Abstract
Although research has identified age-related changes in neural recruitment during emotional memory encoding, it is unclear whether these differences extend to retrieval. In this study, participants engaged in a recognition task during an fMRI scan. They viewed neutral titles and indicated whether each title had been presented with an image during the study phase. Neural activity and connectivity during retrieval of titles associated with positive and negative images were compared, with age (treated as a continuous variable) included as a regressor of interest. Aging was associated with increased prefrontal activation for retrieval of positive and negative memories, but this pattern was more widespread for negative memories. Aging also was associated with greater negative connectivity between a left hippocampal seed region and multiple regions of prefrontal cortex, but this effect of age occurred during negative retrieval only. These findings demonstrate that age-related changes in prefrontal recruitment and connectivity during retrieval depend on memory valence. The use of a lifespan approach also emphasized both continuities and discontinuities in recruitment and connectivity across the adult lifespan, highlighting the insights to be gained from using a full lifespan sample.
Keywords: Memory, Retrieval, Aging, fMRI, Connectivity, Prefrontal Cortex
1. Introduction
Healthy aging, even in the absence of dementia, is associated with cognitive declines, including memory retrieval (Salthouse, 2011). However, it has been suggested that memory impairments in older adults can be mitigated by the presence of emotional arousal (e.g., Kensinger, 2009b). Critically, some studies have found that older adults’ emotional enhancement is particularly strong when the information is of positive valence (e.g., Charles et al., 2003), suggesting that valence, and not only emotional arousal, can influence memory processes in healthy aging. This age-related enhancement of positive information has been of great interest in the cognitive aging literature, as it represents a special circumstance in which age-related cognitive declines may be reduced by specific task-related factors. Further, both young and older adults may process positive and negative information differently, with negative information associated with more visual processing and positive information associated with more conceptual processing (Kensinger, 2009b). As such, age-related changes to positive and negative event retrieval may reflect changes on a number of dimensions relevant to cognitive processing.
One important question that has emerged from the examination of emotional memory in healthy aging is how emotion may alter neural recruitment associated with specific mnemonic processes, and how these influences may differ as a function of age. Identifying the neural correlates of this effect could help researchers understand the underlying cognitive mechanisms contributing to emotional enhancement in older adults. Prior studies have suggested that healthy aging is associated with increased prefrontal cortex (PFC) activity during encoding of emotional relative to neutral information (see St. Jacques, Bessette-Symons, & Cabeza, 2009 for review), and of positive relative to negative information (Leclerc & Kensinger, 2008). In addition to age-related differences in neural recruitment, previous studies have also identified age-related changes in neural connectivity during emotional encoding. Specifically, healthy aging influences connectivity within the medial temporal lobe (MTL) and between the MTL and PFC. Amygdala-hippocampal connections may be weakened in older adults during the encoding of negative information (St. Jacques, Dolcos, & Cabeza, 2009) and older adults show stronger connectivity between the hippocampus, amygdala, and mPFC during the encoding of positive information than do young adults (Addis et al., 2010). Age-related changes in neural activity and connectivity during emotional encoding have been explained as potentially revealing age-related shifts in self-referential processing, where older adults interpret positive stimuli in a more self-relevant way (Kensinger & Leclerc, 2009), as well as age-related increases in emotional regulation strategies in healthy older adults (e.g., St. Jacques, Dolcos, & Cabeza, 2009).
Although most research examining the effects of age on the neural correlates of emotional memory has focused on encoding processes, several behavioral studies suggest that healthy aging also has an effect on processes associated with retrieval. Specifically, older participants exhibit greater increases in ratings of positive valence for personal memories (Kennedy et al., 2004), even when the age of encoding is held constant, and they perceive their recall of positive events to be more vivid than negative ones (Petrican et al., 2008). Investigating the effect of emotional valence on the neural correlates of memory retrieval may elucidate the reasons for these differences. Previous studies have shown increased activity in the amygdala (e.g., Dolan et al., 2000; Murty et al., 2009; but see, Taylor et al., 1998) and lateral frontal lobes (Murty et al., 2009) during retrieval of emotional relative to neutral events, thought to reflect the retrieval of affective content or the re-experience of an affective response, and the monitoring and elaboration of the memory, respectively. However, the effect of valence and healthy aging on these processes is still unknown. The current study extends prior work by examining age-related changes in neural recruitment during retrieval of positive and negative information.
Behavioral studies of memory retrieval reveal linear declines in performance starting as early as in one’s early 30s (Salthouse, 2011). Similarly, behavioral studies with continuous age designs suggest that age-related changes in emotional experience (Pasupathi & Carstensen, 2003) and emotional memory (Carstensen & Charles, 1994) may occur gradually over time. Despite this evidence, previous studies examining the effects of healthy aging on neural recruitment during memory tasks have often compared young adults (typically 18–35 years old) to older adults (often ages 60 and older), ignoring individuals between the ages of 35 and 60. Therefore, it currently is unknown whether the neural changes of middle-aged adults are similar to those of older adults. In addition, while previous research has shown that the emotional valence can have opposite effects on neural recruitment in young and older adults (Leclerc & Kensinger, 2008), it is unknown whether this change is discrete (i.e., valence influences neural recruitment for young and middle-aged adults in the same way until a certain age, then this effect is reversed) or gradual (i.e., middle-aged adults exhibit a pattern of activity that is in-between that of young and older adults). To answer these questions, the current study utilizes a lifespan assessment to examine the effects of emotion on the memory network.
One potential difficulty with examining the neural activity associated with emotional episodic memory retrieval is that re-presenting participants with studied emotional and neutral stimuli could lead to neural differences stemming from the processing of the retrieval cues, in addition to those related to remembering the encoding event. A number of recent fMRI studies have avoided this potential confound of on-line emotional processing by having participants encode a neutral item in a neutral or emotional study context and using the neutral item as the retrieval cue (e.g., Maratos et al., 2001; Sterpenich et al., 2006). This method helps ensure that valence differences at retrieval are related to the mnemonic content and not the retrieval cue. In the current study, we utilize a paradigm that has been reported previously (Ford et al., in press), in which participants encode positive, negative, and neutral images presented with neutral titles. During a scanned retrieval session, participants view the neutral titles – to avoid confounds associated with an emotional retrieval cue -- and retrieve the related emotional or neutral image.
The current study examines age-related changes in neural recruitment, particularly in prefrontal regions, and in MTL-PFC connectivity during retrieval of emotional events. Based on prior evidence that older adults shift to prefrontal rather than sensory based processing (e.g., Davis et al., 2008), we hypothesize that healthy aging will be associated with increased prefrontal and decreased posterior activation during successful retrieval. We are particularly interested in how emotional valence interacts with aging to influence prefrontal activity and connectivity. During encoding, healthy older adults recruit prefrontal regions to a greater extent during positive relative to negative events, whereas young adults exhibit the reverse activation pattern (i.e., negative > positive; Leclerc & Kensinger, 2008), and healthy aging is associated with increased MTL-PFC connectivity during encoding of positive events, but not negative (Addis et al., 2010). Similar age-related increases in prefrontal activity and connectivity during positive event retrieval would demonstrate that age-related changes in emotional processing seen during encoding extend to retrieval. Conversely, age-related changes in neural activity and connectivity that do not replicate these patterns would suggest that emotion influences distinct cognitive processes during encoding and retrieval.
2. Methods
2.1 Participants
Data from sixty-three healthy adults (mean age= 47.92, sd= 19.80, ages 19–85; mean education= 16.56, sd= 2.34) are reported. The ratio of males to females was roughly one-to-one (30 females and 33 males) and was approximately equivalent within each decade (43%–67% male in each decade), with no significant difference in this distribution across decades (x2(6, N=63)=.85, p= .99). Twenty-seven of the young adult subjects from this sample were included in a recent paper examining the interactive effects of emotional valence and memory phase on neural recruitment (Ford et al., in press). Gender distribution was even across the age range and age was not significantly correlated with education (p= .68). Two additional participants were recruited but not scanned due to contraindications for fMRI (ages 50 and 75; both male). Another ten participants were scanned, but were excluded from the current analysis due to equipment malfunction (n=1; age=49, edu=16, male), an abnormal structural scan (n=1, age= 49, edu=17, female), excessive motion (n=1, age=56, edu=16, male), voluntary early termination of the MR session (n=1, age=49, edu=14, female), or low behavioral performance (n=6, mean age= 55.64, sd= 18.12, ages 30–83; mean education= 16.12, sd= 3.49; 2 female). Participants were right-handed native English speakers without psychiatric illness or neurological disorder and were recruited from the greater Boston area. All participants were paid for their participation and gave written informed consent in accordance with the requirements of the Institutional Review Board at Boston College.
All participants completed the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) to examine self-reported symptoms of anxiety, as well as the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the Geriatric Depression Scale (Sheikh & Yesavage, 1986) to evaluate symptoms of depression. In addition, participants engaged in a series of tests intended to examine general cognitive ability, vocabulary, verbal fluency, working memory, and memory (both immediate and delayed). Finally, all participants completed a battery of cognitive tests implemented in CogState, a computerized neuropsychological test battery, that was approximately 30 minutes in duration. The battery included 6 subtests that examine a range of cognitive abilities, including: Detection Task (speed of processing), Identification Task (visual attention), One Card Learning Task (visual learning and memory), One Back Task (attention/working memory), Two Back Task (attention/working memory), and Set-Shifting (executive function); these have acceptable criterion and construct validity in a neuropsychological context (see www.cogstate.com; Maruff et al., 2009). The relations of age with all cognitive variables are reported in Supplementary Table 1. In brief, healthy aging was associated with decreased anxiety, increased vocabulary, and impairments on tasks involving long term memory (e.g., delayed visual pairs) and executive control (e.g., digit/symbol task, mental arithmetic, mental control, digit span, and set shifting). In addition, older adults showed significantly greater retrieval times in four of the six CogState computer tasks.
2.2 Materials
Stimuli were the 480 pictures (160 positive, 160 negative, and 160 neutral) and the neutral titles used in Ford et al. (in press) and selected from the IAPS database, Geneva Affective Picture Database (GAPED) and images used in Waring & Kensinger (2011). Based on normative data available at the time of image selection, arousal ratings were equated for positive and negative images, while positive and negative images were significantly higher in arousal than neutral images. However, arousal ratings given by participants in the current study were higher for negative relative to positive images (see behavioral results section). Neutral titles were selected for each picture to describe the picture as closely as possible. To confirm title neutrality, five pilot participants (4 female; M age = 21.6) were asked to view all titles and determine whether they were neutral, positive, or negative. Titles were replaced if 2 or more participants rated them as either positive or negative (e.g., “Medical Examination” was changed to “Rubber Gloves” and “Alleyway” was changed to “Concrete Arches”). The 480 title-picture pairs were divided into 4 sets of 120 pictures each (40 positive, 40 negative, and 40 neutral) for counterbalancing purposes.
2.3 Procedure
Following instruction and a short practice, participants encoded one set of 120 title-image pairs. Titles (e.g., “Lettuce”) were paired with a positive, negative, or neutral image (e.g., a piece of rotting lettuce with bugs crawling on it as a negative image). In an intentional encoding task (outside of the scanner) participants were given 3 seconds to make a decision regarding the appropriateness of the word as a description of the image (1= poor description, 2= acceptable description, and 3= very good description). After a half-hour delay (M= 34.3 minutes, sd= 7.8), participants took part in a scanned retrieval task. Participants were presented with the 240 titles (120 neutral titles that were studied during the encoding phase and 120 unstudied neutral titles) randomly across 6 retrieval runs of equal length. Participants were given up to 4 seconds to decide whether the word was “old” (i.e., seen previously) or “new” (i.e., not seen previously). The screen was removed following the participant’s button press. Across participants, it was varied which items were studied and which were reserved as foils on the recognition test.
Immediately following an “old” response, 80% of the time, participants were asked to “Elaborate” on the old item (i.e., think about the image presented with the title and the experience with that title and image at encoding) for 5 seconds. Participants then rated (on a 1–5 scale) how well they remembered the image and their own personal thoughts and feelings while encoding the item. Each rating was presented for 3 seconds and the order of the ratings was alternated across participants. To discourage participants from beginning to elaborate during the search phase, and to distinguish activity during search from activity during elaboration, 20% of trials were catch trials; instead of an elaboration phase, the next trial was presented. Following a “new” response, 80% of the time, participants moved on to the next trial. To minimize the likelihood that participants would automatically begin preparing for the next trial after a “new” response, on 20% of the trials, participants were asked to “Imagine” an image that could have accompanied the new item for 5 sec. They then rated (on a 1–5 scale) the vividness of the image they generated for the new item and the vividness of their own personal thoughts and feelings. Following each trial, participants viewed a fixation cross for 0–6 seconds to introduce jitter.
After being removed from the scanner, participants were re-presented with the images from the encoding phase. They rated each image’s valence and arousal on a 1–7 scale and indicated which specific emotions they experienced with each image. This portion was self-paced and participants were encouraged to respond based on their initial reaction.
2.4 Data Acquisition
Participants’ heads were stabilized in a Siemens Tim Trio 3 Tesla scanner. A localizing scan and auto-align scout were followed by a high resolution multi-echo T1 structural scan for anatomical visualization (176 1mm slices, TR=2200ms, TE1=1.64ms, TE2= 3.5ms, TE3= 5.36ms, TE4= 7.22ms). Six runs of whole brain, gradient-echo, echo planar images (31 3mm slices aligned along the line between the anterior and posterior commissures, 20% skip, TR=2s, TE=30ms, Flip angle=90) were acquired during memory retrieval using interleaved slice acquisition. A diffusion weighted scan was collected but will not be discussed. Response data were collected using a magnet-safe button response box.
2.5 Preprocessing and Data Analysis
Images were preprocessed and analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. Images were co-registered, realigned, normalized (resampled at 3 mm at the segmentation stage and written at 2mm at the normalization stage) and smoothed using a Gaussian 8 mm kernel. The current analysis focused on effects of valence and healthy aging on memory search, modeled as an event-related response at cue-onset.
The first level fMRI analysis examined the effect of emotional valence (i.e., positive or negative events) on neural activity during accurate “old” responses to studied items (i.e., “hits”). Neutral hits, incorrect responses and correct “new” responses to positive, negative, and neutral items, although not relevant for the current analysis, were included in each model as five separate nuisance variables. Contrasts were used to examine neural recruitment during successful retrieval of positive events relative to baseline, negative events relative to baseline, positive relative to negative events, and negative relative to positive events.
One-sample t-tests were used at the random-effects level to examine neural recruitment during successful retrieval of positive and negative events. Age was included as a continuous variable of interest, allowing for identification of regions in which activity increased as a function of age (i.e., regions showing increased activity for older relative to younger participants) and regions in which activity decreased as a function of age (i.e., regions showing increased activity for younger relative to older participants). Conjunction analyses were conducted to identify regions that exhibited significant age effects in both positive and negative event retrieval. Conjunction analyses were performed by creating explicit masks of one contrast (e.g., the effect of aging during positive event retrieval) and applying this mask to the second contrast (e.g., the effect of aging during negative event retrieval). This joint analysis allows us to identify regions that show significant age effects, regardless of valence. Activity within a 5mm sphere around peak voxels in several regions of interest (ROIs) was extracted using the REX toolbox (downloaded from http://web.mit.edu/swg/software.htm). Activity in these ROIs was entered into a repeated-measures ANOVA with valence (i.e., positive and negative) as a within-subjects variable and age as a continuous variable of interest.
The significance threshold for all analyses was set at p < .005 (uncorrected). Monte Carlo simulations (Slotnick et al., 2003), run with the normalized voxel size of 2×2×2, determined that a 29-voxel extent corrected results to p < .05. Therefore, we discuss all clusters that reach this threshold. However, because this relatively large voxel extent may put us at risk for type 2 error (see Lieberman & Cunningham, 2009), we report all clusters with a voxel extent of 10 or more in the tables, as these results may be relevant for the purposes of future reviews and meta-analyses. Clusters reaching significance were overlaid on anatomical images from MRICron. For visualization purposes, activity within a 5 mm sphere around peak voxels was extracted using the REX (downloaded from http://web.mit.edu/swg/software.htm) toolbox. For all analyses, reported coordinates reflect the peak activity within active regions. These coordinates were converted from MNI coordinates to Talairach space, localized using the Talairach Client, and confirmed with the Talairach and Tournoux atlas (Talairach & Tournoux, 1988).
The current study examined connectivity between the medial temporal lobe and prefrontal regions during successful retrieval of positive and negative events, utilizing the generalized psychophysiological interactions (gPPI; http://brainmap.wisc.edu/PPI; McLaren et al., 2012) toolbox in SPM8. The gPPI toolbox, which is configured to automatically accommodate multiple task conditions in the same PPI model, compares functional connectivity to a single seed region across tasks. Based on prior research conducted at encoding (Addis et al., 2010), we had an a priori hypothesis that healthy aging would be associated with alterations in prefrontal-MTL connectivity. Therefore, we selected our gPPI seed region from within the medial temporal lobe and identified prefrontal regions in which MTL connectivity was influenced by an age-by-valence interaction.
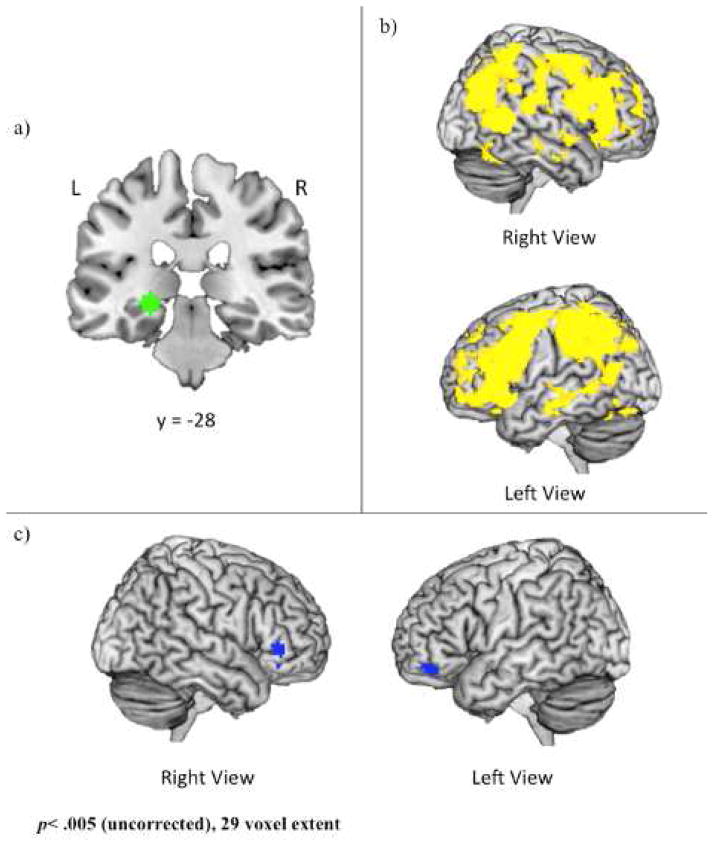
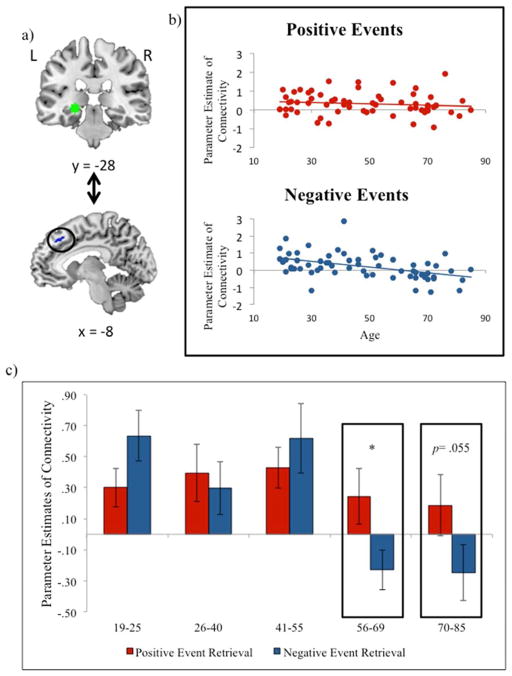
To identify an MTL seed region, we ran an omnibus contrast examining retrieval (hits) of both positive and negative events relative to baseline, controlling for age. This contrast identified clusters within bilateral hippocampus and parahippocampal gyus at p<.005, with no suprathreshold voxels in the amygdala. The most significant cluster was within the left hippocampus, a region that may be critically involved in emotion memory, particularly in its interactions with the amygdala (LaBar & Cabeza, 2006). Therefore, a peak voxel within the left hippocampus (−24, −28, −6) was used to create volumes of interest (VOIs) for each subject. Specifically, for each subject, a VOI was generated by creating a 6mm sphere around this voxel (Figure 3a). Within each subject, the gPPI toolbox was used to estimate functional connectivity across the entire brain with this 6mm VOI in the two memory conditions (i.e., positive hits and negative hits) and to calculate the two contrasts of interest (i.e., positive hits>negative hits, negative hits>positive hits) as well as a conjunction (i.e., positive hits AND negative hits).
Figure 3.
a) 6mm sphere used as the volume of interest (VOI) for psychophysiological analysis. The center of this VOI (−24, −28, −6; left hippocampus) was selected via an omnibus contrast that identified regions associated with retrieval (i.e., Hits) of positive and negative events, controlling for age. VOIs were created and defined functionally at the single-subject level; b) Regions showing age-related decreases in functional connectivity with the left hippocampal seed region during both negative and positive events; c) Regions showing age-related decreases in functional connectivity with the left hippocampal seed region during negative relative to positive events. Coordinates are presented in MNI space.
At the group level, contrast files from each of the three individual-subject gPPIs were entered into separate, single-sample t-tests, with age as a continuous variable of interest. The continuous age variable allowed us to examine regions in which the differences in correlation values increased or decreased as a function of age. The first contrast looked at regions that were more positively correlated with left hippocampal activity in both positive and negative hits. The second identified regions that were more positively - or less negatively - correlated with left hippocampal activity for positive than negative memory. The final contrast identified regions that were more positively - or less negatively - correlated with left hippocampal activity for negative than positive memory. Two additional group analyses were conducted for each of the memory conditions (i.e., positive hits and negative hits) to disambiguate the underlying effects identified in these contrasts. As with the prior analysis, the significance threshold was set at p < .005 with a 29-voxel extent (correcting results to p < .05), with a voxel extent of 10 reported in the tables. In addition, prefrontal regions of a prior interest were examined as this more liberal threshold.
3. Results
3.1 Behavioral Results
The appropriateness of each image’s verbal title was evaluated during the encoding phase of the current task. Participants rated the titles for negative images as less appropriate than positive (t(62)= 11.08, p< .001) and neutral (t(62)= 12.46, p< .001) image titles. Age was not correlated with ratings of appropriateness for any emotion condition (p>.5 for all three conditions). Memory accuracy and retrieval time were entered into a repeated measures ANOVA, with age as a covariate of interest and emotional valence as a within-subjects factor. Accuracy did not differ as a function of valence (p=.64), but was associated with age (F(1,61)=11.75, p<.001). Specifically, increased age was associated with decreased accuracy for neutral (r= −.50, p<.001), positive (r= −.50, p<.001), and negative events (r= −.49, p<.001). There was a trend for valence to affect retrieval time (p= .09) with participants responding faster to retrieval cues for neutral relative to positive (t(62)= 2.73, p<.01) and negative events (t(62)= 3.33, p<.001), and to positive relative to negative events (t(26)= 2.15, p<.05). Healthy aging was associated with increased retrieval times (F(1,61)=9.23, p<.005) for neutral (r= .39, p<.001), positive (r= .32, p<.05), and negative events (r= .35, p<.005). Importantly, the age-by-valence interaction was not significant for either variable (p>.1 for both comparisons).
Ratings collected after the memory test confirmed that positive images were judged as more positive than neutral images (t(62)=12.35, p<.001) which were judged as more positive than negative images (t(62)= 15.89, p<.001). Additionally, negative and positive images were judged as more arousing than neutral images (p<.001 for both contrasts), and negative images were more arousing than positive (t(62)= 3.97, p<.001). Age was not associated with ratings of arousal or valence for any emotion condition (p>.1 for all contrasts).
3.2 Imaging Results
3.2.1 Effects of emotional valence on neural recruitment during retrieval
When controlling for the effect of age by inclusion of the regressor for age, retrieval of negative events was associated with increased activity in ventrolateral prefrontal cortex (vlPFC), lateral temporal lobes, inferior parietal lobe, posterior and anterior cingulate, medial temporal lobes (MTL), and visual cortex compared to positive event retrieval (Table 1 and Supplementary Figure). Relative to negative events, positive event retrieval was associated with increased activity in the premotor cortex (Table 1).
Table 1.
Effects of emotional valence on neural recruitment, collapsing across ages.
| Region of Interest | Hemisphere | BA | MNI Coordinates | p-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Negative > Positive Events | |||||||
| Frontal | |||||||
| Ventrolateral Prefrontal Cortex | L | 11 | −44 | 36 | −16 | 0.000 | 162 |
| R | 47 | 32 | 24 | −14 | 0.000 | 151 | |
| Dorsal Prefrontal Cortex | R | 6 | 6 | 42 | 40 | 0.001 | 26T |
| Temporal | |||||||
| Superior Temporal Gyrus | L | 39 | −44 | −58 | 32 | 0.001 | local maxima within cluster at: −48, −62, 38 |
| Middle Temporal Gyrus | R | 21 | 68 | −26 | −4 | 0.001 | 31 |
| Medial Temporal | |||||||
| Parahippocampal Gyrus/Hippocampus | L | 27 | −22 | −36 | −6 | 0.000 | 90 |
| Parahippocampal Gyrus | L | 36 | −32 | −28 | −18 | 0.002 | 10T |
| Hippocampus | R | na | 30 | −28 | −16 | 0.003 | 10T |
| Parietal | |||||||
| Inferior Parietal Lobe | L | 40 | −48 | −62 | 38 | 0.000 | 236 |
| Occipital | |||||||
| Cuneus | R | 18 | 18 | −88 | 12 | 0.000 | 130 |
| Lingual Gyrus | L | 18 | −16 | −70 | −2 | 0.000 | 108 |
| Cuneus | L | 18 | −8 | −76 | 14 | 0.001 | 97 |
| Limbic | |||||||
| Posterior Cingulate | R | 29 | 12 | −48 | 18 | 0.000 | 194 |
| L | 31 | −8 | −46 | 28 | 0.000 | 169 | |
| L | 30 | −8 | −56 | 10 | 0.003 | 10T | |
| Anterior Cingulate | L | 24 | −10 | −6 | 28 | 0.000 | 140 |
| R | 24 | 10 | −2 | 28 | 0.000 | local maxima within cluster at: −10, −6. 28 | |
| Other | |||||||
| Insula | L | 13 | −40 | −20 | 2 | 0.002 | 18T |
| Positive > Negative Events | |||||||
| Frontal Lobe | |||||||
| Premotor Cortex | L | 6 | 0 | −26 | 64 | 0.000 | 44 |
| Dorsal Prefrontal Cortex | R | 9 | 34 | 46 | 36 | 0.001 | 23T |
Clusters significant at an uncorrected threshold of p<.005, k ≥ 10 voxels
Up to 3 local maxima that are at least 8mm apart reported for each cluster; when peaks are local maxima within larger clusters, source cluster peak is reported instead of cluster size BA= approximate Brodmann Area; L=Left, R=Right
Indicates a cluster size smaller than the designated cluster size of 29
3.2.2 Effects of healthy aging on neural recruitment during emotional memory retrieval
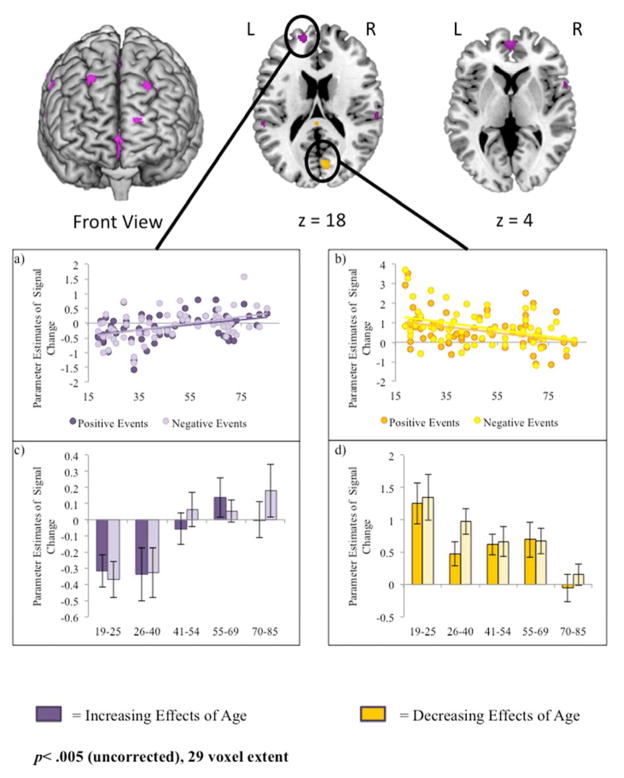
To examine the effects of healthy aging on neural recruitment, we conducted two conjunction analyses: a) a conjunction analysis that identified regions showing increased activity as a function of age in both positive and negative events, and b) a conjunction analysis that identified regions showing decreased activity as a function of age in both positive and negative events. During retrieval of both positive and negative events, healthy aging was associated with increased activity in ventral and dorsal prefrontal regions, inferior parietal lobe, left lateral temporal lobe, posterior cingulate, and bilateral insula (Table 2). Increased age was associated with decreased activity in posterior regions associated with visual processing, such as the bilateral lingual gyrus, left fusiform gyrus, and right cuneus (See Table 2).
Table 2.
Regions exhibiting effects of healthy aging on neural recruitment in both positive and negative events
| Region of Interest | Hemisphere | BA | MNI Coordinates | p-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increased activity as a function of healthy aging | |||||||
| Frontal Lobe | |||||||
| Premotor Cortex | L | 6 | −10 | −10 | 60 | 0.000 | 101 |
| L | 6 | −26 | −20 | 62 | 0.001 | 53 | |
| Dorsal Prefrontal Cortex | L | 9 | −30 | 20 | 38 | 0.000 | 288 |
| L | 9 | −24 | 42 | 42 | 0.001 | 26T | |
| R | 8 | 22 | 28 | 46 | 0.000 | 94 | |
| Anterior Prefrontal Cortex | L | 10 | −34 | 36 | 14 | 0.000 | 21T |
| Ventromedial Prefrontal Cortex | L | 10 | 0 | 58 | 4 | 0.000 | 296 |
| Medial Prefrontal Cortex | L | 10 | −16 | 56 | 14 | 0.000 | 64 |
| Precentral Gyrus | R | 4 | 32 | −20 | 52 | 0.000 | 88 |
| Ventrolateral Premotor Cortex | R | 44 | 60 | 12 | 8 | 0.001 | 86 |
| L | 47 | −30 | 30 | −12 | 0.001 | 11T | |
| Parietal Lobe | |||||||
| Inferior Parietal Lobe | L | 40 | −60 | −34 | 36 | 0.000 | 25T |
| Precuneus | L | 31 | −8 | −58 | 34 | 0.000 | 62 |
| Postcentral Gyrus | R | 2 | 60 | −32 | 42 | 0.001 | 23T |
| Temporal Lobe | |||||||
| Superior Temporal Gyrus | L | 39 | −40 | −60 | 32 | 0.000 | 96 |
| Inferior Temporal Gyrus | L | 20 | −58 | −26 | −20 | 0.001 | 19T |
| Middle Temporal Gyrus | L | 21 | −60 | −14 | −18 | 0.001 | 16T |
| Limbic Lobe | |||||||
| Posterior Cingulate | L | 31 | −6 | −26 | 42 | 0.000 | 965 |
| Other | |||||||
| Insula | R | 13 | 52 | −22 | 26 | 0.000 | 67 |
| R | 13 | 40 | −30 | 16 | 0.002 | 16T | |
| L | 13 | −56 | −34 | 18 | 0.001 | 12T | |
| Decreased activity as a function of healthy aging | |||||||
| Temporal | |||||||
| Fusiform Gyrus | L | 37 | −34 | −50 | −16 | 0.000 | 86 |
| Inferior Temporal Gyrus | L | 19 | −46 | −56 | −8 | 0.003 | 13T |
| R | 18 | 22 | −68 | −4 | 0.000 | 20T | |
| Occipital | |||||||
| Lingual Gyrus | L | 18 | −20 | −72 | −6 | 0.000 | 103 |
| Cuneus | R | 18 | 6 | −74 | 18 | 0.001 | 147 |
| Limbic | |||||||
| Posterior Cingulate | L | 23 | −4 | −30 | 22 | 0.000 | 190 |
Clusters significant at an uncorrected threshold of p<.005, k ≤ 10 voxels
Up to 3 local maxima that are at least 8mm apart reported for each cluster. BA= approximate Brodmann Area; L=Left, R=Right
Indicates a cluster size smaller than the designated cluster size of 29
To better understand the relations between healthy aging and neural activity in regions identified in this analysis, we selected two clusters as regions of interest (ROIs) from which parameter estimates could be extracted. Estimates for negative and positive retrieval were extracted from a 5mm sphere surrounding the peak voxel of each cluster (Figure 1a–1d) and repeated-measures ANOVAs were conducted with valence as a within-subjects variable and age as a covariate of interest. For visualization purposes, the continuous age range was split into five age groups that were approximately equivalent in size (N=12, 14, 13, 12, and 12) whose average parameter estimates have been graphed for each region (See Figure 1c–1d). One of these peaks (medial prefrontal cortex, BA 10; - 16, 56, 14) was selected from regions that exhibited increased activity as a function of age (Figures 1a & 1c). Activity did not differ as a function of emotional valence and there was no age-by-valence interaction (p>.1). However, activity increased as a function age (F(1,61)= 14.49, p< .001). A second peak within the cuneus (BA 18; 6, -74, 18) was selected from regions that exhibited decreased activity as a function of age (Figure 1b and 1d). Estimates for both positive and negative events decreased as a function of age (F(1,61) = 10.38, p< .005), but the effect of valence and the age-by-valence interaction were not significant (p>.1 for both contrasts).
Figure 1.
Regions showing age-related increases (purple) or decreases (yellow) in activity for both positive and negative event retrieval. Parameter estimates for activity are plotted from 5mm spheres surrounding peak voxels from two regions of interest: a) -16, 56, 14 (medial PFC) and b) 6, -74, 18 (cuneus). For visualization purposes, parameter estimates during positive (dark bars) and negative (light bars) event retrieval are also plotted for 5 age groups that were approximately equivalent in size (12, 14, 13, 12, and 12 participants, c & d) and represented a continuous age range. Coordinates are presented in MNI space.
3.2.3 Differential effects of valence on neural recruitment as a function of healthy aging
Two additional analyses examined the effects of healthy aging on valence-related differences in neural activity. Specifically, this analysis identified regions that exhibited age-related increases in the Positive > Negative event contrast and in the Negative > Positive event contrast. No regions were associated with age-related increases in activity during positive relative to negative event retrieval. However, healthy aging was associated with increased activity during negative relative to positive event retrieval in a number of frontal and parietal regions, as well as the left lateral temporal lobe and posterior cingulate (Table 3).
Table 3.
Differential effects of valence on neural recruitment as a function of healthy aging.
| Region of Interest | Hemisphere | BA | MNI Coordinates | p-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Age-related increases in activity during Positive > Negative events | |||||||
| No significant voxels | |||||||
| Age-related increases in activity during Negative > Positive events | |||||||
| Frontal | |||||||
| Precentral Gyrus | R | 6 | 36 | −4 | 34 | 0.000 | 320 |
| Ventrolateral Prefrontal Cortex | R | 45 | 58 | 22 | 8 | 0.000 | 38 |
| R | 47 | 44 | 46 | −14 | 0.001 | 24T | |
| L | 47 | −42 | 36 | −18 | 0.001 | 69 | |
| Orbitofrontal Cortex | L | 11 | −18 | 32 | −12 | 0.001 | 34 |
| R | 11 | 22 | 34 | −12 | 0.001 | 45 | |
| Dorsal Prefrontal Cortex | R | 46 | 36 | 36 | 20 | 0.001 | 166 |
| L | 46 | −38 | 30 | 18 | 0.002 | 29 | |
| Anterior Prefrontal Cortex | R | 47 | 26 | 46 | 2 | 0.002 | 27T |
| L | 46 | −28 | 54 | 14 | 0.003 | 17T | |
| Parietal | |||||||
| Postcentral Gyrus | R | 2 | 32 | −28 | 38 | 0.003 | local maxima within cluster at: 20, −34, 42 |
| L | 3 | −24 | −34 | 48 | 0.002 | local maxima within cluster at: −24, −26, 46 | |
| Inferior Parietal Lobe | L | 40 | −44 | −56 | 42 | 0.001 | 123 |
| R | 40 | 44 | −34 | 34 | 0.001 | 86 | |
| Precuneus | L | 31 | −18 | −58 | 36 | 0.001 | 125 |
| L | 7 | −20 | −50 | 50 | 0.002 | 13T | |
| Temporal | |||||||
| Superior Temporal Gyrus | L | 41 | −38 | −40 | 10 | 0.001 | 90 |
| Limbic | |||||||
| Posterior Cingulate | L | 23 | −12 | −32 | 32 | 0.000 | 108 |
| L | 31 | −24 | −26 | 46 | 0.000 | 123 | |
| R | 31 | 20 | −34 | 42 | 0.000 | 159 | |
| Cerebellum | |||||||
| Culmen | L | −4 | −54 | −28 | 0.000 | 42 | |
| R | 20 | −28 | −22 | 0.001 | 27T | ||
| Other | |||||||
| Caudate | L | −12 | −6 | 28 | 0.000 | 161 | |
| R | 18 | −12 | 30 | 0.001 | 71 | ||
| R | 18 | −30 | 28 | 0.001 | 49 | ||
| Putamen | R | 22 | −10 | 4 | 0.000 | 88 | |
| L | −32 | −20 | 4 | 0.000 | 131 | ||
| Thalamus | R | 22 | −16 | 10 | 0.001 | local maxima within cluster at: 22, −10, 4 | |
| L | −24 | −22 | 18 | 0.001 | local maxima within cluster at: −32, −20, 4 | ||
| Claustrum | L | −30 | −14 | 16 | 0.001 | local maxima within cluster at: −32, −20, 4 | |
| L | −28 | 4 | 14 | 0.001 | 26T | ||
| R | 26 | 20 | 2 | 0.001 | 32 | ||
| Insula | L | 13 | −44 | −28 | 26 | 0.001 | 26T |
| R | 13 | 42 | −6 | 2 | 0.003 | 10T | |
Clusters significant at an uncorrected threshold of p<.005, k ≥ 10 voxels
Up to 3 local maxima that are at least 8mm apart reported for each cluster; when peaks are local maxima within larger clusters, source cluster peak is reported instead of cluster size
BA= approximate Brodmann Area; L=Left, R=Right;
Indicates a cluster size smaller than the designated cluster size of 29
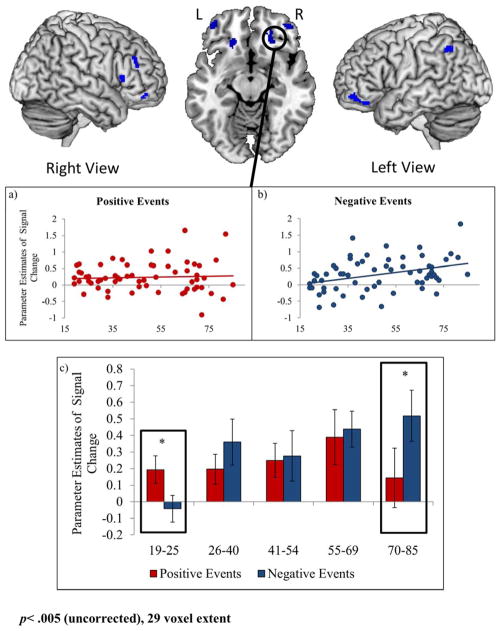
One cluster (orbitofrontal cortex, BA 11; 22, 34, -12) was selected as an ROI from which to extract parameter estimates (Figure 2a–c). For visualization purposes, the continuous age range was split into five age groups whose average parameter estimates have been graphed (Figure 2c). Repeated-measures ANOVAs were conducted and there was a strong trend toward a main effect of age (p=.053). The effect of valence was significant (F(1,61) = 5.01, p<.05) and this effect was qualified by an age-by-valence interaction (F(1,61) = 8.09, p<.01).
Figure 2.
Regions showing age-related increases in activity during negative relative to positive events (blue regions). Parameter estimates for activity during a) positive and b) negative event retrieval are plotted from a 5mm sphere surrounding the peak voxels from one region of interest: 22, 34, -12 (orbitofrontal cortex). For visualization purposes, parameter estimates are also plotted for 5 age groups that were approximately equivalent in size (2c) and represented a continuous age range. When the difference between negative and positive events is significant, this is noted with an asterisk. Coordinates are presented in MNI space.
Additional analyses revealed that the age-by-valence interaction occurred because age was correlated with activity during retrieval of negative events (r=.36, p<.005), but not of positive events (p>.6). To better understand this interaction, t-tests were conducted comparing activity during positive and negative events in each age group. These t-tests suggest that the age difference in positive and negative events was driven by valence effects in the youngest and oldest individuals in our sample. Specifically, activity during negative events was significantly greater than positive events for the oldest age group (70–85) for and activity during positive events was greater than negative events for the youngest age group (19–25; Figure 2c).
3.2.4 Regions exhibiting differential neural connectivity with the left hippocampus
In the gPPI analysis, we examined the effects of age on neural connectivity with the left hippocampus during positive and negative event retrieval. During both positive and negative event retrieval, healthy aging was associated with decreased functional connectivity between the left hippocampus and a widespread, bilateral network of regions including dorsal and ventral PFC, lateral and medial temporal lobe (including the amygdala), parietal lobe, and posterior visual regions (Table 4; Figure 3b). Age was not associated with increased connectivity of the left hippocampus with any neural regions.
Table 4.
Regions exhibiting differential neural connectivity with the left hippocampus as a function of valence or healthy aging
| Region of Interest | Hemisphere | BA | MNI Coordinates | p-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Age-related Increases in Connectivity | |||||||
| None | |||||||
| Age-related Decreases in Connectivity | |||||||
| Frontal | |||||||
| Premotor Cortex | L | 6 | −36 | 4 | 46 | 0.000 | local maxima within cluster at: 34, −48, 58 |
| Parietal | |||||||
| Postcentral Gyrus | R | 5 | 34 | −48 | 58 | 0.000 | 61333 |
| Temporal | |||||||
| Middle Temporal Gyrus | R | 21 | 54 | −14 | −22 | 0.000 | 576 |
| Superior Temporal Gyrus | R | 22 | 54 | −14 | −2 | 0.000 | |
| Middle Temporal Gyrus | R | 21 | 58 | −4 | −4 | 0.001 | |
| Fusiform Gyrus | L | 37 | −38 | −50 | −24 | 0.000 | 170 |
| Fusiform Gyrus | L | 37 | −44 | −62 | −18 | 0.000 | |
| Superior Temporal Gyrus | L | 41 | −52 | −34 | 8 | 0.001 | 12 |
| Occipital | |||||||
| Middle Occipital Gyrus | 37 | −48 | −68 | −12 | 0.001 | local maxima within cluster at: −38, −50, −24 | |
| Other | |||||||
| Posterior Cingulate | R | 30 | 22 | −48 | 10 | 0.000 | 30 |
| Parahippocampal Gyrus | L | 28 | −16 | −16 | −12 | 0.002 | 10 |
| Age-Related Decreases in Connectivity during retrieval of Negative relative to Positive Events | |||||||
| Frontal | |||||||
| Dorsolateral Prefrontal Cortex | L | 9 | −52 | 16 | 40 | 0.000 | 18T |
| L | 8 | −38 | 16 | 54 | 0.003 | 11T | |
| R | 46 | 50 | 32 | 30 | 0.002 | 17T | |
| Ventrolateral Prefrontal Cortex | R | 11 | 42 | 34 | −12 | 0.001 | 93 |
| R | 45 | 54 | 24 | 14 | 0.004 | 10T | |
| L | 11 | −46 | 44 | −14 | 0.001 | 46 | |
| L | 47 | −48 | 20 | 2 | 0.002 | 22T | |
| L | 47 | −42 | 30 | −8 | 0.002 | 12T | |
| Dorsomedial Prefrontal Cortex | L | 8 | −8 | 30 | 44 | 0.003 | 20T |
| Parietal | |||||||
| Angular Gyrus | L | 39 | −30 | −62 | 32 | 0.002 | 14T |
| Superior Parietal Lobule | L | 7 | −36 | −70 | 46 | 0.003 | 13T |
| Other | |||||||
| Thalamus | L | na | 0 | −16 | 4 | 0.001 | 23T |
| Lentiform Nucleus | R | na | 26 | −10 | −2 | 0.002 | 19T |
| Putamen | R | na | 20 | 8 | 10 | 0.002 | 22T |
| L | na | −16 | 6 | 8 | 0.002 | 24T | |
| Age-Related Decreases in Connectivity during retrieval of Positive relative to Negative Events | |||||||
| Temporal | |||||||
| Superior Temporal Gyrus | L | 21 | −54 | −16 | −4 | 0.001 | 18T |
| Other | |||||||
| Posterior Cingulate | L | 31 | −22 | −40 | 30 | 0.002 | 23T |
| Insula | L | 13 | −30 | −32 | 24 | 0.003 | |
Clusters significant at an uncorrected threshold of p<.005, k ≥ 10 voxels
Up to 3 local maxima that are at least 8mm apart reported for each cluster; when peaks are local maxima within larger clusters, source cluster peak is reported instead of cluster size BA= approximate Brodmann Area; L=Left, R=Right
Indicates a cluster size smaller than the designated cluster size of 29
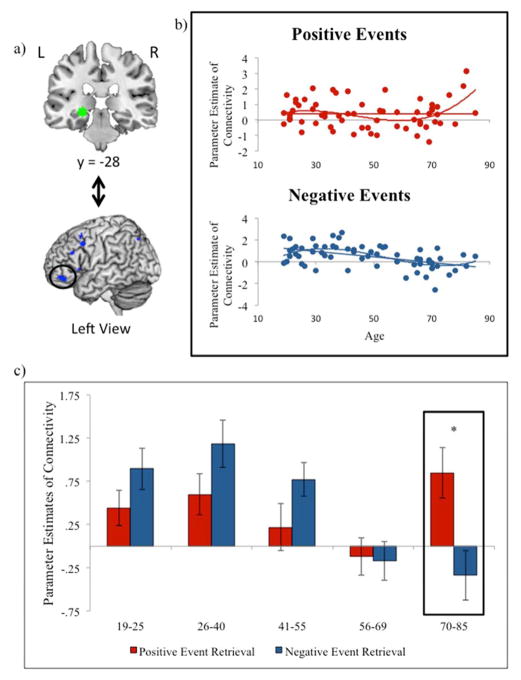
We were particularly interested in regions exhibiting age-related differences in the effects of emotional valence on neural connectivity with the left hippocampus. Healthy aging was not associated with greater decreases in hippocampal connectivity during positive relative to negative event retrieval in any clusters at the 29 voxel threshold (see Table 4). In the reverse contrast, we found that healthy aging was associated with greater decreases in hippocampal connectivity during negative relative positive event retrieval in bilateral lateral prefrontal regions (Table 4; Figure 3c).
To better understand this interaction, beta estimates of functional connectivity were extracted from a 5mm ROI surrounding the peak voxel of the left vlPFC (−46, 44, 14; Figure 4a) for positive and negative event retrieval (Figure 4b). For visualization purposes, the continuous age range was split into five age groups whose average parameter estimates have been graphed (Figure 4c). Healthy aging was negatively correlated with connectivity during negative event retrieval (r= −.48, p<.001), but not positive event retrieval (p= .98). Further, connectivity during positive event retrieval was significantly greater than negative event retrieval in the oldest adult group only (t(11)= 3.13, p<.05).
Figure 4.
a) Beta estimates of functional connectivity between the left hippocampal seed region and the left vlPFC ROI were extracted for each subject; b) Parameter estimates are plotted for negative and positive events with linear and cubic trendlines; c) Parameter estimates are plotted for positive (red bars) and negative (blue bars) for 5 age groups that were approximately equivalent in size (4c & d) and represented a continuous age range. When the difference between negative and positive events is significant, this is noted with an asterisk. Coordinates are presented in MNI space.
Regression analyses were conducted to examine linear, quadratic, and cubic effects of age on connectivity estimates for positive and negative events. As expected, the linear model was significant for negative events (F(1,61)= 18.34, p<.001) but not positive events (p>.1). Adding a quadratic coefficient to the regression did not provide a significant improvement in model fit for either condition. Finally, adding a cubic coefficient significantly improved model fit relative to the linear equation for negative event retrieval (p< .05), and led to a significant model for positive event retrieval (F(3,59)= 3.41, p<.05; Figure 4b).
At the more liberal threshold of p<.005 with a 10 voxel extent, analyses revealed additional age-related decreases in connectivity during negative relative to positive event retrieval in the left dorsomedial prefrontal cortex, bilateral dorsolateral prefrontal cortex, left parietal lobe, and sub-cortical regions such as the thalamus and putamen (Table 4). Prior research has highlighted the dorsomedial prefrontal cortex in both voluntary and automatic emotion regulation (Phillips et al., 2008), and has shown age-by-valence reversals during impression formation (Cassidy et al., 2013). Because of the potential importance of this region in age and emotion processing, we examined its connectivity patterns1.
As with the left vlPFC, age-related decreases in connectivity during negative relative to positive event retrieval in the left dorsomedial prefrontal cortex was driven by a significant decrease in connectivity during negative event retrieval (r= −.46, p<.001; Figure 5) but not positive event retrieval (p=.34). Similarly, the linear model was significant in negative event retrieval only (F(1,61)= 16.53, p<.001). The quadratic and cubic elements did not improve model fit for positive or negative events retrieval. Positive event retrieval was associated with significantly greater connectivity than negative event retrieval in individuals ages 56–69 (t(11)= 2.51, p<.05), with a trend towards significance in the oldest age group (p= .055).
Figure 5.
a) Beta estimates of functional connectivity between the left hippocampal seed region and the left dmPFC ROI were extracted for each subject; b) Parameter estimates are plotted for negative and positive events with linear trendlines; c) Parameter estimates are plotted for positive (red bars) and negative (blue bars) for 5 age groups that were approximately equivalent in size (5c & d) and represented a continuous age range. When the difference between negative and positive events is significant, this is noted with an asterisk. Coordinates are presented in MNI space.
4. Discussion
The current study was the first to investigate the neural processes supporting positive and negative memory retrieval throughout the adult lifespan. The results revealed that although there are main effects of valence and age on episodic memory retrieval, there also are interactions between the factors, with many effects of advancing age depending on the valence of information being retrieved from memory. Specifically, although healthy aging was associated with increased prefrontal activation during retrieval of both positive and negative events, this pattern was more widespread for negative relative to positive event retrieval, with several prefrontal regions exhibiting age-related increases for negative events only.
The use of a lifespan approach also emphasized both continuities and discontinuities across the adult lifespan. While the main effects of age appeared to reflect a gradual change, with no abrupt shift from a “young adult” pattern to an “older adult” pattern, several cases of age-by-valence interactions in both activity and connectivity were driven by opposite patterns in the oldest and youngest participants. In terms of neural activity, negative valence was associated with increased PFC activity for the oldest individuals and positive valence associated with increased activity for the youngest individuals. Valence-related effects on PFC-MTL connectivity also differed as a function of age, and again were driven primarily by differences between the youngest and oldest participants. Specifically, during negative event retrieval, the PFC and MTL were positively correlated in the youngest participants but were negatively correlated in the oldest participants. These findings extend prior research by demonstrating that age-related changes in prefrontal recruitment and connectivity differ depending upon the valence of memory retrieved and demonstrate the insights to be gained from using a full lifespan sample.
Prior studies with continuous age designs suggest that age-related changes in emotional experience (Pasupathi & Carstensen, 2003) and emotional memory (Carstensen & Charles, 1994) may occur gradually over time; however, other research suggests that older adults may be qualitatively different from young and middle-aged adults (Charles, Mather, & Carstensen, 2003). In addition, Gruhn and colleagues (2010) found that happiness, contentment, and surprise demonstrate a U-shaped pattern, such that these emotions were experienced more by young and older adults than middle-aged individuals. Similarly, sadness was experienced more by middle-aged participants than young and older adults, exhibiting an inversed U-shaped pattern (Gruhn et al., 2010). Such complex age-related changes in emotion processing across the lifespan may help explain the non-linear neural effects identified in the current study. Future research should be used to determine how experience of particular emotions, and cognitive responses to such emotions, may contribute to unique patterns of neural activity and connectivity in the youngest and oldest adults in our sample.
4.1 Effects of Valence and Healthy Aging on Neural Recruitment During Episodic Retrieval
The current study was specifically designed to examine the independent and interactive effects of age and emotional valence on episodic memory retrieval. Controlling for age, negative event retrieval was associated with increased activity in bilateral ventrolateral PFC and left posterior hippocampus. Increased posterior hippocampal activation during negative event retrieval is consistent with literature documenting detail-focused processing for negative events (reviewed by Kensinger, 2009a), as previous work has implicated the posterior hippocampus in more local, detail-focused representations relative to the gist-based representations associated with the anterior hippocampus (see Poppenk et al., 2013 for review). Similarly, a recent meta-analysis revealed that the vlPFC activity was associated with objective measures of recollective memory (Spaniol et al., 2009). These findings suggest that, regardless of age, initial retrieval of negative events was associated with more specific item detail than of positive events.
To assess the main effect of age on memory retrieval, the current research took a lifespan perspective rather than using an extreme age-group design. This study extended the prior literature examining effects of age on retrieval of neutral experiences (e.g., Davis et al., 2008) to reveal age-related increases in medial prefrontal activation and decreases in posterior activation during retrieval of emotional events. Of particular interest was the fact that age changes were gradual across age groups, with no distinct point where neural recruitment rapidly transitioned from looking like “young adults” to looking like “older adults”. Such continuous shifts may represent a natural response to neural changes that occur over the course of the adult lifespan.
The current study was also interested in how emotional valence might interact with this natural aging process. Our analysis revealed lateral prefrontal and orbitofrontal regions in which age-related increases were stronger for negative relative to positive event retrieval, suggesting that the presence of negative emotional valence, more so than positive valence, led older adults to engage these regions during retrieval. In addition, our findings suggest that these age-by-valence interactions were driven by age-related reversals in valence effects at the most extreme ends of our sample. In other words, the effect of positive valence on neural recruitment appears to be unique to the youngest individuals in the sample, and the effect of negative valence is unique to the oldest individuals. We have previously reported increased prefrontal activation during positive relative to negative event retrieval in a subset of young adults from the current sample (Ford et al., in press), but the current study extended this finding by showing that this valence-related effect was unique to the youngest age group, with middle-aged adults showing no valence-related differences and older adults exhibiting a reversed effect. The age-by-valence interaction in the extreme ends of our sample is of particular interest when one contrasts this pattern with the more continuous main effect of age.
The current findings mirror prior research from our laboratory that has revealed age-related reversals during encoding of positive and negative events, but with healthy older adults recruiting prefrontal regions to a greater extent during positive relative to negative events and young adults exhibiting the reverse pattern (Leclerc & Kensinger, 2008). In other words, the current study reveals an age-by-valence interaction at retrieval that is the opposite of the interaction previously found at encoding. Together, these two studies suggest that emotional valence influences age-related changes in memory processes at both encoding and retrieval, but not in the same way. It has been proposed that the age-by-valence interaction at encoding represents age-related changes in self-referential processing, with older adults more likely to relate positive information to themselves during encoding (Leclerc & Kensinger, 2008). One possibility is that the effect of age at retrieval reflects a continued influence of these differences present during encoding: The items processed in a more self-referential manner may require less prefrontal processing for their retrieval because they were encoded more deeply or effectively. Alternatively, the opposite interaction may reflect the fact that emotion influences a different memory process at retrieval.
It has been suggested that older adults are motivated to regulate their emotional state to a greater extent than young adults, potentially altering the way in which they interact with to-be-remembered stimuli (Mather & Carstensen, 2005). It is possible that the reversed patterns of activation during positive and negative event retrieval in young and older adults reflects a tendency for older adults to preferentially recruit prefrontal regions in an effort to decrease activity in regions involved in negative memory retrieval, reducing the emotional impact of the image. Such shifts would be associated with motivational changes, as opposed to structural or capacity changes, making the age-by-valence interaction more likely. As we discuss next, the PFC-MTL connectivity results support this regulatory interpretation.
4.2 Effects of Valence and Healthy Aging on Functional Connectivity
When using functional connectivity to examine PFC-MTL interactions, the left hippocampus was selected as a seed region in this analysis, as it was significantly active during both positive and negative event retrieval relative to baseline and, therefore, represented a region involved in retrieval of these events. The connectivity analyses revealed that, during both positive and negative memory retrieval, aging was associated with decreased connectivity between the left hippocampus and a widespread network of regions. These findings suggest an overall decrease in neural connectivity in the memory retrieval network as a function of aging. This reduction could be driven by age-related breakdowns in the structural integrity of the white matter tracts between regions in the network, or by other changes (e.g., in neuronal oscillations) that may affect the ability for disparate regions to act as part of a connected network. Interestingly, the relation of the left hippocampus with bilateral vlPFC exhibited a significant age-by-valence interaction, where the effect of emotional valence on functional connectivity differed as a function of age and showed a pattern reversal in the youngest and oldest age groups. In this analysis, negative valence led to a stronger positive connectivity relative to positive valence in young adults, but more negative connectivity in older adults.
The vlPFC is involved in voluntary regulation of emotions in a variety of tasks and conditions (See Phillips et al., 2008 for a review). Specifically, the vlPFC may regulate emotions by guiding retrieval of emotional details and either increasing or decreasing neural activity in limbic regions associated with emotional responses and memory retrieval (Ochsner & Gross, 2005). Such a role suggests that older adults may be recruiting prefrontal regions during retrieval of negative events in order to reduce their negative emotion, a pattern consistent with the theory that older adults are motivated to regulate negative emotional states during cognitive tasks (Mather & Carstensen, 2005). Notably, we also saw a cubic effect of age on connectivity during positive event retrieval, driven by increased connectivity in the oldest individuals in our sample. Although preliminary, this finding suggests that healthy aging is also associated with an increase in MTL-PFC connectivity, as least in the most extreme end of the age range. Increased connectivity during positive event retrieval would also be consistent with the age-related motivation account.
The current study also identified an age-by-valence interaction in dmPFC-MTL connectivity, although the cluster was below our corrected threshold. The dmPFC has been implicated in both automatic and voluntary emotion regulation (See Phillips et al., 2008 for a review). Ochsner and Gross (2007) proposed two top-down cognitive control systems that could be involved in emotion regulation. The description-based appraisal system (DBAS), which involves the dorsomedial and dorsolateral PFC, generates mental representations of affective states and directs reappraisals to regulate emotion. Importantly, the dmPFC is particularly involved in generating an emotional response when one anticipates an emotional event, as might be the case when trying to remember the details of a negative image associated with a neutral cue. It has also been suggested that, due to the lack of direct connections with regions involved in bottom-up appraisal (such as the amygdala), the DBAS must influence these responses indirectly. One proposed avenue for such indirect influence is by influencing regions involved in long-term memory, such as the hippocampus, which are directly connected to affective appraisal systems. The results of the current study suggest that older adults use the dmPFC to down-regulate hippocampal activity during negative event retrieval, while young adults use the same region to increase hippocampal activity in this same condition. Future research is required to examine age-related changes in the potential role of the dmPFC in emotion regulation during emotional memory retrieval.
4.3 Limitations
When examining the behavioral and neural effects associated with emotion and emotional valence, it is important to consider other potential differences unrelated to the factors of interest. The retrieval cues in the current study were neutral titles that had been paired with positive, negative, and neutral images during encoding. The encoding task required participants to evaluate the appropriateness of each title for the associated image. Although positive and neutral titles were rated as equally appropriate, negative titles were rated as less appropriate, overall, than the other two conditions. It is possible that these differences across retrieval cues could lead to differences in memory performance. However, ratings of title appropriateness were not correlated with memory accuracy in the neutral or negative emotion conditions (p>.2 for both relations) or with age in any emotion condition (p>.5 for all three conditions).
Negative events were also associated with increased arousal ratings relative to positive events. This confound presents the possibility that the effects we see in the current analysis are being driven by an arousal effect rather than a valence effect. In other words, greater age-related increases in prefrontal regions for negative relative to positive events may reflect increased age effects for high relative to low arousal images. To examine this possibility, we conducted an additional analysis in which we examined regions showing greater arousal-related increases in activity as a function of age. Specifically, we conducted a parametric modulation analysis of increased arousal and identified regions in which this effect increased with age (Supplementary Table 2). Critically, none of the regions reported in the valence contrast reached significance in this analysis, suggesting that increased arousal for negative events.
Finally, as with all studies examining healthy aging, it is important to consider potential selection biases in our sample. The older adults in our sample were highly educated individuals who have been screened for potential health problems and dementia. In addition, they are older adults who are interested in research and motivated to come to the MR scanner for a 3-hour study session. In the current study, we must also consider the generalizability of the middle-aged adults, as it is often difficult for individuals in this age range to take the time to come into labs for study sessions. As such, it is possible that these findings do not generalize to all middle-aged and older adults, but rather reflect a particular subsample.
4.4 Conclusions
The current results revealed that aging was associated with increased prefrontal activation in both positive and negative event retrieval, but that this pattern was more widespread for negative memories. Aging also was associated with decreased hippocampal connectivity in a number of neural regions, although in bilateral vlPFC and left dmPFC connectivity was influenced by the interaction between valence and age. These findings extend prior research by demonstrating that age-related changes in prefrontal recruitment and connectivity differ depending upon the valence of memory retrieved. The use of a lifespan approach also revealed both continuities and discontinuities in recruitment and connectivity across the adult lifespan. While the main effects of age exhibit a gradual change, with no abrupt shift from a “young adult” pattern to an “older adult” pattern, the activity and connectivity patterns showing age-by-valence interactions were driven by opposite patterns in the oldest and youngest participants. Such findings suggest that some aging effects are continuous, while others may reflect discrete shifts in emotional or mnemonic processing in adults of a certain age.
Supplementary Material
Highlights.
Aging is associated with significant functional changes, regardless of valence.
Age-related functional changes are greater during negative event retrieval.
Valence-dependent age effects are exaggerated in the oldest adults in the sample.
Acknowledgments
The authors would like to thank Katherine Mickley Steinmetz for her assistance designing the current study and Halle Zucker for her assistance creating presentation scripts and running participants. Magnetic resonance data were collected at the Harvard Center for Brain Science. We thank the staff there, particularly Tammy Moran and Ross Mair, for their assistance with data collection and quality assurance. This work was supported by a Memory and Cognitive Disorders grant from the McKnight Endowment Fund for Neuroscience (EAK), and NIH grant MH080833 (EAK).
Footnotes
Notably, Monte Carlo simulations (Slotnick et al., 2003) run with an uncorrected threshold of p<.01 determined that a 54-voxel extent corrected results to p < .05. At p< .01, the dmPFC cluster spanned 82 clusters, well surpassing the threshold.
Disclosure statement: The authors report no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Leclerc CM, Muscatell KA, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2010;46(4):425–433. doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Turk-Charles S. The salience of emotion across the adult life span. Psychology and Aging. 1994;9:259–264. [PubMed] [Google Scholar]
- Cassidy BS, Leshikar ED, Shih JY, Aizenman A, Gutchess AH. Valence-based age differences in medial prefrontal activity during impression formation. Social Neuroscience. 2013;8:462–473. doi: 10.1080/17470919.2013.832373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher PC. Dissociable temporal lobe activations during emotional episodic memory retrieval. NeuroImage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Ford JH, Morris JA, Kensinger EA. Effects of emotion and emotional valence on the neural correlates of episodic memory search and elaboration. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_00529. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhn D, Kotter-Gruhn D, Rocke C. Discrete affects across the adult lifespan: Evidence for multidimensionality and multidirectionality of affective experiences in young, middle-aged and older adults. Journal of Research in Personality. 2010;44:492–500. [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15(3):208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. 2009a;1:99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA. How emotion affects older adults’ memories for event details. Memory. 2009b;17:208–219. doi: 10.1080/09658210802221425. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. European Journal of Cognitive Psychology. 2009;21(2–3):192–215. [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive and Affective Behavioral Neuroscience. 2008;8(2):153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social, Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, Pietrzak RH. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Science. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized from of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. Journal of Cognitive Neuroscience. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. [Google Scholar]
- Pasupathi M, Carstensen LL. Age and emotional experience during mutual reminiscing. Psychology and Aging. 2003;18:430–442. doi: 10.1037/0882-7974.18.3.430. [DOI] [PubMed] [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychology and Aging. 2008;23:585–594. doi: 10.1037/a0013176. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends in Cognitive Science. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Clinical Gerontology : A Guide to Assessment and Intervention. NY: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analysis using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Bessette-Symons B, Cabeza R. Functional neuroimaging studies of aging and emotion: fronto-amygdalar differences during emotional perception and episodic memory. Journal of the International Neuropsychological Society. 2009;15(6):819–825. doi: 10.1017/S1355617709990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of fMRI data. Psychological Science. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewall G, Degueldre C, Luxen A, Collete F, Maquet P. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. Journal of Neuroscience. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Taylor SF, Liberzon I, Fig LM, Decker LR, Minoshima S, Koeppe RA. The effect of emotional content on visual recognition memory: A PET activation study. NeuroImage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.