C, Liu X, Liao B, Pan X, Ren Y, Fan M, Li M, He Z, Wu J, Wu Z. AGEs increased migration and inflammatory responses of adventitial fibroblasts via RAGE, MAPK and NF-κB pathways. Atherosclerosis. 2010;208:34–42. doi: 10.1016/j.atherosclerosis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Zou J, Zhang B, Yuan Z. Dengue virus subgenomic RNA induces apoptosis through the Bcl-2-mediated PI3k/Akt signaling pathway. Virology. 2014;448:15–25. doi: 10.1016/j.virol.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Liu Y-W, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL. Isoform and splice-variant specific functions of Dynamin-2 revealed by analysis of conditional Knock-Out cells. Mol.r Biol. Cell. 2008;19:5347–5359. doi: 10.1091/mbc.E08-08-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im H-J, Bursch LS, Yan SD. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthr. Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- Luan P, Yang L, Glaser M. Formation of membrane domains created during the budding of vesicular stomatitis virus. A model for selective lipid and protein sorting in biological membranes. Biochemistry. 1995;34:9874–9883. doi: 10.1021/bi00031a008. [DOI] [PubMed] [Google Scholar]
- Maluquer de Motes C, Cooray S, Ren H, Almeida GM, McGourty K, Bahar MW, Stuart DI, Grimes JM, Graham SC, Smith GL. Inhibition of apoptosis and NF-kappaB activation by vaccinia protein N1 occur via distinct binding surfaces and make different contributions to virulence. PLoS Pathog. 2011;7:e1002430. doi: 10.1371/journal.ppat.1002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen A, Saksela K. HIV-1 Nef interacts with Inositol Trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 2002;195:1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marovich M, Grouard-Vogel G, Louder M, Eller M, Sun W, Wu SJ, Putvatana R, Murphy G, Tassaneetrithep B, Burgess T, Birx D, Hayes C, Schlesinger-Frankel S, Mascola J. Human dendritic cells as targets of dengue virus infection. J. Investig. Dermatol. Symp. Proceed. 2001;6:219–224. doi: 10.1046/j.0022-202x.2001.00037.x. (the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research) [DOI] [PubMed] [Google Scholar]
- .Marsh M, Kielian MC, Helenius A. Semliki forest virus entry and the endocytic pathway. Biochem. Soc. Trans. 1984;12:981–983. doi: 10.1042/bst0120981. [DOI] [PubMed] [Google Scholar]
- Martens JH, Kzhyshkowska J, Falkowski-Hansen M, Schledzewski K, Gratchev A, Mansmann U, Schmuttermaier C, Dippel E, Koenen W, Riedel F, Sankala M, Tryggvason K, Kobzik L, Moldenhauer G, Arnold B, Goerdt S. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J. Pathol. 2006;208:574–589. doi: 10.1002/path.1921. [DOI] [PubMed] [Google Scholar]
- Martinez MG, Cordo SM, Candurra NA. Characterization of Junín arenavirus cell entry. J. Gen. Virol. 2007;88:1776–1784. doi: 10.1099/vir.0.82808-0. [DOI] [PubMed] [Google Scholar]
- McIntire JJ, Umetsu DT, DeKruyff RH. TIM-1, a novel allergy and asthma susceptibility gene. Springer Semin. Immunopathol. 2004;25:335–348. doi: 10.1007/s00281-003-0141-3. [DOI] [PubMed] [Google Scholar]
- Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Segawa K, Nagata S. Synergistic effect of Tim4 and MFG-E8 null mutations on the development of autoimmunity. Int. Immunol. 2012;24:551–559. doi: 10.1093/intimm/dxs064. [DOI] [PubMed] [Google Scholar]
- Miyasaka K, Hanayama R, Tanaka M, Nagata S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur. J. Immunol. 2004;34:1414–1422. doi: 10.1002/eji.200424930. [DOI] [PubMed] [Google Scholar]
- Mizui M, Shikina T, Arase H, Suzuki K, Yasui T, Rennert PD, Kumanogoh A, Kikutani H. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int Immunol. 2008;20:695–708. doi: 10.1093/intimm/dxn029. [DOI] [PubMed] [Google Scholar]
- Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. 2013;87:8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Tank S, Albritton LM, Rennert PD, Maury W. Characterizing functional domains for TIM-mediated enveloped virus entry. J. Virol. 2014;88:6702–6713. doi: 10.1128/JVI.00300-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Morizono K, Chen IS. The role of phosphatidylserine receptors in enveloped virus infection. J. Virol. 2014 doi: 10.1128/JVI.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, Chen IS. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9:286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE, Whitmore AC, Shabman RS, Lidbury BA, Mahalingam S, Heise MT. Characterization of Ross river virus Tropism and virus-induced inflammation in a mouse model of viral Arthritis and Myositis. J. Virol. 2006;80:737–749. doi: 10.1128/JVI.80.2.737-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B, Desai DD, Downie MP, Chen Y, Yan SF, Herold K, Schmidt AM, Clynes R. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J.Immunol. 2007;179:8051–8058. doi: 10.4049/jimmunol.179.12.8051. [DOI] [PubMed] [Google Scholar]
- Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherkar N, Raaben M, de la Torre JC, Whelan SP, Chandran K. The Ebola virus glycoprotein mediates entry via a non-classical dynamin-dependent macropinocytic pathway. Virology. 2011;419:72–83. doi: 10.1016/j.virol.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Kohda D, Hatanaka H, Ichikawa S, Matsuda S, Yamamoto T, Suzuki A, Inagaki F. Solution structure of the epidermal growth factor-like domain of heregulin-alpha, a ligand for p180erbB-4. EMBO J. 1994;13:3517–3523. doi: 10.1002/j.1460-2075.1994.tb06658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J. Leukoc. Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi-Oda C, Tahara-Hanaoka S, Honda S, Shibuya K, Shibuya A. Identification of phosphatidylserine as a ligand for the CD300a immunoreceptor. Biochem. Biophys. Res. Commun. 2012;417:646–650. doi: 10.1016/j.bbrc.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Nakano N, Fukuhara-Takaki K, Jono T, Nakajou K, Eto N, Horiuchi S, Takeya M, Nagai R. Association of advanced glycation end products with A549 cells, a human pulmonary epithelial cell line, is mediated by a receptor distinct from the scavenger receptor family and RAGE. J. Biochem. 2006;139:821–829. doi: 10.1093/jb/mvj092. [DOI] [PubMed] [Google Scholar]
- Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, Halfmann P, Kawaoka Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010;6:e1001121. doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Silva R, Peruzzi G, Alvarez Y, Simhadri VR, Debell K, Coligan JE, Borrego F. Human Th1 cells that express CD300a are polyfunctional and after stimulation up-regulate the T-box transcription factor eomesodermin. PLoS One. 2010;5:e10636. doi: 10.1371/journal.pone.0010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi C, Toda S, Segawa K, Nagata S. Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol. Cell Biol. 2014;34:1512–1520. doi: 10.1128/MCB.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto KP, Laust AK, Wang K, Kamrud KI, Hubby B, Smith JF, Nelson EL. Restricted and selective tropism of a Venezuelan equine encephalitis virus-derived replicon vector for human dendritic cells. Viral Immunol. 2007;20:88–104. doi: 10.1089/vim.2006.0090. [DOI] [PubMed] [Google Scholar]
- Nour AM, Li Y, Wolenski J, Modis Y. Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS Pathog. 2013;9:e1003585. doi: 10.1371/journal.ppat.1003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and Mannose binding lectin engagement of cell surface Calreticulin and cd91 initiates Macropinocytosis and uptake of Apoptotic cells. J.Exp. Med. 2001;194:781–796. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Takahashi HK, Mori S, Liu K, Wake H, Sadamori H, Matsuda H, Yagi T, Yoshino T, Nishibori M, Tanaka N. Advanced glycation end products enhance monocyte activation during human mixed lymphocyte reaction. Clin. Immunol. 2010;134:345–353. doi: 10.1016/j.clim.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Oie M. Reversible inactivation and reactivation of vaccinia virus by manipulation of viral lipid composition. Virology. 1985;142:299–306. doi: 10.1016/0042-6822(85)90338-1. [DOI] [PubMed] [Google Scholar]
- Olejnik J, Alonso J, Schmidt KM, Yan Z, Wang W, Marzi A, Ebihara H, Yang J, Patterson JL, Ryabchikova E, Muhlberger E. Ebola virus does not block apoptotic signaling pathways. J. Virol. 2013;87:5384–5396. doi: 10.1128/JVI.01461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Aoki N, Negi M, Kishi M, Kitajima K, Matsuda T. Lactation-dependenteExpression of an mRNA splice variant with an Exon for a multiplyO-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem. Biophys. Res. Commun. 1999;254:522–528. doi: 10.1006/bbrc.1998.0107. [DOI] [PubMed] [Google Scholar]
- Oshima K, Aoki N, Kato T, Kitajima K, Matsuda T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur. J. Biochem. 2002;269:1209–1218. doi: 10.1046/j.1432-1033.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- Ozden S, Huerre M, Riviere J-P, Coffey LL, Afonso PV, Mouly V, de Monredon J, Roger J-C, El Amrani M, Yvin J-L, Jaffar M-C, Frenkiel M-P, Sourisseau M, Schwartz O, Butler-Browne G, Desprès P, Gessain A, Ceccaldi P-E. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS One. 2007;2:e527. doi: 10.1371/journal.pone.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 2009;19:346–351. doi: 10.1016/j.cub.2009.01.042. [DOI] [PubMed] [Google Scholar]
- Park H, Adsit FG, Boyington JC. The 1.5 A crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J. Biol. Chem. 2010;285:40762–40770. doi: 10.1074/jbc.M110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Kim S-Y, Jung M-Y, Bae D-J, Kim I-S. Epidermal growth factor-like domain repeat of stabilin-2 recognizes Phosphatidylserine during cell corpse clearance. Mol. Cell. Biol. 2008;28:5288–5298. doi: 10.1128/MCB.01993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V, Kzhyshkowska J, Kim IS. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J. Cell Sci. 2009;122:3365–3373. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- Peng T, Wang JL, Chen W, Zhang JL, Gao N, Chen ZT, Xu XF, Fan DY, An J. Entry of dengue virus serotype 2 into ECV304 cells depends on clathrin-dependent endocytosis, but not on caveolae-dependent endocytosis. Can. J. Microbiol. 2009;55:139–145. doi: 10.1139/w08-107. [DOI] [PubMed] [Google Scholar]
- Pollreisz A, Hudson BI, Chang JS, Qu W, Cheng B, Papapanou PN, Schmidt AM, Lalla E. Receptor for advanced glycation endproducts mediates pro-atherogenic responses to periodontal infection in vascular endothelial cells. Atherosclerosis. 2010;212:451–456. doi: 10.1016/j.atherosclerosis.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Mol. Cell. Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp. Neurol. 2000;425:295–314. [PubMed] [Google Scholar]
- Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J. Exp. Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, Farzan M, Choe H. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446:92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajotte I, Hasanbasic I, Blostein M. Gas6-mediated signaling is dependent on the engagement of its gamma-carboxyglutamic acid domain with phosphatidylserine. Biochem. Biophys. Res. Commun. 2008;376:70–73. doi: 10.1016/j.bbrc.2008.08.083. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc. Natl. Acad. Sci. USA. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Sanchez AB, Nguyen NT, de la Torre J-C, Kunz S. Different mechanisms of cell entry by human-pathogenic Old World and New World arena viruses. J. Virol. 2008;82:7677–7687. doi: 10.1128/JVI.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Perez M, Kunz S. Cellular entry of lymphocytic choriomeningitis virus. J..Virol. 2008;82:1505–1517. doi: 10.1128/JVI.01331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MBA, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Ryabchikova EI, Kolesnikova LV, Luchko SV. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J. Infect. Dis. 1999;179:S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of Ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6:e1001110. doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Knyazev PG, Cheburkin Y, Gohring W, Tisi D, Ullrich A, Timpl R, Hohenester E. Crystal structure of a C-terminal fragment of growth arrest-specific protein Gas6. Receptor tyrosine kinase activation by laminin G-like domains. J. Biol. Chem. 2002;277:44164–44170. doi: 10.1074/jbc.M207340200. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Knyazev PG, Clout NJ, Cheburkin Y, Gohring W, Ullrich A, Timpl R, Hohenester E. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J. Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- Shabman RS, Morrison TE, Moore C, White L, Suthar MS, Hueston L, Rulli N, Lidbury B, Ting JP, Mahalingam S, Heise MT. Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J. Virol. 2007;81:237–247. doi: 10.1128/JVI.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Novakovic VA, Head JF, Seaton BA, Gilbert GE. Crystal structure of lactadherin C2 domain at 1.7 A resolution with mutational and computational analyses of its membrane-binding motif. J. Biol. Chem. 2008;283:7230–7241. doi: 10.1074/jbc.M705195200. [DOI] [PubMed] [Google Scholar]
- Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M, Stroher U, Ebihara H, Feldmann H, Kawaoka Y. Identification of cell surface molecules involved in dystroglycan-independent Lassa virus cell entry. J. Virol. 2012;86:2067–2078. doi: 10.1128/JVI.06451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi A, Kaido M, Takizawa T, Nakanishi Y. Phosphatidylserine-mediated Phagocytosis of influenza A virus-infected cells by mouse peritoneal macrophages. J. Virol. 2000;74:9240–9244. doi: 10.1128/jvi.74.19.9240-9244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi T, Nishimori H, Ichise H, Nakamura Y, Tokino T. Cloning and characterization of BAI2 and BAI3, novel genes homologous to brain-specific angiogenesis inhibitor 1 (BAI1) Cytogenet. Cell Genet. 1997;79:103–108. doi: 10.1159/000134693. [DOI] [PubMed] [Google Scholar]
- Silva R, Moir S, Kardava L, Debell K, Simhadri VR, Ferrando-Martínez S, Leal M, Peña J, Coligan JE, Borrego F. CD300a is expressed on human B cells, modulates BCR-mediated signaling, and its expression is down-regulated in HIV infection. Blood. 2011;117:5870–5880. doi: 10.1182/blood-2010-09-310318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S, Clergue M, Duriez M, Merval R, Levy B, Tedgui A, Amigorena S, Mallat Z. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- Simhadri VR, Mariano JL, Zhou Q, DeBell KE, Borrego F. Differential expression of CD300a/c on human TH1 and TH17 cells. BMC Immunol. 2011;12:62. doi: 10.1186/1471-2172-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119:2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, Dobles M, Tarilonte L, Miklasz S, Majeau G, Godbout K, Scott ML, Rennert PD. Epitope-dependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J. Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat. Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota A, Strzelecka-Kiliszek A, Gładkowska E, Yoshida K, Mrozińska K, Kwiatkowska K. Binding of IgG-Opsonized particles to FcγR is an active stage of phagocytosis that involves receptor clustering and phosphorylation. JImmunol. 2005;175:4450–4457. doi: 10.4049/jimmunol.175.7.4450. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Nobles SL, Heffron DS, Park D, Ravichandran KS, Mandell JW. Brain-specific angiogenesis inhibitor-1 expression in astrocytes and neurons: Implications for its dual function as an apoptotic engulfment receptor. Brain, Behav. Immun. 2011;25:915–921. doi: 10.1016/j.bbi.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Roux KL, Prevost M-C, Fsihi H, Frenkiel M-P, Blanchet F, Afonso PV, Ceccaldi P-E, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saïb A, Rey FA, Arenzana-Seisdedos F, Desprès P, Michault A, Albert ML, Schwartz O. Characterization of reemerging Chikungunya virus. PLoS Pathog. 2007;3:e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Stubbs JD, Lekutis C, Singer KL, Bui A, Yuzuki D, Srinivasan U, Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc.Natl. Acad.. Sci. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H-L, Liao C-L, Lin Y-L. Japanese Encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J. Virol. 2002;76:4162–4171. doi: 10.1128/JVI.76.9.4162-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Welch MJ, Lemke G, Oldstone MB. Is the TAM receptor Axl a receptor for lymphocytic choriomeningitis virus? J. Virol. 2013;87:4071–4074. doi: 10.1128/JVI.03268-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Makino H. Increased expression of receptor for advanced glycation end products by synovial tissue macrophages in rheumatoid arthritis. Arthr. Rheum. 2006;54:97–104. doi: 10.1002/art.21524. [DOI] [PubMed] [Google Scholar]
- Swairjo MA, Concha NO, Kaetzel MA, Dedman JR, Seaton BA. Ca(2+)-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 1995;2:968–974. doi: 10.1038/nsb1195-968. [DOI] [PubMed] [Google Scholar]
- Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell. Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibrewal N, Wu Y, D’mello V, Akakura R, George TC, Varnum B, Birge RB. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of Lipopolysaccharide-inducible NF-κB transcriptional activation. J. Biol. Chem. 2008;283:3618–3627. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- Tietjen GT, Gong Z, Chen CH, Vargas E, Crooks JE, Cao KD, Heffern CT, Henderson JM, Meron M, Lin B, Roux B, Schlossman ML, Steck TL, Lee KY, Adams EJ. Molecular mechanism for differential recognition of membrane phosphatidylserine by the immune regulatory receptor Tim4. Proc. Natl. Acad. Sci. USA. 2014;111:E1463–1472. doi: 10.1073/pnas.1320174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Mol Cell Biol. 2012;32:118–125. doi: 10.1128/MCB.05993-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse SML, Furuya W, Gold E, Schreiber AD, Sandvig K, Inman RD, Grinstein S. Differential role of Actin, Clathrin, and Dynamin in Fcγ receptor-mediated Endocytosis and Phagocytosis. J. Biol. Chem. 2003;278:3331–3338. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- Uehara K, Uehara A. Integrin αvβ5 in endothelial cells of rat splenic sinus: an immunohistochemical and ultrastructural study. Cell Tissue Res. 2014;356:183–193. doi: 10.1007/s00441-014-1796-x. [DOI] [PubMed] [Google Scholar]
- Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- Vanlandschoot P, Leroux-Roels G. Viral apoptotic mimicry: an immune evasion strategy developed by the hepatitis B virus? Trends. Immunol. 2003;24:144–147. doi: 10.1016/s1471-4906(03)00026-7. [DOI] [PubMed] [Google Scholar]
- Vlach J, Saad JS. Trio engagement via plasma membrane phospholipids and the myristoyl moiety governs HIV-1 matrix binding to bilayers. Proc. Natl. Acad. Sci. USA. 2013;110:3525–3530. doi: 10.1073/pnas.1216655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen Y, Ge Y, Ma P, Ma Q, Ma J, Wang H, Xue S, Han D. Immunoexpression of Tyro 3 family receptors--Tyro 3, Axl, and Mer--and their ligand Gas6 in postnatal developing mouse testis. J. Histochem. Cytochem.: Off. J. Histochem. Soc. 2005;53:1355–1364. doi: 10.1369/jhc.5A6637.2005. [DOI] [PubMed] [Google Scholar]
- Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MD, Yamashina S, Froh M, Rusyn I, Thurman RG. Adenoviral gene delivery can inactivate Kupffer cells: role of oxidants in NF-κB activation and cytokine production. J. Leukoc. Biol. 2001;69:622–630. [PubMed] [Google Scholar]
- Wong K, Valdez PA, Tan C, Yeh S, Hongo JA, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc. Natl. Acad. Sci. USA. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, Yeste A, Quintana FJ, Ichimura T, Sobel RA, Bonventre JV, Kuchroo VK. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc. Natl. Acad. Sci. USA. 2012;109:12105–12110. doi: 10.1073/pnas.1120914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. pathology. J. Infect. Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- Xiong J-P, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of Integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am.J.jReprod. Immunol. 2012;67:184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MR, Lee SR, Oh W, Lee EW, Yeh JY, Nah JJ, Joo YS, Shin J, Lee HW, Pyo S, Song J. West Nile virus capsid protein induces p53-mediated apoptosis via the sequestration of HDM2 to the nucleolus. Cell. Microbiol. 2008;10:165–176. doi: 10.1111/j.1462-5822.2007.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Li B, Subramanian V, Choi B-H, Liang Y, Harikishore A, Chakraborty G, Baek K, Yoon HS. NMR solution structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochim. et Biophys. Acta (BBA) – Biomembr. 2013;1828:1083–1093. doi: 10.1016/j.bbamem.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang F, Kent KC, Yamanouchi D, Zhang Y, Kato K, Tsai S, Nowygrod R, Schmidt AM, Liu B. Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann. Surg. 2009;250:416–423. doi: 10.1097/SLA.0b013e3181b41a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Frey TK, Yang JJ. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46:1–17. doi: 10.1016/j.ceca.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Ren M, Yang C, Hu Y-X, Ran J-M, Yan L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012;21:123–129. doi: 10.1111/j.1600-0625.2011.01408.x. [DOI] [PubMed] [Google Scholar]