Abstract
Objectives:
We evaluated neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C in comparison to established markers of renal function in patients with heart failure (HF).
Background:
Patients with advanced HF develop progressive renal dysfunction. The renal biomarkers NGAL and cystatin C might improve prognostic assessment of patients with HF.
Methods:
Serum samples were collected from 40 patients with stable HF (58±8 yrs, BMI 29.4±4.2 kg/m2), 40 HF patients undergoing ventricular assist device (VAD) implantation (53±11 yrs, BMI 26.8±5.5 kg/m2) and VAD removal at cardiac transplantation, and 24 controls (48±7 yrs, BMI 29.4±4.2 kg/m2). Clinical data were collected from institutional medical records. NGAL and cystatin C levels were measured by ELISA and eGFR calculated using MDRD formula.
Results:
Patients with stable HF showed elevated NGAL and cystatin C levels compared to controls (114.2±52.3 ng/mL vs. 72.0±36.1 ng/mL, p<0.0001; cystatin C: 1490.4±576.1 ng/mL vs. 986.3±347.5 ng/mL, p=0.0026). Unlike cystatin C, NGAL increased in advanced HF requiring VAD implantation (158.7±74.8 ng/mL, p<0.001). On VAD, NGAL levels decreased (127.1±80.4 ng/mL, p=0.034). NGAL was higher in patients who developed right ventricular failure (187.8±66.0 vs. 130.9±67.0 ng/mL, p=0.03) and irreversible renal dysfunction (190.0±73.8 ng/mL vs. 133.8±54.2 ng/mL, p<0.05) while cystatin C, creatinine and eGFR were not different. NGAL correlated with eGFR (r=−0.2188, p=0.01).
Conclusions:
NGAL levels correlate with HF severity and hemodynamic improvement after VAD placement. Our findings suggest a role of this novel biomarker as a marker of severity and prognosis in patients with HF.
Keywords: Heart Failure, Renal Function, NGAL
Heart failure (HF) is a growing public health concern, affecting nearly 5.8 million Americans and is associated with poor prognosis 1. The therapeutic gold standard for end-stage HF remains cardiac transplantation, but the prevalence of HF continues to rise and the supply of available donor hearts has become increasingly inadequate 2. Thus, the use of ventricular assist devices (VADs) has become commonplace for end-stage HF as a bridge-to-transplantation or as destination therapy 2. As such, more information is needed on the effects VAD placement, subsequent mechanical unloading and increased peripheral perfusion, has on other important organ systems.
Cardiovascular morbidity and mortality correlate strongly with impaired renal function 3 and HF is accompanied by progressive renal dysfunction. Such pathophysiology is often further complicated by neurohormonal derangements that favor overproduction of vasoconstrictors with concurrent pathologic alterations in mediators of vasodilation. 4. Together, these hormonal fluctuations, plus the genetic, inflammatory, and biochemical changes that occur during the onset and progression of HF weaken the myocardium directly and negatively impact the function of the kidneys 3, 5.
Renal function is routinely assessed using serum creatinine concentration and calculation of glomerular filtration rate (eGFR). However, because creatinine is a product of muscle breakdown, its interpretation as a marker of kidney function is confounded by age, gender, ethnicity and body mass 6. Other renal function measures, such as the Modification of Diet in Renal Disease (MDRD) eGFR equation compensate for these variables but are limited due to its development in a population diagnosed with chronic kidney disease 7. As such, it is desirable to seek more accurate biomarkers of renal dysfunction that also have prognostic and predictive value for clinical events.
Recently, the novel serum biomarkers, neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C, have shown promise in providing rapid diagnosis of kidney injury and improved prognostic assessment in various patient populations at risk for renal dysfunction 8-12. NGAL is a 25kDa glycoprotein and is expressed at low levels in the normal kidney, trachea and colon, among other organs 8, 13, 14.NGAL expression is induced systemically (liver and spleen) and in immune cells in response to ischemic damage or other kidney insult14, 15. NGAL is elevated in HF patients and thought to be superior to creatinine as an early predictor of acute and chronic kidney injury and thus, renal dysfunction 8, 11, 16.
Cystatin C is an endogenous 13 kDa cysteine protease inhibitor expressed by nucleated cells of the body 6, 17. Increased levels of cystatin C are linked to impaired kidney function and associated with a corresponding decrease in eGFR 17. Previous research has found cystatin C to be potentially superior to creatinine in predicting renal dysfunction and is an independent cardiovascular risk factor 6, 17.
As uncertainty exists as to whether NGAL or cystatin C is the superior diagnostic and prognostic biomarker, our study aimed to characterize the role of NGAL and cystatin C in comparison to established markers of renal function (creatinine and GRF) in patients with HF, and investigate the influence of VAD placement on renal dysfunction as measured by these serum biomarkers. Since VAD implantation improves hemodynamic physiology, we hypothesized that VAD-placement will remove excess physiological stress on the kidneys and reverse renal dysfunction, providing a subsequent decrease of all measured biomarkers.
METHODS
Study design
We completed a retrospective, cross-sectional study of 138 patients at Columbia University Medical Center which included controls without HF (n=24), stable HF patients (NYHA II-III, n=40), patients with advanced HF undergoing VAD implantation (NYHA IV, n=40), and patients undergoing VAD removal (n=40).Paired samples were obtained from 22 individual patients before and after VAD support. Demographic and clinical information was extracted from the institutional medical records of Columbia University Medical Center. In patients undergoing VAD placement, creatinine, eGFR, cystatin C, and NGAL were correlated to the development of right ventricular failure (RVF) defined by the Interagency Registry for Mechanically Assisted Circulatory Support criteria and dynamics in renal function 18.
The Institutional Review Board approved this study and all patients provided written informed consent.
Serum analysis
Blood was drawn from controls and all stable HF subjects at the time of enrollment, while blood samples were collected at time of surgery for VAD implant and explant. Whole blood was collected into serum separator tubes, centrifuged, and harvested serum was stored at −80°C until the biomarker assays were performed.
Serum concentrations of NGAL and cystatin C were determined by commercially available enzyme-linked immunosorbent assay (ELISA, Quantikine, R&D Systems, Minneapolis, MN, USA). Optical densities corrected for background noise and biomarker concentration was calculated using a unique standardized curve. Circulating levels of creatinine were analyzed by the institutional core laboratory. The eGFR was calculated using MDRD Study equation. Renal dysfunction was defined by a creatinine value >1.5 mg/dL. Improvement of renal function was defined as a decrease in creatinine by >25% of baseline values in patients with elevated creatinine levels at the time of VAD implantation.
Statistical analyses
Data were analyzed using GraphPad Prism, Version 5.0b (San Diego, CA, USA), and are presented as means±SD. For categorical variables, results are presented as relative frequency. Normality was evaluated for each variable by the Kolmogorov-Smirnov test. Biomarker values and eGFR calculations before and after VAD placement were assessed using Student’s paired t-test or Wilcoxon signed rank test. Unpaired analyses of the renal markers were completed using unpaired Student’s t-test or Mann Whitney test. Correlations were determined using the Spearman coefficient. All tests were two-tailed. Differences among three or more groups were analyzed using one-way analysis of variance (ANOVA) (parametric) or Kruskal-Wallis test. Tukey and Dunn’s multiple comparison post-hoc tests were used. Sensitivity and specificity were assessed using conventional receiver-operating curves, as well as calculation of the areas under the curve (AUC). A p value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Clinical characteristics of all patients are summarized in Table 1. Significant differences are shown among the groups with regard to body mass index (BMI) .In patients with severe HF before VAD implantation, HF duration ranged from 31 to 6800 days (median 1637 days). The duration of VAD support ranged from 28 days to 508 days (mean 164±123 days). 18 patients (45%) received a pulsatile and 22 patients (55%) received a continuous flow VAD. Table 2 summarizes laboratory examinations comparing controls, stable and severe HF patients before and after VAD placement.
Table 1.
Patient Characteristics.
| Controls (n=24) |
Stable HF (n=40) |
VAD Implant (n=40) |
VAD Explant (n=40) |
|
|---|---|---|---|---|
| Age (yrs) | 48±7.1 | 58±8.4 | 53±11.2 | 53.5±11 |
| Gender (% male) | 11 (61%) | 25 (83%) | 28 (82%) | 27 (82%) |
| BMI (kg/m2) | 28.4±6.4 | 29.4±4.2 | 26.8±5.5 | 26.1±4.3 |
| Etiology of HF (n,%) | ||||
| Dilated CMY | NA | 18 (60%) | 21 (62%) | 19 (58%) |
| Ischemic CMY | 11 (37%) | 13 (38%) | 13 (39%) | |
| Other | 1 (3%) | 0 (0%) | 1 (3%) |
Data show means±SD. Significance tested using ANOVA post-hoc Tukey analysis.
Abbreviations not defined in the text: BMI - body mass index.
Table 2.
Laboratory Data.
| Controls (n=24) |
Stable HF (n=40) |
VAD Implant (n=40) |
VAD Explant (n=40) |
|
|---|---|---|---|---|
| BUN (mg/dL) | 14.9±3.4 | 23.7±11.9 | 40.0±25.7†‡ | 26.7±15.5 |
| Crea (mg/dL) | 1.0±0.2 | 1.2±0.4 | 1.5±0.6† | 1.2±0.4 |
| eGFR (mL/min/1.73 m2) | 76.5±17 | 66.0±22 | 53±33† | 69±25 |
Data show means±SD. Significance tested using ANOVA with post-hoc Tukey analysis.
Abbreviations not defined in text: BUN - blood urea nitrogen and Creat - creatinine.
p<0.05 for NYHA HF Class IV pre-VAD vs Control;
p<0.05 for NYHA HF Class IV pre-VAD vs NYHA HF Class II-III.
Serum levels of biomarkers of renal dysfunction
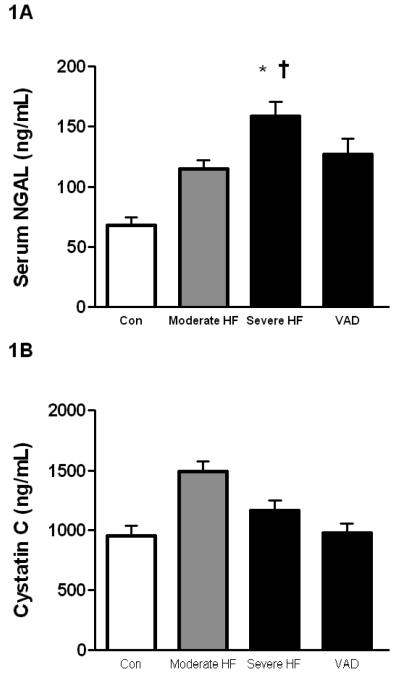
To characterize the impact of HF on renal dysfunction, we analyzed circulating levels of NGAL and correlated values to creatinine and eGFR. We found that all patients with HF (NYHA II-IV) showed elevated NGAL levels compared to controls (137.3±66.0 ng/mL vs. 72.0±36.1 ng/mL, p<0.0001). This increase was even stronger in the subgroup of patients with advanced HF pre-VAD implantation (NYHA IV) as compared to controls (158.7±74.8 ng/mL vs. 72.0±36.1 ng/mL, p<0.0001, Figure 1A), indicating a stepwise increase.
Figure 1. Serum levels of NGAL and Cystatin C in controls, patients with stable and severe HF, and on VAD support.
(1A) NGAL levels increase in correlation to clinical severity of HF and decrease following hemodynamic improvement after VAD placement. (*p<0.01 versus control; †p<0.05 versus moderate HF).
Similar to the results seen for NGAL, patients with stable HF showed elevated cystatin C levels compared to controls (1490.4±576.1 ng/mL vs. 954.7±414.2 ng/mL, p=0.0026, Figure 1B). Unlike NGAL, however, when NYHA Class IV patients were compared to controls, no difference was shown (1163.7±531.7 ng/mL vs. 954.7±414.2 ng/mL, p=NS, Figure 1B) indicating cystatin C does not follow a similar increase with disease severity
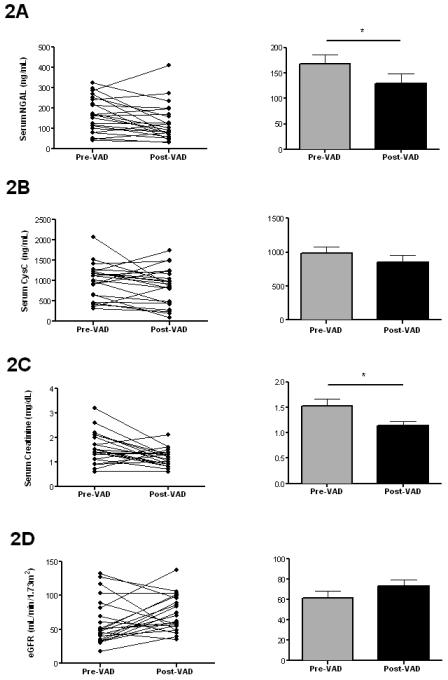
Further, when matched-pair analysis was performed for severe HF patients who underwent both VAD implantation and removal (n=22), we found that post-VAD NGAL decreased compared to pre-VAD levels (129.1±91.1 ng/mL vs. 168.0±85.6 ng/mL pre-VAD, p=0.0296, Figure 2A). No differences in changes of NGAL during VAD support were detectable between the group of patients on continuous compared to patients on pulsatile VADs (68.3±88.5 versus 25.2±72.0 ng/mL in pulsatile VADs, p=NS). Matched-pair analysis revealed that VAD placement had no significant effect on cystatin C levels (855.0±452.9 ng/mL vs. 985.5±433.5 ng/mL pre-VAD, p=NS, Figure 2B). No differences in changes of cystatin C levels during VAD support were detectable between the group of patients on continuous compared to patients on pulsatile VADs (+31.7±389.9 versus −206.1±409.1 ng/mL in pulsatile VADs, p=NS).
Figure 2. Effects of ventricular assist device (VAD) placement on serum NGAL, cystatin C and routine markers of renal function.
NGAL (A) and creatinine (C) improve during VAD support in patients with advanced HF (both p<0.05 versus pre-VAD) while cystatin C (B) and eGFR (D) show only a trend towards improvement post-VAD. Individual data shown for matched pairs on the left, average group data shown on the right (n=22 matched pairs, *p<0.05 versus pre-VAD).
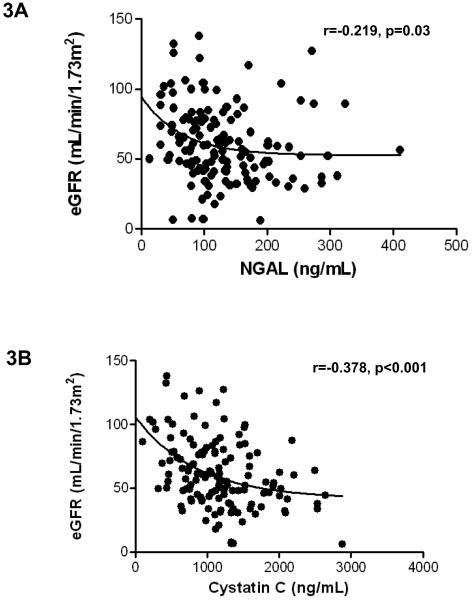
Of the entire cohort, available creatinine values and eGRFs were examined. Patients with advanced HF have higher levels of creatinine compared to controls (Table 2). In matched pairs, a decrease in creatinine levels post-VAD placement was observed (1.5±0.7 mg/dL vs. 1.1±0.4 mg/dL, p=0.01, Figure 2C). A trend towards better recovery of renal dysfunction was noted in patients supported by pulsatile devices compared to continuous flow VADs (0.1±0.6 versus 0.5±0.6 ng/mL in pulsatile VADs, p=0.1). Our data further illustrate significant correlations between eGFR and NGAL and cystatin C. NGAL correlated with eGFR (r=−0.2188 p=0.01, Figure 3A), and cystatin C correlated with eGFR (r=−0.3781, p<0.0001, Figure 3B)
Figure 3. Correlation of NGAL and cystatin C with eGFR.
Analysis of predictors of renal functional recovery
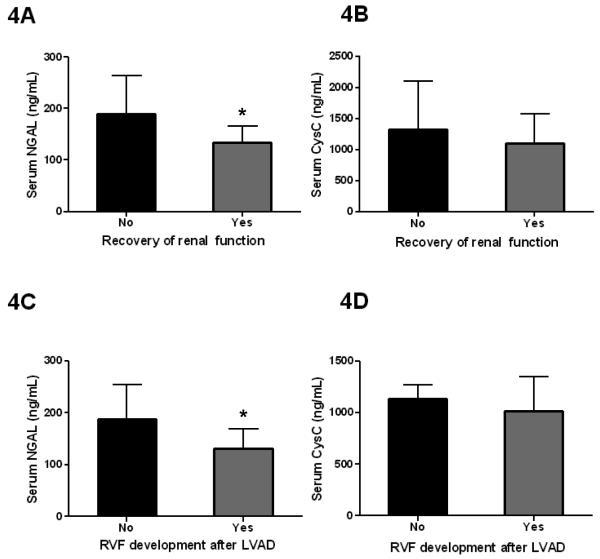
In severe HF patients receiving LVAD support, we found that levels of NGAL are predictive for reversibility of renal dysfunction (Figure 4A). Improvement of renal function was defined as a decrease in creatinine by >25% of baseline values in patients with elevated creatinine levels at the time of VAD implantation. Patients who did not recover from renal dysfunction had elevated NGAL levels as compared to those who did recover (190.0±73.8 ng/mL vs. 133.8±54.2 ng/mL, p=0.0483, Figure 4A). Receiver-operating characteristic (ROC) curve analysis revealed an area under the curve (AUC) of 0.7273. From this data, we show that NGAL levels greater than 200 ng/mL indicate irreversible renal dysfunction with 88.9% certainty, while patients with NGAL levels below 200 ng/mL have a 45.5% chance of recovery.
Figure 4. Analysis of NGAL and cystatin C levels for prediction of worsening renal function and right ventricular failure.
(A) Patients who did not recover from renal failure had elevated NGAL levels as compared to those who did show reversibility. At NGAL levels over 200 ng/mL, renal failure is unlikely reversible (88.9% sensitivity); however, at NGAL levels under 200 ng/mL, there is a 45.5% chance of recovery. Black bar indicates the values in patients without recovery of renal function and the gray bar indicates the values in those with recovery of renal function. (B) Cystatin C levels could not predict recovery of renal function after LVAD surgery. (C) Patients who developed right ventricular failure had elevated NGAL levels as compared to those who did not. At NGAL levels greater than 100 ng/mL, patients were likely to develop right ventricular failure (93.3% sensitivity); however, at NGAL levels under 100 ng/mL, there is only a 40.0% chance of development of right ventricular failure. Black bar indicates the values in patients without right ventricular (RV) failure after LVAD surgery and the gray bar indicates the values in those who developed RV failure (*p<0.05). (D) Unlike NGAL, cystatin C levels did not predict development of right ventricular failure after LVAD surgery.
By the same analysis, cystatin C (1331.0±772.2 ng/mL vs. 1103.9±475.0 ng/mL, Figure 4B) and creatinine (1.5±0.4 mg/dL vs. 1.8±0.5 mg/dL) were not predictive of recovery of renal failure.
Analysis of predictors of right ventricular dysfunction following VAD placement
One of the most severe complications following VAD implantation is the development of RVF, which is associated with high morbidity and mortality. As with renal function, in advanced HF patients receiving left VAD therapy, NGAL was elevated in those who developed RVF compared to those who did not develop RVF (187.8±66.0 ng/mL vs. 130.9±67.0 ng/mL, p=0.0265, Figure 4C). ROC analysis revealed an AUC of 0.7244. Our data indicate that NGAL levels greater than 100 ng/mL predict the development of RVF with 93.3% certainty, while patients with NGAL levels below 100 ng/mL have only a 40.0% chance of development of RVF.
By the same analysis, cystatin C (1135.4±231.3 ng/mL vs. 1016.9±580.8 ng/mL, Figure 4D), creatinine (1.7±0.7 mg/dL vs. 1.5±0.6 mg/dL) and eGFR (53.4±33.0 mL/min/1.73m2 vs. 60.4±29.6 mL/min/1.73m2) showed no difference between patients who developed right ventricular dysfunction and those who did not.
DISCUSSION
In the current study, we demonstrate that circulating levels of NGAL, a novel biomarker of renal dysfunction, increases in patients with HF, correlate with impairment of renal function in HF patients and decrease following VAD implantation likely due to hemodynamic improvement. Cystatin C, another novel biomarker of renal function, shows a similar increase in patients with HF. However, a matched pair analysis of patients before and after VAD placement failed to show a significant change in cystatin C levels. NGAL outperformed cystatin C, creatinine and eGFR as predictor of recovery of renal dysfunction and occurrence of RVF following VAD implantation. Thus, NGAL is a novel and sensitive biomarker of renal dysfunction in patients with HF.
To date, both NGAL and Cystatin C are shown to be promising biomarker indicators of renal injury and dysfunction 9-12, 14, 16. NGAL is a glycoprotein that exists at low levels under normal physiologic conditions, but its expression is induced from various cell types during inflammation, or in response to ischemic damage or other kidney insult 13, 15. NGAL is filtered by the glomeruli and taken up at the proximal tubule 14. Yndestad et al. have described increased NGAL levels in patients with both acute and chronic HF and following myocardial infarction, indicative of a decline in renal function 19. Prior studies also suggested that increased levels of NGAL predict acute kidney injury and renal dysfunction in various patient populations 9-12, 16. In fact, NGAL has been described as superior to creatinine for both acute kidney injury 10, 12 and chronic kidney disease 11. In large part, because NGAL levels increase within hours to indicate worsening renal function whereas creatinine must first reach a steady state, a process which can take up to 48 hours 12. Our results support each of the above assertions as we show elevated NGAL levels in patients affected by HF, an increase that is mitigated by VAD placement due to hemodynamic improvement, increased perfusion at the kidney, and other physiological factors that remain to be elucidated.
Cystatin C is a cysteine protease inhibitor continuously produced by all nucleated cells 6, 17. Physiologically, serum cystatin C concentration is a direct result of decreased glomerular filtration rate (GFR) as the marker is freely filtered at the glomerulus, completely taken up at the tubules, and immediately degraded, preventing its return to circulation and making it a good proxy of GFR 17. It is an ideal biomarker because blood samples are readily obtained from patients and cystatin C is easily measured in the laboratory. However, there appears to be some disagreement whether cystatin C levels are affected by age, gender, and BMI 16, 20 – all of which confound the interpretation of creatinine – nevertheless, the association of cystatin C with GFR is unaffected by these factors 6. Cystatin C has been suggested as superior or equal to eGFR in assessment of renal dysfunction 17 and may be more sensitive to small changes in eGFR than creatinine 6. One prior study further asserts that increased circulating levels of the marker were shown to be an independent predictor of HF in adults over 65 years of age 6. Others have also associated high cystatin C levels with left ventricular hypertrophy, atherosclerosis, cardiovascular events, low-grade inflammation and long term mortality 16, 20, 21. Overall, our data is consistent with previous findings in that cystatin C is elevated in HF patients, and strongly correlates with current laboratory markers of renal function.
Our study validates NGAL and cystatin C as markers of renal dysfunction in a patient population affected by HF, and characterizes the potential use of these novel biomarkers for assessing renal function before and after VAD placement, predicting associated clinical events. Furthermore, we introduce NGAL as a novel predictive biomarker for clinical events such as recovery of renal dysfunction and incidence of RVF in advanced HF patients undergoing VAD placement. Our data show that NGAL outperforms both established markers of renal function and cystatin C but the underlying biologic mechanisms explaining this advantage are unclear. The prognostic value of these findings is clinically relevant, as we provide a concentration cutoff of 200 ng/mL, the level at which it is 88.9% certain that a patient’s renal dysfunction is no longer reversible during VAD support. Likewise, we provide evidence illustrating elevated NGAL levels in patients undergoing VAD placement who developed subsequently RVF as compared to those who did not develop right ventricular dysfunction. Our results provide a concentration cutoff of 100 ng/mL, the level at which it is 93.3% certain that a patient will develop RVF during VAD support. At NGAL concentrations lower than 100 ng/mL, patients have only a 40.0% chance of developing RVF. Due to the established high morbidity and mortality associated with RVF post-VAD placement, the predictive value of NGAL is clinically important and could have future implications in improving the management of patients with HF and VAD placement. Further analyses evaluating the prognostic value of cystatin C, creatinine and eGFR showed no predictive power in regard to recovery of renal dysfunction or development of RVF, confirming that NGAL is superior to conventionally accepted markers of renal function.
Another interesting finding of the present study is the correlation of changes in NGAL and creatinine in patients with HF before and after VAD implantation. NGAL levels decrease in patients with advanced HF following VAD placement to levels close to that of normal individuals. In contrast, creatinine and to some extent also eGFR improve in patients on VAD support. One might postulate that increases in NGAL levels are related to the low cardiac output state and potential tissue hypoxygenation in advanced HF which is partially reversible with LVAD placement.
Our study has several limitations. First, the analysis is limited by its retrospective and observational nature, which compares patients with HF at different stages and interventions performed over various time periods. Second, the patients were not consecutively selected. We evaluated only patients whose blood samples were collected at the time of LVAD implantation and device removal and our data are restricted to the time points of LVAD implantation and explantation. Third, our study was not controlled for concurrent inflammatory states including bacterial infections or autoimmune diseases, both known to increase NGAL levels 19. Lastly, we studied patients from a single medical center, and though our findings support NGAL as a novel and sensitive biomarker of renal dysfunction and clinical outcomes for HF patients, additional research is needed in larger and more diverse patient populations to further evaluate and validate the marker.
In summary, we show elevated NGAL levels in patients with HF correlate with impairment of renal function. VAD placement induces hemodynamic improvement and subsequent decrease in serum NGAL illustrating the reversibility of renal dysfunction in advanced HF. Further, elevated levels of NGAL predict irreversible renal dysfunction and the development of RVF following VAD implantation. Our results indicate that NGAL is superior to standard markers of renal function and proves to be a novel and sensitive biomarker of renal dysfunction and clinical events in patients with advanced HF.
Acknowledgements / Funding Sources
This work was supported by grants from the National Heart, Lung and Blood Institute (K23 HL095742-01, P30 HL101272-01, UL1 RR 024156, HL073029) and the Herbert and Florence Irving Scholar Award to Dr. Schulze.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
REFERENCES
- 1.Heart Failure Society of A. Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010;16(6):e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Lund LH, Matthews J, Aaronson K. Patient selection for left ventricular assist devices. European journal of heart failure. 2010;12(5):434–443. doi: 10.1093/eurjhf/hfq006. [DOI] [PubMed] [Google Scholar]
- 3.Pokhrel N, Maharjan N, Dhakal B, et al. Cardiorenal syndrome: A literature review. Experimental and clinical cardiology. 2008;13(4):165–170. [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco C, Maisel A. Volume overload and cardiorenal syndromes. Congestive heart failure. 2010;16(Suppl 1):Si–iv. doi: 10.1111/j.1751-7133.2010.00176.x. quiz Svi. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E. Biomarkers in heart failure. The New England journal of medicine. 2008;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Katz R, Stehman Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Annals of internal medicine. 2005;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 7.Murata K, Baumann NA, Saenger AK, et al. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(8):1963–1972. doi: 10.2215/CJN.02300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology : JASN. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 9.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 10.Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105(3):485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatric nephrology. 2007;22(1):101–108. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 12.Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Annals of internal medicine. 2008;148(11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malyszko J, Bachorzewska-Gajewska H, Sitniewska E, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2-4 chronic kidney disease. Renal failure. 2008;30(6):625–628. doi: 10.1080/08860220802134607. [DOI] [PubMed] [Google Scholar]
- 14.Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Critical care. 2007;11(6):R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt-Ott KM, Mori K, Kalandadze A, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Current opinion in nephrology and hypertension. 2006;15(4):442–449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 16.Yanavitski M, Givertz MM. Novel biomarkers in acute heart failure. Current heart failure reports. 2011;8(3):206–211. doi: 10.1007/s11897-011-0065-5. [DOI] [PubMed] [Google Scholar]
- 17.Lassus J, Harjola VP. Cystatin C: a step forward in assessing kidney function and cardiovascular risk. Heart failure reviews. 2012;17(2):251–261. doi: 10.1007/s10741-011-9242-6. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28(6):535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Yndestad A, Landro L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. European heart journal. 2009;30(10):1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 20.Evangelopoulos AA, Vallianou NG, Bountziouka V, et al. Association between serum cystatin C, monocytes and other inflammatory markers. Internal medicine journal. 2012;42(5):517–522. doi: 10.1111/j.1445-5994.2011.02500.x. [DOI] [PubMed] [Google Scholar]
- 21.Koenig W, Twardella D, Brenner H, et al. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clinical chemistry. 2005;51(2):321–327. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]