Abstract
The continuing challenge of HIV-1 treatment resistance in patients creates a need for the development of new antiretroviral inhibitors. The HIV nucleocapsid (NC) protein is a potential therapeutic target. NC is necessary for viral RNA packaging and in the early stages of viral infection. The high level of NC amino acid conservation among all HIV-1 clades suggests a low tolerance for mutations. Thus, NC mutations that could arise during inhibitor treatment to provide resistance may render the virus less fit. Disruption of NC function provides a unique opportunity to strongly dampen replication at multiple points during the viral life cycle with a single inhibitor. Although NC exhibits desirable features for a potential antiviral target, the structural flexibility, size, and the presence of two zinc fingers makes small molecule targeting of NC a challenging task. In this review, we discuss the recent advances in strategies to develop inhibitors of NC function and present a perspective on potential novel approaches that may help to overcome some of the current challenges in the field.
Keywords: HIV-1, Nuclocapsid protein, Inhibitors, RNA
1. Introduction
The HIV nucleocapsid (NC) is a small, highly basic, zinc binding protein that interacts with the viral RNA to facilitate numerous processes in the viral life cycle (Darlix et al., 2007; Darlix et al., 2011; Muriaux and Darlix, 2010). When NC is contained within the precursor Gag polyprotein, the NC domain is involved in genomic RNA packaging, Gag-Gag multimerization and possibly initiation of particle production during virus assembly. The NC domain also plays a pivotal role in recruiting host cellular factors such as the members of the endosomal sorting complex required for transport (ESCRT) machinery that promote viral release (Sette et al., 2012). The mature NC protein, which is formed upon protease-mediated cleavage of the Gag polyprotein, facilitates encapsidation of the nucleic acids and is found in tight association with viral RNA in the viral core. During viral replication, NC chaperones the reverse transcriptase (RT) mediated synthesis of viral DNA and cooperates with the integrase for maintenance and integration of viral DNA (reviewed in (Godet et al., 2012; Sleiman et al., 2012)).
The NC protein is structurally flexible and is characterized by the presence of two highly conserved zinc fingers with a Cys-X2-Cys-X4-His-X4-Cys (CCHC) motif and each of which coordinates a zinc ion (Fig 1). Aided by the electrostatic complementarity offered by the flanking basic residues, as well as by other hydrophobic and hydrogen bond interactions, the zinc fingers provide a hydrophobic platform for direct interactions with the viral nucleic acids. The NC residue Trp37 is reported to form the key π-stacking and hydrogen bond interactions with the guanine base of viral RNA. The inherent plasticity of the NC protein permits it to adapt to sequence and structural variability of the various nucleic acids it chaperones (Darlix et al., 2011; Godet et al., 2013). Point mutations in NC significantly reduce the viral load and infectivity, thereby further underlining the importance of NC in the viral life cycle when evaluated in cell lines in tissue culture (Bombarda et al., 2005; Houzet et al., 2008; Wu et al., 2013a; Wu et al., 2013b).
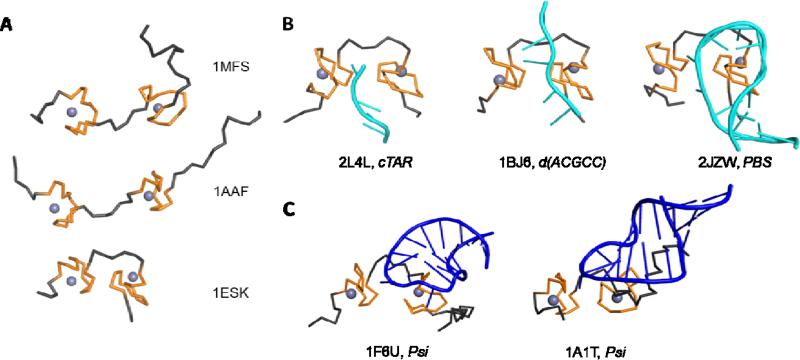
Figure 1.
Structure and flexibility of nucleocapsid protein. Solution structures of apo-NC (A) demonstrate that the zinc fingers (orange ribbons) show some structure conformation due to coordination with the zinc ions (purple spheres), but the flanking regions (grey ribbons) are highly flexible (Lee et al., 1998; Summers et al., 1992). Upon binding nucleic acids (B & C) DNA (cyan cartoon) and RNA (blue cartoon), respectively, both zinc fingers anchor against the nucleobases and the basic flanking regions and make electrostatic contacts with the nucleic acids (Amarasinghe et al., 2000; Bazzi et al., 2011; Bourbigot et al., 2008; De Guzman et al., 1998; Morellet et al., 1998). The NC protein-nucleic acid complex is much less flexible than the apo-NC protein. The first model in the available PDB file has been represented here; PDB codes of individual structures and names of the bound nucleic acids are provided next to the figure. cTAR - complementary trans-activating response element, PBS - primer binding site, Psi – packaging signal.
Moreover, NC is conserved throughout all clades, further underscoring it as a promising target for HIV therapy. Nevertheless, no approved drugs or investigational agents targeting NC are available in the clinic. The highly flexible nature, high basicity and the presence of zinc-coordinating zinc fingers make it challenging to identify therapeutics with specific activity and low cellular cytotoxicity.
Because of the necessity of the NC-nucleic acid interactions in the viral life cycle, the focus of older strategies to disrupt NC function was to target small molecules to the zinc fingers or RNA. In this review, we discuss recent advances in disrupting NC function as well provide a perspective on potential novel approaches that might help overcome the current challenges in the field.
2. Zinc Finger Binders
Zinc fingers are a highly conserved feature found in many viruses such, as herpes virus (Lium and Silverstein, 1997), ebola virus (Modrof et al., 2003), HIV and other viruses. Therefore, targeting viral zinc fingers is an obvious strategy. However, since zinc fingers are present (~10%) in the human proteome (Andreini et al., 2006), viral zinc finger antiviral compounds must be highly specific to the virus otherwise off-target effects, causing cellular toxicity, can arise. The approaches to targeting the NC zinc fingers include small molecules that result either in removal of the zinc ion or directly interfere with nucleic acid binding without promoting zinc ejection.
2.1. Zinc Ejectors
2.1.1. Covalent binders
The most common category of investigated NC zinc ejectors are soft electrophiles that react covalently with the sulfhydryl group of the nucleophilic cysteine residues in the zinc fingers, leading to the release of the coordinated zinc ion. Ejection of the zinc ion destroys the structure, thereby disrupting the nucleic acid interacting function of NC. Virally infected cells treated with compounds that eject zinc result in the accumulation of aggregated, unprocessed Gag proteins (Grigorov et al., 2007). Because of the critical role of the cysteines in the zinc fingers, covalent binding zinc ejectors are not susceptible to the development of resistance (Jenkins et al., 2010). Various categories of compounds in this class were reported during the 1990s, including 3-nitrosobenzamide (NOBA) (Rice et al., 1993), 2,2’-dithiobisbenzamide (DIBA) (Rice et al., 1995), cyclic 2,2’- dithiobisbenzamide (Witvrouw et al., 1997), 1,2-dithiane-4,5-diol-1,1-dioxide (Rice et al., 1997a), azadicarbonamide (ADA) (Rice et al., 1997b) and pyridinioalkanoyl 2-mercaptobenzamide thioesters (PATE) (Turpin et al., 1999). The structure and activity of these zinc ejectors has been reviewed by Rocquigny et al (de Rocquigny et al., 2008). Some of these molecules, such as DIBA-1, dithiane and ADA, were selective to HIV NC, with limited interaction with endogenous cellular zinc fingers (Huang et al., 1998). DIBA-4 (renamed CI-1012) and ADA entered Phase I/II clinical trials (Goebel et al., 2001; Huang et al., 1998). None of these compounds reached the clinic due to cellular toxicity.
DIBAs are unstable compounds due to the presence of a disulfide bond that is susceptible to reduction, resulting in loss of activity and reduced half-life. The disulfide was substituted with a less reactive thioester bridge, resulting in PATEs, which are resistant to glutathione reduction (Turpin et al., 1999). The higher stability of the PATEs provides a better in-cell therapeutic index due to the reduced cellular toxicity (Turpin et al., 1999). Replacing the thioester bridge by thiolcarbamate (TICA) resulted in improved retroviral potency over that of PATEs (Goel et al., 2002). However, PATEs and TICAs carry a positive charge, which makes them very hygroscopic and hydrophilic. These chemical properties result in difficult isolation and purification and when administered cause decreased absorption and increased excretion making them poor candidates for oral administration (Inman et al., 2009).
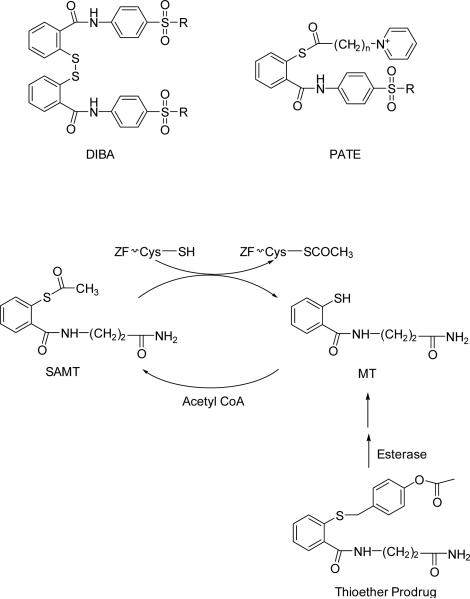
Subsequently uncharged thioesters such as S-acyl-2-mercaptobenzamide thioesters (SAMTs) were reported (Fig 2) (Inman et al., 2009; Srivastava et al., 2004). The disulfides and the thioesters differ in their mechanism of action for ejecting zinc from the zinc fingers. The disulfides, such as the DIBA-like compounds, covalently interact with the cysteines of the zinc fingers to eject zinc and form mixed disulfides within the cysteine residues. The advantages of this class of compounds are their preferential selectivity for NC, over that of cellular zinc fingers, although they do interact with cellular zinc fingers (Bombarda et al., 2001; Huang et al., 1998; Jenkins et al., 2007). While DIBAs sequentially interact with both the carboxyl- and the amino-terminal zinc fingers of NC, the thioesters like PATEs and SAMTs react only with the carboxyl-terminal zinc finger. The thioesters first acetylate Cys36 through an intermolecular nucleophilic attack, which is followed by an intramolecular acyl transfer to the Lys33 and Lys38 residues promoting zinc finger conformation changes and the subsequent ejection of zinc. The requirement of having specific residues in the proximity of the reactive cysteine endows specificity to the 2-mercaptobenzamide thioesters (Jenkins et al., 2007; Jenkins et al., 2010). In the case of SAMTs, the intermolecular acyl transfer from SAMTs to the cysteine residue results in release of mercaptobenzamide thiol (MT). The cellular enzyme acetyl-CoA acetylates MT, thereby effectively recycling SAMT (Jenkins et al., 2010). Thioethers which intracellularly hydrolyze to form MT followed by acetylation to SAMT have been developed as prodrugs for SAMTs (Fig 2) (Appella et al., 2013). The SAMTs and their thioether prodrugs have been demonstrated to be stable in synthetic vaginal fluid and effective as microbicides in non-human primates (Appella et al., 2013; Wallace et al., 2009). Such selective, recyclable zinc ejectors could aid in improving the therapeutic index of the molecules by reducing the EC50 and require less frequent dosage due to increased half-life. Since continuous recycling may make it difficult to clear the drug from the system, an optimal balance between recycling and excretion should be established.
Figure 2.
Representative structures of covalent zinc ejectors. Disulfides: 2,2’-dithiobisbenzamide (DIBA). Thioesters: Pyridinioalkanoyl 2-mercaptobenzamide thioester (PATE) and S-acyl-2-mercaptobenzamide thioester (SAMT). The intracellular recycling of SAMT with 2-mercaptobenzamide thioester (MT) and activation of thioether prodrug are also shown. ZF=Zinc Finger.
2.1.2. Non-covalent binders
Another relatively recently reported category of zinc ejectors are the thiadiazaole derivatives that act by zinc chelation (Fig 3) (Pannecouque et al., 2010; Vercruysse et al., 2012). Unlike the thioesters, thiadiazaole derivatives do not form covalent bonds with the NC protein. Treatment of infected cells with the phenyl-thiadiazolidene amine derivative does not result in accumulation of unprocessed Gag (Vercruysse et al., 2012). Therefore these molecules probably act at a different step in the viral life cycle than the thioesters. Since the mechanism and possibly the time of action of these molecules are different from that of thioesters, they might offer a potential new class of antiviral agents.
Figure 3.
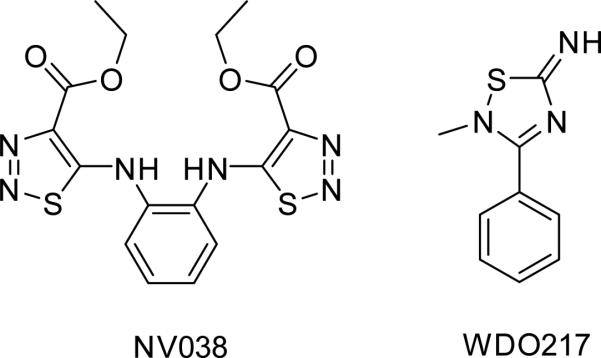
Non-covalent binding zinc ejectors.
2.2. Non-Zinc ejectors
Small molecules
Some of the first compounds identified to bind NC zinc fingers without ejecting the coordinated zincs were gallein derivatives, which contained a xanthenyl ring substituted with two hydroxyl groups. These compounds were found to interact with the apo-NC and interfered with NC- nucleic acid binding. However, the compounds did not compete with the pre-bound DNA on NC. The two hydroxyl substitutions on the xanthenyl ring, which are thought to interact with the backbone atoms of Lys33, Gly35 and Gly40, were found to be critical for binding to NC and for providing protection against HIV infection (de Rocquigny et al., 2008).
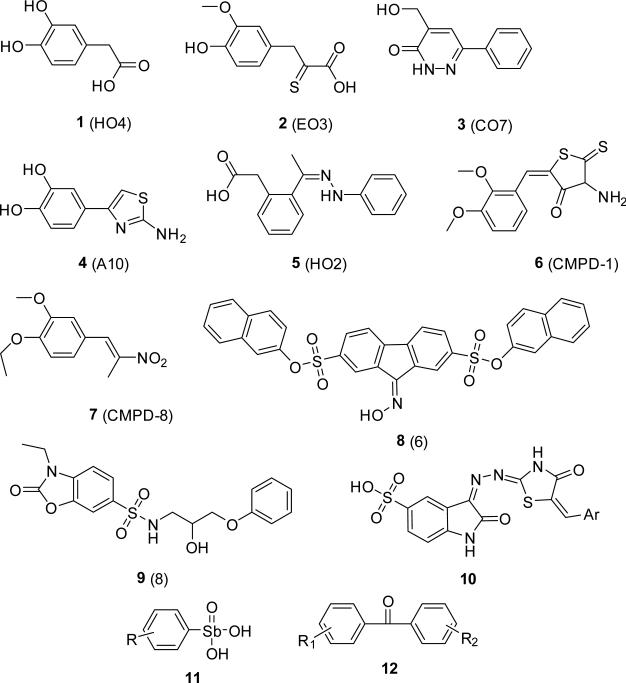
Subsequently a fluorescence resonance energy transfer (FRET) based assay, exploiting the cTAR DNA stem-loop melting property of NC was developed for identifying inhibitors of NC-mediated DNA chaperoning. To establish the assay, cTAR was labeled with 6-carboxyrhodamine and 4-(4’- dimethylaminophenylazo)benzoic acid on the 5’ and 3’ ends. In the closed form of cTAR, the proximity of the two labels quenches the fluorescence signal. However, on addition of functional NC(12-55) peptide, the DNA partly melts, separating the labels and causing a large increase in the fluorescence signal (Shvadchak et al., 2009). High-throughput screening of 4,800 molecules by this assay followed by mass-spectrometry and fluorescence anisotropy assays resulted in identification of 5 positive compounds, 1-5, (Fig 4) with the inhibition constant values in micromolar range (Ki=8.5-15 μM). However, the cellular antiviral activity of these compounds was not tested (Shvadchak et al., 2009). The screening assay did not preferentially select for non-zinc ejectors; nevertheless, all 5 identified molecules inhibited NC–DNA binding without removing zinc from the NC zinc fingers. Four of the five compounds identified were substituted phenyl or pyridazine derivatives with at least one hydroxyl substitution similar to the previously identified gallein derivatives with two hydroxyls, but unlike the gallein derivatives, these molecules could displace bound DNA from NC. Docking-based molecular modeling studies used to predict the binding mode of these molecules suggest π-π interaction with and binding in the lipophilic pocket flanking the side chain of Trp37, which is an important residue for nucleic acid binding. The receptor used for docking was developed by optimizing the NMR-based solution structures of NC-nucleic acids complexes available in the PDB through a combination of density functional theory and molecular dynamics simulations. Based on the binding mode obtained for guanine, the new models were found to be better suited for docking studies than the original NMR structures (Mori et al., 2011). Subsequent virtual screening efforts using the optimized structure coordinates of NC, followed by mass spectrometry and cell based assays, led to the identification of two non-zinc ejecting inhibitors 8, 9 (Fig 4). These aromatic molecules are predicted to stack against Trp37, while the sulfonate/sulfonamide hydrogen bond with the neighboring Lys47 or Arg32 residues (Mori et al., 2012).
Figure 4.
Non-covalent, non-zinc ejecting zinc finger binders. The designation of the compounds in the original report is given in parentheses. R=aliphatic group(s), Ar=aryl group
Through the use of an in vitro capsid assembly assay employing a recombinant capsid-p2-NC protein immobilized on a TG rich DNA oligonucleotide, Goudreau and colleagues identified highly conjugated imino-oxindole derivatives as inhibitors of NC-DNA interaction, compound 10(Fig 4) (Goudreau et al., 2013). NMR-based solution structure of the inhibitor-NC complex revealed that both zinc fingers bound one molecule each, resulting in a 2:1 ligand:NC stoichiometry. The oxindole rings of the ligands stack against the Phe16 and Trp37 of the amino and carboxyl terminal zinc fingers, respectively. Not only do these molecules not eject zinc from the zinc fingers, the presence of intact structured zinc-fingers of NC is essential for their interaction (Goudreau et al., 2013).
Breuer and colleagues recently utilized a fluorescence polarization assay to identify inhibitors, which disrupted p2-NC-nucleic acid interaction. By using 6-carboxyfluorescein labeled SL2-DNA (bases corresponding to SL2 RNA) as the tracer, 101 compounds from a commercial library of 14,400 compounds were identified which disrupted the p2-NC-SL2 DNA interaction (Breuer et al., 2012). Further evaluation by differential scanning fluorimetry identified compounds that bound to NC and enzymatic assays were employed to remove promiscuous NC and Gag binders. Biological evaluation of the remaining compounds for antiviral activity and the lack of cellular toxicity identified two compounds, 6, 7 (Fig 4), with cellular EC50s in the low micromolar range (0.3-3.5 μM). Furthermore, these compounds were found to dislodge pre-bound DNA from the NC protein. It is noteworthy that these molecules are structurally similar to the independently identified non-zinc ejectors, imino- oxindole derivatives (Goudreau et al., 2013) and the sulfonate/sulfonamides (Mori et al., 2011). In all cases, the aromatic ring is conjugated with an electro deficient heterocycle or an electron-withdrawing group. Through the use of a Gag cytometric bead array assay (Breuer et al., 2011), it was demonstrated that unlike the zinc ejectors, the identified NC inhibitors did not interfere with protease mediated processing of Gag (Breuer et al., 2012).
Arylstibonic acids, 11, and diphenyls, 12, compounds (Fig 4) constitute another series of NC-DNA interaction inhibitors that were identified by high-throughput screening (Shoemaker et al., 2009). These compounds, identified in a competition assay, are able to dislodge bound DNA from NC as well as from Gag proteins. However, since arylstibonic acids are known to bind basic residues in other DNA binding proteins (Heyerdahl et al., 2010; Zhao et al., 2012), it is plausible that these compounds interact preferentially with the positively charged residues in the flanking regions of the zinc fingers. The activity of these compounds in infected cells has not been elucidated.
Methylated oligonucleotides
GU rich methylated oligoribonucleotides (mONs) have been shown to target nucleocapsid, which alters DNA chaperoning and/or viral DNA synthesis resulting in inhibition of viral infection and replication. mONs appear to act at the reverse transcription stage in the viral life cycle, since non-nucleoside reverse transcriptase inhibitor related resistance mutations, L100I, K103N in reverse transcriptase and R32K, M46I in NC, result in resistance to inhibitory mONs (Avilov et al., 2012; Grigorov et al., 2011).
While the covalent zinc ejectors have a long history of development and have been tested for viral specificity and antiviral activity in non-human primate studies, the non-covalent inhibitors are a relatively new class of inhibitors. The non-covalent NC inhibitors have advantages over the covalent zinc ejectors in that they do not destroy the structure of the zinc fingers and appear to be less cytotoxic. However, before consideration for continued development of this class of compounds, their mechanism of actions, viral-specificity over endogenous cellular zinc fingers, cellular antiviral activity, and cytotoxicity must be carefully evaluated.
3. RNA binders
Research efforts in the field of targeting nucleic acids have increased, with RNA being recognized as an emerging drug target (Aboul-ela, 2010; Foloppe et al., 2006; McKnight and Heinz, 2003). In HIV genomic RNA, the highly conserved, functionally important and structured 5’ untranslated region (5’UTR), provides a potential target for drug development. The 5’UTR consists of a transactivation responsive element (TAR), poly A tract, primer-binding site (PBS) and packaging domain. The packaging domain folds into four stem loop motifs (SL1-SL4) containing a dimerization initiation site (DIS), a splice donor site (SD), a packaging signal (Psi) and the AUG initiation codon respectively (Reyes-Darias et al., 2012; Sleiman et al., 2012). This domain functions as molecular switch to control viral activities, such as packaging, splicing and translation. The various structural motifs in 5’UTR interact within themselves and with various viral proteins such as NC, matrix, tat, reverse transcriptase and host transcription factors; each of these viral proteins presents a potential drug target (Al-Harthi and Roebuck, 1998; Turner et al., 2006a). NC is known to interact with the TAR and SL1-SL3 domains in the viral 5’UTR.
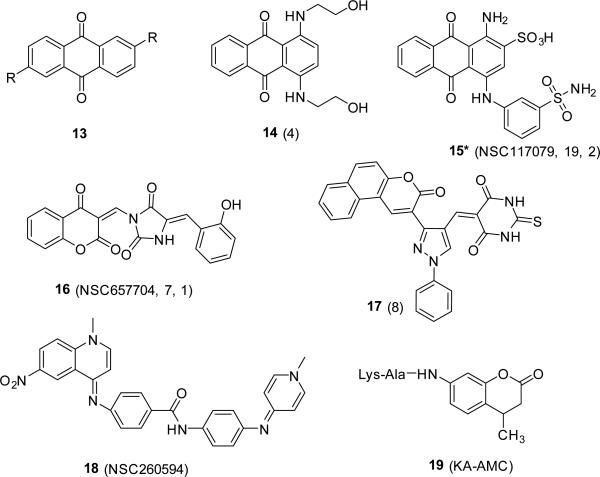
TAR has been extensively studied and targeted for its interaction with the tat protein, which is necessary for viral transcription (Richter and Palu, 2006). The interaction of TAR with NC, plays a critical role in the minus strand transfer during reverse transcription. NC reduces the melting temperature of the stable stem-loops of TAR and its complementary DNA cTAR, reducing the energy barrier for the formation of the extended heteroduplex (Bernacchi et al., 2002; Urbaneja et al., 2002). Compounds that bind to TAR can interfere with this important NC interaction, contributing to an antiviral effect. Indeed, a recent study characterizing the effect of positively charged anthraquinone derivatives, compound 13 (Fig 5), on TAR and cTAR melting and hybridization in presence of NC demonstrates that targeting these sequences inhibits the NC chaperoning function in vitro (Sosic et al., 2013).
Figure 5.
Nucleic acid binders. The designation of the compounds in the original report is given in parentheses. R=aliphatic group. *The identity of compound 15 was not established.
Interaction of NC with viral genomic RNA mediates NC dimerization and packaging of the genomic RNA into the virus particles (Lu et al., 2011; Webb et al., 2013). A continuing strategy has been to identify peptides, oligonucleotides, or compounds that disrupt the interaction of viral RNA to NC. Some of the initial efforts to identify agents that disrupted viral RNA binding to NC, and thus functioned as antivirals, resulted in the identification of peptides (Dietz et al., 2008; Park et al., 2004; Pustowka et al., 2003), cyclic peptides (Druillennec et al., 1999), antisense oligonucleotides (Elmen et al., 2004) and aminoglycosides (Turner et al., 2006b). More recently, virtual and high-throughput screening identified 4 compounds, 14-17 (Fig 5), which bound to SL3 (the packaging signal Psi) and inhibited SL3-NC complex formation (Warui and Baranger, 2009; Warui and Baranger, 2012). The SL3 Kds of compounds that disrupted SL3-NC complex formation ranged from 1-68 μM. Other SL3 binders with similar Kds were identified, but could not inhibit the SL3-NC complex formation. Thus, these findings suggest that the compounds bind to a specific site on the SL3 that interacts with NC. One possibility could be the GGAG tetraloop of SL3, Fig 1 (De Guzman et al., 1998), which is required for NC interaction. The structural composition of compound 15, the most efficacious of the compounds identified, could not be confirmed, thus bringing into question the chemical structure of the compound that was the most active. Curiously, the compounds identified in the screen that were selective for the SL3 RNA, rather than a double stranded or single stranded RNA control, were not effective in disrupting SL3-NC complex formation. Rather, compound 15 (Fig 5), which recognized multiple RNA species, was the best inhibitor (Warui and Baranger, 2012).
A FRET based SL3 RNA melting assay, similar to the cTAR DNA melting assay (Shvadchak et al., 2009) where the 3’ and 5’ ends of the oligonucleotide are labeled with a TET fluorophore and a black hole quencher (BHQ1), was used by Bell et al. to identify a SL3-Gag interaction to identify compound 18 (Fig 5) (Bell et al., 2013). Using NMR studies, compound 18 was shown to interact with the tetraloop of the RNA, which is the region that is required for NC binding. Unlike other reports that used NC or zinc-finger containing peptides for screening, Bell and colleagues relied on the GagΔP6 protein. They had shown earlier that NC is sufficient to bind SL3 RNA, however, the chaperoning effect is significant only in the presence of the flanking domains of the Gag polyprotein (Bell et al., 2012). Bell and colleagues hypothesized that the specific ability of Gag polyprotein, rather than mature NC, could effect structural changes in SL3 which may aid in genomic viral RNA packaging into the virus particle though avoiding binding by prematurely cleaved NC (Bell et al., 2013). This is in contrast with the significant chaperoning of NC alone on the TAR/cTAR oligonucleotides (Godet and Mely, 2010; Levin et al., 2010; Shvadchak et al., 2009; Sosic et al., 2013). Unlike SL3, the TAR/cTAR chaperoning is required during reverse transcription, which requires mature NC after cell infection. Taken together, these independent reports might indicate that NC has evolved to accommodate specific nucleic acid sequences and to chaperone them “time selectively” depending on the viral life cycle stage.
In their pursuit to identify compounds that bind to SL1 and inhibit SL1-NC interaction, Chung and colleagues have identified a peptide-based coumarin derivative compound, 19 (Fig 5), that binds the SL1 stem-bulge region, which appears to mimic the chaperoning effect of NC for SL1 (Chung et al., 2010). Although the identified compound is much less efficient at chaperoning than NC, the unique compounds and identification strategy opens a new avenue for identifying compounds that compete for NC and bind viral RNA. Since the time and rate of NC mediated chaperoning is essential for viral survival, NC mimetics would be predicted to disturb the rhythm of the viral life cycle, therefore effectively interrupting viral spread.
4. Perspective and future directions
During the past 7 years there has been an increasing number of publications on the identification of novel inhibitors of NC function. In some part, this has to do with the fundamental role NC plays in HIV replication that reinforces the validity of NC as a potential antiviral target. NC is a multifunctional protein necessary for binding and chaperoning nucleic acids at various stages of the viral life cycle. It functions when present in the Gag polyprotein as well as a mature, separated protein. The highly conserved nature of amino acids that compose NC, which are found across all viral clades, suggests that it maybe be less tolerant to inhibitor escape mutations than other HIV proteins. Furthermore, engineering mutations in NC have confirmed the strict requirement for the inherent amino acids to be maintained to provide function. However, there are structural and biological caveats that must be considered for antiviral targeting of NC, which are the high structural flexibility of NC and presence of zinc fingers. Furthermore, compounds that target NC zinc finger structures could well cross-react with cellular zinc fingers, promoting cellular toxicity.
A second change in the field that has resulted in the reemergence of NC as a potential therapeutic target are the novel methods for screening for inhibitors of NC function, improvements in structural and biophysical methodology and the availability of diverse chemical libraries, all of which were touched on in the review. The compounds that have been reported thus far to disrupt NC function, as discussed in the review, reaffirm that the field is rapidly moving forward. Moreover, there appears to be a focus on fine-tuning antiviral efficacy, while decreasing the cellular toxicity of the compounds identified. We anticipate the identification of compounds with high-affinity NC binding and potent antiviral efficacy. Those in the NC field (we included!) would like to lay claim, as our raison d'être for selecting NC for antiviral targeting is the structural and functional characteristics of NC that potentially may result in it being less tolerant to inhibitor escape mutations. However, this concept has not been carefully evaluated as it has been for protease, integrase, and RT inhibitors. If a barrier exists for NC inhibitor escape mutations, what are the viral fitness and NC functional consequences of the viral escape mutants? As NC inhibitors improve in their antiviral efficacy and cellular toxicity profiles, the “best” compounds should be evaluated as to their capacity to generate viral escape mutants.
Although our group has developed and reported on methods to identify inhibitors of NC and Gag function (Breuer et al., 2012; Breuer et al., 2011), we would like to propose an as of yet unexploited strategy to disrupt NC function utilizing small molecules. We posit that it would be possible to chemically target the NC-nucleic acid complex, where the compound acts as a NC-RNA complex stabilizer, unlike the NC inhibitors identified thus far, which were discussed in the review. From the various solution structures of apo- and complexed-NC and RNAs that have been solved so far, it is evident that the complex is better structured than either molecule alone (Fig 1). Furthermore, since a viral nucleic acid-NC complex would be required for compound binding, off-target effects may be lessened. Like NC ‘functional mimetics’, a nucleic acid-NC complex stabilizer would alter the efficiency and rate of NC chaperoning, effectively stalling replication and reducing viral production and spread. Furthermore, given the requirements for the exacting NC and viral RNA interaction, we would argue that inhibitor escape mutations in NC or the viral RNA may generate a less fit virus. However, this notion must be directly evaluated.
In summary, during the past 7 the HIV nucleocapsid protein and assembly field has made significant advancements. This current edition of Virus Research attests to this point by providing selected and cutting edge reviews of the field presented at the 9th International Retroviral Nucleocapsid Protein and Assembly Symposium. What we find exciting are the insights into retroviral nucleocapsid protein function and assembly resulting from innovative research. Moreover, the advancements in the NC field have led to novel methods for identifying small molecules that disrupt nucleocapsid protein function. In turn, small molecules identified from the chemical biology methodology should allow for further interrogation of viral function and may provide therapeutic leads.
Highlights.
The nucleocapsid protein is required for the HIV life cycle.
The nucleocapsid protein is conserved among all viral clades.
Functional disruption of nucleocapsid may be an effective antiviral strategy.
The biology and requirements for nucleocapsid in the HIV life cycle are discussed.
Small molecule targeting strategies to disrupt nucleocapsid function are highlighted.
Acknowledgments
Funding: Research reported in this publication was supported by the NIGMS of the National Institutes of Health under the award number: P50GM103368 (B.E.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This is internal publication MEM #27005 from The Scripps Research Institute.
Abbreviations
- 5′UTR
5′ Untranslated Region
- FRET
Fluorescence Resonance Energy Transfer
- NC
Nucleocapsid
- PDB
Protein Data Bank
- RT
Reverse Transcriptase
- TAR
Transactivation Responsive Element
- SL1-SL4
Stem Loop Motif1-Stem Loop Motif4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: No conflicts of interest.
Author Contributions: D.G. and B.E.T. conceived the primary idea and wrote and edited the review
REFERENCES
- Aboul-ela F. Strategies for the design of RNA-binding small molecules. Future Med. Chem. 2010;2(1):93–119. doi: 10.4155/fmc.09.149. [DOI] [PubMed] [Google Scholar]
- Al-Harthi L, Roebuck KA. Human immunodeficiency virus type-1 transcription: role of the 5′-untranslated leader region (review). Int. J. Mol. Med. 1998;1(5):875–881. doi: 10.3892/ijmm.1.5.875. [DOI] [PubMed] [Google Scholar]
- Amarasinghe GK, De Guzman RN, Turner RB, Chancellor KJ, Wu ZR, Summers MF. NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the psi-RNA packaging signal. Implications for genome recognition. J. Mol. Biol. 2000;301(2):491–511. doi: 10.1006/jmbi.2000.3979. [DOI] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5(1):196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- Appella D, Appella E, Inman JK, L.M.M. J, Hayashi R, Wang D. Thioether prodrug compositions as anti-HIV and anti retroviral agents. 2013 U.S.A. Patent No. 20130096092.
- Avilov SV, Boudier C, Gottikh M, Darlix JL, Mely Y. Characterization of the inhibition mechanism of HIV-1 nucleocapsid protein chaperone activities by methylated oligoribonucleotides. Antimicrob. Agents Chemother. 2012;56(2):1010–1018. doi: 10.1128/AAC.05614-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi A, Zargarian L, Chaminade F, Boudier C, De Rocquigny H, Rene B, Mely Y, Fosse P, Mauffret O. Structural insights into the cTAR DNA recognition by the HIV-1 nucleocapsid protein: role of sugar deoxyriboses in the binding polarity of NC. Nucleic Acids Res. 2011;39(9):3903–3916. doi: 10.1093/nar/gkq1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell NM, Kenyon JC, Balasubramanian S, Lever AM. Comparative structural effects of HIV-1 Gag and nucleocapsid proteins in binding to and unwinding of the viral RNA packaging signal. Biochemistry. 2012;51(15):3162–3169. doi: 10.1021/bi2017969. [DOI] [PubMed] [Google Scholar]
- Bell NM, L'Hernault A, Murat P, Richards JE, Lever AM, Balasubramanian S. Targeting RNA-Protein Interactions within the Human Immunodeficiency Virus Type 1 Lifecycle. Biochemistry. 2013;52(51):9269–9274. doi: 10.1021/bi401270d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi S, Stoylov S, Piémont E, Ficheux D, Roques BP, Darlix JL, Mély Y. HIV-1 nucleocapsid protein activates transient melting of least stable parts of the secondary structure of TAR and its complementary sequence. J. Mol. Biol. 2002;317(3):385–399. doi: 10.1006/jmbi.2002.5429. [DOI] [PubMed] [Google Scholar]
- Bombarda E, Morellet N, Cherradi H, Spiess B, Bouaziz S, Grell E, Roques BP, Mély Y. Determination of the pKa of the four Zn2+-coordinating residues of the distal finger motif of the HIV-1 nucleocapsid protein: Consequences on the binding of Zn2+. J. Mol. Biol. 2001;310(3):659–672. doi: 10.1006/jmbi.2001.4770. [DOI] [PubMed] [Google Scholar]
- Bombarda E, Roques BP, Mely Y, Grell E. Mechanism of zinc coordination by point-mutated structures of the distal CCHC binding motif of the HIV-1 NCp7 protein. Biochemistry. 2005;44(19):7315–7325. doi: 10.1021/bi047349+. [DOI] [PubMed] [Google Scholar]
- Bourbigot S, Ramalanjaona N, Boudier C, Salgado GF, Roques BP, Mely Y, Bouaziz S, Morellet N. How the HIV-1 nucleocapsid protein binds and destabilises the (−)primer binding site during reverse transcription. J. Mol. Biol. 2008;383(5):1112–1128. doi: 10.1016/j.jmb.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Breuer S, Chang MW, Yuan J, Torbett BE. Identification of HIV-1 inhibitors targeting the nucleocapsid protein. J. Med. Chem. 2012;55(11):4968–4977. doi: 10.1021/jm201442t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer S, Sepulveda H, Chen Y, Trotter J, Torbett BE. A cleavage enzyme-cytometric bead array provides biochemical profiling of resistance mutations in HIV-1 Gag and protease. Biochemistry. 2011;50(20):4371–4381. doi: 10.1021/bi200031m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Ulyanov NB, Guilbert C, Mujeeb A, James TL. Binding characteristics of small molecules that mimic nucleocapsid protein-induced maturation of stem-loop 1 of HIV-1 RNA. Biochemistry. 2010;49(30):6341–6351. doi: 10.1021/bi100660r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix JL, Garrido JL, Morellet N, Mely Y, de Rocquigny H. Properties, functions, and drug targeting of the multifunctional nucleocapsid protein of the human immunodeficiency virus. Adv. Pharmacol. 2007;55:299–346. doi: 10.1016/S1054-3589(07)55009-X. [DOI] [PubMed] [Google Scholar]
- Darlix JL, Godet J, Ivanyi-Nagy R, Fosse P, Mauffret O, Mely Y. Flexible nature and specific functions of the HIV-1 nucleocapsid protein. J. Mol. Biol. 2011;410(4):565–581. doi: 10.1016/j.jmb.2011.03.037. [DOI] [PubMed] [Google Scholar]
- De Guzman RN, Wu ZR, Stalling CC, Pappalardo L, Borer PN, Summers MF. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science. 1998;279(5349):384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- de Rocquigny H, Shvadchak V, Avilov S, Dong CZ, Dietrich U, Darlix JL, Mely Y. Targeting the viral nucleocapsid protein in anti-HIV-1 therapy. Mini Rev. Med. Chem. 2008;8(1):24–35. doi: 10.2174/138955708783331603. [DOI] [PubMed] [Google Scholar]
- Dietz J, Koch J, Kaur A, Raja C, Stein S, Grez M, Pustowka A, Mensch S, Ferner J, Moller L, Bannert N, Tampe R, Divita G, Mely Y, Schwalbe H, Dietrich U. Inhibition of HIV-1 by a peptide ligand of the genomic RNA packaging signal Psi. ChemMedChem. 2008;3(5):749–755. doi: 10.1002/cmdc.200700194. [DOI] [PubMed] [Google Scholar]
- Druillennec S, Dong CZ, Escaich S, Gresh N, Bousseau A, Roques BP, Fournie-Zaluski MC. A mimic of HIV-1 nucleocapsid protein impairs reverse transcription and displays antiviral activity. Proc. Natl. Acad. Sci. U.S. A. 1999;96(9):4886–4891. doi: 10.1073/pnas.96.9.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmen J, Zhang HY, Zuber B, Ljungberg K, Wahren B, Wahlestedt C, Liang Z. Locked nucleic acid containing antisense oligonucleotides enhance inhibition of HIV-1 genome dimerization and inhibit virus replication. FEBS Lett. 2004;578(3):285–290. doi: 10.1016/j.febslet.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foloppe N, Matassova N, Aboul-Ela F. Towards the discovery of drug-like RNA ligands? Drug Discov. Today. 2006;11(21-22):1019–1027. doi: 10.1016/j.drudis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Godet J, Boudier C, Humbert N, Ivanyi-Nagy R, Darlix JL, Mely Y. Comparative nucleic acid chaperone properties of the nucleocapsid protein NCp7 and Tat protein of HIV-1. Virus Res. 2012;169(2):349–360. doi: 10.1016/j.virusres.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet J, Kenfack C, Przybilla F, Richert L, Duportail G, Mely Y. Site-selective probing of cTAR destabilization highlights the necessary plasticity of the HIV-1 nucleocapsid protein to chaperone the first strand transfer. Nucleic Acids Res. 2013;41(9):5036–5048. doi: 10.1093/nar/gkt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet J, Mely Y. Biophysical studies of the nucleic acid chaperone properties of the HIV-1 nucleocapsid protein. RNA Biol. 2010;7(6):687–699. doi: 10.4161/rna.7.6.13616. [DOI] [PubMed] [Google Scholar]
- Goebel FD, Hemmer R, Schmit JC, Bogner JR, de Clercq E, Witvrouw M, Pannecouque C, Valeyev R, Vandevelde M, Margery H, Tassignon JP. Phase I/II dose escalation and randomized withdrawal study with add-on azodicarbonamide in patients failing on current antiretroviral therapy. AIDS. 2001;15(1):33–45. doi: 10.1097/00002030-200101050-00007. [DOI] [PubMed] [Google Scholar]
- Goel A, Mazur SJ, Fattah RJ, Hartman TL, Turpin JA, Huang M, Rice WG, Appella E, Inman JK. Benzamide-Based Thiolcarbamates: A New Class of HIV-1 NCp7 Inhibitors. Bioorg. Med. Chem. Lett. 2002;12(5):767–770. doi: 10.1016/s0960-894x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Goudreau N, Hucke O, Faucher AM, Grand-Maitre C, Lepage O, Bonneau PR, Mason SW, Titolo S. Discovery and structural characterization of a new inhibitor series of HIV-1 nucleocapsid function. NMR solution structure determination of a ternary complex involving a 2:1 inhibitor/NC stoichiometry. J. Mol. Biol. 2013;425(11):1982–1998. doi: 10.1016/j.jmb.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Grigorov B, Bocquin A, Gabus C, Avilov S, Mely Y, Agopian A, Divita G, Gottikh M, Witvrouw M, Darlix JL. Identification of a methylated oligoribonucleotide as a potent inhibitor of HIV-1 reverse transcription complex. Nucleic Acids Res. 2011;39(13):5586–5596. doi: 10.1093/nar/gkr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorov B, Decimo D, Smagulova F, Pechoux C, Mougel M, Muriaux D, Darlix JL. Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers. Retrovirology. 2007;4:54. doi: 10.1186/1742-4690-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyerdahl SL, Rozenberg J, Jamtgaard L, Rishi V, Varticovski L, Akah K, Scudiero D, Shoemaker RH, Karpova TS, Day RN, McNally JG, Vinson C. The arylstibonic acid compound NSC13746 disrupts B-ZIP binding to DNA in living cells. Eur. J. Cell Biol. 2010;89(7):564–573. doi: 10.1016/j.ejcb.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Morichaud Z, Didierlaurent L, Muriaux D, Darlix JL, Mougel M. Nucleocapsid mutations turn HIV-1 into a DNA-containing virus. Nucleic Acids Res. 2008;36(7):2311–2319. doi: 10.1093/nar/gkn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Maynard A, Turpin JA, Graham L, Janini GM, Covell DG, Rice WG. Anti-HIV agents that selectively target retroviral nucleocapsid protein zinc fingers without affecting cellular zinc finger proteins. J. Med. Chem. 1998;41(9):1371–1381. doi: 10.1021/jm9708543. [DOI] [PubMed] [Google Scholar]
- Inman JK, Goel A, Appella E, Turpin J, Schito M. Acylthiols and component thiol compositions as anti-HIV and anti-retroviral agents. 2009 U.S.A. Patent No. 20090247473.
- Jenkins LMM, Hara T, Durell SR, Hayashi R, Inman JK, Piquemal JP, Gresh N, Appella E. Specificity of acyl transfer from 2-mercaptobenzamide thioesters to the HIV-1 nucleocapsid protein. J. Am. Chem. Soc. 2007;129(36):11067–11078. doi: 10.1021/ja071254o. [DOI] [PubMed] [Google Scholar]
- Jenkins LMM, Ott DE, Hayashi R, Coren LV, Wang D, Xu Q, Schito ML, Inman JK, Appella DH, Appella E. Small-molecule inactivation of HIV-1 NCp7 by repetitive intracellular acyl transfer. Nat. Chem. Biol. 2010;6(12):887–889. doi: 10.1038/nchembio.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BM, De Guzman RN, Turner BG, Tjandra N, Summers MF. Dynamical behavior of the HIV-1 nucleocapsid protein. J. Mol. Biol. 1998;279(3):633–649. doi: 10.1006/jmbi.1998.1766. [DOI] [PubMed] [Google Scholar]
- Levin JG, Mitra M, Mascarenhas A, Musier-Forsyth K. Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription. RNA Biol. 2010;7(6):754–774. doi: 10.4161/rna.7.6.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lium EK, Silverstein S. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 1997;71(11):8602–8614. doi: 10.1128/jvi.71.11.8602-8614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Heng X, Summers MF. Structural determinants and mechanism of HIV-1 genome packaging. J. Mol. Biol. 2011;410(4):609–633. doi: 10.1016/j.jmb.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight KL, Heinz BA. RNA as a target for developing antivirals. Antivir. Chem. Chemother. 2003;14(2):61–73. doi: 10.1177/095632020301400201. [DOI] [PubMed] [Google Scholar]
- Modrof J, Becker S, Muhlberger E. Ebola virus transcription activator VP30 is a zinc-binding protein. J. Virol. 2003;77(5):3334–3338. doi: 10.1128/JVI.77.5.3334-3338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellet N, Demene H, Teilleux V, Huynh-Dinh T, de Rocquigny H, Fournie-Zaluski MC, Roques BP. Structure of the complex between the HIV-1 nucleocapsid protein NCp7 and the single-stranded pentanucleotide d(ACGCC). J. Mol. Biol. 1998;283(2):419–434. doi: 10.1006/jmbi.1998.2098. [DOI] [PubMed] [Google Scholar]
- Mori M, Manetti F, Botta M. Predicting the Binding Mode of Known NCp7 Inhibitors To Facilitate the Design of Novel Modulators. J. Chem. Inf. Mod. 2011;51(2):446–454. doi: 10.1021/ci100393m. [DOI] [PubMed] [Google Scholar]
- Mori M, Schult-Dietrich P, Szafarowicz B, Humbert N, Debaene F, Sanglier-Cianferani S, Dietrich U, Mely Y, Botta M. Use of virtual screening for discovering antiretroviral compounds interacting with the HIV-1 nucleocapsid protein. Virus Res. 2012;169(2):377–387. doi: 10.1016/j.virusres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Muriaux D, Darlix JL. Properties and functions of the nucleocapsid protein in virus assembly. RNA Biol. 2010;7(6):744–753. doi: 10.4161/rna.7.6.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannecouque C, Szafarowicz B, Volkova N, Bakulev V, Dehaen W, Mely Y, Daelemans D. Inhibition of HIV-1 replication by a bis-thiadiazolbenzene-1,2-diamine that chelates zinc ions from retroviral nucleocapsid zinc fingers. Antimicrob. Agents Chemother. 2010;54(4):1461–1468. doi: 10.1128/AAC.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MY, Kwon J, Lee S, You J, Myung H. Selection and characterization of peptides specifically binding to HIV-1 psi (psi) RNA. Virus Res. 2004;106(1):77–81. doi: 10.1016/j.virusres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Pustowka A, Dietz J, Ferner J, Baumann M, Landersz M, Konigs C, Schwalbe H, Dietrich U. Identification of peptide ligands for target RNA structures derived from the HIV-1 packaging signal psi by screening phage-displayed peptide libraries. Chembiochem. 2003;4(10):1093–1097. doi: 10.1002/cbic.200300681. [DOI] [PubMed] [Google Scholar]
- Reyes-Darias JA, Sanchez-Luque FJ, Berzal-Herranz A. HIV RNA dimerisation interference by antisense oligonucleotides targeted to the 5′ UTR structural elements. Virus Res. 2012;169(1):63–71. doi: 10.1016/j.virusres.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Rice WG, Baker DC, Schaeffer CA, Graham L, Bu M, Terpening S, Clanton D, Schultz R, Bader JP, Buckheit RW, Jr., Field L, Singh PK, Turpin JA. Inhibition of multiple phases of human immunodeficiency virus type 1 replication by a dithiane compound that attacks the conserved zinc fingers of retroviral nucleocapsid proteins. Antimicrob. Agents Chemother. 1997a;41(2):419–426. doi: 10.1128/aac.41.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WG, Schaeffer CA, Harten B, Villinger F, South TL, Summers MF, Henderson LE, Bess JW, Jr., Arthur LO, McDougal JS, et al. Inhibition of HIV-1 infectivity by zinc-ejecting aromatic C-nitroso compounds. Nature. 1993;361(6411):473–475. doi: 10.1038/361473a0. [DOI] [PubMed] [Google Scholar]
- Rice WG, Supko JG, Malspeis L, Buckheit RW, Jr., Clanton D, Bu M, Graham L, Schaeffer CA, Turpin JA, Domagala J, Gogliotti R, Bader JP, Halliday SM, Coren L, Sowder RC, 2nd, Arthur LO, Henderson LE. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science. 1995;270(5239):1194–1197. doi: 10.1126/science.270.5239.1194. [DOI] [PubMed] [Google Scholar]
- Rice WG, Turpin JA, Huang M, Clanton D, Buckheit RW, Jr., Covell DG, Wallqvist A, McDonnell NB, DeGuzman RN, Summers MF, Zalkow L, Bader JP, Haugwitz RD, Sausville EA. Azodicarbonamide inhibits HIV-1 replication by targeting the nucleocapsid protein. Nat. Med. 1997b;3(3):341–345. doi: 10.1038/nm0397-341. [DOI] [PubMed] [Google Scholar]
- Richter SN, Palu G. Inhibitors of HIV-1 Tat-mediated transactivation. Curr. Med. Chem. 2006;13(11):1305–1315. doi: 10.2174/092986706776872989. [DOI] [PubMed] [Google Scholar]
- Sette P, Dussupt V, Bouamr F. Identification of the HIV-1 NC binding interface in Alix Bro1 reveals a role for RNA. J. Virol. 2012;86(21):11608–11615. doi: 10.1128/JVI.01260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RH, Currens M, Rein A, Feng Y, Yuan H, Fisher R, Stephen A, Worthy K, Sei S, Crise B, Henderson LE. Identification of anti-HIV compounds inhibiting virus assembly and binding of nucleocapsid protein to nucleic acid. 2009 U.S.A. Patent No. 7572828.
- Shvadchak V, Sanglier S, Rocle S, Villa P, Haiech J, Hibert M, Van Dorsselaer A, Mely Y, de Rocquigny H. Identification by high throughput screening of small compounds inhibiting the nucleic acid destabilization activity of the HIV-1 nucleocapsid protein. Biochimie. 2009;91(7):916–923. doi: 10.1016/j.biochi.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Sleiman D, Goldschmidt V, Barraud P, Marquet R, Paillart JC, Tisne C. Initiation of HIV-1 reverse transcription and functional role of nucleocapsid-mediated tRNA/viral genome interactions. Virus Res. 2012;169(2):324–339. doi: 10.1016/j.virusres.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Sosic A, Frecentese F, Perissutti E, Sinigaglia L, Santagada V, Caliendo G, Magli E, Ciano A, Zagotto G, Parolin C, Gatto B. Design, synthesis and biological evaluation of TAR and cTAR binders as HIV-1 nucleocapsid inhibitors. MedChemComm. 2013;4(10):1388–1393. [Google Scholar]
- Srivastava P, Schito M, Fattah RJ, Hara T, Hartman T, Buckheit RW, Jr., Turpin JA, Inman JK, Appella E. Optimization of unique, uncharged thioesters as inhibitors of HIV replication. Bioorg. Med. Chem. 2004;12(24):6437–6450. doi: 10.1016/j.bmc.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Summers MF, Henderson LE, Chance MR, Bess JW, Jr., South TL, Blake PR, Sagi I, Perez-Alvarado G, Sowder RC, 3rd, Hare DR, et al. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992;1(5):563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JJ, Fabani M, Arzumanov AA, Ivanova G, Gait MJ. Targeting the HIV-1 RNA leader sequence with synthetic oligonucleotides and siRNA: chemistry and cell delivery. Biochim. Biophys. Acta. 2006a;1758(3):290–300. doi: 10.1016/j.bbamem.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Turner KB, Hagan NA, Fabris D. Inhibitory effects of archetypical nucleic acid ligands on the interactions of HIV-1 nucleocapsid protein with elements of Psi-RNA. Nucleic Acids Res. 2006b;34(5):1305–1316. doi: 10.1093/nar/gkl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin JA, Song Y, Inman JK, Huang M, Wallqvist A, Maynard A, Covell DG, Rice WG, Appella E. Synthesis and biological properties of novel pyridinioalkanoyl thiolesters (PATE) as anti-HIV-1 agents that target the viral nucleocapsid protein zinc fingers. J. Med. Chem. 1999;42(1):67–86. doi: 10.1021/jm9802517. [DOI] [PubMed] [Google Scholar]
- Urbaneja M.a.A., Wu M, Casas-Finet JR, Karpel RL. HIV-1 Nucleocapsid Protein as a Nucleic Acid Chaperone: Spectroscopic Study of its Helix-destabilizing Properties, Structural Binding Specificity, and Annealing Activity. J. Mol. Biol. 2002;318(3):749–764. doi: 10.1016/S0022-2836(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Vercruysse T, Basta B, Dehaen W, Humbert N, Balzarini J, Debaene F, Sanglier-Cianferani S, Pannecouque C, Mely Y, Daelemans D. A phenyl-thiadiazolylidene-amine derivative ejects zinc from retroviral nucleocapsid zinc fingers and inactivates HIV virions. Retrovirology. 2012;9:95. doi: 10.1186/1742-4690-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GS, Cheng-Mayer C, Schito ML, Fletcher P, Jenkins LMM, Hayashi R, Neurath AR, Appella E, Shattock RJ. Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection. J. Virol. 2009;83(18):9175–9182. doi: 10.1128/JVI.00820-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warui DM, Baranger AM. Identification of specific small molecule ligands for stem loop 3 ribonucleic acid of the packaging signal Psi of human immunodeficiency virus-1. J. Med. Chem. 2009;52(17):5462–5473. doi: 10.1021/jm900599v. [DOI] [PubMed] [Google Scholar]
- Warui DM, Baranger AM. Identification of small molecule inhibitors of the HIV-1 nucleocapsid-stem-loop 3 RNA complex. J. Med. Chem. 2012;55(9):4132–4141. doi: 10.1021/jm2007694. [DOI] [PubMed] [Google Scholar]
- Webb JA, Jones CP, Parent LJ, Rouzina I, Musier-Forsyth K. Distinct binding interactions of HIV-1 Gag to Psi and non-Psi RNAs: implications for viral genomic RNA packaging. RNA. 2013;19(8):1078–1088. doi: 10.1261/rna.038869.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvrouw M, Balzarini J, Pannecouque C, Jhaumeer-Laulloo S, Este JA, Schols D, Cherepanov P, Schmit JC, Debyser Z, Vandamme AM, Desmyter J, Ramadas SR, de Clercq E. SRR-SB3, a disulfide-containing macrolide that inhibits a late stage of the replicative cycle of human immunodeficiency virus. Antimicrob. Agents Chemother. 1997;41(2):262–268. doi: 10.1128/aac.41.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Mitra M, McCauley MJ, Thomas JA, Rouzina I, Musier-Forsyth K, Williams MC, Gorelick RJ. Aromatic residue mutations reveal direct correlation between HIV-1 nucleocapsid protein's nucleic acid chaperone activity and retroviral replication. Virus Res. 2013a;171(2):263–277. doi: 10.1016/j.virusres.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Mitra M, Naufer MN, McCauley MJ, Gorelick RJ, Rouzina I, Musier-Forsyth K, Williams MC. Differential contribution of basic residues to HIV-1 nucleocapsid protein's nucleic acid chaperone function and retroviral replication. Nucleic Acids Res. 2013b;42(4):2525–2537. doi: 10.1093/nar/gkt1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Stagno JR, Varticovski L, Nimako E, Rishi V, McKinnon K, Akee R, Shoemaker RH, Ji X, Vinson C. P6981, an arylstibonic acid, is a novel low nanomolar inhibitor of cAMP response element-binding protein binding to DNA. Mol. Pharmacol. 2012;82(5):814–823. doi: 10.1124/mol.112.080820. [DOI] [PMC free article] [PubMed] [Google Scholar]