Abstract
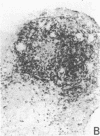
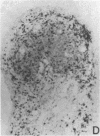
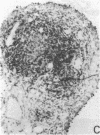
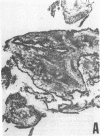
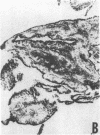
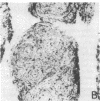
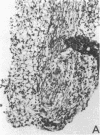
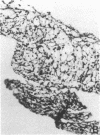
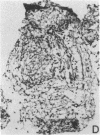
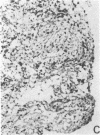
The immunohistology of synovium from a tender, swollen knee and peripheral blood cellular immune function were correlated in 24 clinically similar patients with active, seropositive rheumatoid arthritis who were not taking cytotoxic or long-acting antirheumatic drugs. The patients were classified as anergic (n = 6) or nonanergic (n = 18) on the basis of peripheral blood mononuclear cell proliferative responses to a battery of soluble recall antigens. The peripheral blood mononuclear cells of anergic patients failed to respond significantly to any soluble recall antigen, whereas cells from nonanergic patients responded to at least one such antigen. Multiple pieces of synovial tissue were obtained from each patient at arthroscopy. To minimize intrajoint variability, all pieces were analyzed and averaged to determine a composite profile of abnormalities. Synovial specimens from all six anergic patients had "high intensity" lymphocytic infiltration (group A). In sharp contrast, synovial specimens from 15 of 18 nonanergic patients had "low intensity" lymphocytic infiltration (group B) (P = 0.002). Group A tissues typically showed higher intensity T cell and plasma cell infiltration, more synovial lining layer hyperplasia, more HLA-DR bearing cells, and a higher ratio of Leu 3A/Leu 2A T cells than did group B. Group B tissues had fewer infiltrating cells (most of which were OKM1 and HLA-DR bearing), more extensive fibrin deposition, and far fewer T and plasma cells. Although these data do not imply that synovium from different joints in an individual patient are immunohistologically identical, they do provide evidence that peripheral blood mononuclear cell immune function reflects immunopathologic events in the biopsied joint. Moreover, the data further support the view that clinically active rheumatoid arthritis is, like certain other chronic inflammatory conditions, a heterogeneous disorder with polar subgroups.
Full text
PDF



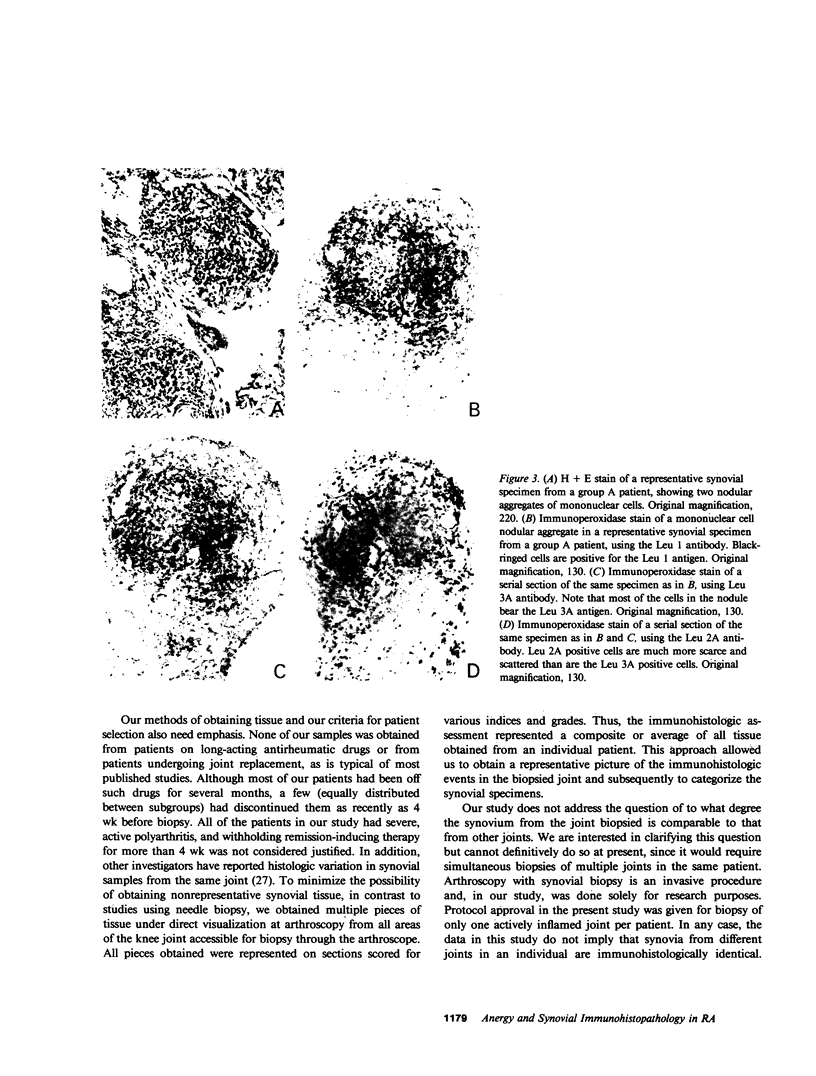
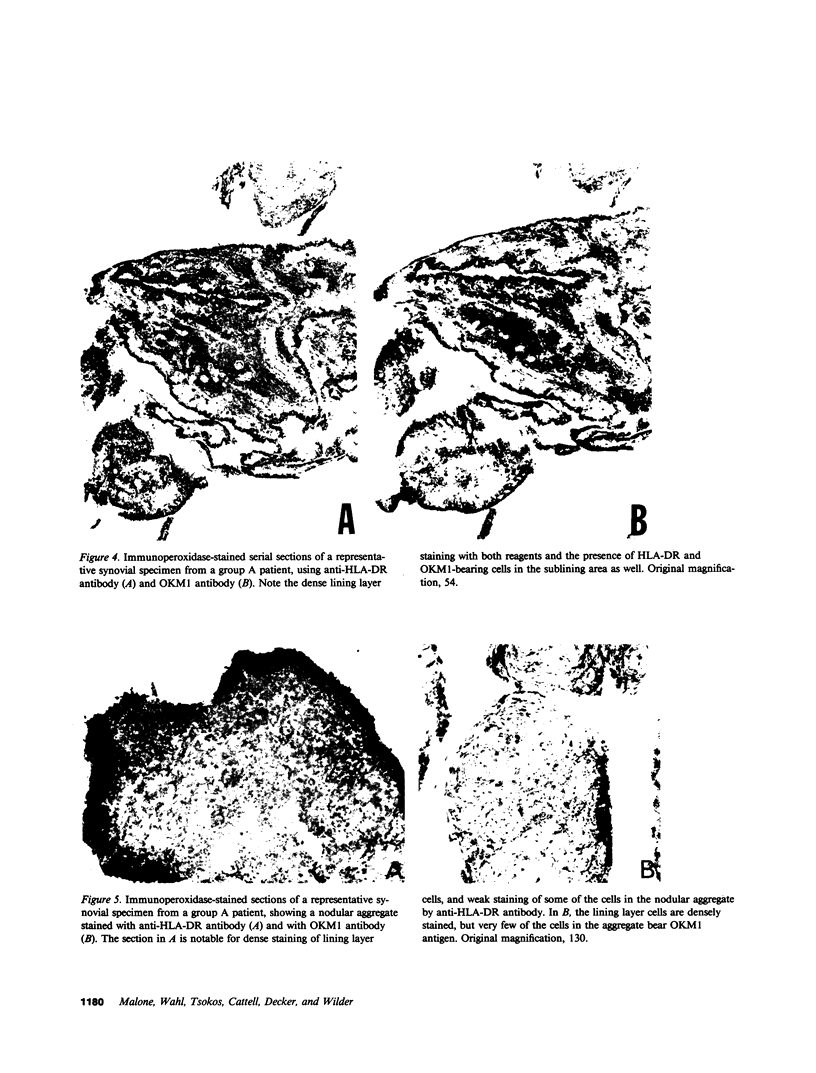
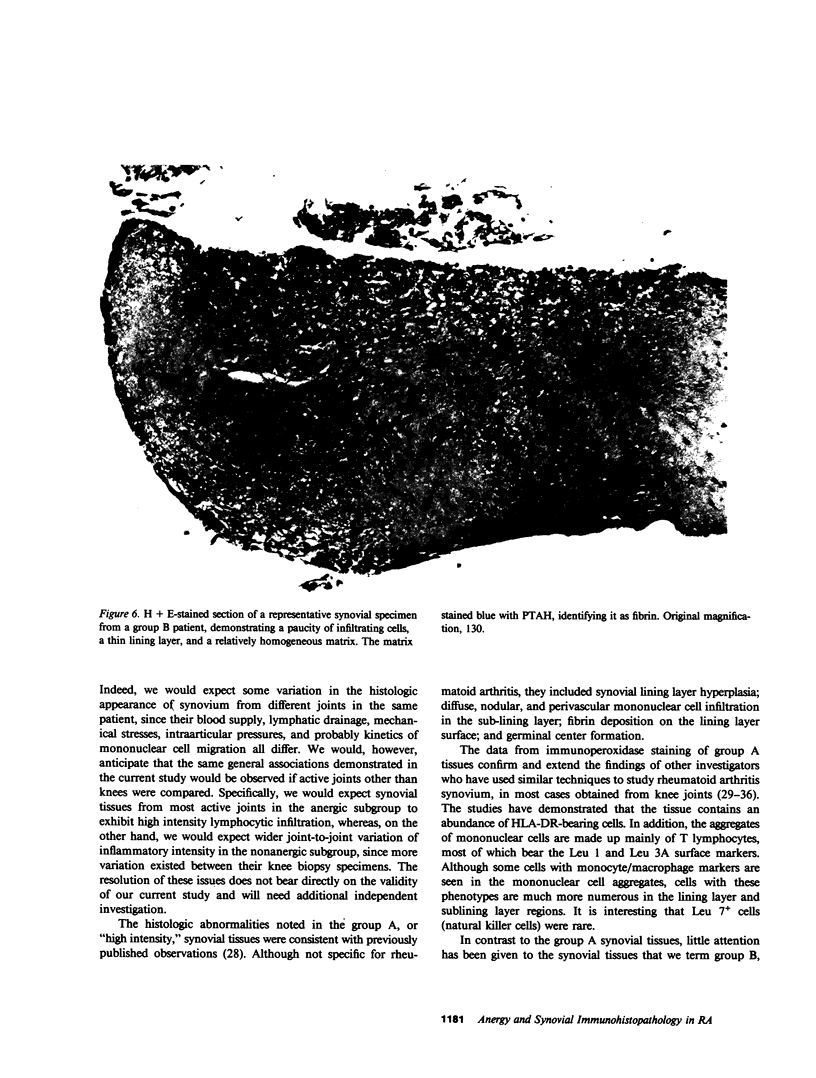




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Bankhurst A. D., Husby G., Williams R. C., Jr Predominance of T cells in the lymphocytic infiltrates of synovial tissues in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):555–562. doi: 10.1002/art.1780190307. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- CRUICKSHANK B. Interpretation of multiple biopsies of synovial tissue in rheumatic diseases. Ann Rheum Dis. 1952 Jun;11(2):137–145. doi: 10.1136/ard.11.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens J. L., Goodwin J. S., Searles R. P. The presence of Ia antigen on human peripheral blood T cells and T-cell subsets: analysis with monoclonal antibodies and the fluorescence-activated cell sorter. Cell Immunol. 1981 Nov 1;64(2):277–292. doi: 10.1016/0008-8749(81)90480-9. [DOI] [PubMed] [Google Scholar]
- Cooper N. S., Soren A., McEwen C., Rosenberger J. L. Diagnostic specificity of synovial lesions. Hum Pathol. 1981 Apr;12(4):314–328. doi: 10.1016/s0046-8177(81)80141-4. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Lazarus H., Penta A. C., Schlossman S. F. Two functionally distinct subpopulations of human T cells that collaborate in the generation of cytotoxic cells responsible for cell-mediated lympholysis. J Immunol. 1978 Apr;120(4):1423–1428. [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Førre O., Dobloug J. H., Natvig J. B. Augmented numbers of HLA-DR-positive T lymphocytes in the synovial fluid and synovial tissue of patients with rheumatoid arthritis and juvenile rheumatoid arthritis: in vivo-activated T lymphocytes are potent stimulators in the mixed lymphocyte reaction. Scand J Immunol. 1982 Feb;15(2):227–231. doi: 10.1111/j.1365-3083.1982.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Førre O., Thoen J., Dobloug J. H., Egeland T., Kvien T. K., Mellbye O. J., Natvig J. B. Detection of T-lymphocyte subpopulation in the peripheral blood and the synovium of patients with rheumatoid arthritis and juvenile rheumatoid arthritis using monoclonal antibodies. Scand J Immunol. 1982 Feb;15(2):221–226. doi: 10.1111/j.1365-3083.1982.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Malone D. G., Allen J. B., Katona I. M., Wahl S. M. Immune function in severe, active rheumatoid arthritis: a relationship between peripheral blood mononuclear cell proliferation to soluble antigens and mononuclear cell subset profiles. J Immunol. 1984 Aug;133(2):697–701. [PubMed] [Google Scholar]
- Helder A. E., Feltkamp-Vroom T. M., Nienhuis R. L. Electron and light microscopical observations and serological findings in rheumatoid arthritis. Ann Rheum Dis. 1973 Nov;32(6):515–523. doi: 10.1136/ard.32.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Ree H. J. Self-sandwich method. An improved immunoperoxidase technic for the detection of small amounts of antigens. Am J Clin Pathol. 1980 Jul;74(1):32–40. doi: 10.1093/ajcp/74.1.32. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Huth F., Soren A., Klein W. Structure of synovial membrane in rheumatoid arthritis. Curr Top Pathol. 1972;56:55–78. doi: 10.1007/978-3-642-65324-7_2. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Wigren A., Wigzell H. Relationship between HLA-DR-expressing cells and T lymphocytes of different subsets in rheumatoid synovial tissue. Scand J Immunol. 1981 May;15(5):501–507. doi: 10.1111/j.1365-3083.1982.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of lymphoid cells in the rheumatoid synovial membrane. Arthritis Rheum. 1973 Jul-Aug;16(4):471–486. doi: 10.1002/art.1780160407. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Reitamo S., Ranki A., Häyry P., Kankaanapä U., Wegelius O. Characterization of the immunocompetent cells of rheumatoid synovium from tissue sections and eluates. Arthritis Rheum. 1981 Jan;24(1):71–79. doi: 10.1002/art.1780240112. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Marin D., Negoescu M., Stoia I., Pierrette A., Petrescu A., Constantinescu S. The morphology of the synovial tissue and articular fluid cells in rheumatoid polyarthritis--studied with the optical and electronic microscope. Acta Rheumatol Scand. 1969;15(2):126–134. doi: 10.3109/rhe1.1969.15.issue-1-4.19. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Melendro E. I., Saldate C., Rivero S. J., Alarcon-Segovia D. T-cell subpopulations in the peripheral blood of patients with connective tissue diseases as determined by flow cytometry using monoclonal antibodies. Clin Immunol Immunopathol. 1983 Jun;27(3):340–347. doi: 10.1016/0090-1229(83)90086-7. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Gebhard J. F., Taylor C. R., Rea T. H. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol. 1983 Jul;53(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Scheynius A., Klareskog L., Forsum U. In situ identification of T lymphocyte subsets and HLA-DR expressing cells in the human skin tuberculin reaction. Clin Exp Immunol. 1982 Aug;49(2):325–330. [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum H., Pinkus G. S., Anderson L. G., Schur P. H. Immunologic characterization of the mononuclear cell infiltrates in rheumatoid synovia, in rheumatoid nodules, and in lip biopsies from patients with Sjögren's syndrome. Arthritis Rheum. 1975 Jul-Aug;18(4):305–314. doi: 10.1002/art.1780180403. [DOI] [PubMed] [Google Scholar]
- Ugolini V., Nunez G., Smith R. G., Stastny P., Capra J. D. Initial characterization of monoclonal antibodies against human monocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6764–6768. doi: 10.1073/pnas.77.11.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Goldstein G., Kung P., Schindler J., Van Wauwe J. Determination of T lymphocyte subpopulations by monoclonal antibodies in rheumatoid arthritis. Influence of immunomodulating agents. Int J Immunopharmacol. 1981;3(3):313–319. doi: 10.1016/0192-0561(81)90025-4. [DOI] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Verbruggen G., Schindler G., Goldstein G. T lymphocytes in blood and synovial fluid in rheumatoid arthritis. Lancet. 1982 Jan 23;1(8265):225–226. doi: 10.1016/s0140-6736(82)90791-7. [DOI] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Verbruggen G., Schindler J., Goldstein G. Evaluation of T cell subsets with monoclonal antibodies in synovial fluid in rheumatoid arthritis. J Rheumatol. 1982 Nov-Dec;9(6):821–826. [PubMed] [Google Scholar]
- Wahl S. M., Wilder R. L., Katona I. M., Wahl L. M., Allen J. B., Scher I., Decker J. L. Leukapheresis in rheumatoid arthritis. Association of clinical improvement with reversal of anergy. Arthritis Rheum. 1983 Sep;26(9):1076–1084. doi: 10.1002/art.1780260904. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]