Summary
Aim
The aim of the present in vitro study was the evaluation of two products: a CPP-ACP paste (GC Tooth Mousse, GC Corp.) and a desensitizing toothpaste (Colgate Sensitive Pro Relief, Colgate-Palmolive) on preventing enamel erosion produced by a soft drink (Coca Cola) by using Atomic Force Microscopy (AFM).
Methods
Thirty enamel specimens were assigned to 6 groups of 5 specimens each. 1: intact enamel, 2: enamel + soft drink, 3: intact enamel + Colgate Sensitive Pro Relief, 4: enamel + soft drink + Colgate Sensitive Pro Relief, 5: intact enamel + GC Tooth Mousse, 6: enamel + soft drink + GC Tooth Mousse. The surface of each specimen was imaged by AFM. The root mean-square roughness (Rrms) was obtained from the AFM images and the differences in the averaged values among the groups were analyzed by ANOVA test.
Results
Comparing groups 4 and 6 (soft drink + toothpastes) with group 2 (eroded enamel) a statistical difference (P<0.05) was registered, suggesting effectiveness in protecting enamel against erosion of the products investigated.
Conclusions
The use of new formulation toothpastes can prevent enamel demineralization.
Keywords: AFM, enamel, remineralization, SEM, soft drinks, surface roughness
Introduction
Dental erosion has been defined commonly as the chemical dissolution of the hard tissues of teeth by acids of non-microbiological origin (1). Dental erosion is a common problem in modern societies, owing to the increased consumption of acid drinks such as soft drinks, sport drinks, fruit juices (2). The development of erosion involves a chemical process in which the inorganic phase of the tooth is demineralized, thereby reducing the hardness of the tooth substrates. Subsequent abrasive challenges through brushing increase the loss of the tooth substrates (3). The early stages of dental erosion are characterized by a softening of the enamel surface to a depth of the order of 1–10 microns. Many studies have been carried out to understand the process of enamel demineralization at the early stages, but there are still little known about if these early stages are reversible (4). Biological and chemical factors in the oral environment influence the progress of dental erosion. Saliva provides protective effects by neutralizing and clearing the acids; it is also a source of inorganic ions necessary for the remineralization process (5). Enamel has no spontaneous capability to repair when affected by specific dental pathologies such as caries, abrasions or fractures because it contains no cells (6). Therefore, when enamel is exposed to oral environment the only possibility to be reconstructed depends on the application of alloplastic materials. Toothpastes have been considered effective and accessible vehicles to improve enamel resistance to erosive attacks (7). The incorporation of protecting agents in toothpastes has become increasingly more common, because sensitivity is a common complaint among patients. Many types of toothpaste recently introduced are claimed to prevent erosion. Fluoride dentifrices have some protective effect on enamel eroded to brushing abrasion when immersed in vitro in a cola drink (8). Currently, conventional fluoride-containing toothpastes do not appear to be able to protect efficiently against erosion (9). Colgate Sensitive Pro Relief (Colgate-Palmolive, New York, NY, USA) is based on arginine 8% (Pro-Argin™). Pro-Argin ™ technology contains arginine, an amino acid naturally found in saliva, and a compound of insoluble calcium in the form of calcium carbonate. Arginine and calcium carbonate bind to the dentin-enamel negatively charged surface, creating a protective layer, resistant to acid stimuli, that covers the surface defects and occludes the exposed dentinal tubules with a consequently reduced sensitivity (10). GC Tooth Mousse (GC Corp., Tokio, Japan) is based on CPP-ACP. Casein phosphopeptides (CPP) containing the cluster sequence – Ser(P)-Ser(P)- Ser(P)-Glu-Glu – have a remarkable ability to stabilize calcium phosphate (ACP) in metastable solution. Through the multiple phosphoseryl residues, the CPP binds to forming nanoclusters of ACP, preventing their growth to the critical size required for nucleation and phase transformation (11). CPP-ACP has been demonstrated to have anticariogenic activity in laboratory, animal and human in situ experiments (12). The CPPACP solutions have shown to significantly remineralize enamel subsurface lesions in vitro (13). CPP-ACP has been successfully incorporated into oral health products such as a mouth rinses, chewing gums and sports drink to reduce enamel erosion (14). Changes in tooth structure due to extrinsic factors have been widely investigated. Whereas the material losses (absolute erosions) have been carefully characterized, only minor attention has been devoted to the investigation of tooth surface change during erosion and demineralization (15). The kinetic roughening of a surface is the process that takes place when material is removed from or added to the surface. Theoretical and experimental studies showed that this process might be often interpreted in terms of self-affinity concepts and simple scaling laws. However, whereas many works have been devoted to the investigation of film growth by chemical or physical vapor deposition methods, less attention has been reserved to the inverse problem of surface etching, polishing, or erosion (16). Atomic Force Microscopy (AFM) is capable of giving images with atomic resolution with minimal sample preparation. This technique has been widely used to characterize the erosion of enamel and dentine (17). More recently, also AFM nanoindentation has been applied to the study of enamel erosion (18). The aim of the present in vitro study was the evaluation of a CPP-ACP paste and of a desensitizing toothpaste (Colgate Sensitive Pro Relief, Colgate-Palmolive) on preventing enamel erosion produced by a soft drink (Coca Cola) by using Atomic Force Microscopy (AFM).
Materials and methods
Specimens’ preparation
Specimens were prepared from 30 human incisors free of caries and defects, extracted for periodontal reasons. After the extraction, the teeth were cleansed of soft tissue debris and inspected for cracks, hypoplasia and white spot lesions; they were disinfected in 5.25% sodium hypochlorite solution for one hour and stored in artificial saliva (pH 7.0, 14.4 mM NaCl; 16.1 mM KCl; 0.3m mM Cl2.6H20; 2.9 mM K2HPO4; 1.0 mM CaCl2.2H2O; 0.10 g/100 ml sodium carboxymethylcellulose) during the whole experimentation (19). The specimens were cut at the enameldentin junction, with a high-speed diamond rotary bur with a water-air spray. The labial surfaces near the enamel dentin junction were ground using silicon carbide papers (grades 600 to 1200) under water irrigation to produce flat enamel surfaces. Samples were placed into Teflon moulds measuring 10 × 8 × 2 mm, embedded in flowable composite resin and polymerized. The baseline root mean-square roughness, Rrms, was measured for all the specimens before starting experimentation. No statistical difference in Rrms values (150±5) was recorded, suggesting that the specimens may be comparable.
Demineralization and remineralization
A soft drink (Coca Cola, Coca Cola Company, Milano, Italy) was chosen for the demineralization process. The pH at 20°C, buffering capacity, concentration of calcium and phosphate of the beverage were measured (20). Measurements were performed in triplicate and average values calculated. Two remineralizating agents were used: GC Tooth Mousse and, as reference, Colgate Sensitive Pro Relief, a desensitizing toothpaste. The samples were then assigned to 6 groups, each made of 5 teeth:
- group 1: intact enamel
- group 2: enamel + soft drink
- group 3: enamel + Colgate Sensitive Pro Relief
- group 4: enamel + soft drink + Colgate Sensitive Pro Relief
- group 5: enamel + GC Tooth Mousse.
- group 6: enamel + soft drink + GC Tooth Mousse.
The control specimens (group 1) were taken on storage for the whole experimentation and they did not receive any treatment. The specimens of groups 2, 4 and 6 were immersed in 6mL of the soft drink for 2 min at room temperature before rinsing with deionized water. Four consecutive intervals of the immersion procedure were carried out at 0, 8, 24 and 36 h for a total of 8 minutes (21). The toothpastes were applied neat onto the surface of the specimens of groups 3, 4, 5 and 6 without brushing for 3 min at 0, 8, 24 and 36 h and then wiped off with distilled water washing. In groups 4 and 6 the toothpastes were applied after demineralization with Coca Cola.
Atomic Force Microscopy (AFM) observations
After 24 hours from the last procedure of demineralization and remineralization (15), specimens were observed with an atomic force microscopy AutoProbe CP 100 (Thermomicroscopes, Veeco, Plainview, NY, USA), equipped with a piezoelectric scanner, which can cover an area of 100 × 100 μm2 with a range of 7 μm in the z-direction. The most common topographical parameters were determined, such as the surface roughness (Rrms). Rrms is given by the standard deviation of the heights, obtained from the AFM images by testing, for each sample, at least 10 different film areas of 30 × 30 μm2 with a resolution of 256 × 256 pixels. From the analyses of the AFM height profiles, it was also possible to estimate the erosion cavities depth of the enamel surface. The data were obtained by averaging on at least 20 selected lines of the image. Measurements were performed on the treatment specimens and on the matching controls.
Statistical analysis
Differences in the averaged values among the groups were analyzed by ANOVA test. Statistical difference was set at P<0.05. Post hoc Bonferroni test was performed to assess the differences between the different groups.
Results
The surface roughness (Rrms) is an index of the surface quality. The mean Rrms values recorded before experimentation was not statistically different when compared with the mean Rrms value of group 1 (intact enamel) after immersion in artificial saliva. Table 1 reports mean Rrms values with related standard deviations obtained in the six groups. Comparing untreated specimens (group 1) with demineralized specimens (group 2), a statistically significant difference (P<0.05) in Rrms values was registered, with an increase of surface roughness passing from intact enamel to enamel exposed to Coca Cola. A significant difference (P < 0.05) was registered when comparing the Rrms values of group 3 (enamel treated with Colgate Sensitive Pro Relief) with intact enamel (group 1). Significant higher Rrms values are registered with GC Tooth Mousse (P < 0.05). Comparing the Rrms values of groups 4 and 6 (soft drink + toothpastes) with group 2 (enamel only demineralized) a statistical difference (P<0.05) was registered, suggesting a protective effect of the toothpastes investigated against enamel demineralization.
Table 1.
Mean roughness values (Rrms) ± standard deviation obtained in the six groups. Different superscript letters means significant differences between the groups (P < 0.05).
| Groups | Mean roughness values (Rrms) |
|---|---|
| group 1: intact enamel | 148 ± 19a |
| group 2: enamel + soft drink | 261 ± 28b |
| group 3: intact enamel + Colgate Sensitive Pro Relief | 59 ± 5c |
| group 4: enamel + soft drink + Colgate Sensitive Pro Relief | 93 ± 7d |
| group 5: intact enamel + GC Tooth Mousse | 155 ± 14a |
| group 6: enamel + soft drink + GC Tooth Mousse | 167 ± 12a |
Discussion and conclusion
In the present in vitro study, AFM was used to verify the protective effect of a CPP-ACP paste on enamel exposed to the erosive action of a soft-drink. AFM was used to study tooth surfaces in order to compare the pattern of particle distribution in the outermost layer of the tooth surfaces (19). It was found that AFM gives high-contrast, high-resolution images and is an important tool as a source of new structural information: tapping mode AFM (TM-AFM) images are able to show net differences between exposed and unexposed enamel areas (4). There is a clear relationship between erosion and temperature of the beverages (2). In this study, the beverage was kept at a constant temperature of 20° C. Although erosion proceeds more slowly in vivo than in vitro owing to the protective effect of saliva and acquired pellicle, the effect of temperature can be expected to be significant (22). In order to stress their demineralizing potential, the soft drink was replenished every 2 min to ensure that it was carbonated and to reduce the buffering effect from ions dissolved from the enamel surface (21). Enamel surface is often aprismatic and more highly mineralized than enamel subsurface. This prismless enamel arises at the end of amelogenesis. Although this layer of enamel is more frequent on the surface of deciduous teeth, it can also be found on the surface of permanent teeth. It is known that the prism-free enamel is gradually worn off during mastication but it is retained in protected areas. Flat and polished specimens were used in the present study in an attempt to standardize specimens and remove natural variations in surface enamel between teeth and between different tooth sites and types, which may result in different responses to acid dissolution (1). However, it should be noted that natural tooth surfaces erode more slowly than polished surfaces (22). The specimens were cleaned with 5% NaOCl for 1 hour, which could not alter enamel surface. The erosive potential of orange juice modified by food additives in enamel and dentine was evaluated; it was concluded that only the combination of calcium lactate pentahydrate and sodium linear polyphosphate reduced erosion (23).
CPP-ACP clusters seem to be anticariogenic in laboratory animal in situ experiments (24) and on human caries in vitro (13). A CPP-ACP crème was effective in remineralizing early enamel lesions of the primary teeth (25), while recently a CCP-ACP paste was found to be effective in promoting enamel remineralization (20). Finally CPP-ACP complex has been successfully added to chewing-gums: the efficacy of three commercially available sugar-free chewing gums was compared, concluding that the superior remineralization activity of the gum was attributed to the presence of casein phosphopeptide-amorphous calcium phosphate nanocomplexes (CCP-ACP) (26). Que et al., (27) study demonstrated that the new Pro-Argin formula toothpaste provided a significant reduction in dentin hypersensitivity. The reduced sensitivity recorded after a single application can be maintained over time with daily use of the product for a period of 24 weeks (28). In addition the desensitizing pastes based on argine and calcium carbonate have proven to be useful in reducing dentinal sensitivity if applied before a session of professional oral hygiene (29).
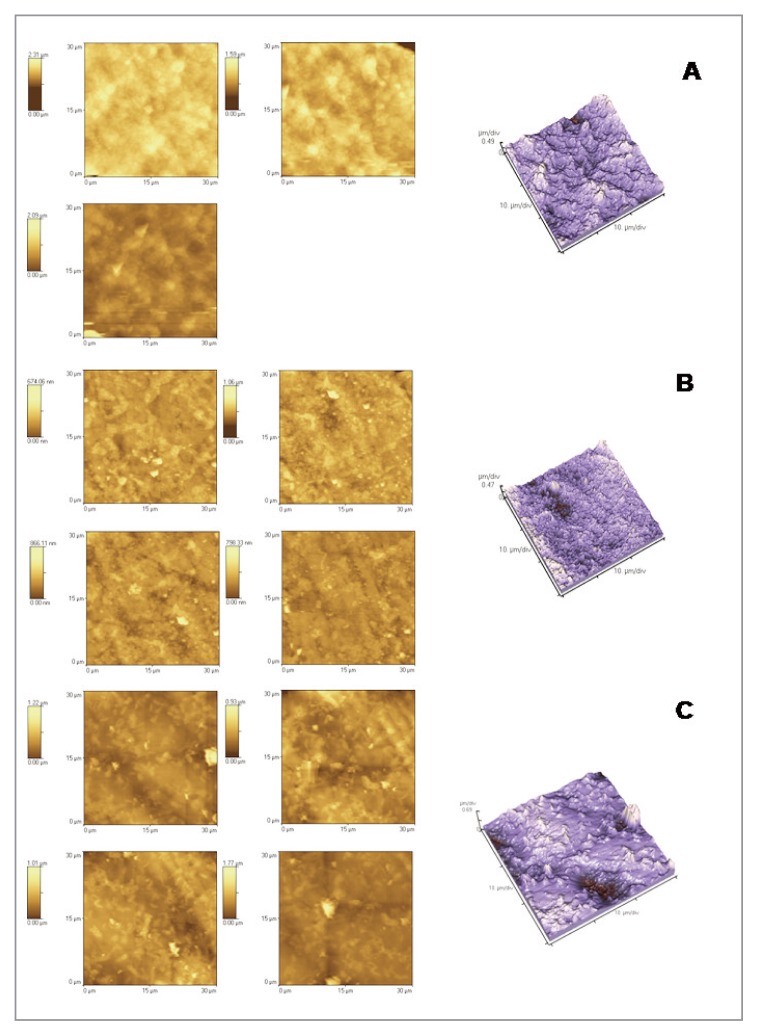
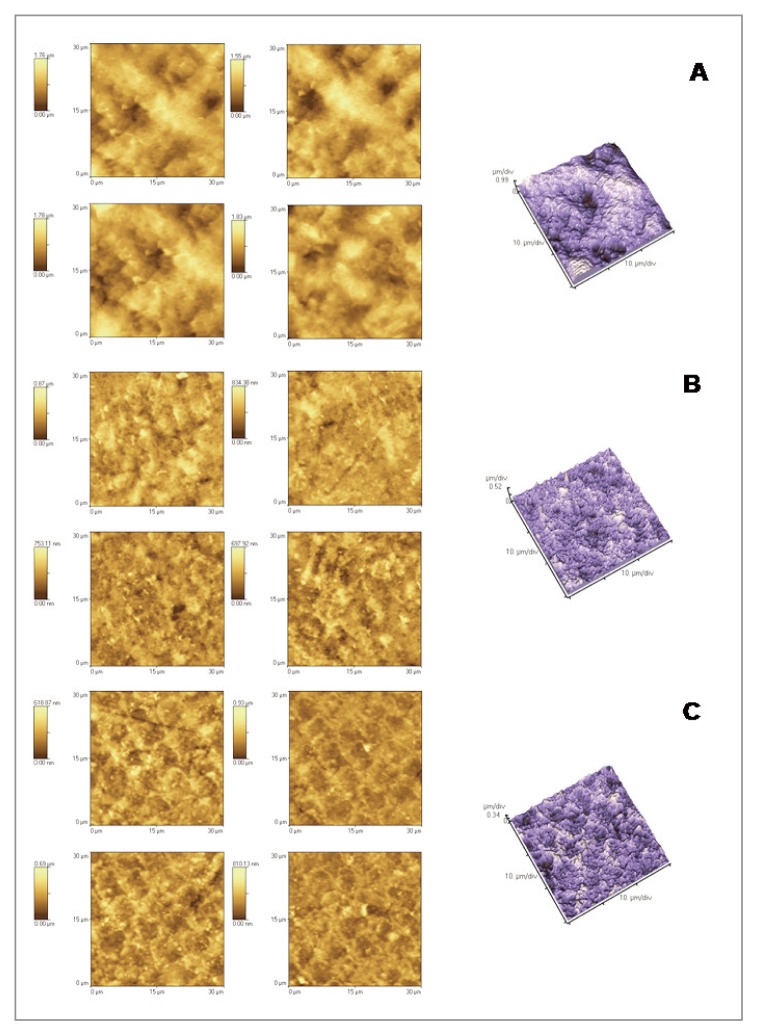
The Atomic Force Microscopy could analyze the topographical aspects of enamel samples and the effects of the demineralization/remineralization processes on the enamel morphology, in the presence of the studied toothpastes. The most common topographical parameter was therefore determined, such as the surface roughness (Rrms), to quantitatively evaluate the surface aspect. After demineralization with an acidic substance such as Coca Cola, the surface should appear much rougher. The process of erosion causes, in fact, an increase of Rrms. The demineralizing process was investigated by comparing the fraction of intact enamel (Fig. 1) with the one treated with the soft drink (Fig. 2). After the demineralization process, cavities of diameter between 4 and 7 microns were formed. This is reflected in the values of Rrms, which increased from 148 to 261 nm. As regards the effect of the toothpastes in the process of enamel remineralization, several considerations can be made. Comparing the Rrms values of groups 3 (enamel + Colgate Sensitive Pro Relief) with intact enamel (group 1) a statistical significant (P < 0.05) decrease of surface roughness suggests a remineralizating effect on intact enamel of the product used. In groups 3, in fact, enamel shows the typical aprismatic surface layer, on which it is possible to identify the precipitated crystals of the remineralizating agents (Fig. 1). Instead significant higher Rrms values are registered in group 5 (enamel + GC Tooth Mousse) (P < 0.05). However, in group 5, AFM images showed a random distribution along the surface of the protective agent to form globular aggregates and/or precipitates of mineral substances, more or less abundant (as seen in the lighter areas of images). This may justify roughness values, significantly higher than those recorded for the other product tested (Fig. 1). Comparing the Rrms values of groups 4 and 6 (enamel + soft drink + toothpastes) with group 2 (eroded enamel) a statistical difference (P<0.05) was registered, suggesting a remineralizing power on eroded enamel of the two products. In group 4 the prismatic structure of hydroxyapatite, which typically becomes evident as a result of erosion, was not observed. Therefore Colgate Sensitive Pro Relief seems to able to regenerate a homogeneous, very compact, thick and uniform surface layer (Fig. 2). As regards the effects of GC Tooth Mousse on eroded enamel (group 6), the prismatic structure is slightly evident but the cavities seems to be well-filled. In this case the superficial layer of the remineralizing agent is probably constituted by a globular arrangement of the mineral substances (Fig. 2).
Figure 1.
2D and 3D AFM images: intact enamel (A) and enamel treated with Colgate Sensitive Pro Relief (B) and with GC Tooth Mousse (C).
Figure 2.
2D and 3D AFM images: enamel exposed to soft drink (A) and enamel demineralised and then remineralised with Colgate Sensitive Pro Relief (B) and with GC Tooth Mousse (C).
Under the limitations of the present in vitro study, the application of the tested toothpastes can be considered effective on preventing enamel erosion produced by a soft drink.
Acknowledgments
Nothing to declare.
Footnotes
Conflict of interest statement
The authors of this study have no conflict of interest to disclose.
References
- 1.Hemingway CA, Parker DM, Addy M, Barbour ME. Erosion of enamel by non-carbonated soft drinks with and without toothbrushing abrasion. Br Dent J. 2006;201:447–50. doi: 10.1038/sj.bdj.4814073. [DOI] [PubMed] [Google Scholar]
- 2.Barbour ME, Finke M, Parker DM, Hughes JA, Allen GC, Addy M. The relationship between enamel softening and erosion caused by soft drinks at a range of temperatures. J Dent. 2007;34:207–13. doi: 10.1016/j.jdent.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Lippert F, Parker DM, Jandt KD. Toothbrush abrasion of surface softened enamel studied with tapping mode AFM and AFM nanoindentaion. Caries Res. 2004;38:464–72. doi: 10.1159/000079628. [DOI] [PubMed] [Google Scholar]
- 4.Finke M, Jandt KM, Parker DM. The early stages of native enamel dissolution studied with atomic force microscopy. J Colloid Interface Sci. 2000;232:156–64. doi: 10.1006/jcis.2000.7200. [DOI] [PubMed] [Google Scholar]
- 5.Lussi A, Hellwig E, Zero D, Jaeggi T. Erosive tooth wear: diagnosis, risk factors and prevention. Am J Dent. 2006;19:319–25. [PubMed] [Google Scholar]
- 6.Oshiro M, Yamaguchi K, Takamizawa T, Inage H, et al. Effect of CPP-ACP paste on tooth mineralization: an FE-SEM study. J Oral Sci. 2007;49:115–20. doi: 10.2334/josnusd.49.115. [DOI] [PubMed] [Google Scholar]
- 7.Kato MT, Lancia M, Sales-Peres SH, Buzalaf MA. Preventive effect of commercial desensitizing toothpastes on bovine enamel erosion in vitro. Caries Res. 2010;44:85–9. doi: 10.1159/000282668. [DOI] [PubMed] [Google Scholar]
- 8.Ganss C, Schulze K, Schlueter N. Toothpaste and erosion. Monogr Oral Sci. 2013;23:88–99. doi: 10.1159/000350475. [DOI] [PubMed] [Google Scholar]
- 9.Moron BM, Miyazaki SS, Ito N, Wiegand A, Vilhena F, Buzalaf MA, Magalhães AC. Impact of different fluoride concentrations and pH of dentifrices on tooth erosion/abrasion in vitro. Aust Dent J. 2013;23:106–11. doi: 10.1111/adj.12016. [DOI] [PubMed] [Google Scholar]
- 10.Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent. 2009;22 Pro-Argin™ Special Issue. [PubMed] [Google Scholar]
- 11.Reynolds EC. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: a review. Spec Care Dent. 1998;18:8–16. doi: 10.1111/j.1754-4505.1998.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 12.Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–70. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 14.Adebayo OA, Burrow MF, Tyas MJ. An SEM evaluation of conditioned and bonded enamel following carbamide peroxide bleaching and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) treatment. J Dent. 2007;37:297–306. doi: 10.1016/j.jdent.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Bertassoni LE, Habelitz S, Pugach M, et al. Evaluation of surface structural and mechanical changes following remineralization of dentin. Scanning. 2010;32:312–19. doi: 10.1002/sca.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour ME, Rees JS. The laboratory assessment of enamel erosion: a review. J Dent. 2004;32:591–602. doi: 10.1016/j.jdent.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.De-Deus G, Paciornik S, Pinho Mauricio MH, Prioli R. Realtime atomic force microscopy of root dentin during demineralization when subjected to chelating agents. Int Endod J. 2000;39:683–92. doi: 10.1111/j.1365-2591.2006.01128.x. [DOI] [PubMed] [Google Scholar]
- 18.Lippert F, Parker DM, Jandt KD. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J Colloid Interface Sci. 2004;280:442–48. doi: 10.1016/j.jcis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Farina M, Schemmel A, Weissmuller G, Cruz R, Kachar B, Bisch PM. Atomic force microscopy study of tooth sufaces. J Struct Biol. 1999;125:39–49. doi: 10.1006/jsbi.1998.4069. [DOI] [PubMed] [Google Scholar]
- 20.Poggio C, Lombardini M, Vigorelli P, Ceci M. Analysis of Dentin/Enamel Remineralization by a CPP-ACP Paste: AFM and SEM Study. Scanning. 2013 doi: 10.1002/sca.21077. in press. [DOI] [PubMed] [Google Scholar]
- 21.Tantbirojin D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. J Dent. 2008;36:74–79. doi: 10.1016/j.jdent.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Ranjitkar S, Rodriguez JM, Kaidonis JA, Richards LC, Townsend GC, Bartlett DW. The effect of casein phosphopeptide-amorphous calcium phosphate on erosive enamel and dentin wear by toothbrush abrasion. J Dent. 2009;37:250–54. doi: 10.1016/j.jdent.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Scaramucci T, Sobrai MA, Eckert GJ, et al. In situ evaluation of the erosive potential of orange juice modified by food additives. Caries Res. 2012;46:55–61. doi: 10.1159/000335572. [DOI] [PubMed] [Google Scholar]
- 24.Santosh BP, Jethmalani P, Shashibushan KK, Subba VV. Effect of casein phosphopeptide amorphous calcium phosphate containing chewing gum on salivary concentration of calcium and phosphorus: an in vivo study. J Indian Soc Pedod Prev Dent. 2012;30:146–150. doi: 10.4103/0970-4388.99990. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G, Zou J, Yang R, Zhou X. Remineralization effects of casein phosphopeptide-amorphous calcium phosphate crème on artificial early enamel lesions of primary teeth. Int J Paediatr Dent. 2011;21:374–81. doi: 10.1111/j.1365-263X.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Manton DJ, Walker GD, Fai C. Remineralization of enamel subsurface lesions in situ by the use of three commercially available sugar-free gums. Int J Paediatr Dent. 2008;18:284–90. doi: 10.1111/j.1365-263X.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 27.Que K, Fu Y, Hu D, et al. Dentin hypersensitivity reduction of a new toothpaste containing 8% arginine and 1450 ppm fluoride an 8-week clinical study on Chinese adults. Am J Dent. 2010;23:28–35. [PubMed] [Google Scholar]
- 28.Hamlin D, Mateo LR, Dibart S, Delgado E, Devizio W. Comparative efficacy of two treatment regimens combining in-office and at-home programs for dentin hypersensitivity relief: a 24-week clinical study. Am J Dent. 2012;25:146–152. [PubMed] [Google Scholar]
- 29.Hamlin D, Phelan Williams K, Delgado E, Zhang YP, Devizio W, Mateo LR. Clinical evaluation of the efficacy of a desensitizing paste containing 8% arginine and calcium carbonate for the in-office relief of dentin hypersensitivity associated with dental prophylaxis. Am J Dent. 2009;22 Pro-Argin™ Special Issue. [PubMed] [Google Scholar]