Abstract
The purpose of this study was to determine if intake of the antioxidants coenzyme Q10 (CoQ10) or α-tocopherol (Toc), either alone or in combination, could ameliorate cognitive and psychomotor impairments of aged mice, as well as reduce oxidative burden in tissues. For a period of 10 weeks, male C57BL/6J mice (3 or 18 months) were fed either a control diet, or one of three diets supplemented with Toc, CoQ10 or their combination, and were tested for cognitive and psychomotor function. Old mice on the Toc or Toc/CoQ10 diets showed improved coordinated running performance. Mice on the diet containing Toc/CoQ10 demonstrated improved performance in the discriminated avoidance task. CoQ10 and Toc alone also resulted in improved performance, albeit to a lesser degree. Protein damage was decreased especially when the mice received Toc + CoQ10 combination. Overall, these results suggest that, Toc and CoQ supplementation can ameliorate age-related impairment and reduce protein oxidation. Moreover, concurrent supplementation of CoQ10 and Toc may be more effective than either antioxidant alone.
Keywords: Behavior, antioxidants and mitochondria
1. Introduction
Age-related losses of cognitive and psychomotor performance are closely associated with oxidative stress and molecular damage within regions of the brain critical to those functions (Forster et al. 2000; Nicolle et al. 2001; Serrano and Klann 2004). The link between oxidative damage and brain aging suggested that experimental interventions attenuating oxidative stress could reverse age-related brain dysfunction, yet rodent studies of antioxidant supplementation have provided inconsistent support for this prediction (Shukitt-Hale et al. 1999; Sumien et al. 2004; Carney et al. 1991; Shetty et al. 2012). Moreover, both human and animal studies have suggested that supplementation of a single antioxidant can generate negative outcomes (Sumien et al. 2004; Kristal et al. 2014; Saremi and Arora 2010).
Interestingly, antioxidant mixtures or antioxidant-rich foods appear to produce more consistent improvements in brain function when implemented during senescence. Senescent dogs supplemented with an antioxidant mixture exhibited improved learning after only 14 days of –treatment (Milgram et al. 2002). More recently, aged beagles given a medical food cocktail for 3 months had improved spatial attention and reduced motivation deficits (Head et al. 2012). Furthermore, studies of two- and three-way combinations of specific antioxidants also suggest improved effectiveness. For example, supplementation with vitamins E, C and PBN in combination resulted in improved cognitive performance of aged rats (Socci et al. 1995), and in aged mice, supplementation with α-tocopheryl acetate and coenzyme Q10 in combination was determined to improve learning of an active avoidance response (McDonald et al. 2005). Lipoic acid and acetyl-L-carnitine were more effective than either of the two compounds alone in reducing oxidative stress, restoring mitochondrial function and preventing age-associated decline in spatial memory (Hagen et al. 2002; Liu et al. 2002). Similarly, vitamins E and C in combination significantly improved the performance of old mice in a passive avoidance task (Arzi et al. 2004). Additionally, clinical studies have indicated that antioxidants may be beneficial in certain neurodegenerative diseases. The combination of vitamins E and C delayed the onset of need for L-dopa treatment when administered to patients with Parkinson’s disease (Buhmann et al. 2004). Patients with mild to moderate dementia receiving supplementation with vitamins C and E and α-lipoic acid for 16-weeks had a significant reduction in F2-isoprostane levels in the cerebrospinal fluid and a significant improvement in their cognitive abilities relative to placebo (Galasko et al. 2012).
Taken together these studies suggest that combination of antioxidants allows for additive and perhaps synergistic interactions among the antioxidants affording a more balanced protection against reactive oxygen species and reducing the probability of deleterious outcomes. Based on in vivo and in vitro data suggesting a sparing/regenerative interaction between vitamin E and coenzyme Q10, the combination of these two compounds is expected to lead to an additive interaction. Lipid peroxidation reactions can be stopped by vitamin E, which acts as a primary donor of hydrogen and gets in turn oxidized to tocopheroxyl radical. Ubiquinol reduces the tocopheroxyl radical back to tocopherol and in turn forms ubisemiquinone. This latter form will then be reduced back to ubiquinol by reducing equivalents supplied by the electron transport chain (Wang and Quinn 1999). Because reduced coenzyme Q10 regenerates α-tocopherol from the tocopheroxyl radical in the mitochondria, it plays a significant role in modulating oxidant production (Stoyanovsky et al. 1995; Kagan et al. 1998; Lass and Sohal 1998). Supplementation of α-tocopherol, in the absence of commensurate amounts of coenzyme Q, may limit the maximum protective effect achieved and could possibly result in excess tocopheroxyl radicals, resulting in deleterious effects.
Three separate studies were performed addressing the ability of α-tocopherol (Toc) and coenzyme Q10 (CoQ) to concurrently (i) modify neurobehavioral deficits in old mice, (ii) attenuate oxidative stress/damage, and (iii) to assess the effect of formulation on experimental outcomes. These studies are detailed below and summarized in Table 1.
Table 1.
Summary of route of administration, duration of treatment and number of mice used for each study
| Study | Route | Pre-treatment prior to behavioral testing |
Length of behavioral testing |
Total time on antioxidant supplementation |
N |
|---|---|---|---|---|---|
| 1 | Gavage | N/A | N/A | 3 weeks | 35 |
| 2 | Diet | 3 weeks | 6 weeks | 10 weeks | 119 |
| 3 | Diet | 3 weeks | 10 weeks | 13 weeks | 43 |
2. Materials and Methods
2.1. Animals and treatment
Study 1
This preliminary study was done to determine whether a short-term intervention (3 weeks) can reverse accumulation of protein oxidation in various brain regions of old mice. A total of 35 C57BL/6 J male mice were obtained from the National Institute on Aging at 20 months of age, and were subsequently maintained in the UNT Health Science Center vivarium. The mice were housed in groups of 3 or 4 in clear polycarbonate cages (28 × 17 × 12.5 cm), and had ad libitum access to food and water. The ambient temperature was maintained at 23 ± 1 °C, under a 12-h light/dark cycle starting at 0600. The animals were allowed to acclimate for a two-week period, following which they were randomly assigned to one of the four treatment groups: vehicle (436 mg/kg/d γ-cyclodextrin (γ-CD)), Toc (250 mg/kg/d α-tocopheryl acetate + vehicle), CoQ (109 mg/kg/d CoQ10 + vehicle) or Toc+CoQ (250 mg/kg/d α-tocopheryl acetate + 109 mg/kg/d CoQ10 + vehicle). A group of 4-month-old mice receiving only the vehicle was used as a young control group. The mice were gavaged daily between 5 and 6pm with their respective treatments for a period of 3 weeks after which they were euthanized and protein oxidative damage (protein carbonyls) was measured in brain region homogenates (cerebral cortex, hippocampus, striatum, cerebellum, midbrain and hindbrain).
Study 2
This study was designed to assess the effect of CoQ, Toc or their combination on reversing age-related motor and cognitive dysfunction.
Separate groups of male C57BL/6J were obtained from the National Institute on Aging at 3 or 18 months of age (N=119) and subsequently maintained in the UNT Health Science Center vivarium (By 3 months of age diet changes can be implemented without affecting developmental stages of the mice, and by the end of the study the mice were still young to detect age differences with the old group. By 18 months, motor and cognitive declines are already present and necessary for studying the reversal power of the intervention used in this study). The mice were housed in groups of 3 or 4 in clear polycarbonate cages (28 × 17 × 12.5 cm), and had ad libitum access to food and water except during the testing sessions. The ambient temperature was maintained at 23 ± 1 °C, under a 12-h light/dark cycle starting at 0600. The animals were allowed to acclimate for a two-week period, following which they were randomly assigned to one of four treatment groups. The mice were weighed weekly, and survival was monitored throughout the study. Food and water intake was measured daily during the week prior to behavioral testing. The mice were maintained on the supplemented diets throughout the period of testing and, upon completion, were euthanized for collection of tissues and preparation of mitochondria.
The mice were fed, ad libitum, either a control diet (NIH-31), or one of three diets supplemented with antioxidants alone or in combination [D- α-tocopheryl acetate (Toc), 1.65 mg/g diet; coenzyme Q10 (CoQ), 0.72 mg/g diet, or Toc + CoQ in the same concentrations]. The diets were formulated by Purina Mills Test Diets (Richmond, IN, Cat. Nos. 1810691, 1810688, 1810689, and 1810690 respectively). Each of the diets, including the control diet, contained 0.288% γ-cyclodextrin, which was a component of the CoQ10 formulation provided by Tishcon Inc. (Westbury, NY). The formulation of this compound in the diet was expected to enhance the bioavailability of CoQ10 (Chopra and Bhagavan 2006; Bhagavan and Chopra 2007). Based on the food intake measured in the different age groups of the mice, the daily delivered doses were approximately 241 mg/kg body weight for Toc and 108 mg/kg body weight for CoQ10. A previous study determined that Toc supplementation had no effect on behavioral outcomes in young mice; therefore this experimental group was omitted from the current study (Sumien et al. 2004). Only 3 mice died during the study (2 young controls and 1 old Toc + CoQ).
Study 3
A follow-up study was conducted to determine whether γ-cyclodextrin had any effects on any of the behavioral measures used in the antioxidant studies. A total of 43 C57BL/6J male mice age 18.5 months were obtained from NIA and were randomly assigned to either an NIH-31 diet or NIH-31 supplemented with 0.288% γ-cyclodextrin. The mice were on their respective diets for 3 weeks prior to behavioral testing, and throughout the study for a total of 13 weeks.
2.2. Neurobehavioral measures
In study 2 the mice were maintained on the control and antioxidant-supplemented diets for a period of 3 weeks, following which they were subjected to a series of behavioral tests.
Throughout the period of testing the mice remained on their respective diets. The behavioral tests were conducted over a period of ~7 weeks in the following order: locomotor activity, wire suspension, bridge-walking, coordinated running, spatial learning and memory, auditory and shock startle (sensory reactivity), and discriminated avoidance. Brains and tissues of the young and old mice were harvested for biochemical measurements at the end of behavioral testing, when they were 5.5 and 20.5 months, respectively. In study 3 during which behavioral tests were also performed, the mice were also supplemented for 3 weeks prior to the tests. In this study, the same tests were done in the following order: locomotor activity, wire suspension, bridge-walking, spatial learning and memory, coordinated running auditory and shock startle (sensory reactivity), and discriminated avoidance. By the end of the behavioral testing, the mice were ~21.5 months.
2.2. 1. Locomotor activity
Spontaneous locomotor activity was measured using a Digiscan apparatus (Omnitech Electronics, model RXYZCM-16), as described previously (Forster and Lal 1991). Each mouse was placed in a clear acrylic test cage (40.5 × 40.5 × 30.5 cm) that was surrounded by a metal frame lined with photocells. The test cage was enclosed in a dimly-lit, sound-attenuating chamber equipped with a fan that provided background noise (80 dB). During a 16-min period, movements in the horizontal plane, as well as a vertical plane 7.6 cm above the floor, were detected by the photocells and processed by a software program to yield different variables describing distance, vertical, and spatial components of spontaneous activity in the apparatus.
2.2.2. Wire suspension
The mouse was allowed to grip a horizontal wire with the front paws when suspended 27 cm above a padded surface. The latency to tread (reach the wire with their hind legs) and the latency to fall were recorded and averaged over four consecutive daily sessions (2 trials per day).
2.2.3. Bridge walking
Each mouse was tested for the latency to fall or reach a safe platform after being placed on one of four acrylic bridges, each mounted 50 cm above a padded surface. The bridges differed in diameter (small or large) and shape (round or square), providing four levels of difficulty. Each bridge was presented three times, and the measure of performance was the average latency to fall (up to a maximum of 60 s) across all bridges.
2.2.4. Coordinated running
Motor learning and maximum running performance were measured using an accelerating rotorod test described previously (Forster and Lal 1999). The apparatus was a motor-driven treadmill (Accuscan Instruments, Model # AIO411RRT525M) that consisted of a 3-cm diameter nylon cylinder mounted horizontally at a height of 35 cm above a padded surface. The cylinder was separated into four 11 cm wide compartments, formed by 5 white round plastic dividers, each with a radius of 30 cm. The cylinder rotated with an acceleration of 0.5 revolutions per minute (rpm) per second. Acceleration continued until either 75 rpm was reached (in 150s) or the mouse is unable to perform the running response and fell.
In a given trial, the mouse was placed on the cylinder, which then began rotating with increasing speed until the animal fell to a well-padded surface. Ability of the mice to improve running performance was assessed in a series of training sessions (two per day), each consisting of four trials separated by a 10-min interval. The training sessions continued until the running performance (the average latency to fall from the cylinder) failed to show improvement over three consecutive sessions (on average this test required 8–10 sessions (4–5days) for all mice to reach their maximum performance). The treatment groups were compared for their average latency to fall on the first seven sessions, and for the final session on which each mouse had reached its maximum stable level of performance.
2.2.5. Startle response
The musculoskeletal startle reflex to auditory or shock stimuli of various intensities was measured using a standard testing system (SA Lab, San Diego Instruments, San Diego CA). For the auditory startle test, a mouse was placed inside an acrylic cylinder and presented with a series of mixed-frequency noise bursts (0, 90, 100, 110, 120, or 140 dB). Each acoustic signal (lasting 20 ms) was presented 12 times in a counterbalanced series, for a total of 72 trials. For the shock startle test, a mouse was placed inside the same acrylic cylinder, and a series of shocks (0, 0.02, 0.04, 0.08, 0.16, 0.24, 0.32, 0.64 mA) was delivered. Each shock stimulus (100 ms in duration and scrambled across 8 inputs to the grid floor of the acrylic cylinder) was given five times, for a total of 40 trials. The amplitude of the startle reflex was defined as the peak response to each auditory or shock stimulus within a 250-ms time window that began with the stimulus presentation (modified from Sumien et al. 2006).
2.2.6. Morris water maze
Spatial learning and memory were measured using a Morris water maze (MWM) test as described previously (Sumien et al. 2009; Shetty et al. 2012). A computerized tracking system recorded the length of the path taken by the mouse to reach the platform, as well as the swimming speed (San Diego Instruments, San Diego CA, Model # SA-3). During a pre-training phase, the tank was covered by a black curtain to prevent pre-exposure of the mice to visual cues present outside of the tank. In this way, mice learned the motor components of swimming and climbing onto the platform without learning its location in the tank. On each trial, the mouse was placed at one end of a 10 × 65-cm (W × L) straight alley that had a platform at the other end, and allowed to swim until it reached the platform or a maximum latency of 60 s had elapsed. The mice were given four sessions of pre-training (two per day), each consisting of five trials spaced at 5-min intervals.
After pre-training, the black curtain was removed from above the tank, and the mice were tested for their ability to learn the location of the platform using spatial cues. Testing was divided into three phases: acquisition (eight sessions with the platform in a fixed location), retention (two additional sessions after a 66-h delay interval), and reversal (four sessions with the platform at a new, fixed location). Each session consisted of five trials, at 10-min intervals, during which the mouse had to swim to the platform from one of four different starting points in the tank. Two sessions were conducted per day, separated by a period of at least 2 h, during which the mice were returned to the home cages. After the fifth trial of session 8, a probe trial was given in which the platform was submerged to a depth that prevented the mice from climbing onto it. The platform was raised after 30 s, and the trial was ended when the mouse successfully located it. On this trial, spatial bias for the platform location was evaluated in terms of the percentage of time spent within a 40-cm diameter annulus surrounding the platform location.
A criterion was used to confirm that all mice in the study used a spatial strategy for locating the platform position in the tank. According to this inclusion criterion, the mouse had to develop a spatial bias for the platform location within 10 training sessions, as evidenced by at least 1 entry in to the previous location of the platform on the first trial of reversal (session 11). The mice that did not reach this criterion were excluded from the swim maze data analysis. Two mice in the young and 7 mice in the old group did not reach criterion in this study, representing less than 8% of the mice being tested.
Path length (the distance taken by the mouse to reach the platform) over sessions was used as the primary measure of swim maze performance. The path-independent swim speed was calculated by dividing distance by the latency to reach the platform.
2.2.7. Discriminated avoidance test
A T-maze constructed of acrylic (black for the sides and clear for the top) was utilized for the discriminated avoidance task (Forster and Lal 1992; McDonald et al. 2005). The maze was divided into three compartments: a start box (10 × 6.3 × 6 cm), a stem (17.5 × 6.3 × 6 cm), and two goal arms (14.5 × 6.3 × 6 cm), each separated by clear acrylic doors. The maze rested on a grid floor wired to deliver 0.27-mA scrambled shock to the feet.
The test consisted of two sessions separated by 24 h. On each training trial, the mouse was placed in the start box, and the start door was removed to signal the beginning of the trial. On the first trial of the first session, the mouse received shock in the first arm entered and was permitted to escape shock by running to the opposite arm, which was then designated the correct arm for the remainder of the session. On subsequent trials, shock was initiated 5 s after the opening of the start door if the mouse had not entered the correct goal arm, or immediately upon entry into the incorrect arm. In either case, the shock continued until the correct goal arm was entered or a maximum of 60 s had elapsed. Upon the mouse’s entry into the correct arm, the door was closed (to prevent departure) and, after 10 s, the mouse was removed (by detaching the goal arm) and allowed to enter a holding cage for 1 min. Training in this fashion continued at 1-min intervals until the mouse had met the criterion of a correct avoidance (defined as running directly to the correct arm within 5 s) on four of the last five training trials. The second session of avoidance training was a reversal such that the mice were required to run to the goal arm opposite that to which they had been trained on the previous day. Two measures were considered to show the ability of the mice to learn the discrimination and avoidance components of the task. Their ability to learn was considered inversely proportional to the number of trials required to reach the avoidance criterion aforementioned and the number of trials required to reach the discrimination criterion (4 out 5 correct turns regardless of the time taken).
2.3. Animal dissection and tissue collection
Study 1
Mice from the gavage study did not receive behavioral testing and were euthanized after 3 weeks on their respective treatment. Each brain was dissected into six regions: cortex, hippocampus, striatum and cerebellum, midbrain and brainstem, and stored at −80°C in antioxidant buffer.
Study 2
Mice were euthanized one week after the active avoidance tests, and mitochondria were isolated from the whole brain (Sims 1993), liver (Lash and Sall 1993), heart (Arcos et al. 1968) and skeletal muscle (Trounce et al. 1989) by differential centrifugation. The mitochondrial pellets were re-suspended in appropriate volume of buffer and stored at −80°C for later analysis.
2.4. Protein carbonyl concentration
Carbonyl concentrations were measured as previously described (Levine et al. 1994). The samples were incubated in the dark at room temperature for 1 h with either 2,4-dinitrophenyl hydrazine (DNPH) for the experimentals or HCl for the blanks. The difference in absorbance at 366nm between DNPH-treated and HCL-treated samples was determined and the results were expressed as nmol carbonyls / mg protein using an extinction coefficient of 22.0 mM−1 cm−1.
2.5. Statistical analysis of data
For the gavage study, carbonyl concentration in the various brain regions was considered in a two-way ANOVA with experimental groups and regions as the factors. The data were further analyzed with one-way ANOVA with experimental groups as the factor, followed by planned individual comparisons between young and old vehicle groups, and treatment groups amongst the old group. The alpha level was set at 0.05 for all analyses.
Performance on the functional tests and biochemical measurements were assessed using two-way analyses of variance (ANOVA) with Age and Treatment as between-groups factors. The control diet, CoQ alone, and Toc + CoQ diet were the levels of Treatment considered in a balanced ANOVA that did not include data from mice on the Toc diet (as this treatment was not administered to young mice in this study). To assess whether the Toc diet had an effect on any of the measures, one-way ANOVAs were performed on behavioral and biochemical tests with Group as the factor, followed by planned individual comparisons between different age groups (young vs. old control) and treatment groups (i.e., each diet group vs. age-matched control). These comparisons were performed using single degree of freedom F tests involving the error term from the overall ANOVA. For the Morris water maze and weight data, three-way ANOVAs were performed for each dependent variable, with Sessions as the repeated measure. The alpha level was set at 0.05 for all analyses.
For the γ-cyclodextrin study, behavioral measures were analyzed using Student’s t-test, with an alpha level set at 0.05.
3. Results
3.1Carbonyl concentrations in brain region homogenates (Study 1)
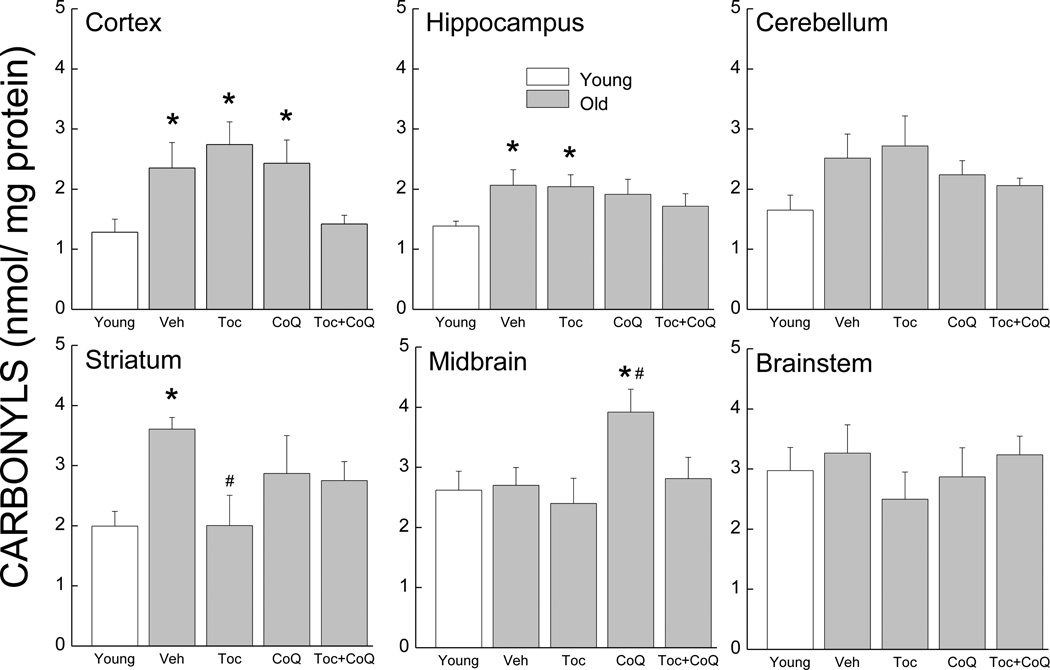
Protein carbonyl content was measured in the homogenates from six different brain regions collected from the treated and control mice from the gavage study (Fig.1). An analysis of the whole brain using weighed data (wet tissue weight) from each region revealed a main effect of group (p=0.02). Overall, carbonyl concentration was increased with age by 50–80% in the cerebellum, cortex, hippocampus and striatum but it remained unchanged in the midbrain and brain stem. In the cortex, carbonyl levels were 40% lower in the Toc + CoQ groups compared to the vehicle and did not differ from the young group. In the hippocampus and cerebellum, carbonyl concentrations were lower 17–18% lower in the Toc + CoQ groups compared to the vehicle and did not differ from the young group. In the striatum, only the Toc group had significantly lower carbonyl levels (44%) when compared to vehicle group. Interestingly, in the midbrain CoQ increased the levels of carbonyls by 45%. Main effects of groups were significant after one-way analyses of the data for cortex, and midbrain only (All ps<0.04).
Figure 1.
Effects of age and antioxidant supplementation on carbonyl concentration in homogenates from brain regions: cortex, hippocampus, cerebellum, striatum, midbrain, brainstem. * indicates a significant difference from young control. # indicates a significant difference from old control. Each value represents the mean ± SEM (Young, n=4–7; Old Veh, n=5–7; Old Toc, n=4–7; Old CoQ, n=4–7; Old Toc+CoQ, n=5–7).
3.2. Body weight and food intake (Study 2)
There was no change in body weight in either young or old control mice from the start of the diet implementation to the beginning of behavioral measurements (data not shown). The young mice weighed less than the old mice, but none of the antioxidant diets had a significant effect on the body weights. A three-way ANOVA indicated a significant main effect of Age and Weeks (p < 0.001), but not of Treatment or the interaction of Age with Treatment or with Week (all ps > 0.135). Furthermore, a one-way ANOVA indicated a main effect of group which was mainly due to the effect of age on weight (p=0.044). A one-way ANOVA using only the old groups did not reveal any effect of the diets, including the Toc diet, on the weight of the mice (All ps>0.219). Food intake measurements one and six weeks after the start of the diet were not affected by Age or Treatment, as supported by lack of main effects of Age or Treatment (all ps>0.351). Individual differences following a one-way ANOVA with Group as a factor indicated that the TOC diet also had no effect on the food intake at one and six weeks (All ps>0.848).
3.3. Neurobehavioral measures (Study 2)
3.3.1. Locomotor Activity
Distance (cm) and rearing (counts) were selected as measures of spontaneous locomotor activity (Table 2). There were no age-related alterations in rearing activity or distance traveled, and supplementation with the antioxidants alone or their combination did not significantly affect either measured variables in the young or old mice. A two-way ANOVA failed to indicate significant effects of Age, Treatment, or the interaction of those factors (all ps> 0.554).
Table 2.
Effects of age and/or antioxidant supplementation on psychomotor and sensory function
| Young | Old | ||||||
|---|---|---|---|---|---|---|---|
| Behavioral measures | Control | CoQ | Toc+CoQ | Control | Toc | CoQ | Toc +CoQ |
| Spontaneous Activity | |||||||
| Distance (cm) | 796 ± 89 | 754 ± 49 | 712 ± 60 | 805 ± 74 | 701 ± 70 | 731 ± 51 | 753 ± 97 |
| Rearing (counts) | 88 ± 15 | 92 ± 19 | 92 ± 20 | 106 ±11 | 90 ± 13 | 88 ± 15 | 97 ± 15 |
| Motor | |||||||
| Wire tread (s) | 17.7 ± 4.7 | 20.9 ± 4.1 | 20.5 ± 5.2 | 40.5 ± 4.8* | 41.1 ± 3.6 | 45.1 ± 3.9 | 46.1 ± 2.4 |
| Wire fall (s) | 37.2 ± 3.2 | 31.3 4.1 | 29.9 ± 2.8 | 23.2 ± 2.7* | 21.4 ± 2.7 | 19.8 ± 3.1 | 17.5 ± 1.7 |
| Bridge fall (s) | 48.1 ± 3.5 | 49.3 ± 1.4 | 51.5 ± 1.9 | 31.2 ± 3.0* | 33.6 ± 2.4 | 34.4 ± 3.2 | 33.9 ± 2.5 |
| Sensory (force units) | |||||||
| Auditory | |||||||
| low intensities1 | 102 ± 12 | 114 ± 22 | 104 ± 11 | 47 ± 9* | 48 ± 10 | 55 ± 8 | 64 ± 7 |
| high intensities2 | 108 ± 17 | 110 ±30 | 90 ± 13 | 94 ± 13 | 96 ± 16 | 107 ± 15 | 105 ± 12 |
| Shock | |||||||
| low intensities3 | 101 ± 16 | 148 ± 30 | 143 ± 30 | 49 ± 11* | 60 ± 11 | 42 ± 6 | 43 ± 7 |
| high intensities4 | 652 ± 75 | 686 ± 60 | 663 ± 55 | 485 ± 51* | 504 ± 55 | 438 ±41 | 421 ± 41 |
All values are the group means ± S.E.M (Young Con, n=11–12; Young CoQ, n=12; Young Toc+CoQ, n=12; Old Con, n=18; Old Toc, n=21; Old CoQ, n=18–19; Old Toc+CoQ, n=23–25)
Average startle response to 90, 100 and 110 dB noise bursts
Average startle response to 120 and 140 dB noise bursts
Average startle response to 0.02, 0.04, 0.08 mA shocks
Average startle response to 0.32 and 0.64 mA shocks
p<0.05 different from young controls
3.3.2. Wire Suspension
Performance of each mouse on the wire suspension test was measured by the average over a period of 4 sessions of the latencies to tread or fall (Table 2). When suspended from the wire, the latency to tread of old control mice was 130% higher than the young ones. Additionally, their latency to fall was 38% lower than the young ones. However, none of the antioxidant-enriched diets affected the latency to tread or fall of the young or old mice. A two- way ANOVA indicated a significant main effect of Age for both latency to fall and latency to tread (All ps < 0.001), but did not indicate an effect of Treatment or an interaction of Age and Treatment (All ps>0.073).
3.3.3. Bridge walking
Ability of the mouse to balance on a bridge was measured by the average latency to fall from 4 different bridges, each representing a different level of difficulty (Table 2). The latency to fall from the bridges was 35% lower for the old control mice than the young ones. Antioxidant treatment, alone or in combination had no effect on the latency to fall of either young or old mice. A two-way ANOVA confirmed a significant main effect of Age (p<0.001), and yielded no main effect of Treatment or interaction of Age and Treatment (All ps> 0.572).
3.3.4. Coordinated running
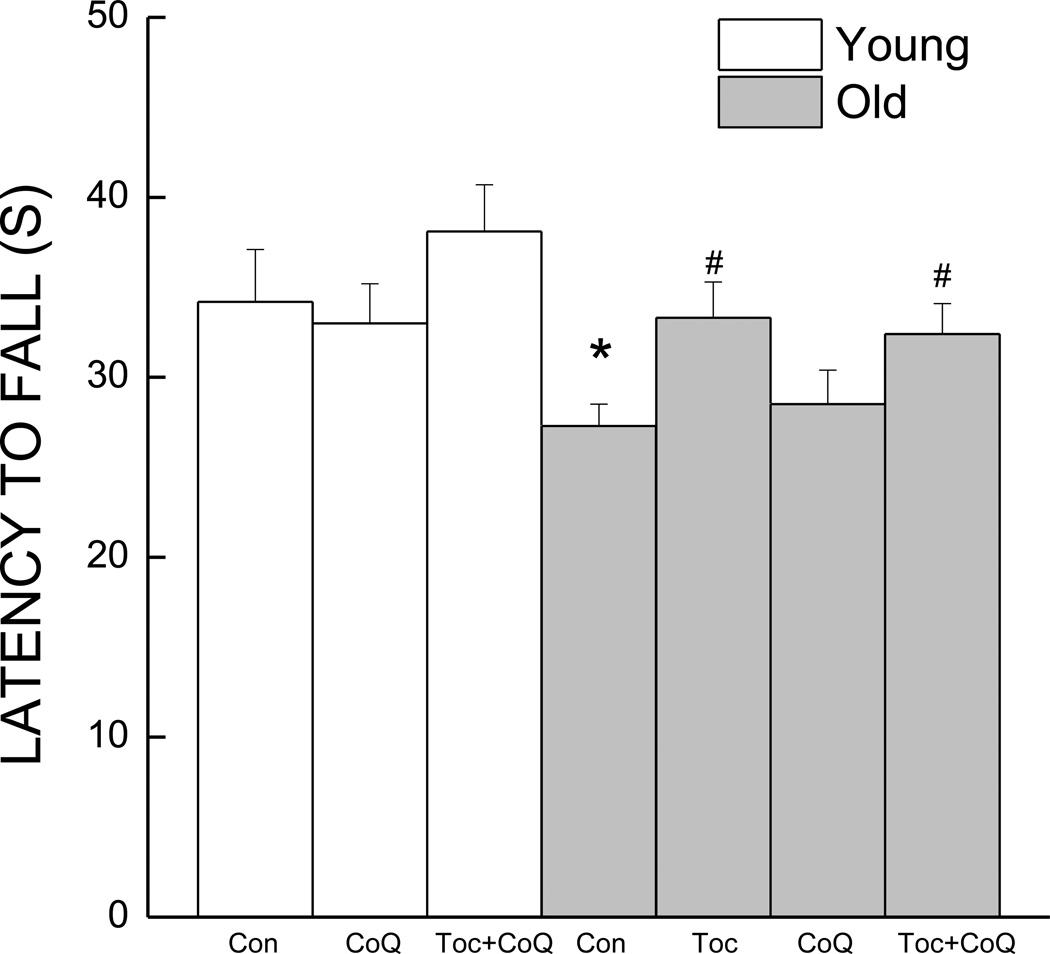
The effects of age and supplementation on the ability of the mice to reach a criterion of stable running performance are indicated in Fig. 2. There was an improvement in performance of both young and old mice over a period of seven sessions; however, overall the older mice performed worse than the young mice (data not shown). When the mice reached criterion, the latency to fall of old control mice from the rotating cylinder was reduced by 20% when compared to young control mice. While supplementation with antioxidants failed to improve performance in young mice, old mice supplemented with either the Toc diet or the combination diet exhibited performance to a level similar to that of the young mice. A two-way ANOVA indicated significant main effects of Age and Treatment (All ps<0.041) but not a significant interaction (p >0.852).
Figure 2.
Effects of age and antioxidant supplementation on rotorod performance as measured by latency to fall. The latency to fall represents the maximum performance attained by a mouse over 7–10 sessions. * indicates a significant difference from young control. # indicates a significant difference from age-matched control. Each value represents the mean ± SEM (Young Con, n=11; Young CoQ, n=12; Young Toc+CoQ, n=12; Old Con, n=18; Old Toc, n=21; Old CoQ, n=17; Old Toc+CoQ, n=25).
3.3.5. Sensory response
Response curves for auditory and shock startle response were summarized and analyzed separately for low and high sound and shock intensities (Table 2). Control mice had a 44% age-related decline in response to low sound intensities and a 13% decrease in their responses to high intensities. Supplementation with antioxidants alone or in combination did not have any effects on sensory reactivity. Control mice exhibited a lower response with age at both low (52%) and high (26%) intensities of shock. Despite a trend of CoQ and Toc + CoQ to increase the startle response of the young mice, there was no significant effect of antioxidant supplementation in young and old mice. All observations were confirmed by two-way ANOVA on auditory and shock (low and high intensities) indicating significant main effects of Age (All ps <0.001) and failing to suggest effects involving Treatment (All ps > 0.203).
3.3.6. Morris water maze
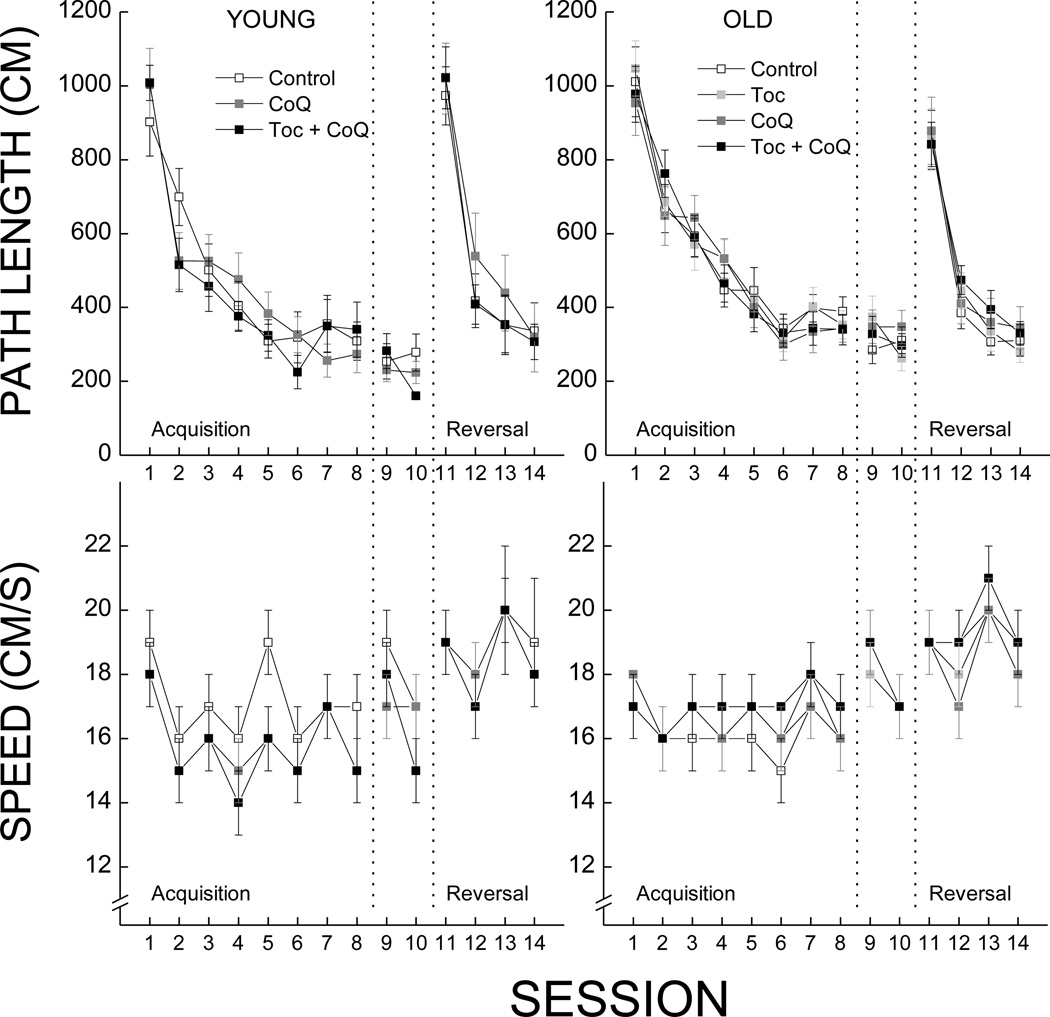
The efficiency of the mice to locate the hidden platform was assessed by the path length taken to reach the platform. Path lengths of both young and old mice decreased as a function of sessions during the acquisition phase (Fig.3-top panels), reached a maximum level of performance maintained during the retention phase, and attained the same level of efficiency after learning a new platform position during the reversal phase (Fig.3- top panels). Analysis of the data confirmed the effect of testing session on path length for the acquisition and reversal phases (p<0.001) and the lack of an effect for the retention phase (p=0.386).
Figure 3.
Effects of age and antioxidant supplementation on Morris water maze performance as measured by path length (cm ± SE) and speed (cm/s ± SE) during the acquisition (sessions 1–8), retention (sessions 9–10), and reversal (sessions 11–14) phases. Each value represents the mean ± SEM (Young Con, n=11; Young CoQ, n=10; Young Toc+CoQ, n=12; Old Con, n=18; Old Toc, n=20; Old CoQ, n=15; Old Toc+CoQ, n=20).
In the acquisition and retention phases, young mice reached the platform with shorter path length than the old mice, which was supported by two-way ANOVAs indicating main effects of Age (All ps<0.034). During the reversal phase, the two-way ANOVA yielded no effect of Age or Age by Session (All ps>0.076). There was no effect of Treatment on the performance of the young or old mice during any of the measured phases (p>0.124). The analysis of the pathindependent swim speed data (Fig.2- bottom panels) yielded significant effects of Session during all phases (p<0.001), a significant interaction of Age with Session during acquisition (p=0.016), but no effect of Treatment was found during any of the phases (p>0.066).
Accuracy for spatial memory was measured by conducting a probe trial as the last trial of the last session during the acquisition phase (data not shown). All the mice tested in the swim maze task developed a strong bias for the platform location, however there was no difference between young and old or amongst the supplemented groups as supported by a two-way ANOVA (ps > 0.227).
3.2.7. Discriminated avoidance test
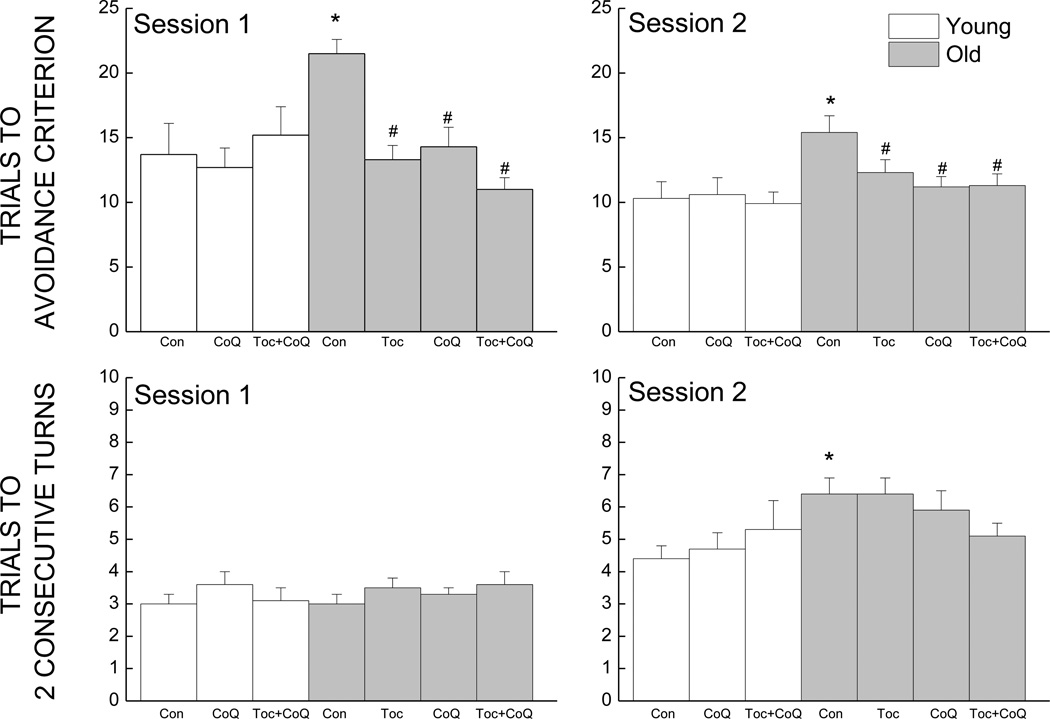
Two components of discriminated avoidance learning were considered for effects of age and antioxidant treatment. Learning of the preemptive response is shown in the top panels of Fig. 4, whereas the discriminative component is shown in the bottom panels of Fig. 4. For mice maintained on a control diet, there was an increase with age in the mean number of trials taken to reach the avoidance criterion as indicated by a 57 % increase for learning during acquisition (session 1) and 49 % increase during reversal (session 2). During session 1, all of the groups of old mice receiving antioxidant supplementation learned the avoidance response in fewer trials when compared to the old control group, and their level of learning was comparable to that of the young control group. Number of trials taken to reach the criterion of correct avoidance was reduced, by 38% in old mice supplemented with Toc-enriched diet, by 34% with CoQ10 and by 49% with a combination diet in comparison to their age-matched controls. Analysis of the trials to avoidance criterion data for session 1 revealed a significant main effect of Treatment as well as an interaction of Age and Treatment (ps<0.001), the latter reflecting that the antioxidant treatments had effects only in the old mice. In the reversal session (session 2; Fig. 3, top right panel), a similar effect of age antioxidant can be observed though the differences were not as large. Old mice maintained on the Toc diet took 19% fewer trials to reach the avoidance criterion, while old mice supplemented with CoQ took 27% fewer trials. Old mice maintained on the combination diet took fewer trials to reach criterion when compared to their age-matched controls; a reduction of 27%. Analysis of the data from session 2 indicated only a significant main effect of Age (p =0.015) and did not reveal a significant Age × Treatment interaction (p>0.135).
Figure 4.
Effects of age and antioxidant supplementation on discriminated avoidance task : number of total trials taken to reach criterion on day 1 (top left panel)and on day 2 (top right panel), and number of total trials to two consecutive correct turns for day 1 (left bottom panel) and day 2 (right bottom panel). * indicates a significant difference from young control. # indicates a significant difference from age-matched control. Each value represents the mean ± SEM (Young Con, n=10; Young CoQ, n=12; Young Toc+CoQ, n=12; Old Con, n=18; Old Toc, n=21; Old CoQ, n=19; Old Toc+CoQ, n=24).
Perusal of the data for the discriminative component of the avoidance response (Fig. 4, bottom panels) failed to suggest any age-related impairment during session 1, however during session 2, the ability of the old mice to learn the correct side of the maze was impaired as there was a 44% increase in the number of trials to 2 consecutive correct turns when compared to the young mice on the control diet. Only the mice on the combination diet took less trials to reach this criterion (21% reduction) but it failed to reach significance (p=0.054). Analyses of the data for trials to reach the criterion of 2 consecutive turns on sessions 1 and 2 did not indicate significant interactions of Age and Treatment (ps >0.145), and only a main effect of Age on session 2 (p=0.037).
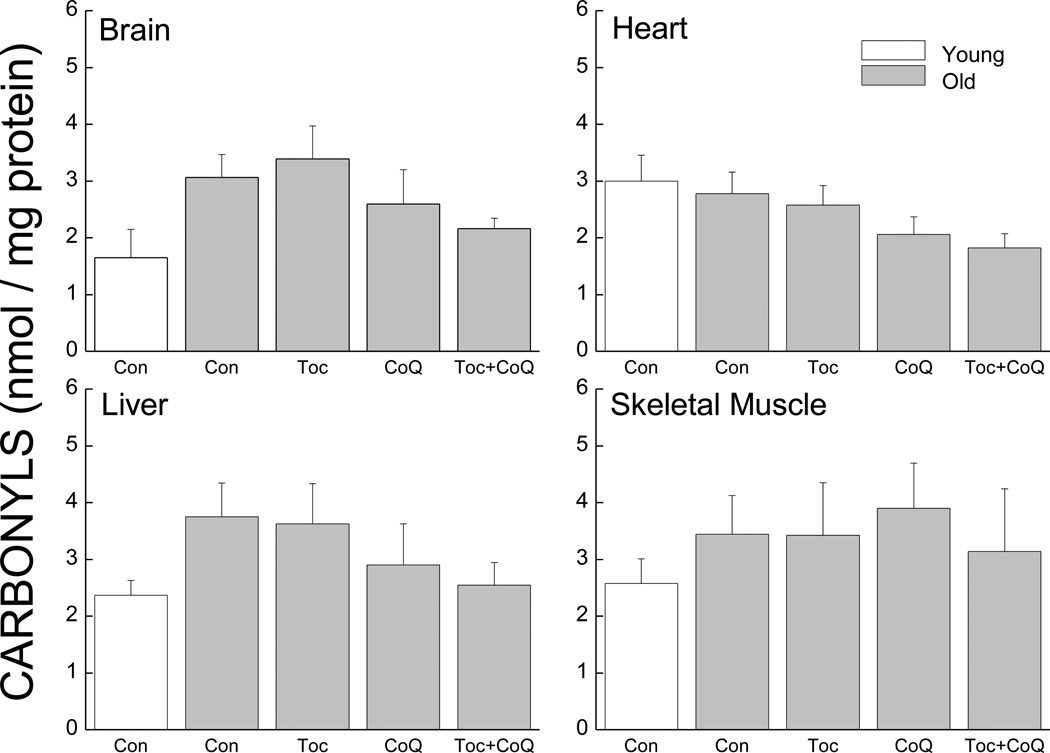
3.4 Carbonyl concentrations in mitochondrial fraction from tissues (Study 2)
Protein carbonyl content was measured in the mitochondria from brain, heart, liver and skeletal muscle from the young control and old control and treated mice (Fig.5). Age-related increases in carbonyl content were observed in the brain (86%), liver (58.5%) and skeletal muscle (37%) mitochondria. Carbonyl concentration was decreased in the mitochondria from brain, heart and muscles from mice supplemented with either CoQ or the combination of CoQ and Toc (decreases ranged from 15 to 34%). However, one-way ANOVA did not show a significant effect of Group (ps>0.213) and individual comparisons also did not yield significant differences.
Figure 5.
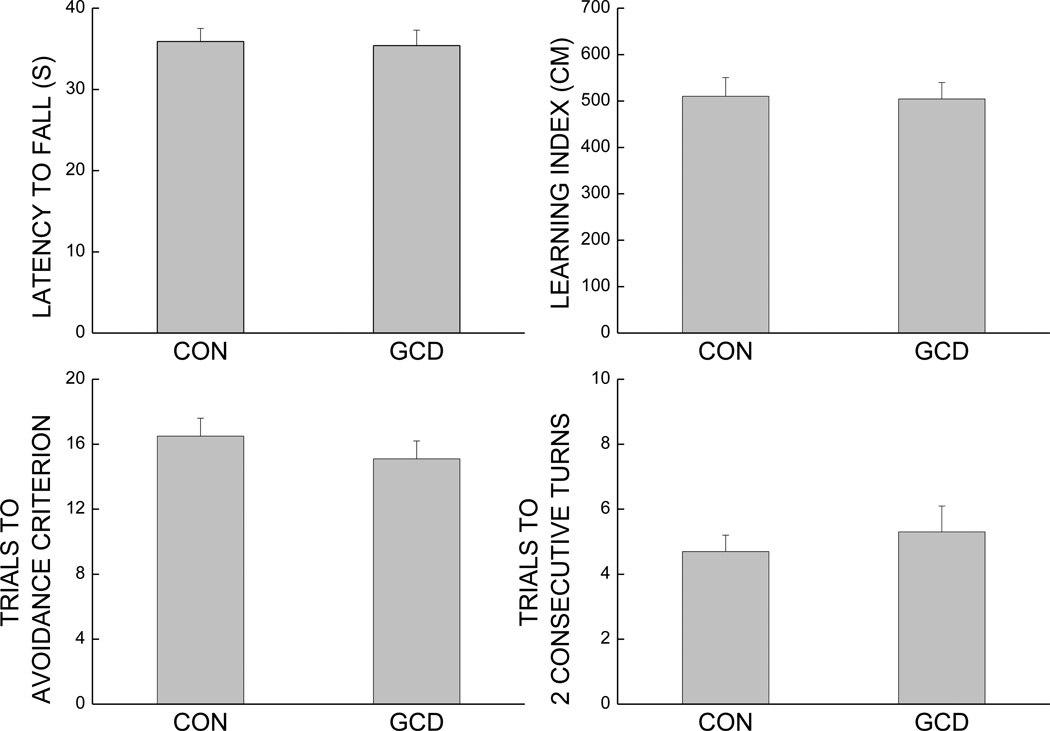
Effect of gamma-cyclodextrin supplementation on rotorod performance (top left panel), on Morris water maze performance (top right panel), and on performance on a discriminated avoidance task (bottom left panel: number of total trials taken to reach criterion; bottom right panel: number of total trials to two consecutive correct turns. Each value represents the mean ± SEM of 21–22 mice.
3.5 Effect of γ-cyclodextrin on behavioral measures (Study3)
The effects of a 3-week supplementation with γ-cyclodextrin are presented in Fig.6 for the main measures where effects of antioxidant supplementation were observed. Overall, there was no effect of the supplementation with γ-cyclodextrin on any of the measures. Student’s t-test yielded no significant effect of the addition of γ-cyclodextrin to the diet on maximum performance on the rotorod (p=0.654), on learning index in swim maze (composite measure averaging the path length in sessions 2, 3 and 4; p=0.917), on the avoidance (p=0.414) and discriminative (p=0.558) criteria during Session 1 of the active avoidance task.
Figure 6.
Effects of age and antioxidant supplementation on carbonyl concentration in mitochondria from brain, heart, liver and skeletal muscles. Each value represents the mean ± SEM (Young Con, n=3–5; Young CoQ, n=5–7; Young Toc+CoQ, n=4–6; Old Con, n=5–8; Old Toc, n=3–7; Old CoQ, n=4–7; Old Toc+CoQ, n=4–9). * indicates a significant difference from young control.
4. Discussion
The main findings of the study were: 1) Toc, but not CoQ, was sufficient for a full reversal of age-related impairments measured in a test of coordinated running; 2) Both Toc and CoQ improved the performance of aged mice in a learning task, although a combination of these antioxidants may be more effective than either one alone; 3) Toc + CoQ was more effective than either antioxidant alone at decreasing protein oxidation in peripheral tissues and certain brain regions; 4) The beneficial effects of the antioxidant supplementation were not generalized, but instead involved performance within specific domains of cognitive and psychomotor function.
A major finding of this study was the significant improvement of the coordinated running ability of old mice supplemented with Toc or the combination of Toc + CoQ when compared to their age-matched controls. This finding was in contrast to the previous findings from our laboratory where in one study supplementation with either Toc or a combination of Toc + CoQ did not benefit old mice in coordinated running (McDonald and Forster 2005), while yet another suggested that supplementation with Toc in aging mice was deleterious (Sumien et al. 2004). While the duration of the supplementation (14 months vs. 10 weeks) could explain the divergent outcomes, it is noteworthy that the formulation of the antioxidant used in the studies was different: synthetic form of Toc (dl-α-tocopheryl acetate; McDonald and Forster 2005; Sumien et al. 2004vs. natural form of Toc (d-α-tocopheryl acetate; current studies). Furthermore, the coenzyme Q10 used in this study was provided by Tischon Corp. (Westbury, NY) and was formulated with cyclodextrin to enhance its bioavailability. Therefore in order to maintain the same amount of additives, an equivalent amount of cyclodextrin was added to the other diets. Huang and colleagues have established that the water solubility of a highly lipophilic antioxidant like Toc can be dramatically increased by adding a 7% randomly methylated β-cylcodextrin (Huang et al. 2002). The doughnut shaped inner cavity of a cyclodextrin molecule can host large hydrophobic molecules like Toc and CoQ facilitating their dissolution in aqueous environment thereby increasing their bioavailability. Another study suggested that complexation with cyclodextrins improved the antioxidant activity of flavonols by reducing malondialdehyde formation in rat liver microsomes (Calabro et al. 2004). The current study is the first study of its kind to report that old mice fed a diet formulated with cyclodextrin and Toc for a short-term resulted in an improvement in performance in a coordinated running task in aged mice.
Despite being established as age-sensitive measures for motor functions (Sumien et al. 2006), performance on the bridge and wire suspension tasks was not affected by the Toc alone and CoQ + Toc diet in contradiction with the major effect of the antioxidants on rotorod performance of old mice. Performance on a rotorod task requires adequate visual cues, vestibular function to balance on the rotating rod, learning and memory when the task is performed over multiple trials and muscle strength, and depends on the cerebellum, motor cortex and striatum which are involved in learning and coordination (Altman and Bayer 1996). Further, Sumien and colleagues reported that latency to fall from the bridge is correlated with performance on the wire suspension test but none of these motor tasks are correlated with rotorod performance (Sumien et al. 2006). Therefore it appears that improvement in performance in rotorod of old mice fed a combination diet reflects an improvement in maximum capacity, rather than a simple improvement in strength, and balance. Another factor that could influence performance on the rotorod vs. other motor test is endurance; however, the motor tests were designed with limited duration and long rest period between trials and sessions to minimize its influence on motor outcomes. Evidence that the influence of endurance on outcome is negligible is that the performance of the mice, young or old, did not decrease as a function of trial or session. Furthermore, many exercise studies suggest that old mice tolerate treadmill running regimens well in excess of motor test requirements administered in these studies. Thus, endurance, defined in the conventional sense as limitation of performance due to cardiac or skeletal muscle fatigue, seems unlikely (though not impossible) as an explanation of the impaired performance of older mice in the current studies.
The results of this study revealed a robust age-related decline in the ability of the control groups to learn the preemptive component of a discriminated avoidance task during the initial session, and in the ability to reverse the direction of responding during the second session. These findings are in accordance with several previous investigations (Forster and Lal 1992; McDonald and Forster 2005). In the current study, the performance of the old mice supplemented with the antioxidants by themselves or in combination was improved in the initial acquisition of the discriminated avoidance task. However, the degree of improvement was most pronounced for mice on the CoQ+Toc diet. In the second session of the task, the impairment of old mice on the control diet is likely reflective of a cognitive inflexibility, or perseveration, that can be linked to impaired frontal cortical function (Schoenbaum et al. 2006). When supplemented with either Toc, CoQ or the combination of Toc + CoQ, the old mice were improved in their ability to reverse their performance on session two. While in session one the ability of the mice to discriminate was not affected by age or diet, in session two the old control mice had an impaired discrimination, which seemed to be reversed by antioxidant supplementation, especially Toc + CoQ. Although data for both discriminated avoidance sessions indicated significant improvements resulting from supplementation of a single antioxidant, it is noteworthy that combination of the two antioxidants yielded the most robust effects. The latter finding is consistent with an additive or synergistic interaction between Toc and CoQ when supplemented in combination. However, statistical analyses of the data did not support the observation. A power analysis indicated that we had enough mice to detect an additive effect of Toc + CoQ. However, a synergistic effect would have required about 10 more mice per group. The lack of a significant additive or synergistic effect could also be explained by the supplemented mice reaching a plateau of performance on the active avoidance task, and no further improvement can be made.
While antioxidant supplementation improved performance in the avoidance task that relies on cortical function, there was no effect on spatial learning and memory (Morris water maze) which relies more on hippocampal function. These findings are consistent with previously published studies in our laboratories. When old mice were fed Toc (synthetic form; Sumien et al. 2004) and CoQ10 (powder form; Shetty et al. 2013) for short period of time (12–15 weeks vs. 10 weeks in the current study), spatial learning and memory was not affected. However, when a higher dose of CoQ10 was used (~ 450 mg/kg/d), a significant improvement in spatial learning was observed (Shetty et al. 2013).
The exact mechanism of the cooperative effect of the combination of antioxidants is not clear. However, there is considerable evidence suggesting that the two antioxidants work in tandem to provide greater antioxidant potential and are more effective in providing protection against increased oxidative stress. Ubiquinol (reduced form of CoQ) regenerates Toc from the tocopheroxyl radical in the mitochondria, and the level of Toc is inversely proportional to the superoxide production in the mitochondria (Stoyanovsky et al. 1995; Lass 1999). Lass and colleagues concluded that only supplementation of combination of Toc and CoQ increases the levels of Toc in the brain mitochondria and such increases in the brain were not found with single antioxidant supplementation (Lass et al. 1999).
Our study revealed that indeed the antioxidant supplementation, especially CoQ10+Toc decreased protein oxidative damage in brain, heart and liver mitochondria. Toc supplementation did not produce a decrease in protein damage in these tissues, which is consistent with previous studies in our laboratory using a similar dose and duration of treatment (Sumien et al. 2004; Sumien et al. 2006). Though CoQ alone did not significantly reduce the levels of oxidative damage, there was a trend in mitochondria from all tissues which is consistent with our previous study of short-term supplementation of CoQ, using a similar dose but different CoQ formulation (Shetty et al. 2012).
Furthermore, our preliminary study determined the protein damage in 6 distinct brain regions after 3 weeks of daily intake of a combination of CoQ10 and Toc alone or in combination (120 mg/kg/day and 275 mg/kg/day). The evidence of a synergistic effect of the combination of antioxidants on protein damage was observed more specifically in the cortex, but were also observed in other regions. While it is not feasible to correlate these data to the outcomes from the behavioral study, it is noteworthy that the behavioral test that were most responsive to the treatments were the discriminated avoidance task and the rotorod test, which rely on cortical and striatal function respectively. Previous studies have determined that cognitive and motor performances are associated with protein oxidation measured by carbonyl content in different regions of the brain (Dubey et al. 1996). Therefore, one can conjecture that a decrease in oxidative damage in these regions could lead to improve behavioral outcomes on tests relying on these brain regions.
In conclusion, short-term supplementation with Toc, CoQ10 or Toc+CoQ10 had selective effects on various domains of motor and cognitive function that may be associated with their ability to reduce oxidative damage in specific brain regions, irrespective of an association between function and protein oxidation. Additionally, while the predicted synergistic or additive pattern of effect between Toc and CoQ10 was clearly present for protein oxidation, it was not as clearly determined when the functional outcomes were considered.
Highlights- Revised manuscript (EXG-14–145).
Toc, but not CoQ, reversed age-related impairments in coordinated running.
Both Toc and CoQ improved cognitive performance of aged mice.
Combination of Toc and CoQ may be more effective than either one alone.
Toc + CoQ was effective at decreasing protein oxidation in brain regions.
The beneficial effects of the antioxidants were brain domain specific.
Acknowledgment
This research was supported by grants R01 AG027353 and P01 AG022550 from the National Institutes of Health - National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Development of the cerebellar system: In relation to its evolution, structure, and functions. CRC Press; 1996. [Google Scholar]
- Arcos JC, Sohal RS, Sun SC, Argus MF, Brunch GE. Changes in ultrastructure and respiratory control in mitochondria of rat heart hypertrophied by exercise. Exp. Mol. Pathol. 1968;8:49–65. doi: 10.1016/0014-4800(68)90005-1. [DOI] [PubMed] [Google Scholar]
- Arzi A, Hemmati AA, Razian A. Effects of vitamins C and E on cognitive function in mouse. Pharmacological Research. 2004;49:249–252. doi: 10.1016/j.phrs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7(Suppl):S78–S88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Arlt S, Kontush A, Moller-Bertram T, Sperber S, Oechsner M, Stuerenburg HJ, Beisiegel U. Plasma and CSF markers of oxidative stress are increased in Parkinson's disease and influenced by antiparkinsonian medication. Neurobiol Dis. 2004;15(1):160–170. doi: 10.1016/j.nbd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Calabro ML, Tommasini S, Donato P, Raneri D, Stancanelli R, Ficarra P, Ficarra R, Costa C, Catania S, Rustichelli C, Gamberini G. Effects of alpha- and beta-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3-hydroxyflavones. J Pharm Biomed Anal. 2004;35(2):365–377. doi: 10.1016/j.jpba.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Flyod RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin trapping compound N-tert-butyl-α-phenylnitrone. Proceedings of the National Academy of Science USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra RK, Bhagavan HN. On the bioequivalence and bioavailability of three coenzyme Q10 products. J Med Food. 2006;9(1):131–132. doi: 10.1089/jmf.2006.9.131. author reply 133–134. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, Sumien N, Forster MJ, de Fiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: Age sensitivity and reliability of individual differences. AGE. 2006;28(3):235–253. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333(1):189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93(10):4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Neurobehavioral biomarkers of aging: influence of genotype and dietary restriction. Biomed Environ Sci. 1991;4(1–2):144–165. [PubMed] [Google Scholar]
- Forster MJ, Lal H. Within-subject behavioral analysis of recent memory in aging mice. Behav Pharmacol. 1992;3(4):337–349. [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20(2):167–176. doi: 10.1016/s0197-4580(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J Gerontol A Biol Sci Med Sci. 2000;55(11):B522–B529. doi: 10.1093/gerona/55.11.b522. [DOI] [PubMed] [Google Scholar]
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, Aisen P. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69(7):836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Liu JK, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):1870–1875. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Murphey HL, Dowling AL, McCarty KL, Bethel SR, Nitz JA, Pleiss M, Vanrooyen J, Grossheim M, Smiley JR, Murphy MP, Beckett TL, Pagani D, Bresch F, Hendrix C. A combination cocktail improves spatial attention in a canine model of human aging and Alzheimer's disease. J Alzheimers Dis. 2012;32(4):1029–1042. doi: 10.3233/JAD-2012-120937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Deemer EK. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated beta-cyclodextrin as the solubility enhancer. Journal of Agricultural & Food Chemistry. 2002;50(7):1815–1821. doi: 10.1021/jf0113732. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Witt E. Role of coenzyme Q and superoxide in vitamin E cycling. Subcell Biochem. 1998;30:491–507. doi: 10.1007/978-1-4899-1789-8_20. [DOI] [PubMed] [Google Scholar]
- Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Jr, Goodman GE, Minasian LM, Parnes HL, Lippman SM, Klein EA. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106(3) doi: 10.1093/jnci/djt456. djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Sall JM. Mitochondrial isolation from liver and kidney: strategy, techniques, and criteria for purity. In: Lash LH, Jones DP, editors. Methods in Toxicology: Mitochondrial Dysfunction. Vol. 2. San Diego: Academic Press; 1993. pp. 8–12. [Google Scholar]
- Lass A, Forster MJ, Sohal RS. Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: elevation of mitochondrial alpha-tocopherol by coenzyme Q10. Free Radic Biol Med. 1999;26(11–12):1375–1382. doi: 10.1016/s0891-5849(98)00330-x. [DOI] [PubMed] [Google Scholar]
- Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch. Biochem. Biophys. 1998;352(2):229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- Lass A, Sohal RS. Comparisons of coenzyme Q bound to mitochondrial membrane proteins among different mammalian species. Free Radical Biology & Medicine. 1999;27(1/2):220–226. doi: 10.1016/s0891-5849(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha - lipoic acid. Proc Natl Acad Sci U S A. 2002;99(4):2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SR, Forster MJ. Lifelong vitamin E intake retards age-associated decline of spatial learning ability in apoE-deficient mice. AGE. 2005;27(1):5–16. doi: 10.1007/s11357-005-4003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38(6):729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Muggenburg B, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified food, and cognitive strategy. Neuroscience & Biobehavioral Reviews. 2002;26(6):679–695. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-imparied rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther. 2010;17(3):e56–e65. doi: 10.1097/MJT.0b013e31819cdc9a. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. Journal of Neurophysiology. 2006;95(3):1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Research Reviews. 2004;3(4):431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Shetty RA, Forster MJ, Sumien N. Coenzyme Q(10) supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age (Dordr) 2012 doi: 10.1007/s11357-012-9484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty RA, Forster MJ, Sumien N. Coenzyme Q(10) supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age (Dordr) 2013;35(5):1821–1834. doi: 10.1007/s11357-012-9484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Smith DE, Meydani M, Joseph JA. The effects of dietary antioxidants on psychomotor performance in aged mice. Experimental Gerontology. 1999;34(6):797–808. doi: 10.1016/s0531-5565(99)00039-x. [DOI] [PubMed] [Google Scholar]
- Sims NR. Methods in Toxicology: Mitochondrial Dysfunction. San Diego: Academic Press; 1993. [Google Scholar]
- Socci DJ, Crandall BM, Arendash GW. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Research. 1995;693:88–94. doi: 10.1016/0006-8993(95)00707-w. [DOI] [PubMed] [Google Scholar]
- Stoyanovsky DA, Osipov AN, Quinn PJ, Kagan VE. Ubiquinone-dependent recycling of vitamin E radicals by superoxide. Arch Biochem Biophys. 1995;323(2):343–351. doi: 10.1006/abbi.1995.9955. [DOI] [PubMed] [Google Scholar]
- Stoyanovsky DA, Osipov AN, Quinn PJ, Kagan VE. Ubiquinone-dependent recylcing of vitamin E radicals by superoxide. Arch. Biochem. Biophys. 1995;323:343–351. doi: 10.1006/abbi.1995.9955. [DOI] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, Shetty RA, Sohal RS, Forster MJ. Prolonged intake of coenzyme Q10 impairs cognitive functions in mice. J Nutr. 2009;139(10):1926–1932. doi: 10.3945/jn.109.110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, Sohal RS, Forster MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36(11):1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Sumien N, Sims MN, Taylor HJ, Forster MJ. Profiling psychomotor and cognitive aging in four-way cross mice. AGE. 2006;28:265–282. doi: 10.1007/s11357-006-9015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochrondrial respiratory chain functions: possible factor in aging. Lancet. 1989:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38(4):309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]