Abstract
Currently, there are no available approaches to cure or slow down the progression of Alzheimer’s disease (AD), which is characterized by the accumulation of extracellular amyloid-β (Aβ) deposits and intraneuronal tangles composed of hyperphosphorylated tau. β2 adrenergic receptors (β2ARs) are expressed throughout the cortex and hippocampus and play a key role in cognitive functions. Alterations in the function of these receptors have been linked to Alzheimer’s disease; however these data remain controversial as apparent contradicting reports have been published. Given the current demographics of growing elderly population and the high likelihood of concurrent beta-blocker use for other chronic conditions, more studies into the role of this receptor in AD animal models are needed. Here we show that administration of ICI 118,551, a selective β2AR antagonist, exacerbates cognitive deficits in a mouse model of AD, the 3xTg-AD mice. Neuropathologically, ICI 118,551 increased Aβ levels and Aβ plaque burden. Concomitantly, ICI 118,551-treated 3xTg-AD mice showed an increase in tau phosphorylation and accumulation. Mechanistically, these changes were linked to an increase in amyloidogenic APP processing. These results suggest that under the conditions used here, selective pharmacological inhibition of β2ARs has detrimental effects on AD-like pathology in mice. Overall, these studies strengthen the notion that the link between β2ARs and AD is likely highly complex and suggest caution in generalizing the beneficial effects of beta-blockers on AD.
Keywords: Aβ, amyloid-β, APP, tau, tangles, NFT, β-blockers
Introduction
Alzheimer’s disease (AD) is the most common form of dementia and currently affects 5.2 million people in the US (Thies, et al., 2013). Currently, there is no cure or effective therapies to slow down the progression of the disease. The vast majority of AD cases (>95%) are sporadic and of unknown etiology. Aging is the major known risk factor for AD and it is estimated that about 20% of people 75–84 years of age and 50% of people 85 and older have AD. Given that people 75 and older represent the fastest growing segment of the population in the US, the number of individuals with AD is likely to reach 20 million by 2050 in the US alone (Thies, et al., 2013).
One of the first clinical manifestations of AD is the inability to recall past experiences; as the disease progresses, memory loss becomes more severe and it is accompanied by deficits in language, spatial orientation, attention and executive function (Artero, et al., 2003; Perry and Hodges, 1999; Welsh, et al., 1992). Neuropathologically, AD is characterized by the presence of two hallmarks, extracellular plaques and intracellular neurofibrillary tangles (Querfurth and LaFerla, 2010). The former are mainly composed of amyloid-β (Aβ), a small peptide derived from a series of proteolytic cleavages of a larger precursor known as amyloid precursor protein (Masters, et al., 1985; Querfurth and LaFerla, 2010) . The latter are mainly comprised of the microtubule binding protein tau, which in pathological conditions is hyperphosphorylated (Ballatore, et al., 2007; Feany and Dickson, 1996). A proper diagnosis of AD can only be made postmortem if both hallmarks are present throughout the brain. Overwhelming evidence indicates that the accumulation of Aβ and tau play a key role in the pathogenesis of the disease (Querfurth and LaFerla, 2010).
The beta2-adrenergic receptors (β2ARs) are G-protein coupled receptors involved in a wide range of physiological functions ranging from control of vascular tone and cardiac performance to metabolism and memory formation (Ameredes, 2011; Gibbs and Summers, 2002; McIntyre, et al., 2012; Sato, et al., 2011; Woo and Xiao, 2012). The β-AR subfamily comprises three members: β1, β2, and β3, all coupling to adenylate cyclase through the subunit of the heterotrimeric Gs-protein (Kolinski, et al., 2012). While β1 subtype is the cardiovascular receptor and β3 is by far the predominating subtype in adipose tissue, β2 subtype is found in the brain, abundantly in hippocampus and cortex, the two brain regions involved in AD pathogenesis (Daly and McGrath, 2011). Specifically, dendritic spines are enriched in β2ARs further highlighting their role in memory formation (Joiner, et al., 2010; Wang, et al., 2010). Stimulation of β2ARs activates cAMP/PKA signaling, which in turn leads to phosphorylation and activation of other pathways, including immediate early genes known for their primary role in learning and memory (Insel, 2011).
Alterations in the function of β2ARs have been linked to AD; however these data remain controversial as apparent contradicting reports have been published. For example, epidemiological studies suggest that the use of non-selective βAR antagonists for the treatment of hypertension correlates with a lower incidence of AD (Khachaturian, et al., 2006). Furthermore, genetic studies indicate that polymorphisms in the gene encoding the β2AR are linked to a higher risk of late onset AD (Yu, et al., 2008). Consistent with these data, chronic treatment with β2AR agonists increases amyloid-β (Aβ) load in transgenic mice, while the use of β2AR blockers conversely decreases acute stress-induced Aβ production (Ni, et al., 2006; Yu, et al., 2010). In contrast, it is well established that activation of β2ARs is required for normal learning and memory. Indeed, β2ARs activation promotes various forms of long-term potentiation (LTP) (Connor, et al., 2011; Gelinas and Nguyen, 2005; Lin, et al., 2003; Qian, et al., 2012; Thomas, et al., 1996; Walling and Harley, 2004). Consistently, activation of β2ARs modulates memory formation and consolidation (Gibbs, et al., 2010; Zhou, et al., 2013). Furthermore, two independent reports have shown that activation of β2ARs can overcome the detrimental effects of Aβ on LTP (Li, et al., 2013; Wang, et al., 2009).
Taken together these studies clearly support an association between β2ARs and AD but also highlight the need for more studies to clearly detail the role of β2ARs signaling in the pathogenesis of AD. Here, we used a mouse model of Alzheimer’s disease to determine the effect of a selective β2AR antagonist on Aβ and tau pathology and the associated cognitive deficits.
Results
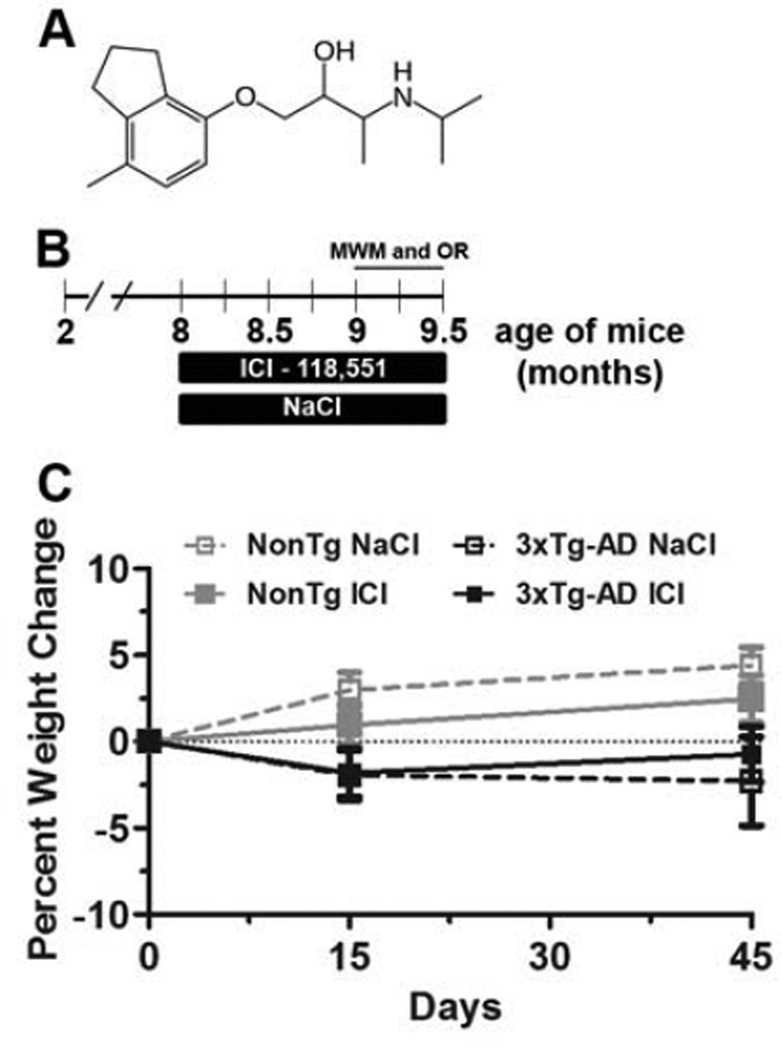
To determine the effects of chronic administration of selective β2AR antagonist on Aβ and tau pathology, we treated 8-month-old female 3xTg-AD (n=9) and NonTg (n=12) mice with 1 mg/kg ICI 118,551 (hereafter referred to as ICI; Fig. 1A), a selective β2AR antagonist (Summerhill, et al., 2008), which was delivered via daily intraperitoneal (i.p.) injections for 6 weeks (Fig. 1B). Age and gender-matched 3xTg-AD (n=9) and NonTg (n=12) mice were injected with 0.9% NaCl and used a control groups. Mice were weighed before the ICI 118,551 administration, two weeks after and at the end of the experiments. Notably, no statistically significant changes in body weight were found for any of the groups of mice (Fig. 1C).
Figure 1. Drug administration has no effect on body weight.
(A) Chemical structure of ICI 118,551 (hereafter referred to as ICI), a selective β2AR antagonist. (B) Schematic representation of the treatment paradigm. 8-month-old female 3xTg-AD and NonTg mice were administered 1 mg/kg ICI or 0.9% NaCl (control) via daily i.p. injections for 6 weeks (3xTg-AD mice, n=9/drug group; NonTg mice, n=12/drug group). During the last two weeks of injections, mice were tested in MWM and object recognition tests. (C) Percent weight change throughout the treatment. No statistically significant changes in body weight were detected for any of the groups of mice. Data are presented as means ± SEM.
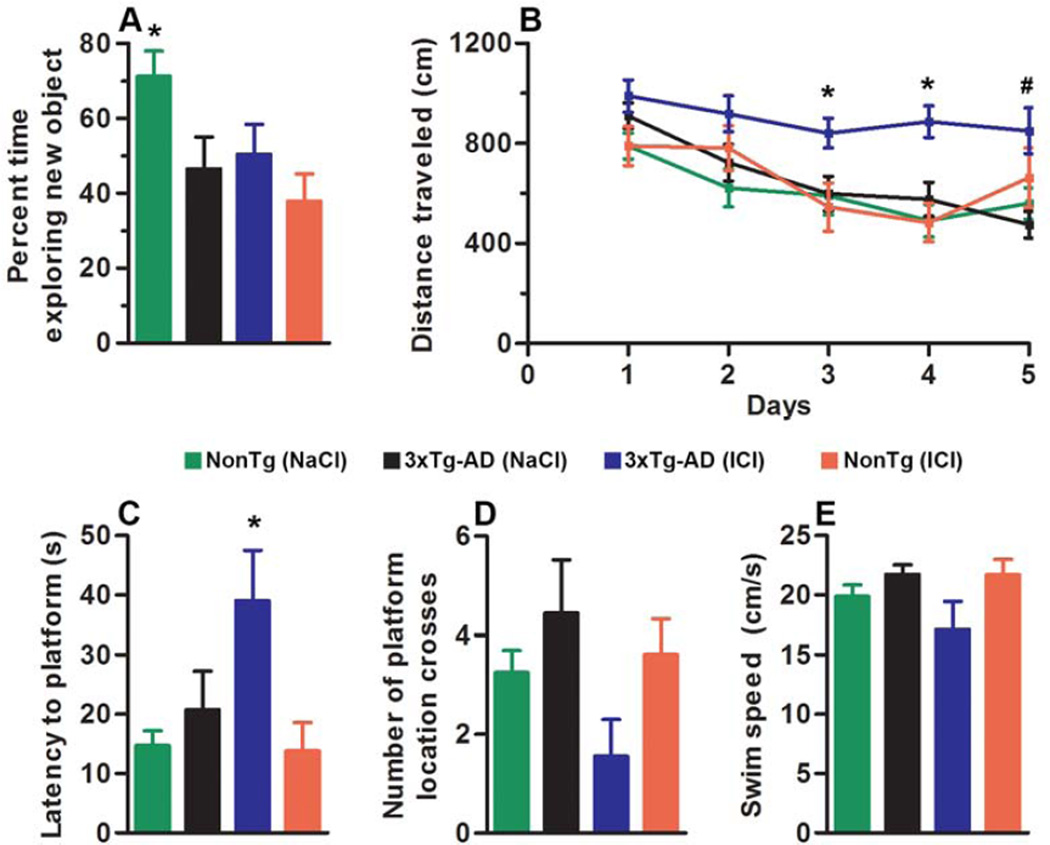
During the last two weeks of i.p. injections, mice were tested in the Morris water maze (MWM) and the novel object recognition task. The former is a spatial task mainly dependent on the hippocampus, while the latter relies on natural mouse behavior to explore a novel object (Antunes and Biala, 2012). During training sessions of the object recognition, mice were exposed to two small toys and were left free to explore for 5 minutes. During the probe trials, one of the two toys was replaced with a new toy, similar in size and overall appearance. We found that the NonTg mice treated with NaCl (NonTg/NaCl) spent 71.25 ± 6.75% of their time exploring the new toy (Fig. 2A). In contrast, the 3xTg-AD mice treated with NaCl (3xTg-AD/NaCl) spent 46.57% ± 8.50 of their time exploring the new toy, indicating that they were not able to discriminate between the new and the old toy. We found that ICI administration disrupted this form of working memory in the NonTg mice. Indeed, the ICI-treated NonTg mice (NonTg/ICI) perform at chance levels (Fig. 2A). In contrast, ICI administration had no detectable effect on the performance of the 3xTg-AD mice (Fig. 2A). These changes were statistically significant as indicated by one-way ANOVA (p = 0.017). A Bonferroni’s post-hoc test showed that the NonTg/ICI were significantly different from NonTg/NaCl (p < 0.05; Fig. 2A).
Figure 2. ICI administration disrupts working memory in NonTg mice and impairs spatial memory in 3xTg-AD mice.
(A) During novel object recognition, 3xTg-AD/NaCl mice are not able to discriminate between objects, and ICI administration has no detectable effect on mouse performance. In contrast, NonTg/NaCl mice spend ~71% of their time exploring the new object, and ICI administration disrupts this form of working memory in the NonTg mice, as they perform at chance levels. These changes were statistically significant as indicated by one-way ANOVA with a Bonferroni’s post-hoc test (p = 0.017). (B) Learning curve of mice trained in the spatial reference version of the MWM. Mice were trained to swim to a hidden platform in a tank using extramazal visual cues. Each day represents the average of four training trials. All genotypes showed significant improvements over the 5 days of training. However, the 3xTg-AD/ICI mice performed significantly worse than the other three groups at day 3 and day 4 as indicated by a greater distance traveled to find the hidden platform (*; p < 0.05 and 0.01, respectively). Additionally, 3xTg-AD/ICI mice are significantly impaired compared to both NonTg groups at day 4 and 5 (#; p < 0.05). (C–D) Probe trials, indicative of spatial memory were conducted 24 hours after the last training trail. The latency to reach the platform location during a 60-second trial was significantly higher for the 3xTg-AD/ICI mice compared to the other three groups (p = 0.01). We also found a strong trend toward an exacerbation of performance in the 3xTg-AD/ICI mice when measuring the number of platform location crosses during the duration of the trial (p = 0.09; Fig. 2D). (E) Average swim speed during the probe trials was not statistically significant among the four groups (p = 0.11). Data are presented as means ± SEM.
For the MWM training, mice received four training trials per day for 5 consecutive days. To analyze the learning data, we used a mixed-model repeated measures ANOVA with treatment and genotype as categorically fixed effects, days as a numeric covariate, and animals as the random effect; distance traveled during learning was the dependent variable. We found a significant effect for days (p < 0.0001; F = 8.766), indicating a significant difference in performance improvement across the sessions. We also found that the group: drug interaction was significant (p < 0.0001; F = 14.26), indicating that one or more of the groups were different from each other (Fig. 2B). To find the group(s) most responsible for the differences, we performed a post-hoc test with a Bonferroni correction and compared all groups to each other. We found that the 3xTg-AD/ICI mice were significantly impaired at day 3 and day 4 compared to 3xTg-AD/NaCl mice, indicated by a greater distance traveled to find the hidden platform (p < 0.05 and 0.01, respectively; Fig. 2B). Also, the 3xTg-AD/ICI mice were significantly impaired compared to both NonTg groups at day 4 and 5 (p < 0.05). In contrast, ICI did not alter learning in the NonTg mice, that is, NonTg/ICI and NonTg/NaCl performed similarly (Fig. 2B). To assess spatial memory, we performed probe trials 24 hours after the last training trial and analyzed the data by one-way ANOVA. We found that reference memory in the 3xTg-AD/ICI mice was significantly worse compared to the other three groups, as indicated by the latency to reach the platform location (p = 0.016; Fig. 2C). We also found a strong trend toward an exacerbation of performance in the 3xTg-AD/ICI mice when measuring the number of platform location crosses during the duration of the trial (p = 0.09; Fig. 2D). Notably, the average swim speed during the probe trials was not statistically significant among the four groups (p = 0.11; Fig. 2E). Given that ICI-treated 3xTg-AD mice performed worse than the other groups during the learning tests, we cannot exclude that some of the deficits that this group of mice showed during the probe trials might be still due to the poor learning performance and not necessarily retention deficits. Overall, these data show that ICI administration significantly impaired cognition in the 3xTg-AD mice but had no effects on NonTg mice.
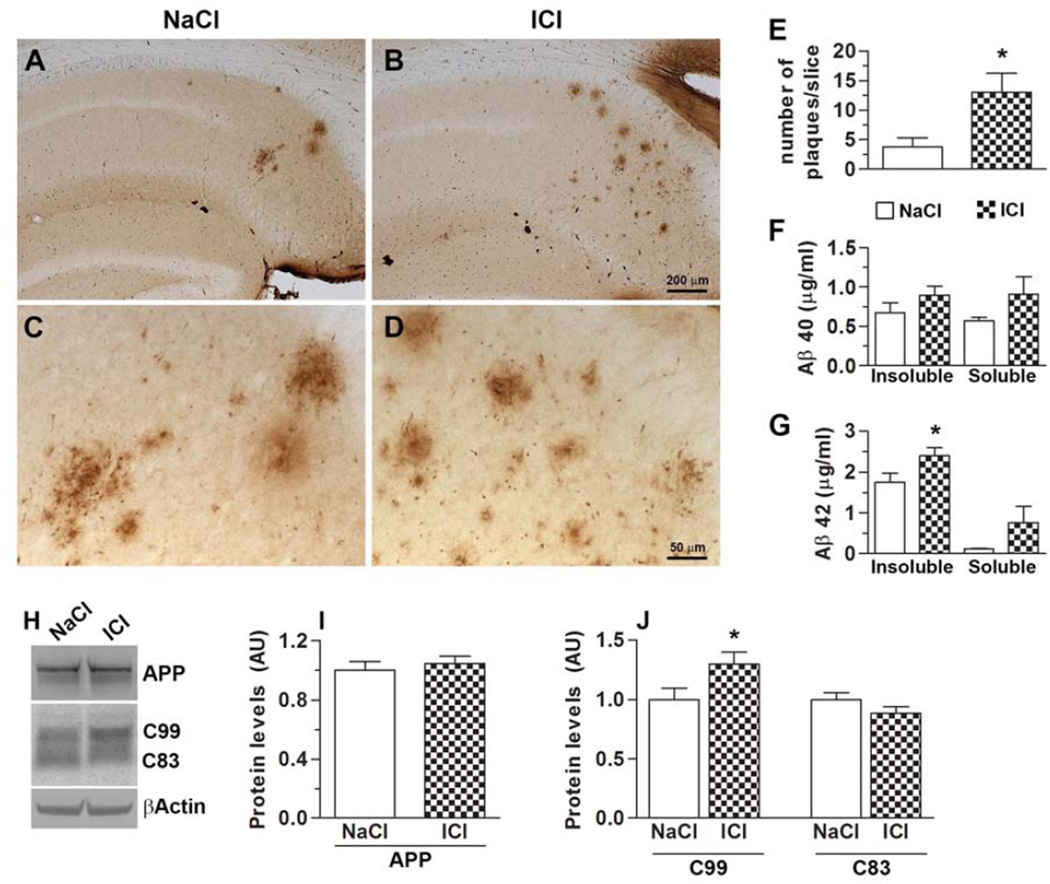
At the end of the behavioral tests, mice were 9.5-month-old. At this age, Aβ plaques are readily detectable in the CA1/subiculum area of female 3xTg-AD mice. Additionally, at this age, female 3xTg-AD mice also show significant tau hyperphosphorylation in CA1 (Oddo, et al., 2003; Oddo, et al., 2008). The presence of both Aβ and tau pathology allowed us to assess the concomitant effect of ICI on both hallmarks of AD. To evaluate the degree of Aβ pathology following ICI administration, we immunostained brain sections from treated and untreated 3xTg-AD mice with an Aβ42-specific antibody (n = 5/group). We focused our analysis to the hippocampus as this brain region plays a key role in learning and memory and is highly affected by AD pathology. We found that ICI treatments significantly increased the number of Aβ plaques in the 3xTg-AD mice compared to mice that received NaCl (Fig. 3A–D). Specifically, semi-quantitative analysis of the number of hippocampal plaques showed that the average number of plaques per slice was 3.75 ± 1.60 in the 3xTg-AD/NaCl mice. In contrast, the average number of plaques in the 3xTg-AD/ICI mice was 13 ± 3.24 (p < 0.05; Fig. 3E). To further evaluate the degree of change in Aβ pathology, we measured Aβ40 and Aβ42 levels by sandwich ELISA (n = 9/group). We found that soluble and insoluble Aβ40 levels were not statistically different between treated and untreated 3xTg-AD mice (Fig. 3F). In contrast, we found a non-significant increase in soluble Aβ42 levels and a significant increase in insoluble Aβ42 levels in the brains of the 3xTg-AD/ICI mice compared to 3xTg-AD/NaCl mice (p < 0.05; Fig. 3G). To study the mechanism underlying the changes in Aβ deposition, we first focused on Aβ production and measured APP processing in brain extracts. We found that full-length APP levels were unchanged between 3xTg-AD/NaCl and 3xTg-AD/ICI mice (Fig. 3H–I). In contrast, we found that the levels of C99, an immediate precursor of Aβ, were significantly higher in the brains of 3xTg-AD/ICI mice compared to 3xTg-AD/NaCl mice (p < 0.05; Fig. 3H, J). Additionally, while the C83 levels were lower in the ICI treated mice, this difference did not reach statistical significance (Fig. 3H, J). Taken together, these data show that, under the conditions used here, ICI administration increases Aβ levels by altering APP processing.
Figure 3. Chronic ICI administration exacerbates Aβ pathology.
(A–D) Representative photomicrographs of 3xTg-AD mice treated with ICI or NaCl. Sections were immunostained with an Aβ42-specific antibody from Millipore. Panels C and D are higher magnification views of panels A and B, respectively. (E) The graph shows average number of plaques in each brain section from treated and untreated 3xTg-AD mice. (F) ELISA measurements from brain lysates revealed no difference in both soluble and insoluble Aβ40 levels. (G) In contrast, insoluble Aβ42 levels were significantly increased in the brains of ICI-treated 3xTg-AD mice (p < 0.05). (H) Representative Western blots of proteins extracted from the brains of treated and untreated 3xTg-AD mice. Blots were probed with the indicated antibodies. (I) Quantitative analysis showed that ICI administration did not change the steady-state levels of full-length APP. (J) In contrast, C99 levels were significantly increased by ICI (p < 0.05). C83 levels were unchanged. Data are presented as means ± SEM.
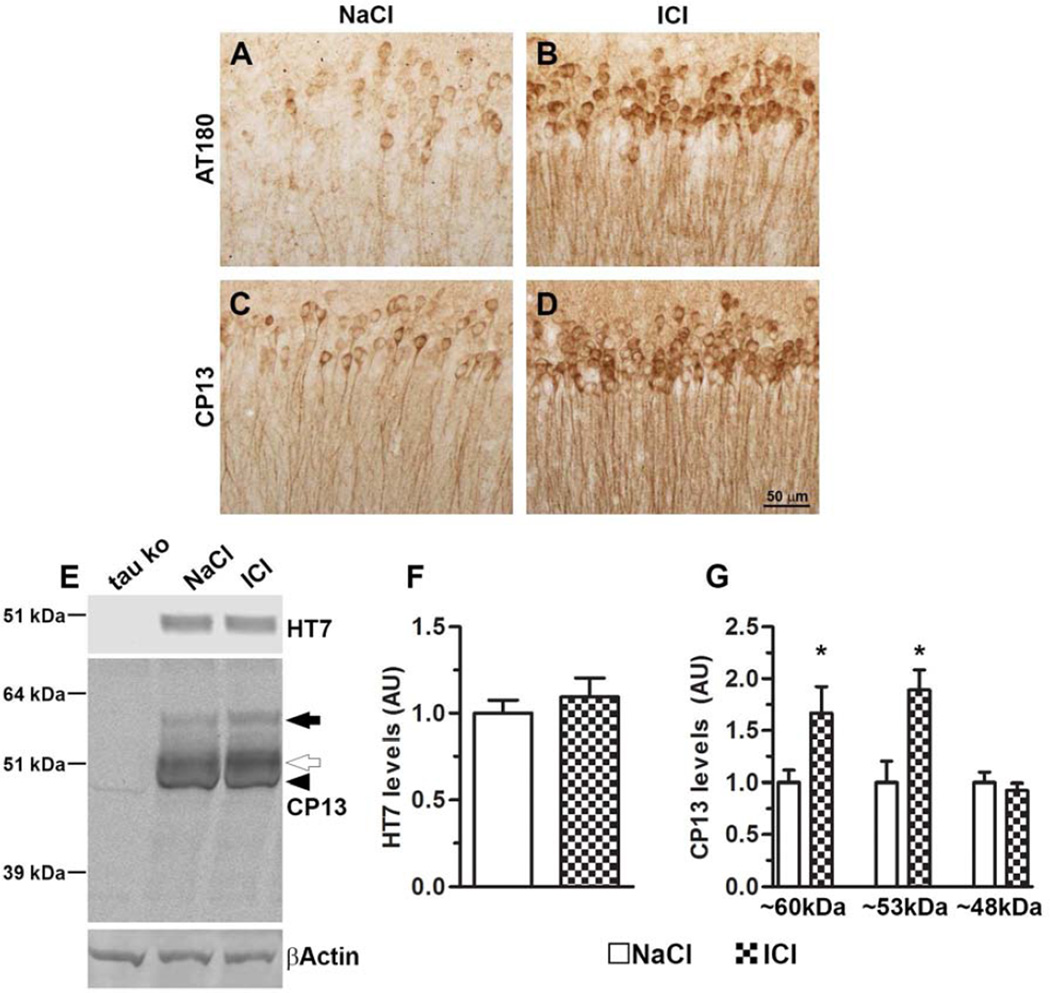
We next immunostained sections with AT180 and CP13, two antibodies that recognize tau phosphorylated at Thr231 and Ser202, respectively. We found that the number of AT180- and CP13-positive neurons was markedly increased in the hippocampi of 3xTg-AD/ICI mice compared to 3xTg-AD/NaCl mice (Fig. 4A–D). To further analyze the effect of ICI on tau pathology, we measured the steady-state levels of tau by Western blot (n = 9/group). We found that total tau levels were unchanged between treated and untreated mice (Fig. 4E–F). In contrast, we found that CP13 recognized three distinctive tau bands in the 3xTg-AD mice, which were not present in lysates from tau knockout mice (Fig. 4E and supplementary Fig. S1). Quantitative analyses of these bands showed that the steady-state levels of the ~60 kDa band (black arrow in Fig. 4E) were 66.9 ± 2.54% higher in the ICI-treated mice (p = 0.005; Fig. 4E, G). Similarly, we found that the steady-state levels of the ~53 kDa band (open arrow in Fig. 4E) were 89.1 ± 1.9% higher in the 3xTg-AD/ICI mice compared to the 3xTg-AD/NaCl mice (p = 0.02; Fig. 4G). Finally, the levels of the ~48 kDa band were similar between the two groups. Overall, these data show that chronic ICI administration increased the levels of tau phosphorylation and accumulation in the hippocampi of the 3xTg-AD mice.
Figure 4. Chronic ICI administration increases tau phosphorylation and accumulation in the hippocampi of the 3xTg-AD mice.
(A–D) Representative photomicrographs of treated and untreated 3xTg-AD brain slices probed with AT180 and CP13 antibodies, which recognize tau phosphorylated at Thr 231 and Ser 202, respectively. (E) Western blot on brain lysates probed with the indicated antibodies. Notably, CP13 reveals three tau bands, which are not present in brains from tau knockout mice. (F) Quantitative analysis of the HT7 blot showed no differences between the two groups. (G) Quantitative analyses of the CP13 bands showed that the steady-state levels of the ~ 60 kDa band (black arrow) were ~67% higher in the ICI-treated mice (p = 0.005), and the steady-state levels of the ~53 kDa band (open arrow) were ~89% higher in the 3xTg-AD/ICI mice compared to the 3xTg-AD/NaCl mice (p = 0.02). The levels of the ~48 kDa band were similar between the two groups. Data are presented as means ± SEM.
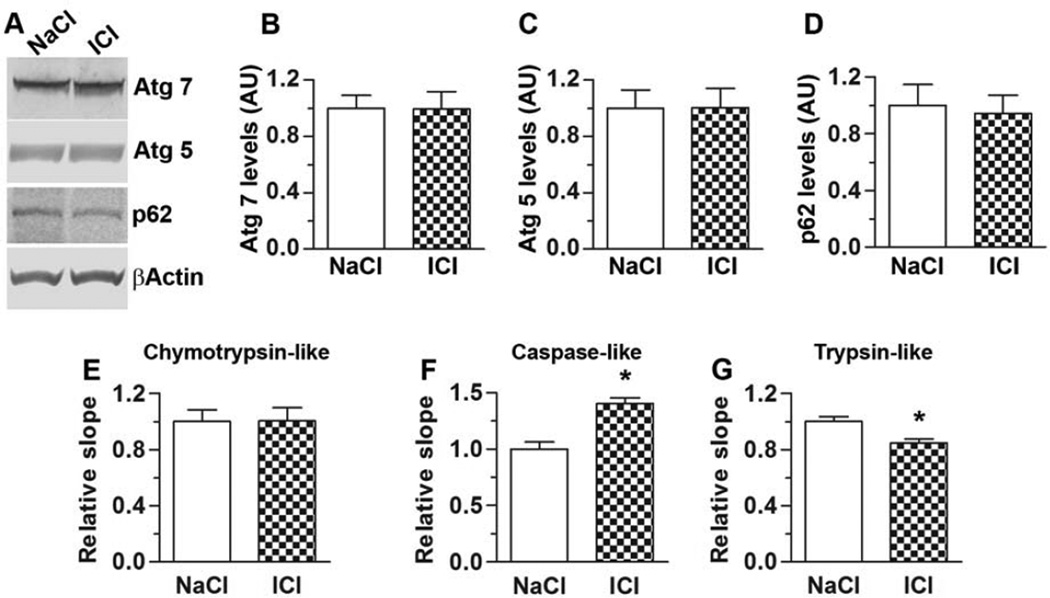
To gain insights into the mechanism underlying the ICI-mediated increase in Aβ levels, we initially focused on Aβ turnover. Autophagy and ubiquitin proteasome system represent the two major cellular protein degradation systems and are known to be involved in Aβ metabolism. Autophagy is a key pathway in Aβ metabolism and its induction is linked to the activation of a series of autophagy related genes (Atg). Among these, Atg7 and Atg5 are necessary for autophagy induction (Mizushima, et al., 1998). We found that the steady-state levels of Atg7 and Atg5 were similar between treated and untreated 3xTg-AD mice (Fig. 5A–C). Another protein often used as a marker of autophagy is p62. Consistent with the Atg results, we found that p62 levels were similar between 3xTg-AD/NaCl and 3xTg-AD/ICI mice (Fig. 5A, D). We then utilized the fluorogenic substrates Bz-VGR-AMC, Suc-LLVY-AMC and Z-LLE-AMC to measure chymotrypsin-like, trypsin-like, and caspase-like activities of the proteasome in the brain of treated and untreated 3xTg-AD mice. We found that the chymotrypsin-like proteasomal activity was similar between these two groups of mice (Fig. 5E). In contrast, ICI significantly increased caspase-like proteasomal activity (Fig. 5F), while decreasing the trypsin-like proteasomal activity (Fig. 5G). While more studies are needed to dissect the differential changes in proteasomal activity, these data suggest that chymotrypsin- and caspase-like activities most likely are not involved in the ICI-mediated increase in Aβ levels. However, the decrease in trypsin-like proteasomal activity might contribute to the increase in Aβ levels in the 3xTg-AD/ICI mice.
Figure 5. Effects on ICI on autophagy and proteasome function.
(A) Representative Western blots of proteins extracted from the brains of treated and untreated 3xTg-AD mice. Blots were probed with the indicated antibodies. (B–D) Quantitative analysis of the blots showed that the steady-state levels of Atg 5, Atg 7 and p62 were similar between 3xTg-AD/NaCl and 3xTg-AD/ICI mice. (E–F) Whole hemi-brain homogenates (without cerebellum) from NaCl- and ICI-treated 3xTg-AD mice were analyzed for proteasome activity. ICI had differential effects on the proteasome activity. While did not alter the chymotrypsin-like activity, it led to an increase in the caspase-like activity and to a decrease in the trypsin-like activities. Data are presented as means ± SEM.
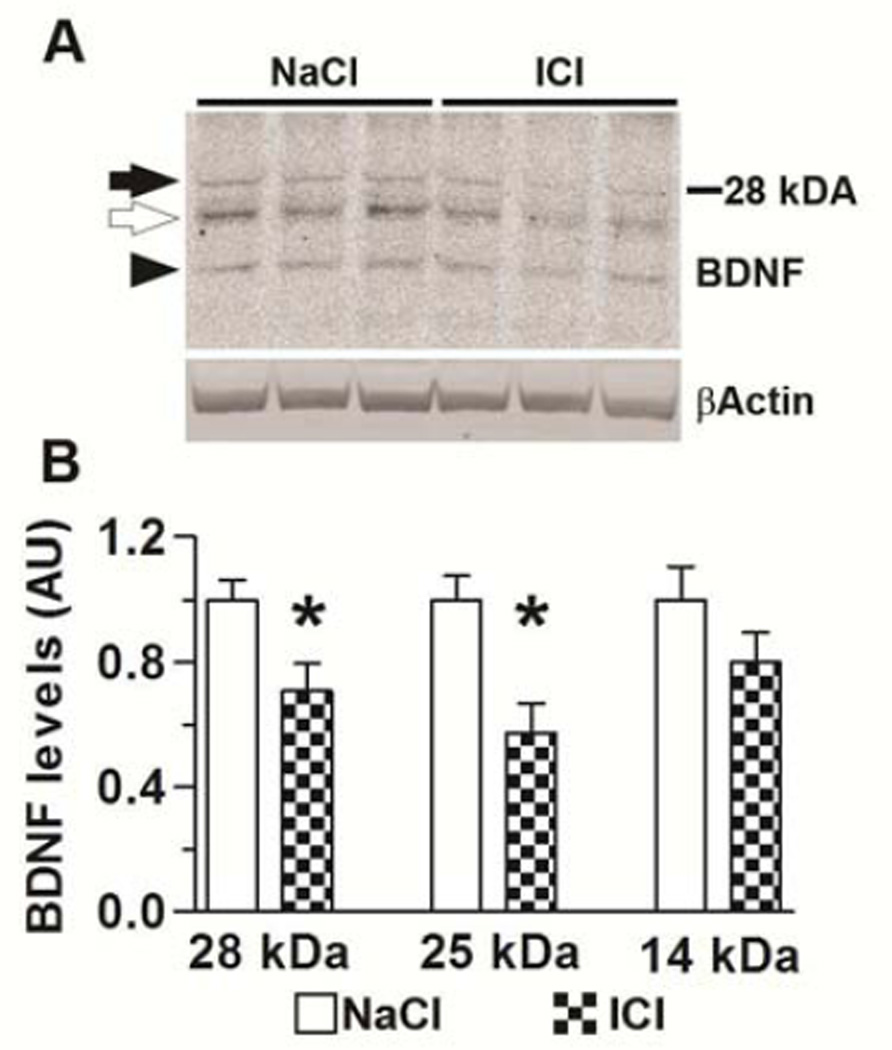
Taken together, the results presented so far indicate that antagonizing the β2ARs increases APP processing and Aβ production, which in turn facilitates tau phosphorylation and exacerbates behavioral deficits in the 3xTg-AD mice. A large body of evidence suggests that the activation of β2ARs elicits the activation of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (Counts and Mufson, 2010; Gleeson, et al., 2010; Juric, et al., 2008). Consistently, β2AR blockade depresses long term potentiation by decreasing BDNF levels (Flores, et al., 2010). To further elucidate the mechanism underlying the ICI-mediated decrease in cognitive function, we focused on BDNF as it is known to facilitate synaptic plasticity and memory formation (Cowansage, et al., 2010), and has been proposed to play a role in the pathogenesis of AD (Hock, et al., 2000). To determine whether administration of ICI alters BDNF levels, we measured the levels of this trophic factor by Western blot. We found that BDNF levels were significantly lower in the brains of the 3xTg-AD/ICI mice compared to 3xTg-AD/NaCl mice (Fig 6). Given the role of BDNF in regulating memory formation, these data suggest that in addition to increasing Aβ levels, ICI exacerbates behavioral deficits by decreasing BDNF levels.
Figure 6. ICI administration decreases BDNF levels.
(A) Representative Western blots of proteins extracted from the brains of treated and untreated 3xTg-AD mice. Blots were probed with the indicated antibodies. (F) Quantitative analysis of the three bands detected by the BDNF antibody showed that the steady-state levels of the ~28 kDa and ~25 kDa bands (black and open arrows) were significantly lower in the 3xTg-AD/ICI mice compared to the 3xTg-AD/NaCl mice. While the levels of the 14kDa band (black arrowhead) were also reduced in the 3xTg-AD/ICI mice, the difference did not reach statistical significance. Data are presented as means ± SEM.
Discussion
People over the age of 85 represent the fastest growing segment of the American population (United States Census Bureau, www.census.gov). Given that aging is the single major risk factor for AD, the prevalence of AD is estimated to quadruple by midcentury (Thies, et al., 2013). Aging is a risk factor for other widely spread disorders as well, including hypertension and cardiovascular diseases; consistently hypertension is a common comorbidity in AD (Csiszar, et al., 2013; Nelson, et al., 2014). Considering the prevalence of AD and hypertension, it is likely that an elderly patient is simultaneously treated for both conditions. Therefore, understanding the role of β-blockers, which represent the first line of treatment for hypertension (Wiysonge, et al., 2012), in the context of AD is highly clinically relevant. In this study, we treated 8-month-old female 3xTg-AD mice with a selective β2 adrenergic receptor antagonist, ICI 118,551. Our results clearly indicate that under the experimental conditions used in this study, ICI exacerbated Aβ and tau pathology and cognitive deficits in 3xTg-AD mice. Overall, the data presented here expand the current knowledge and provide key insights into the role of β2ARs in health and disease.
Published reports on the role of β2ARs in memory and AD have reached apparent contradicting results. Epidemiological studies suggest that the use of non-selective βAR antagonists for the treatment of hypertension correlates with a lower incidence of AD (Khachaturian, et al., 2006). Consistently, genetic studies indicate that polymorphisms in the gene encoding the β2AR are linked to a higher risk of late onset AD (Insel, 2011; Yu, et al., 2008). It has been postulated that the two polymorphisms (Gly16Arg and Gln27Glu) that confer higher AD susceptibility do so by increasing receptor signaling (Insel, 2011; Yu, et al., 2008). It appears that subjects with Gly16Glu27 haplotype have increased responsiveness to endogenous norepinephrine whose production is boosted by stress (Yu, et al., 2008). In fact, Gly16 variant (whether paired with Gln27 or Glu27) shows faster activation in cAMP formation and accelerated signal amplification in this second messenger system (Insel, 2011). It may be that due to this persistent response and the consequent dysregulated signaling downstream of β2AR, these polymorphisms increase the risk of AD. These data are consistent with work in animal models, where chronic treatment with β2AR agonists has been shown to increase Aβ load in transgenic mice, while the use of β-blockers conversely decreases acute stress-induced Aβ production (Ni, et al., 2006; Yu, et al., 2010).
While these data clearly suggest that the use of β-blockers would have beneficial effects on AD, these conclusions appear counterintuitive with the overwhelming data showing that activation of β2ARs is required for normal learning and memory. This has been shown at a cellular level (Flores, et al., 2010; Gelinas and Nguyen, 2005; Lin, et al., 2003; Qian, et al., 2012; Thomas, et al., 1996; Walling and Harley, 2004) and at a whole animal behavior, where activation of β2ARs positively modulates memory formation and consolidation (Gibbs, et al., 2010; Insel, 2011; Kolinski, et al., 2012; Zhou, et al., 2013). Furthermore, two independent reports have shown that activation of β2ARs can overcome the detrimental effects of Aβ on LTP (Li, et al., 2013; Wang, et al., 2009). Specifically, in an elegant work, Selkoe and colleague have reported that wildtype mice chronically fed a β-adrenergic agonist were protected from Aβ-induced hippocampal impairments. They concluded that oral β-adrenergic agonist, and not antagonist, could be efficacious in mitigating the detrimental effect of high Aβ levels in the brain (Li, et al., 2013). Our data are consistent with these observations as we found that the use of a β2AR antagonist leads to memory deficits. However, the effects were selective to the specific memory being tested. Toward this end, in NonTg mice, ICI caused working memory deficits, a memory function mainly controlled by the frontal cortex. In contrast, in the same mice, ICI had no effect on spatial learning and memory, whose function is mainly controlled by the hippocampus. While further studies are needed to explain the lack of effect of ICI on spatial memory in NonTg mice, the effect of ICI on working memory is consistent with data showing that β-adrenergic signaling is necessary for OR performance (Roozendaal, et al., 2008). Our data also showed that under the conditions used here the 3xTg-AD mice treated with NaCl performed as well as NonTg mice treated with NaCl. This is somewhat surprising given the wealth of data showing that at this age the 3xTg-AD mice have spatial memory deficits. Since their development in 2003, the phenotype of 3xTg-AD mice on a mixed genetic background has been drifting and becoming more variable. While more studies are needed to identify why the 3xTg-AD mice used here performed similarly to NonTg, it is likely that these results were restricted to the specific cohort of mice used for this study. Independent of that, we found that ICI exacerbated spatial learning and memory deficits in 3xTg-AD mice, effects that were most likely mediated by the increase in brain pathology. The 3xTg-AD/NaCl mice performed at a random level in the object recognition and significantly worse than NonTg/NaCl mice, suggesting that these transgenic mice have working memory impairments. Given the nature of the task (mice cannot perform worse than chance levels), no conclusions can be drawn on the effects of ICI on working memory in the 3xTg-AD mice.
We reported that ICI increased Aβ pathology by increasing APP processing. Given that in the 3xTg-AD mice, the tau pathology is highly dependent on the Aβ load (Oddo, et al., 2003; Oddo, et al., 2008; Oddo, et al., 2006), it is tempting to speculate that the increase in tau pathology in the ICI-treated 3xTg-AD mice might be triggered by the increase in Aβ levels. It is also plausible that the increase in tau pathology in the ICI-treated mice is due to a direct interaction between β2ARs signaling and tau. Toward this end, we recently showed that genetically reducing β2AR signaling significantly decreased tau pathology in the presence and absence of Aβ (Wang, et al., 2013; Wisely, et al., 2014). While these genetic data could appear counterintuitive with the data presented here, there are several mechanisms that could explain the variation between genetic and pharmacological data. Even though normal β2AR signaling is important for physiological cognitive brain functions such as learning and memory, by completely eliminating the receptor in the diseased brain, the harmful effect of dysregulated β2AR signaling is completely prevented. Indeed, strong evidence suggests that Aβ binds directly to these receptors activating detrimental downstream pathways, some of which increase presenilin activity and further increase Aβ production and neuronal excitotoxicity (Ni, et al., 2006; Wang, et al., 2010; Wang, et al., 2011). By genetically removing the receptor, these pathways cannot contribute to Aβ production while the critical functions of β2ARs are likely fulfilled by other adrenergic receptors that could be upregulated to overcome the lack of β2ARs. Toward this end, despite the critical function of β2ARs, a full β2AR knockout produces viable mice that develop normally (Chruscinski, et al., 1999).
In contrast, blocking a receptor pharmacologically decreases the probability of engaging compensatory mechanisms that could fulfill the function of β2AR in learning and memory. Additionally, ICI binds to a β2AR site different from the site at which Aβ binds, thus allowing for a possibility of competing effects of these two molecules on the receptor functioning (Selvam, et al., 2012; Wang, et al., 2010). Thus, it is likely that the vicious cycle of Aβ binding β2AR and generating more Aβ was not affected by the presence of ICI, at least using administered dose. In summary, these studies strengthen the notion that the link between β2ARs and AD is likely highly complex and suggest caution in generalizing the beneficial effects of beta-blockers on AD. Given the contradicting reports in the literature and the obvious importance of these receptors in AD, more studies are warranted to fully understand how β2ARs are involved in AD pathogenesis and how they could be modulated to produce most beneficial outcomes.
Methods
Mice and drug administration
3xTg-AD mice were generated as previously described (Oddo, et al., 2003). In these studies, only female mice were used as we find that the neuropathological variability in females is small while the pathology in males is widely variable. The selective β2-blocker ICI 118,551 was purchased from Tocris Bioscience (Bristol, United Kingdom) and delivered via daily i.p. injections for 6 weeks, at 1 mg/kg. Control mice were injected with an equal volume of 0.9% NaCl. Mice were weighed immediately before, halfway throughout and upon the completion of the experiments.
Morris Water Maze
Morris water maze tests were conducted as previously described (Caccamo, et al., 2013a). Briefly, mice were tested in a circular tank of 1.5 meters in diameter located in a room with extra maze cues. The platform (14 cm in diameter) location was maintained constant for each mouse during training and was submerged 1.5 cm beneath the surface of the water, which was maintained at 25 °C throughout the duration of the testing. During 5 days of training, the mice underwent 4 trials a day, alternating among 4 pseudorandom starting points. If a mouse failed to find the platform within 60 s, it was guided to the platform by the researcher and kept there for 10 s. The inter-trial interval was 25 s, during which time each mouse was returned to its home cage. Probe trials were conducted 24 hours after the last training trial. During the probe trials, the platform was removed and mice were free to swim in the tank for 60 s. The training and probe trials were recorded by a video camera mounted on the ceiling, and data were analyzed using the EthoVisio XT tracking system (Noldus Information Technology, Leesburg, VA).
Novel Object Recognition
The test was conducted in a clear Plexiglas box (40 × 40 cm) and was recorded with a video camera mounted above the testing box. To allow habituation to the arena, mice were individually placed in the box for 10 minutes of free exploration in the absence of objects, for two days. At day 3, mice were placed in the middle of the box and left free to explore two identical objects for 5 minutes. After a 7 minute delay, during which mice were returned to their home cage, mice were gently placed back into the arena where one of the two objects was replaced with a new object, similar in size. Mice were allowed to explore the two objects for 3 minutes. The percentage of time spent exploring the new object was measured with the EthoVision XT tracking system.
Protein Extraction and Western blots
Mice were sacrificed by CO2 asphyxiation and their brains extracted and cut midsagitally, ½ brain was used for immunohistochemical analyses while the other half was quickly frozen in dry ice for biochemical analysis. Frozen brains were processed as described previously (Caccamo, et al., 2013b). Briefly, brains were homogenized in a solution of tissue protein extraction reagent (Pierce) containing 0.7 mg/ml of Pepstatin A supplemented with a complete Mini protease inhibitor tablet (Roche Applied Science) and phosphatase inhibitors (Invitrogen). The homogenized mixtures were centrifuged at 4 °C for 1 h at 100,000g, and the resulting supernatant was stored as the soluble fraction. The pellet was re-homogenized in 70% formic acid and centrifuged as above. The supernatant was stored as the insoluble fraction. For Western blot analyses we used established protocols (Majumder, et al., 2012). Proteins from the soluble fraction were resolved by 10% BisTris SDS-PAGE (Invitrogen) under reducing conditions and transferred to a nitrocellulose membrane. Membranes were developed as described previously (Orr, et al., 2014). Briefly, membranes were incubated for 1 hour in 5% nonfat milk in TBST (0.1% Tween-20 in tris-buffered saline pH 7.6) then incubated overnight in primary antibody. The folloiwng primary antibodies were used: CP13 (a generous gift from Peter Davies); Atg5, Atg7 and β-Actin (Cell Signaling); 6E10 (Covance); p62 (Millipore) and APP C-terminal (Calbiochem). The blots were rinsed in TBST for 30 minutes then incubated in goat anti-mouse IRDye 680LT or goat anti-rabbit IRDye 800CW LI-COR secondary antibodies (1:10,000) for 1 hour at room temperature. The membranes were rinsed for 30 minutes in TBST and imaged and analyzed using the Odyssey (LI-COR, Lincoln, Nebraska). The protein levels reported in the figures were obtained as a ratio between the band intensity of the protein of interest and the band intensity of β-actin, used as loading control.
Immunohistochemistry
Hemibrains were drop-fixed in 4% paraformaldehyde in phosphate-buffered saline for 48 hours and then transferred into 0.02% sodium azide in phosphate-buffered saline until slicing. 50 µn thick free-floating sections were subsequently obtained using a vibratome. Sections were washed twice with TBS (100 mM Tris pH 7.5; 150 mM NaCI), 5 minutes each, followed by a 30 minute incubation in 3% H2O2, to quench endogenous peroxidase activity. Next, sections were transferred into TBS-A (100 mM Tris pH 7.5; 150 mM NaCI; 0.1% Triton X-100) and TBS-B (100 mM Tris pH 7.5; 150 mM NaCI; 0.1% Triton X-100; 2% BSA) for 15 and 30 minutes, respectively, to block non-specific binding. Finally, the proper primary antibody was applied overnight at 4°C; anti body dilution was made in TBS-B. Aβ42 (1:200) from Millipore; AT180 (1:3000) from Pierce; CP13 (1:1000) was a generous gift from Dr. Peter Davies. Sections were washed 3 times in TBS and incubated with a mouse secondary antibody for 1 hour at room temperature and developed as described previously (Majumder, et al., 2011).
Proteasome activity assay
This assay was performed by incubating 10 µl of brain homogenate with proteasomal substrates Suc-LLVY-AMC, Bz-VGR-AMC and Z-LLE-AMC (Enzo Life Sciences, Plymouth Meeting, PA), which probe for chymotrypsin-like, trypsin-, and caspase-like activities, respectively. Reactions were carried as previously described (Medina, et al., 2014).
ELISA
Aβ40 and Aβ42 ELISA plates were purchased from Invitrogen and experiments were conducted using the manufacturer’s instructions.
Statistical analyses
All data were analyzed using GraphPad Prism, GraphPad Software, San Diego California, USA, www.graphpad.com. Data were analyzed by one- or two-way ANOVA followed by Bonferroni post-hoc analysis. When applicable, data were analyzed by Student t-test, as specified in the results section.
Supplementary Material
Highlights.
Alterations in the function of β2ARs is linked to Alzheimer’s disease.
We report that ICI 118,551, a selective β2AR agonist, increases Aβ pathology.
We report that ICI 118,551 increases tau phosphorylation and accumulation.
Pharmacological inhibition of β2ARs exacerbates cognitive deficits.
We propose caution in generalizing the beneficial effects of beta-blockers on AD.
Acknowledgements
The authors thank Mr. Darren Shaw, Banner Sun Health Research Institute for superb technical assistance and Dr. Marina Pizzi, University of Brescia, Italy for productive discussions. This work was supported by grants to S.O. by the National Institutes of Health (R01 AG037637) and the American Federation for Aging Research. E.V.W. was supported by a training grant from the National Institutes of Health (F30 AG043248).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors declare no competing financial interests. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Ameredes BT. Beta-2-receptor regulation of immunomodulatory proteins in airway smooth muscle. Front Biosci (Schol Ed) 2011;3:643–654. doi: 10.2741/s177. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero S, Tierney MC, Touchon J, Ritchie K. Prediction of transition from cognitive impairment to senile dementia: a prospective, longitudinal study. Acta Psychiatr Scand. 2003;107(5):390–393. doi: 10.1034/j.1600-0447.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, Oddo S. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies. Aging Cell. 2013a;12(3):370–380. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Medina DX, Oddo S. Glucocorticoids exacerbate cognitive deficits in TDP-25 transgenic mice via a glutathione-mediated mechanism: implications for aging, stress and TDP-43 proteinopathies. J Neurosci. 2013b;33(3):906–913. doi: 10.1523/JNEUROSCI.3314-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274(24):16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Connor SA, Wang YT, Nguyen PV. Activation of {beta}-adrenergic receptors facilitates heterosynaptic translation-dependent long-term potentiation. J Physiol. 2011;589(Pt 17):4321–4340. doi: 10.1113/jphysiol.2011.209379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem. 2010;113(3):649–660. doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3(1):12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, Deak F, Sonntag WE, Ungvari Z. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in beta-amyloid generation and Alzheimer's disease. Am J Physiol Heart Circ Physiol. 2013;305(8):H1120–H1130. doi: 10.1152/ajpheart.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CJ, McGrath JC. Previously unsuspected widespread cellular and tissue distribution of beta-adrenoceptors and its relevance to drug action. Trends Pharmacol Sci. 2011;32(4):219–226. doi: 10.1016/j.tips.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40(2):139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- Flores O, Nunez H, Perez H, Morgan C, Soto-Moyano R, Valladares L, Burgos H, Olivares R, Hernandez A. beta-Adrenoceptor blockade depresses molecular and functional plasticities in the rat neocortex. Brain Res Bull. 2010;82(5–6):284–288. doi: 10.1016/j.brainresbull.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25(13):3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Hutchinson DS, Summers RJ. Noradrenaline release in the locus coeruleus modulates memory formation and consolidation; roles for alpha- and beta-adrenergic receptors. Neuroscience. 2010;170(4):1209–1222. doi: 10.1016/j.neuroscience.2010.07.052. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Role of adrenoceptor subtypes in memory consolidation. Prog Neurobiol. 2002;67(5):345–391. doi: 10.1016/s0301-0082(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Gleeson LC, Ryan KJ, Griffin EW, Connor TJ, Harkin A. The beta2-adrenoceptor agonist clenbuterol elicits neuroprotective, anti-inflammatory and neurotrophic actions in the kainic acid model of excitotoxicity. Brain Behav Immun. 2010;24(8):1354–1361. doi: 10.1016/j.bbi.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57(6):846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Insel PA. beta(2)-Adrenergic receptor polymorphisms and signaling: Do variants influence the "memory" of receptor activation? Sci Signal. 2011;4(185):e37. doi: 10.1126/scisignal.2002352. [DOI] [PubMed] [Google Scholar]
- Joiner ML, Lise MF, Yuen EY, Kam AY, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, Burette AC, Weinberg RJ, Law PY, El-Husseini A, Yan Z, Hell JW. Assembly of a beta2-adrenergic receptor--GluR1 signalling complex for localized cAMP signalling. EMBO J. 2010;29(2):482–495. doi: 10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juric DM, Loncar D, Carman-Krzan M. Noradrenergic stimulation of BDNF synthesis in astrocytes: mediation via alpha1- and beta1/beta2-adrenergic receptors. Neurochem Int. 2008;52(1–2):297–306. doi: 10.1016/j.neuint.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol. 2006;63(5):686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- Kolinski M, Plazinska A, Jozwiak K. Recent progress in understanding of structure, ligand interactions and the mechanism of activation of the beta(2)-adrenergic receptor. Curr Med Chem. 2012;19(8):1155–1163. doi: 10.2174/092986712799320547. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ. Environmental novelty activates beta2-adrenergic signaling to prevent the impairment of hippocampal LTP by Abeta oligomers. Neuron. 2013;77(5):929–941. doi: 10.1016/j.neuron.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Min MY, Chiu TH, Yang HW. Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. J Neurosci. 2003;23(10):4173–74181. doi: 10.1523/JNEUROSCI.23-10-04173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11(2):326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36(7):1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DX, Orr ME, Oddo S. Accumulation of C-terminal fragments of transactive response DNA-binding protein 43 leads to synaptic loss and cognitive deficits in human TDP-43 transgenic mice. Neurobiol Aging. 2014;35(1):79–87. doi: 10.1016/j.neurobiolaging.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Nelson L, Gard P, Tabet N. Hypertension and Inflammation in Alzheimer's Disease: Close Partners in Disease Development and Progression! J Alzheimers Dis. 2014 doi: 10.3233/JAD-140024. [DOI] [PubMed] [Google Scholar]
- Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang Z, Song M, Xiong J, Bai Y, Pei G. Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med. 2006;12(12):1390–1396. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, LaFerla FM. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J Neurosci. 2008;28(47):12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281(51):39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Orr ME, Salinas A, Buffenstein R, Oddo S. Mammalian target of rapamycin hyperactivity mediates the detrimental effects of a high sucrose diet on Alzheimer's disease pathology. Neurobiol Aging. 2014;35(6):1233–1242. doi: 10.1016/j.neurobiolaging.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122(Pt 3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Qian H, Matt L, Zhang M, Nguyen M, Patriarchi T, Koval OM, Anderson ME, He K, Lee HK, Hell JW. beta2-Adrenergic receptor supports prolonged theta tetanus-induced LTP. J Neurophysiol. 2012;107(10):2703–2712. doi: 10.1152/jn.00374.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90(3):576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Shirato K, Tachiyashiki K, Imaizumi K. Muscle plasticity and beta(2)-adrenergic receptors: adaptive responses of beta(2)-adrenergic receptor expression to muscle hypertrophy and atrophy. J Biomed Biotechnol. 2011:729598. doi: 10.1155/2011/729598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam B, Wereszczynski J, Tikhonova IG. Comparison of dynamics of extracellular accesses to the beta(1) and beta(2) adrenoceptors binding sites uncovers the potential of kinetic basis of antagonist selectivity. Chem Biol Drug Des. 2012;80(2):215–226. doi: 10.1111/j.1747-0285.2012.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhill S, Stroud T, Nagendra R, Perros-Huguet C, Trevethick M. A cell-based assay to assess the persistence of action of agonists acting at recombinant human beta(2) adrenoceptors. J Pharmacol Toxicol Methods. 2008;58(3):189–197. doi: 10.1016/j.vascn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L, Alzheimer's A. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17(3):475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Walling SG, Harley CW. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel beta-adrenergic- and protein synthesis-dependent mammalian plasticity mechanism. J Neurosci. 2004;24(3):598–604. doi: 10.1523/JNEUROSCI.4426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fu Q, Zhou Y, Xu B, Shi Q, Igwe B, Matt L, Hell JW, Wisely EV, Oddo S, Xiang YK. beta2 adrenergic receptor, protein kinase A (PKA) and c-Jun N-terminal kinase (JNK) signaling pathways mediate tau pathology in Alzheimer disease models. J Biol Chem. 2013;288(15):10298–10307. doi: 10.1074/jbc.M112.415141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Govindaiah G, Liu R, De Arcangelis V, Cox CL, Xiang YK. Binding of amyloid beta peptide to beta2 adrenergic receptor induces PKA-dependent AMPA receptor hyperactivity. FASEB J. 2010;24(9):3511–3521. doi: 10.1096/fj.10-156661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yuen EY, Zhou Y, Yan Z, Xiang YK. Amyloid beta peptide-(1–42) induces internalization and degradation of beta2 adrenergic receptors in prefrontal cortical neurons. J Biol Chem. 2011;286(36):31852–31863. doi: 10.1074/jbc.M111.244335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QW, Rowan MJ, Anwyl R. Inhibition of LTP by beta-amyloid is prevented by activation of beta2 adrenoceptors and stimulation of the cAMP/PKA signalling pathway. Neurobiol Aging. 2009;30(10):1608–1613. doi: 10.1016/j.neurobiolaging.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1992;49(5):448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Wisely EV, Xiang YK, Oddo S. Genetic suppression of beta2-adrenergic receptors ameliorates tau pathology in a mouse model of tauopathies. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD002003.pub4. CD002003. [DOI] [PubMed] [Google Scholar]
- Woo AY, Xiao RP. beta-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin. 2012;33(3):335–341. doi: 10.1038/aps.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JT, Tan L, Ou JR, Zhu JX, Liu K, Song JH, Sun YP. Polymorphisms at the beta2-adrenergic receptor gene influence Alzheimer's disease susceptibility. Brain Res. 2008;1210:216–222. doi: 10.1016/j.brainres.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Yu NN, Wang XX, Yu JT, Wang ND, Lu RC, Miao D, Tian Y, Tan L. Blocking beta2-adrenergic receptor attenuates acute stress-induced amyloid beta peptides production. Brain Res. 2010;1317:305–310. doi: 10.1016/j.brainres.2009.12.087. [DOI] [PubMed] [Google Scholar]
- Zhou HC, Sun YY, Cai W, He XT, Yi F, Li BM, Zhang XH. Activation of beta2-adrenoceptor enhances synaptic potentiation and behavioral memory via cAMP-PKA signaling in the medial prefrontal cortex of rats. Learn Mem. 2013;20(5):274–284. doi: 10.1101/lm.030411.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.