Abstract
Sarcopenia is an age-related decline in skeletal muscle mass and function that is multifactorial in etiology. Age-related changes in the renin-angiotensin system (RAS), increased oxidative stress, and chronic inflammation likely all contribute to its development. Losartan, an angiotensin II type I receptor blocker (ARB) decreases RAS activity and likely influences oxidative stress and inflammation. Given this, we hypothesized that losartan would improve activity levels and parameters related to inflammation and oxidative stress in older mice. We sought to test this hypothesis by comparing functional and molecular parameters between 18-month-old C57BL/6 mice treated with 50-70 mg/kg/day of losartan over a 4 month-period and age- and gender-matched mice receiving placebo. Losartan treatment significantly improved several activity measurements during treatment period compared to placebo controlled group, including increased time on treadmill, traveling activity, standing activity, and decreased grid contacts (p-values < 0.05, 0.001, 0.01; and 0.04 respectively). Grip strength did not improve in treatment group relative to control group over time. Serum IL-6 level in the treated group was significantly lower than that in the control group at the end of treatment, (30.3±12.9 vs. 173.0±59.5 pg/ml, p< 0.04), and mRNA expression of antioxidant enzymes catalase (3.9±0.9 vs. 1.0±0.4) and glutathione peroxidase (4.7±1.1 vs. 1.0±0.4) was significantly higher, P-value: 0.02, and 0.03 respectively) in quadriceps muscle after 4 months of treatment in treated and control groups. These results support the hypothesis that chronic losartan treatment improves skeletal muscle related activity measures in older mice, and that it is associated with more favorable relevant biological profiles in the treatment group. Additional studies are needed to 1) further quantify this functional improvement, 2) further identify mechanisms that influence this improvement, and 3) provide additional rationale for translating these findings into older adults.
Keywords: frailty, sarcopenia, oxidative stress, renin-angiotensin system, angiotensin II receptor blocker, inflammation
1. Introduction
Sarcopenia, an age-related decline in skeletal muscle mass and function with a complex and multifactorial etiology, is closely associated with declines in activity levels, function, metabolic abnormalities and increases in frailty and disability 1. Age-related increases in oxidative stress, decreases in autophagy, altered mitochondrial function, chronic inflammatory pathway activation, and modulation of other signaling pathways involved in skeletal muscle homeostasis likely play a role in its development 2. Age-related alterations in the renin-angiotensin system (RAS) have also been hypothesized to contribute to sarcopenia. The angiotensin II type I receptor (AT1R) increases and type II receptor (AT2R) decreases with aging, likely influencing fibrotic tissue changes, free radical production, and chronic inflammation in multiple tissues 3. Losartan, an angiotensin II type I receptor blocker (ARB) has been proposed to affect the progression of sarcopenia via its impact on RAS signaling 4;5. Modulation of RAS influences inflammation and oxidative stress, with prior studies demonstrating that ARB treatment leads to decreased IL-6 and free radical production in aging mice 6;7. Losartan has already been demonstrated to accelerate muscle healing and slow the development of disuse atrophy in aging mice 8. In addition, an earlier pilot study of chronic losartan treatment in humans with heart failure demonstrated improvement in exercise capacity associated with a shift towards more fatigue resistant skeletal muscle fibers 9. Although multiple hormonal and pharmaceutical treatments have been developed for age-related sarcopenia in humans, none have to date proven effective 10. Based on this background, and the need to further study the potential utility of losartan treatment for age-related sarcopenia and related activity and functional decline, we hypothesized that losartan will improve activity levels and muscle strength in older mice in part by decreasing inflammation and oxidative stress.
2. Materials and methods
2.1. Experimental groups
All mouse protocols were approved by the Animal Care and Use Committee of Johns Hopkins University School of Medicine. These mice were purchased directly from the Jackson laboratories, or they were born to breeders purchased from the Jackson laboratories and aged to 18 months. Helicobacter hepaticus negative, female mice were housed in ventilated racks (Allentown Caging Equipment) with a 14 hour light cycle at the Johns Hopkins University (JHU) specified pathogen-free barrier facility. Mice were maintained on the 2018SX Teklad Global 18% Protein Extruded Rodent Diet (Harlan Teklad, Madison, WI). Eighteen C57BL/6 background control strain mice aged 18 months were equally divided into control group or treated with Losartan in drinking water (0.9 g/L, Cozaar, Merck) for 4 months. Daily water intake was monitored to be 3.5ml/mouse/day. The rationale for using this concentration was derived from detailed studies titrating Losartan doses in mice to achieve a hemodynamic effect of a 10 to 20% decrease in blood pressure and heart rate comparable to the desired response in humans 11, and from our previous studies where this dose was used to demonstrate significantly accelerated muscle healing and slowing of disuse atrophy in older mice 8. The Losartan dose of 50-70 mg/kg/day in mice translates to a dose of 4–6 mg/kg/day in humans when normalized to standard body surface area 12.
2.2. Experimental measurements
Physical Measurements
Because there are no specific health span and activity equivalent measurements in mice that directly compare to those measured in humans, several activity-related measurements were utilized in this pilot study to assess the potential impact of losartan on function and mobility in mice. First, a measure of activity tolerance was ascertained at baseline and at then every month for 4 months using an exercise treadmill with an electric shock grid (Columbus Instruments, Columbus, OH) at the end of the running band. Mice underwent two 3-minute intervals of training in the week before baseline measurements. To measure time on the treadmill, mice were placed in their respective lanes with the shock grid turned off and allowed to adjust to the surroundings for 10 min. The treadmill was then started, and set at 15 meters/minute speed for a total of 30 minutes. A small electric shock was automatically given and recorded when a mouse slowed and came in contact with the grid. When mice reached exhaustion as measured by number of grid contacts > 50 total, rate higher than 20 electrical stimulations per minute, or failure to re-engage on the treadmill despite aversive stimulation for more than 15 sec, the amount of time was measured and recorded as total treadmill time. The number of grid contacts was also recorded during the treadmill period. The total time on treadmill and the number of grid contacts were then utilized as measures of activity tolerance. Other previously described activity measurements for mice were also collected and compared between groups 13. Traveling activity was measured placing a single mouse in a new cage and then counting the number of times the mouse crossed completely over the midline of the cage during a 5-minute period. Standing activity (rearing behavior) was measured by placing a single mouse in a new cage and counting each time the mouse balanced itself on its hind paws while extending its body vertically onto the wall of the cage or without cage support during a 5-minute period. Other physical parameters measured before each treadmill test included body weight and forelimb grip strength using Grip Strength Meter (Columbus Instruments, Columbus, OH, USA).
2.3. Biological Measurements
No baseline tissue or serum samples were taken given the age and related vulnerability of the mice in this study. For biological measures reported here, mice were sacrificed one week after final treadmill recording. Serum was extracted from terminal phlebotomy, and quadriceps and other hind limb muscles were harvested and immediately frozen at −80°C. For systemic inflammation measure, serum interleukin 6 (IL-6), was measured by Quantikine mouse enzyme-linked immunosorbent assay (ELISA) IL-6 kit (R&D Systems, Inc., Minneapolis, MN). For oxidative stress, multiple markers were utilized. 25mg of quadriceps from each mouse was homogenized in cold phosphate buffered saline and samples were prepared to measure thiobarbituric acid reactive substances (TBARS, oxidized lipids), total antioxidant capacity (TAC), and glutathione by BioAssay Systems QuantiChrom™ Assay Kit (BioAssay Systems, Hayward, CA). The linear detection ranges in colorimetric assay are 1–30μM MDA equivalents for TBARS, 1.5 to 1000 μM Trolox equivalents for TAC, and 0.4 – 100 μM for glutathione. Quantitative PCR (MX-3000P System, Stratagene Inc., La Jolla, CA) was utilized for measurement of antioxidant enzyme-related gene. Total RNA was isolated from muscle tissue using an RNeasy fibrous tissue kit (QIAGEN, Valencia, CA), first-strand cDNA was generated with random primers, PCR was optimized using previously published primers, and relative abundance of specific mRNAs was normalized against actin mRNA level.
2.4. Statistical analysis
Summary statistics of sample characteristics at the beginning and the conclusion of the experiment are presented as mean ± standard deviation (SD). The Wilcoxon rank-sum test was used to compare conservatively the distributions of continuous variables between the treated and the control groups at the initial and final assessments. Generalized Estimating Equations (GEE) were used to evaluate and compare between-group changes over time in the repeated measures of study outcomes including body weight, grip strength, treadmill time, number of grid contacts, and standing and travelling activity 14. The Huber-White standard error estimates were used for robust inference. The GEE estimated the average time trajectory of each outcome by treatment status while accounting for within-subject correlation over time. The GEE were fit based on normal-error models with an identity link and an exchangeable correlation structure for all outcomes except for the number of grid contacts. For the latter, a negative-binomial model with a log link was used instead to account for the excess variance in the count data compared to what is expected under a Poisson model. The group-specific time trajectories estimated from the GEE were plotted for each outcome to reveal systematic trends and group difference. Analyses were conducted in STATA version 12.1 (StataCorp LP, College Station, TX)
3. Results
3.1 Losartan treatment improves activity measurements
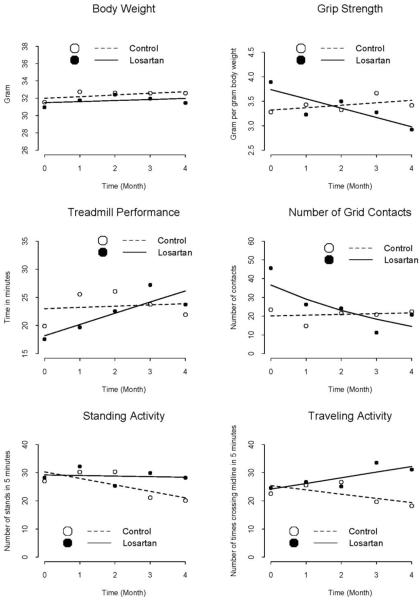
To determine whether losartan treatment positively impacted activity measures, we assessed time on treadmill, number of grid contacts, and traveling and standing activity as described above at baseline and at one month interval for 4 consecutive months for a total of 5 time points. The mean baseline characteristics of treatment and control groups are displayed in tables 1 and 2. The groups were similar at baseline in all measures except that the treatment group mice had on average a significantly lower amount of time on the treadmill and higher numbers of grid contacts before treatment began (table 2). Over the 4 months of intervention, the losartan treated group had a significant increase in the amount of time on the treadmill, increased traveling and standing activity, and decreased contact with electrical grid as compared to the untreated mice (table 2 and figure 1). There was no significant difference in rate of change over time in grip strength or body weight (table 2, figure 1).
Table 1.
Mean cross sectional characteristics of C57BL/6 mice in treatment and control groups*, with standard deviation (SD)
| Control (n=9) | Treatment (n=9) | P-value+ | |
|---|---|---|---|
| Initial Age (months) | 18 | 18 | |
| Initial body weight (gm) | 30.1; 31.5 ± 5.3 | 28.2; 31.0 ± 5.6 | 0.86 |
| Final body weight (gm) | 30.8; 33.5 ± 6.5 | 28.7; 31.5 ± 7.2 | 0.40 |
| Initial grip strength (gm) | 3.4; 3.3±0.7 | 3.8; 3.9±1.0 | 0.27 |
| Final grip strength (gm) | 2.7; 3.4±1.5 | 2.9; 2.9±0.7 | 0.96 |
| Initial treadmill time (min) | 20; 19.9 ± 5.3 | 20; 17.6 ± 3.3 | 0.43 |
| Final treadmill time (min) | 21.8; 21.9 ± 7.7 | 30; 23.7 ± 8.7 | 0.58 |
| Initial number of grid contacts | 29; 23.4 ± 20.6 | 47; 45.6 ± 13.0 | 0.03 |
| Final number of grid contacts | 24; 22.4 ± 15.7 | 12; 20.8 ± 19.4 | 0.69 |
| Initial No. of stands in 5min | 25; 27.0 ± 6.7 | 28; 28.2 ± 8.7 | 0.82 |
| Final No. of stands in 5min | 22; 20.1 ± 8.3 | 28; 28.2 ± 7.5 | 0.05 |
| Initial No. of travelling in 5min | 23; 22.6 ± 7.3 | 20; 24.6 ± 14.1 | 0.72 |
| Final No. of travelling in 5min | 19; 18.2 ± 6.4 | 23; 31.1 ± 16.6 | 0.05 |
| Serum IL-6 at endpoint (Pg/ml) | 173.0 ± 59.5 | 30.3 ± 12.9 | 0.04 |
Data expressed as median; mean ± SD
Based on two-sided Wilcoxon rank-sum test
Table 2.
Effects of Losartan on mean trajectories of physical function over time: results from the GEE fitting in terms of mean (95% confidence interval) baseline score and mean rate of change over time (95% confidence interval) by treatment status as well as difference in means (95% confidence interval) between the losartan treatment and the control groups.
| Outcome | Control | Treatment | Difference in mean baseline score (Losartan-Control) | Difference in mean rate of change (Losartan-Control) | ||
|---|---|---|---|---|---|---|
| Mean baseline score | Mean rate of change (per month) | Mean baseline score | Mean rate of change(per month) | |||
| Body Weight (gm) | 32.0 (28.9, 35.2)# | 0.19 (−0.14, 0.53) | 31.5 (27.9, 35.1)# | 0.12 (−0.39, 0.63) | −0.5 (−5.3, 4.2) | −0.08 (−0.68, 0.53) |
| Grip Strength (gm/gm) | 3.32 (3.06, 3.58)# | 0.05 (−0.12, 0.22) | 3.74 (3.15, 4.34)# | −0.19 (−0.37, −0.01)^ | 0.42 (−0.23, 1.07) | −0.24 (−0.49, 0.01) |
| Time on Treadmill (minutes) | 23.0 (20.6, 25.4)# | 0.22 (−1.17, 1.62) | 18.2 (16.7, 19.7)# | 1.99 (0.91, 3.06)# | −4.8 (−7.6, −2.0)§ | 1.76 (−0.00, 3.52)* |
| Grid contact (log count over 30 minutes | 3.0 (2.6, 3.4)# | 0.02 (−0.13, 0.17) | 3.6 (3.4, 3.8)# | −0.23 (−0.41, −0.04)& | 0.65 (0.18, 1.11)§ | −0.24 (−0.48, −0.01)^ |
| Standing Activity (count over 5 minutes) | 30.3 (26.5, 34.2)# | −2.3 (−3.4, −1.2)# | 29.2 (23.9, 34.6)# | −0.2 (−1.3, 0.8) | −1.1 (−7.7, 5.5) | 2.1 (0.6, 3.5)§ |
| Traveling Activity (count over 5 minutes) | 25.4 (21.3, 29.6)# | −1.5 (−2.3, −0.6)§ | 24.2 (17.4, 31.0)# | 2.0 (0.5, 3.5)¶ | −1.2 (−9.2, 6.7) | 3.5 (1.7, 5.2)# |
p<0.001;
p<0.01
p=0.05;
p=0.04;
p=0.02
p=0.01
Figure 1.
Change in mean body weight, grip strength, treadmill performance, number of grid contacts, and physical activity over time by treatment status; open and solid circles represent observed time-specific sample means for the control and the losartan groups, respectively, with dashed and solid lines representing the corresponding estimated trajectories of change over time based on the GEE models.
3.2 Losartan treatment is associated with reduced serum IL6 and increased catalase and GPX
To assess biological pathways that might link losartan treatment with activity measures, we measured serum IL-6 as a marker of inflammation and found that serum IL-6 level in the treated group was significantly lower than that in the control group (30.3±12.9 vs. 173.0±59.5 pg/ml, p< 0.04). Given prior data that suggests that losartan may reduce oxidative stress, we also measured markers of antioxidant activity and chronic oxidative damage 3. Significantly higher mRNA levels of catalase (3.9±0.9 vs. 1.0±0.4, P-value; 0.02) and GPX (4.7±1.1 vs. 1.0±0.4, P-value: 0.03) were identified in the treated compared to control group. Anti-oxidant enzyme gene expression of MnSOD (3.1±0.6 vs. 1.0±0.3, P<0.06) and cytochrome B (1.5±0.4 vs. 1.0±0.3, P< 0.22) in the skeletal muscle trended in the same direction but did not reach statistical significance. The mean concentration of glutathione (43.3±5.0 vs. 38.4±1.6μM, P = 0.70) and total antioxidant capacity (445.0±21.6 vs 465.6±14.9μM Trolox eq., P= 0.46) in quadriceps of the treated group compared to those in the control group were not different. In addition, no significant difference was observed in mean TBARS level (62.7±8.1 vs. 70.2±3.7 μM MDA eq., p= 0.42), a marker of oxidative stress damage
4. Discussion
In this study, losartan treatment over 4 months significantly improved activity as measured by increased amount of time on the treadmill and decreased number of grid contacts and by increased traveling and standing (rearing) levels in older C57Bl6 losartan treated mice when compared to age matched, untreated control mice. Although prior studies using losartan in older mice have demonstrated improvement in muscle healing and protection against disuse atrophy 8, no prior studies have demonstrated a longitudinal improvement in activity levels related to ARBs. In addition, significant end-point differences in the inflammatory marker serum IL-6 and in gene expression levels of antioxidant enzymes were identified that support the hypothesis that ARBs may work in part through lowering of inflammation and oxidative stress. Losartan has previously been shown to attenuate inflammation and free radical production 15, 3, and both chronic inflammation and lower levels of anti-oxidant enzymes including catalase and GPX likely accelerate sarcopenia 16;17. Although this pilot study could not generate the data necessary to fully characterize the mechanisms that drive the complex biological relationships that connect RAS, inflammation, and oxidative stress in older organisms, it provides important rationale for the further study of the utility of ARBs in maintaining function later in life, and for the study of mechanistic relationships between ARBs, inflammation and oxidative stress.
There are several potential limitations to this study. First, our findings do not allow us to tie the improvement in activity to skeletal muscle improvements and sarcopenia per se. Indeed, we did not find improvement in grip strength over time. Given the broad influence of the RAS on many tissues, and the fact that ARBs such as losartan impact multiple organ systems, we can speculate that some of the observed activity improvement may come from ARB related cognitive and cardiovascular improvements and from mitochondrial functional improvement, in addition to skeletal muscle improvement 3;9;18. Next, the broad array of activity measures that we utilized in this study are not necessarily measuring the same kind of activity that equipment with sensor beams such as the Opto-M4 (Columbus Instruments, Columbus, OH, USA) uses. Although the sensor beam activity measures are useful for calculating activity over hours in a non-stressed state, it does not necessarily introduce any challenge to the aged mouse, which in turn may not reveal changes in function and health span. Hence, the constellation of measures that we utilized could also be considered novel and a strength rather than a limitation. Treadmill measurements are almost certainly stressful to the mice. Standing and traveling activity measurements are taken in an environment that differs from the usual cage environment and hence likely include some cognitive components such as curiosity and anxiety. Hence, the demonstration of improvement in activity through several measures that are taken outside of the `usual' environment may better capture improvements in response to stress or new situations and therefore be more equivalent to human functional measurements. Next, there were baseline differences between groups in some of the activity variables before the intervention started. Although these differences are not ideal for intervention studies, the GEE modeling used to evaluate the trajectory over 4 time points mitigates that difference by comparing change over time between the two groups. Another limitation of our study is the paucity of conclusive biological measures. We did not conclusively demonstrate oxidative stress changes in muscle beyond the alterations in gene expression in anti-oxidant enzymes. This may be in part because the treatment period was not long enough to alter the trajectory of end-point oxidative stress tissue damage. We did not have baseline biological measures in the mice because of the vulnerability of older mice to invasive procedures. Hence, we are not able to estimate change over time in these measures. We also did not have muscle inflammation markers, which reduces our ability to causally link inflammation and activity levels in skeletal muscle.
Despite these limitations, the results identified in this brief report suggest that treatment with losartan improves measures of physical function, decreases the inflammatory cytokine IL-6, and increases protective, antioxidant enzymes. Our results provide rationale for further development of future intervention studies targeting activity and functional improvement or maintenance in older adults and rationale for the further development of mechanistic studies that will help to facilitate the understanding of how inflammation, oxidative stress and the RAS impact health span and activity levels in older adults.
Highlights.
-
1)
Losartan improves several measures of physical activity in older mice.
-
2)
IL-6 levels are lower in older mice losartan treated compared to untreated mice.
-
3)
Anti-oxidant enzyme gene expression is higher in mice treated with losartan.
Acknowledgments
This work is supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, National Institute on Aging (NIA), P30AG021334. The authors gratefully thank Jackie Langdon for performing the mouse husbandry in support of this project, and Denise Baldwin for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Burks TN, Cohn RD. One size may not fit all: anti-aging therapies and sarcopenia. Aging (Albany NY) 2011;3:1142–1153. doi: 10.18632/aging.100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abadir PM, Foster DB, Crow M, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukhanov S, Semprun-Prieto L, Yoshida T, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342:143–147. doi: 10.1097/MAJ.0b013e318222e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter CS, Groban L. Role of the renin-angiotensin system in age-related sarcopenia and diastolic dysfunction. Aging health. 2008;4:37–46. doi: 10.2217/1745509X.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi H, Mizuno Y, Yamamoto H, et al. Effects of telmisartan therapy on interleukin-6 and tumor necrosis factor-alpha levels: a meta-analysis of randomized controlled trials. Hypertens Res. 2013;36:368–373. doi: 10.1038/hr.2012.196. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa S, Mori T, Nako K, et al. Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension. 2006;47:699–705. doi: 10.1161/01.HYP.0000203826.15076.4b. [DOI] [PubMed] [Google Scholar]
- 8.Burks TN, Andres-Mateos E, Marx R, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vescovo G, Dalla LL, Serafini F, et al. Improved exercise tolerance after losartan and enalapril in heart failure: correlation with changes in skeletal muscle myosin heavy chain composition. Circulation. 1998;98:1742–1749. doi: 10.1161/01.cir.98.17.1742. [DOI] [PubMed] [Google Scholar]
- 10.Rolland Y, Dupuy C, Abellan van KG, et al. Treatment strategies for sarcopenia and frailty. Med Clin North Am. 2011;95:427–38. ix. doi: 10.1016/j.mcna.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 13.Walston J, Fedarko N, Yang H, et al. The Physical and Biological Characterization of a Frail Mouse Model. Journal of Gerontology. 2008;63:391–398. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghisletta P, Spini D. An Introductin to Generalilzed Estimating Equations and an Application to Assess Selectivity Effects in a Longitudinal Study on Very Old Individuals. Journal of Educational and Behavioral Statistics. 2004;29:421–437. [Google Scholar]
- 15.Dai Q, Xu M, Yao M, et al. Angiotensin AT1 receptor antagonists exert anti-inflammatory effects in spontaneously hypertensive rats. Br J Pharmacol. 2007;152:1042–1048. doi: 10.1038/sj.bjp.0707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo E, Lee SR, Park BS, et al. Potential Mechanisms Underlying the Role of Chronic Inflammation in Age-Related Muscle Wasting. Aging Clin Exp Res. 2012 doi: 10.3275/8464. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan-Gunn MJ, Lewandowski PA. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013;13:104. doi: 10.1186/1471-2318-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasar S, Xia J, Yao W, et al. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2013;81:896–903. doi: 10.1212/WNL.0b013e3182a35228. [DOI] [PMC free article] [PubMed] [Google Scholar]