Abstract
Macromolecular X-ray crystallography, usually done at cryogenic temperature to limit radiation damage, often requires liquid cryoprotective soaking that can be labor intensive and damaging to crystals. Here we describe a method for cryoprotection that uses vapor diffusion of volatile cryoprotective agents into loop-mounted crystals. The crystal is mounted into a vial containing a small volume of an alcohol-based cryosolution. After a short incubation with the looped crystal sitting in the cryosolution vapor, the crystal is transferred directly from the vial into the cooling medium. Effective for several different protein crystals, the approach obviates the need for liquid soaking and opens up a heretofore underutilized class of cryoprotective agents for macromolecular crystallography.
Keywords: X-ray crystallography, cryoprotection, vapor diffusion
1. Introduction
Data collection at cryogenic temperature has become the normal approach for structure determination via X-ray diffraction. The low temperature (typically 100 K) slows radiation damage and is especially useful at high intensity synchrotron radiation sources (Kmetko et al., 2006; Owen et al., 2006). However, cryogenic cooling itself can damage the crystal and compromise diffraction quality, often due to ice formation (Haas and Rossmann, 1970; Juers and Matthews, 2001; Juers and Matthews, 2004; Kriminski et al., 2002; Low et al., 1966). Cooling-induced damage is typically reduced by cooling faster and/or adding cryoprotective agents such that the system cools through the freezing point of water to the glass transition before ice can form (Chinte et al., 2005; Shah et al., 2011; Warkentin et al., 2013). The use of pressure to prevent the formation of ice I during cooling has also been successfully applied to several systems (Burkhardt et al., 2012; Kim et al., 2005; Thomanek et al., 1973).
Many different cryoprotective agents have been identified (Bujacz et al., 2010; Gulick et al., 2002; Holyoak et al., 2003; Hope, 1988; Marshall et al., 2012; Mueller-Dieckmann et al., 2011; Pemberton et al., 2012; Rubinson et al., 2000; Vera and Stura, 2014), including sugars, linear and branched polyols, salts, organic solvents, amino acids, methylamine osmolytes and viscous hydrocarbons. In some cases, an adequate cryoprotective agent is already present in the crystallization buffer and the crystal can be cryocooled directly from the growth drop. However, very often an additional cryoprotection step is performed by soaking the crystal in a cryosolution, which can be laborious and damaging to crystals due to handling and osmotic stresses. Approaches to cryoprotection that limit such treatments would be advantageous.
Volatile alcohols are known to be efficient cryoprotective agents and have been useful for cryopreservation of microorganisms (Hubalek, 2003) and for low temperature crystallography in the liquid state (Douzou et al., 1975). Recent experiments showed that both methanol and ethanol require lower concentrations (w/v) than traditional cryoprotective agents (e.g. glycerol and ethylene glycol) to prevent ice formation in small volumes of plunge-cooled solution (Warkentin et al., 2013). Despite their effectiveness, volatile alcohols have seen little use for cryoprotection in macromolecular crystallography, due in part to the difficulty of working with their high vapor pressures. Of the ~100,000 structures in the protein data bank, just 0.2% have methanol or ethanol present in the model, while 14% include either glycerol or ethylene glycol (Berman et al., 2000). A recently described vial mounting methods offers the possibility of turning the high vapor pressure into an advantage to deliver the volatile alcohol to a loop-mounted crystal (Farley and Juers, 2014). Here we show the approach is rapid and effective for several different protein crystals. Subsequent cryocooling yields high quality diffraction without ice formation. The approach does not require liquid soaking and opens up a new class of cryoprotective agents for macromolecular crystallography.
2. Materials and Methods
2.1 Crystals
Chemicals were from Hampton Research (Aliso Viejo, California, USA; glucose isomerase #HR7–100) or Sigma-Aldrich (St. Louis, Missouri, USA; all other chemicals). Orthorhombic glucose isomerase crystals were used as provided by the supplier. All other crystals were grown using hanging drop vapor diffusion with 24 well plates (Hampton Research, Aliso Viejo, CA) at 294–298 K (277 K for hexagonal thaumatin) and used within a few months of growth. Tetragonal lysozyme (#L6876) well: 20 mM NaOAc 4.5, 3–5% w/v NaCl; protein: 80–100 mg/mL in 20 mM NaOAc 4.5(Forsythe et al., 1999). Orthorhombic and trigonal trypsin (#T8003) well: 100 mM Tris 8.0, 25% w/v PEG 8000, 0.2 M AmSO4, 0.1 M benzamidine HCl; protein: 50 mg/mL in water (Leiros et al., 2001); Tetragonal thaumatin (#T7638) well: 0.2 M – 0.9 M Na/K tartrate; protein: 35–70 mg/mL in 100mM HEPES 7.3 (Ko et al., 1994). Hexagonal thaumatin (#T7638) well: 0.1 M NaOAc 4.5, 0.175 M AmSO4, 0.1 M LiSO4, 0.1 M MgCl2, 15% (v/v) glycerol, 2% (w/v) PEG 400; protein: 35 mg/mL in 100 mM HEPES 7.3(Charron et al., 2004); Thermolysin (#P1512) well: 30% sat’d AmSO4; protein: 150 mg/mL in 45% v/v DMSO (hexagonal); 100 mg/mL in 45% v/v DMSO, 0.5 M ZnCl2 (tetragonal) (Hausrath and Matthews, 2002). Tetragonal proteinase K (#P6556) well: .3–.4 M Na/K tartrate or 12–15% w/v PEG 8K; protein: 30–50 mg/mL in water. Cubic insulin (#I5523) well: 345–525 mM NaPhosphate dibasic, 10 mM EDTA 9.2; protein: 15 mg/mL in 18 mM NaPhosphate dibasic, 10mM EDTA 10.5(Gursky et al., 1992). In all cases, drop sizes were 6–9 µL and were ½ well/½ protein, except for thermolysin, which used just the protein solution given set up over the well. Prior to cryocooling, some thermolysin crystals were serial diluted (2–3 minutes) into DMSO-free protein solution (i.e. water for hexagonal crystals and 0.5 M ZnCl2 for tetragonal crystals) to ensure the absence of the natural cryoprotective effects of DMSO. Similarly, some glucose isomerase crystals were serial diluted over 2–3 minutes from their 0.9 M AmSO4 solution into 0.25 M AmSO4.
2.2 Cryosolutions
Cryosolutions were based on four volatile alcohols – methanol, ethanol, isopropanol, and tert-butanol. Binary cryosolutions (alcohol/water) were prepared gravimetrically, while well-based cryosolutions were prepared volumetrically using 2X well solution, water and the alcohol. Because the low surface tension can make vial mounting difficult (see below), we also tested a cryosolution of 7.5 % agar, 40% methanol and 52.5 % water (by weight). The agar was dissolved in hot water and pipetted into a cryovial. Then the methanol was added and the solution was mixed, covered with a crystal-cap and O-ring and allowed to cool.
2.3 Vial Mounting and Cryoprotection
Vial mounting proceeded as previously reported (Farley and Juers, 2014). Briefly, a cryovial (Hampton Research, Aliso Viejo, California, USA) was prepared by plugging the liquid nitrogen escape holes with clay and fitting an O-ring (amazon.com, nitrile rubber, 50A durometer hardness; 3/8” ID×1/16” thick) on the crystal cap (SPINE, Hampton Research). Crystals were mounted by placing the crystal growth coverslip in a humid flow of 85–98% RH, looping the crystal using cryoloops of 20 µm diameter nylon with microtubes snapped at the 18 mm notch (Hampton Research) and inserting into a vial containing 500 µL of cryosolution. Crystals were mounted directly from drops without adding extra solution. (Sometime crystals were pushed into the drop.) The vial was allowed to sit for some time period (a few seconds up to 16 hours). Our default condition was 2 minute equilibration against 40% w/w methanol. After equilibrating, the crystal was directly mounted on the diffractometer from the vial. It is recommended that the vial undergo minimal handling and that the crystal cap be manipulated with a thermally insulated wand in order to uniformly maintain the cap-vial system at ambient temperature. The vial mounting technique should be practiced to achieve the smooth motions required to prevent crystals from being dislodged from the loop. The goniometer should be positioned such that the vial is at least horizontal and ideally angled downward as it is removed from the crystal cap, keeping the low surface tension cryosolution towards the bottom of the vial. The cryosolution can also be prepared as an agar gel to limit its movement during mounting (see above).
2.4 X-ray Data Collection
X-ray data were collected using an Agilent Xcalibur X-ray diffractometer with a Nova X-ray source and Onyx detector (Agilent Technologies, Santa Clara, California, USA) using the following parameters: 50 kV, 0.8 mA, crystal to detector distance = 65.000 mm, theta (the detector angle) = 3.5°, oscillation width = 0.25°, number of frames: 2×6, separated by 90 degrees. The detector edge was set to 1.8 Å for all crystals, regardless of their diffraction power. Exposure times were 15 or 30 seconds, the latter if the shorter exposure did not yield 2.0 Å data. Data were processed with CrysalisPro (Agilent) in Pre-experiment mode, which outputs cell parameters, an estimate of the diffraction limit and the mosaicity.
3. Results and Discussion
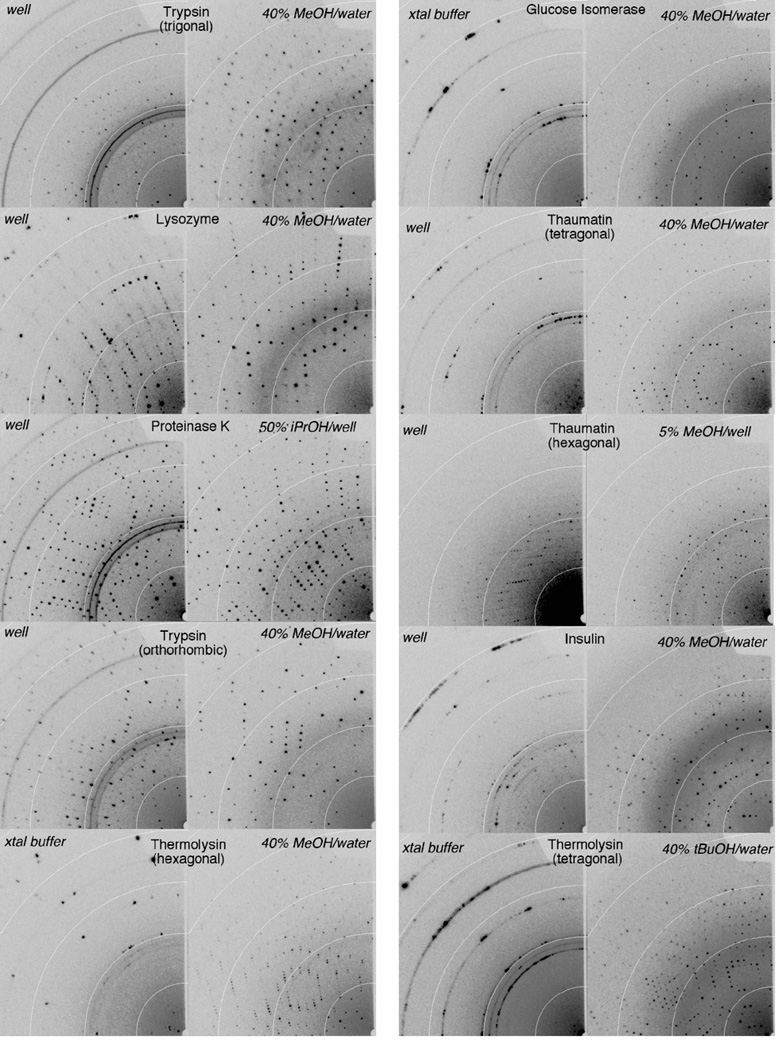
Fig. 1 compares diffraction images from crystals incubated in-vial over crystal growth well solution vs an alcohol-based cryosolution. The crystals equilibrated over alcohols show high quality diffraction to at least 2.0 Å resolution, comparable to crystals cryoprotected by soaking in traditional cryoprotectants (i.e. ethylene glycol, glucose, MPD) while the negative controls show ice and reduced diffraction power. Many of the crystals diffracted to much higher resolution than 2.0 Å, and data sets were collected to 0.95, 1.3, 1.5, 1.5, 1.5, 1.8, and 1.9 Å resolution for trypsin (orthorhombic), proteinase K, lysozyme, glucose isomerase, thaumatin (tetragonal), thermolysin (tetragonal) and insulin respectively. The approach was effective for eliminating ice from well-diffracting crystals as well as the complete cryoprotection of crystals for which the negative control destroyed the crystal lattice. Initially, in-vial equilibration times of tens of minutes were used, since we found previously that small unit cell changes occur on that time scale for vial mounts of thaumatin crystals (Farley and Juers, 2014). Subsequently, for most of the proteins we tested shorter equilibrations (except for trigonal trypsin for which we only had two crystals) finding 10 seconds – 3 minutes produced high quality diffraction.
Figure 1.
Diffraction images from crystals equilibrated in-vial over crystal buffer (left hand image each pair) or an alcohol solution (right). Incubation times for the well solution measurements are a few minutes. Incubation times for the alcohol incubations are 10–120 seconds, except for trigonal trypsin, which was 15 minutes. The detector is set so the resolution at the edge is 1.8 Å and the four inner resolution rings are at 6.6, 3.5, 2.5 and 2.0 Å. All of the alcohol cryoprotected crystals diffract to at least 2.0 Å resolution. (a) trigonal trypsin, lysozyme, proteinase K, orthorhombic trypsin, hexagonal thermolysin (b) glucose isomerase, tetragonal thaumatin, hexagonal thaumatin, insulin, tetragonal thermolysin. While glucose isomerase, and the two thermolysin crystal forms were soaked in xtal buffer (see methods) to reduce the natural cryoprotective effects of their crystallization buffers, they can also be mounted directly from the drop over alcohol solutions with high quality diffraction.
The method was successful with all ten crystals tested Eight crystals could be cryoprotected using the vapor of a simple binary solution of water and alcohol and two crystals required supplementing the crystal growth solution with alcohol. For tetragonal thermolysin, methanol/water produced high mosaicity and an apparent change in space group but tert-butanol/water yielded diffraction nearly equal to a positive control (diffraction to 2.0 Å vs 1.9 Å for a soak in 50% w/w glucose). Hexagonal thaumatin dissolved upon exposure to methanol/water vapor, but using the well solution supplemented with 5% v/v methanol yielded high quality diffraction beyond 2.0 Å. For PEG grown proteinase K, alcohol only solutions usually yielded high mosaicity, as did well solution supplemented with methanol. But well solution supplemented with 60% ethanol or 50% isopropanol yielded high quality diffraction with Bragg spots beyond 1.2 Å. Tartrate grown Proteinase K could be cryoprotected with 40% methanol, but with somewhat higher mosaicity (~0.7° vs ~0.5°) than the ethanol or isopropanol protected PEG grown crystals. Additionally, lysozyme crystals (grown from 5% NaCl) were tested with ethanol (90% w/w), isopropanol (85% w/w), tert-butanol (75% w/w) and methanol/water/agar (see methods), yielding diffraction similar to the 40% w/w methanol/water cryoprotected crystal.
The mechanism of cryoprotection of the volatile alcohols can be understood in the framework of critical droplet theory, in which all standard cryoprotective agents (e.g. methanol, ethanol, glycerol, glucose) function simply by sterically hindering ice nucleation (Warkentin et al., 2013). The presence of these solutes decreases the probability of finding a region of pure water of sufficient size to crystallize, which increases the free energy of ice nucleation. Vapor diffusion apparently delivers the alcohol to high enough concentration to prevent ice nucleation both external and internal to the crystal. Initial examination of electron density maps for lysozyme, thaumatin, proteinase K, glucose isomerase and trypsin indicates the presence of some bound alcohol molecules, consistent with rapid delivery to the crystal and subsequent diffusion into the crystals. Further work is underway to understand the extent of diffusion along the solvent channels and binding to the protein during the short equilibration.
As described, the method includes humid flow for manipulating crystals, an O-ring to help seal the crystal capvial junction, and crystals directly mounted on the cryostream from the vial. These enhancements have clear benefits, including improved reproducibility of cell parameters, the possibility of long in-vial incubations, more time for crystal handling, and more reliable removal of external solution (Farley and Juers, 2014). Simpler approaches were also tested with lysozyme, glucose isomerase, thaumatin and tetragonal thermolysin. Using a vial at ambient humidity without an O-ring yielded high quality diffraction data without ice (Fig. 2). Using vial equilibrated crystals cooled by rapidly removing them from the vials and plunging into liquid nitrogen also yielded data of similar quality.
Figure 2.
Diffraction images from crystals transferred to vials without the use of humid flow or an O-ring on the vial. Liquid nitrogen escape holes were plugged with clay. Crystals were mounted directly from the vial onto the cryostream. Lysozyme: 40% MeOH, mosaicity 0.55°, <I/σ> = 8.0. Glucose isomerase: 40% MeOH, mosaicity 0.56°, <I/σ> = 2.6. Thaumatin: 40% MeOH, mosaicity 0.59°, <I/σ> = 4.9. Thermolysin: 40% tBuOH, mosaicity 0.61°, <I/σ> = 2.9. <I/σ> is given for the 2.0 Å resolution bin.
The main advantage of the method is its ease of use. The large vapor pressure (Table 1) facilitates transport of the cryoprotective agent to the crystal, obviating the need for liquid soaking, which can be laborious if serial soaks are required and damaging from handling and osmotic stresses. Another benefit of organic solvents is that they tend to reduce protein solubility by decreasing the dielectric constant of the medium (McPherson, 1999), unlike glycerol and ethylene glycol, which solubilize proteins (Auton et al., 2011).
Table 1.
Vapor pressures of some cryoprotective agents, in mm Hg at 298 K.
| Molecule |
Vapor Pressure (Yaws, 1999) |
|---|---|
| Methanol | 125 |
| Ethanol | 59 |
| Isopropanol | 45 |
| Tert-butanol | 42 |
| Water | 24 |
| DMF | 4 |
| DMSO | 0.6 |
| Ethylene glycol | 0.1 |
The required conditions will depend on particulars of each crystal/solvent system. Minimum concentrations for cryoprotection were 20% – 40% w/w for the binary cryosolutions and usually somewhat lower for the well-based cryosolutions (Table 2). Well-based cryosolutions were effective for all crystals tested, so a conservative approach would be to start with them. In some cases, the diffraction quality depended on the alcohol concentration, so a range of concentrations should be tested. Minimum equilibration times also varied, but were relatively short – ranging from 30 seconds for lysozyme crystals to 3 minutes for very large – 8003 µm3 – insulin crystals). Longer equilibrations tended to reduce cell parameters (i.e. for insulin from 77.9 Å vs 77.6 Å for 45 second and 30 min incubations respectively). It should be noted that crystal packing changes associated with dehydration can occur slowly (Sanchez-Weatherby et al., 2009) (Farley and Juers, 2014). Therefore, in the event that short equilibrations are unsuccessful, we suggest that overnight incubations be considered, using an appropriately sealed vial/crystal cap.
Table 2.
Some characteristics of the crystals and diffraction conditions.
| Crystal | Solvent Contenta |
Primary component of crystal buffer prior to cryoprotection. |
Binary Cryo (w/w) b |
Xtal buffer Cryo(v/v)b |
Mosaicityc | I/sigma (2.0 Å)d |
|---|---|---|---|---|---|---|
| Trypsin (trigonal) |
40 % | 25% P8K, 0.2 M AmSO4 | - | - | 0.59 | 6.4 |
| Lysozyme | 41 | 0.5 M NaCl | 40% MeOH | 30% MeOH | 0.57 | 12.8 |
| Proteinase K | 44 | 12% PEG 8K | 55% EtOH | 50% EtOH | 0.56 | 15.1 |
| Trypsin (orthorhombic) |
47 | 25% P8K, 0.2 M AmSO4 | 20% MeOH | 5% MeOH | 0.45 | 10.1 |
| Thermolysin (hexagonal) |
50 | water | 30% MeOH | 30% MeOH | 0.57 | 4.0 |
| Glucose isomerase |
57 | 0.25 M AmSO4 | 40% MeOH | 40% MeOH | 0.57 | 3.0 |
| Thaumatin (tetragonal) |
58 | 0.2 M Na/K tartrate | 40% MeOH | 40% MeOH | 0.49 | 2.8 |
| Thaumatin (hexagonal) |
62 | 0.2 M AmSO4, 15% glycerol | Xtal dissolved | 5% MeOH | 0.57 | 3.4 |
| Insulin | 65 | 0.25 M NaPhosphate | 40% MeOH | 40% MeOH | 0.61 | 2.6 |
| Thermolysin (tetragonal) |
66 | 0.5 M ZnCl2 | 30% BuOH | 30% BuOH | 0.58 | 2.8 |
Solvent content is calculated based the cell volume and SEQRES data in the pdb file (Matthews, 1968) (Kantardjieff and Rupp, 2003).
Minimal concentration of volatile alcohol required for cryoprotection using 2 minute in-vial equilibrations. Diffraction characteristics for the images shown in Fig. 1 (right).
The average of e1, e2 and e2 output by CrysalisPRO.
<I/σ> for the highest resolution bin (2.0 Å). Exposure times were 15 seconds, except for tetragonal thermolysin, tetragonal thaumatin, and glucose isomerase, which were 30 seconds. Crystal sizes were ~100–500 µm.
Compared to crystals cryoprotected via liquid soaking with traditional cryoprotectants the unit cell volumes of the volatile alcohol cryoprotected crystals were smaller by 0–2%, which could be due to dehydration or greater thermal contraction of the alcohol solutions (Alcorn and Juers, 2010). Dehydration can increase the extent of crystal contacts and improve diffraction (Kiefersauer et al., 2000) (Russi et al., 2011), but can also be detrimental (Bernal and Crowfoot, 1934). The smaller cell volume may have been part of the reason tetragonal thermolysin required tert-butanol instead of methanol, and follow-up studies are being conducted to further understand this result. Because the alcohols tested and traditional cryoprotective agents sample different ranges of thermal contraction, the approach described may be viewed as complementary to liquid soaking with traditional cryoprotective agents.
4. Conclusions
We have shown that the vial mounting method in concert with a volatile alcohol/water cryosolution combine to yield a new, effective approach for cryoprotecting macromolecular crystals. The approach is rapid, uses simply prepared cryosolutions, limits crystal handling and does not require liquid soaking.
Acknowledgements
This work was supported in part by a grant from the National Institutes of Health (GM090248).
Abbreviations
- DMSO
dimethyl sulfoxide
- AmSO4
ammonium sulfate
- NaOAc
sodium acetate
- PEG
polyethylene glycol
- Tris
tris(hydroxymethyl)aminomethane
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MPD
2-methyl-2,4-pentanediol
- MeOH
methanol
- EtOH
ethanol
- iPrOH
isopropanol
- tBuOH
t-butanol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcorn TA, Juers DH. Progress in Rational Methods of Cryoprotection in Macromolecular Crystallography. Acta Cryst. D. 2010;66:366–373. doi: 10.1107/S090744490903995X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auton M, Rösgen J, Sinev M, Holthauzen LMF, Bolen DW. Osmolyte effects on protein stability and solubility: A balancing act between backbone and side-chains. Biophys. Chem. 2011 doi: 10.1016/j.bpc.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland GL, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal JD, Crowfoot D. X-ray Photographs of Crystalline Pepsin. Nature. 1934;133:794–795. [Google Scholar]
- Bujacz G, Wrzesniewska B, Bujacz A. Cryoprotection properties of salts of organic acids: a case study for a tetragonal crystal of HEW lysozyme. Acta Cryst. D. 2010;66:789–796. doi: 10.1107/S0907444910015416. [DOI] [PubMed] [Google Scholar]
- Burkhardt A, Warmer M, Panneerselvam S, Wagner A, Zouni A, Glockner C, Reimer R, Hohenberg H, Meents A. Fast high-pressure freezing of protein crystals in their mother liquor. Acta Crystallogr F. 2012;68:495–500. doi: 10.1107/S1744309112009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron C, Giege R, Lorber B. Structure of thaumatin in a hexagonal space group: comparison of packing contacts in four crystal lattices. Acta Cryst. D. 2004;60:83–89. doi: 10.1107/s0907444903022613. [DOI] [PubMed] [Google Scholar]
- Chinte U, Shah B, DeWitt K, Kirschbaum K, Pinkerton AA, Schall C. Sample size: an important parameter in flash-cooling macromolecular crystallization solutions. J. Appl. Cryst. 2005;38:412–419. [Google Scholar]
- Douzou P, Hoa GH, Petsko GA. Protein crystallography at sub-zero temperatures: lysozyme-substrate complexes in cooled mixed solvents. J. Mol. Biol. 1975;96:367–380. doi: 10.1016/0022-2836(75)90166-7. [DOI] [PubMed] [Google Scholar]
- Farley C, Juers DH. Improved Reproducibility of Cell Parameters in Macromolecular Cryocrystallography by Limiting Dehydration during Crystal Mounting. Acta Cryst. D. 2014;70 doi: 10.1107/S1399004714012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe EL, Snell EH, Malone CC, Pusey ML. Crystallization of chicken egg white lysozyme from assorted sulfate salts. J. Cryst. Growth. 1999;196:332–343. [Google Scholar]
- Gulick AM, Horswill AR, Thoden JB, Escalante-Semerena JC, Rayment I. Pentaerythritol propoxylate: a new crystallization agent and cryoprotectant induces crystal growth of 2-methylcitrate dehydratase. Acta Crystallogr D Biol Crystallogr. 2002;58:306–309. doi: 10.1107/s0907444901018832. [DOI] [PubMed] [Google Scholar]
- Gursky O, Li Y, Badger J, Caspar DL. Monovalent cation binding to cubic insulin crystals. Biophys J. 1992;61:604–611. doi: 10.1016/S0006-3495(92)81865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DJ, Rossmann MG. Crystallographic Studies on Lactate Dehydrogenase at −75° C. Acta Cryst. B. 1970;26:998–1004. doi: 10.1107/s0567740870003485. [DOI] [PubMed] [Google Scholar]
- Hausrath AC, Matthews BW. Thermolysin in the absence of substrate has an open conformation. Acta Cryst. D. 2002;58:1002–1007. doi: 10.1107/s090744490200584x. [DOI] [PubMed] [Google Scholar]
- Holyoak T, Fenn TD, Wilson MA, Moulin AG, Ringe D, Petsko GA. Malonate: a versatile cryoprotectant and stabilizing solution for salt-grown macromolecular crystals. Acta Crystallogr D Biol Crystallogr. 2003;59:2356–2358. doi: 10.1107/s0907444903021784. [DOI] [PubMed] [Google Scholar]
- Hope H. Cryocrystallography of biological macromolecules: a generally applicable method. Acta Cryst. B. 1988;44:22–26. doi: 10.1107/s0108768187008632. [DOI] [PubMed] [Google Scholar]
- Hubalek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46:205–229. doi: 10.1016/s0011-2240(03)00046-4. [DOI] [PubMed] [Google Scholar]
- Juers DH, Matthews BW. Reversible lattice repacking illustrates the temperature dependence of macromolecular interactions. J. Mol. Biol. 2001;311(4):851–862. doi: 10.1006/jmbi.2001.4891. [DOI] [PubMed] [Google Scholar]
- Juers DH, Matthews BW. Cryo-cooling in macromolecular crystallography: advantages, disadvantages and optimization. Q. Rev. Biophys. 2004;37:105–119. doi: 10.1017/s0033583504004007. [DOI] [PubMed] [Google Scholar]
- Kantardjieff KA, Rupp B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA, protein-nucleic acid complex crystals. Protein Sci. 2003;12:1865–1871. doi: 10.1110/ps.0350503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefersauer R, Than ME, Dobbek H, Gremer L, Melero M, Srobl S, Dias JM, Soulimane T, Huber R. A novel free-mounting system for protein crystals: transformation and improvement of diffraction power by accurately controlled humidity changes. J. Appl. Cryst. 2000;33:1223–1230. [Google Scholar]
- Kim CU, Kapfer R, Gruner SM. High-pressure cooling of protein crystals without cryoprotectants. Acta Crystallogr D Biol Crystallogr. 2005;61:881–890. doi: 10.1107/S090744490500836X. [DOI] [PubMed] [Google Scholar]
- Kmetko J, Husseini NS, Naides M, Kalinin Y, Thorne RE. Quantifying X-ray radiation damage in protein crystals at cryogenic temperatures. Acta Cryst. D. 2006;62:1030–1038. doi: 10.1107/S0907444906023869. [DOI] [PubMed] [Google Scholar]
- Ko TP, Day J, Greenwood A, McPherson A. Structures of three crystal forms of the sweet protein thaumatin. Acta Cryst. D. 1994;50:813–825. doi: 10.1107/S0907444994005512. [DOI] [PubMed] [Google Scholar]
- Kriminski S, Caylor CL, Nonato MC, Finkelstein KD, Thorne RE Laboratory of, A., Solid State Physics, C.U.I.N.Y.U.S.A. Flash-cooling and annealing of protein crystals. Acta Cryst. D. 2002;58((Pt) 3):459–471. doi: 10.1107/s0907444902000112. [DOI] [PubMed] [Google Scholar]
- Leiros HKS, McSweeney SM, Smalas AO. Atomic resolution structures of trypsin provide insight into structural radiation damage. Acta Cryst. D. 2001;57:488–497. doi: 10.1107/s0907444901000646. [DOI] [PubMed] [Google Scholar]
- Low BW, Chen CCH, Berger JE, Singman L, Pletcher JF. Studies of Insulin Crystals at Low Temperatures: Effects on Mosaic Character and Radiation Sensitivity. Proc. Natl. Acad. Sci. U.S. A. 1966;56:1746–1750. doi: 10.1073/pnas.56.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H, Venkat M, Seng NS, Cahn J, Juers DH. The use of trimethylamine N-oxide as a primary precipitating agent and related methylamine osmolytes as cryoprotective agents for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2012;68:69–81. doi: 10.1107/S0907444911050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BW. Solvent content of protein crystals. J. Mol. Biol. 1968;33:491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McPherson A. Crystallization of Biological Macromolecules. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- Mueller-Dieckmann C, Kauffmann B, Weiss MS. Trimethylamine N-oxide as a versatile cryoprotective agent in macromolecular crystallography. J. Appl. Cryst. 2011;44:433–436. [Google Scholar]
- Owen RL, Rudino-Pinera E, Garman EF. Experimental determination of the radiation dose limit for cryocooled protein crystals. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4912–4917. doi: 10.1073/pnas.0600973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton TA, Still BR, Christensen EM, Singh H, Srivastava D, Tanner JJ. Proline: Mother Nature's cryoprotectant applied to protein crystallography. Acta Cryst. D. 2012;68:1010–1018. doi: 10.1107/S0907444912019580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson KA, Ladner JE, Tordova M, Gilliland GL. Cryosalts: suppression of ice formation in macromolecular crystallography. Acta Cryst. D. 2000;56:996–1001. doi: 10.1107/s0907444900007587. [DOI] [PubMed] [Google Scholar]
- Russi S, Juers DH, Sanchez-Weatherby J, Pellegrini E, Mossou E, Forsyth VT, Huet J, Gobbo A, Felisaz F, Moya R, McSweeney SM, Cusack S, Cipriani F, Bowler MW. Inducing phase changes in crystals of macromolecules: status and perspectives for controlled crystal dehydration. J. Struct. Biol. 2011;175:236–243. doi: 10.1016/j.jsb.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Sanchez-Weatherby J, Bowler MW, Huet J, Gobbo A, Felisaz F, Lavault B, Moya R, Kadlec J, Ravelli RBG, Cipriani F. Improving diffraction by humidity control: a novel device compatible with X-ray beamlines. Acta Cryst. D. 2009;65:1237–1246. doi: 10.1107/S0907444909037822. [DOI] [PubMed] [Google Scholar]
- Shah BN, Chinte U, Tomanicek SJ, Hanson BL, Schall CA. Flash Cooling Protein Crystals: Estimate of Cryoprotectant Concentration Using Thermal Properties. Cryst. Growth Des. 2011;11:1493–1501. [Google Scholar]
- Thomanek UF, Parak F, Mossbauer RL, Formanek H, Schwager P, Hoppe W. Freezing of Myoglobin Crystals at High Pressure. Acta Cryst. A. 1973;29:263. [Google Scholar]
- Vera L, Stura EA. Strategies for Protein Cryocrystallography. Cryst. Growth Des. 2014;14:427–435. [Google Scholar]
- Warkentin M, Sethna JP, Thorne RE. Critical Droplet Theory Explains the Glass Formability of Aqueous Solutions. Phys. Rev. Lett. 2013;110 doi: 10.1103/PhysRevLett.110.015703. [DOI] [PubMed] [Google Scholar]
- Yaws CL. Chemical Properties Handbook. McGraw-Hill; 1999. [Google Scholar]