Abstract
C57BL6 mice display non-eupneic breathing and spontaneous apneas during wakefulness and sleep as well as markedly disordered breathing following cessation of a hypoxic challenge. We examined whether (1) C57BL6 mice display marked non-eupneic breathing following hypercapnic or hypoxic-hypercapnic challenges, and (2) compared the post-hypoxia changes in non-eupneic breathing of C57BL6 mice to those of B6AF1 (57BL6 dam × A/J sire) and Swiss-Webster mice, which display different ventilatory responses than C57BL6 mice. C57BL6 mice displayed marked increases in respiratory frequency and non-eupneic breathing upon return to room-air after hypoxic (10% O2, 90% N2), hypercapnic (5% CO2, 21% O2, 74% N2) and hypoxic-hypercapnic (10% O2, 5% CO2, 85% N2) challenges. B6AF1 mice displayed less tachypnea and reduced non-eupneic breathing post-hypoxia, whereas Swiss-Webster mice displayed robust tachypnea with minimal increases in non-eupneic breathing post-hypoxia. These studies demonstrate that non-eupneic breathing increases after physiologically-relevant hypoxic-hypercapnic challenge in C57BL6 mice and suggest that further studies with these and B6AF1 and Swiss-Webster mice will help define the genetics of non-eupneic breathing.
Keywords: Non-eupneic breathing, hypoxic-hypercapnic gas challenge, C57BL6 mice, B6AF1 mice, Swiss-Webster mice
1. Introduction
Sleep-related breathing disorders during sleep states and periods of wakefulness affect 9-14% of the US population (Dempsey et al., 2010). Studies in normal and transgenic mice have been used widely as models of sleep apnea (see Yamauchi et al., 2010). The C57BL6 mouse is a common inbred strain that is widely used in ventilatory and pulmonary function studies (Soliz et al., 2008, 2009) and to produce mice lacking genes for numerous functional proteins (Kline et al., 2002; Liu et al., 2004; Duling et al., 2006; Palmer et al., 2013b). The C57BL6 mouse was presumably chosen to be a model with “normal” physiology and indeed they display many normal traits (see Tankersley et al., 2002a; Campen et al., 2004, 2005; Tewari et al., 2013; Palmer et al., 2013a,b; Gaston et al., 2014). For example, resting systemic and pulmonary arterial blood pressures and cardiovascular responses upon challenges with hypoxic (HX), hypercapnic (HC) and hypoxic-hypercapnic (H-H) gas mixtures are representative of those observed in “normal” mice and rats (Campen et al., 2004, 2005; Tewari et al., 2013). Moreover, the ability of HC to modulate the effects of HX on arterial blood pressure (HX elicits pronounced depressor response, HC elicits a minor pressor response, H-H elicits a minimal response) appears to be intact in C57BL6 mice (Campen et al., 2004, 2005).
Following exposure of mice to a HX challenge, the return to room-air results in respiratory patterns classified as short-term potentiation (STP), in which ventilation remains elevated (Powell et al., 1998) or post-hypoxic frequency decline (PHFD) in which breathing frequency falls below baseline (Dick and Coles, 2000). One mechanism for initiation and propagation of unusual breathing patterns is the disturbed balance between STP and PHFD with the dominance of PHFD (i.e., absence of STP and expression of PHFD) promoting disordered breathing (Powell et al., 1998; Yamauchi et al., 2008a,b,c; Strohl, 2003; Younes, 2008; Yamauchi et al., 2010). Eupnea (quiet breathing) is the term for normal ventilation (see Mitchell et al., 1975). Eupnea reflects the output of a pontomedullary neuronal circuit that drives inspiratory motor neurons (St-John and Paton, 2004; Garcia et al., 2011). During eupneic breathing, neural output to respiratory muscles is regular, with rhythmic bursts of motor nerve activity to the diaphragm and external (but not internal) intercostal muscles causing inspiration. Expiration during eupneic breathing is passive and occurs via simple elastic recoil of the lungs (Mitchell et al., 1975; St-John and Paton, 2004; Garcia et al., 2011). In contrast, disordered breathing is characterized by abnormal breaths (disturbed balance of inspiratory and expiratory volume per breath) during rest, and the presence of recurrent spontaneous apneas (pauses), sighs and sniffs, and irregular breaths due to abrupt movements such as grooming and locomotor activity (Han and Strohl, 2000; Han et al., 2001, 2002; Price et al., 2003; Strohl, 2003; Yamauchi et al., 2007, 20008a,b,c, 2010; Chai et al., 2011; Gillombardo et al., 2012; Strohl et al., 2012).
The mechanisms responsible for post-HX disordered breathing have received considerable attention. At present, evidence is in favor of disturbances in central signaling (Wilkinson, 1997; Strohl, 2003) including the pons (Coles and Dick, 1996; Dick and Coles, 2000) rather than processes within the carotid bodies (Vizek et al., 1987; Brown et al., 1993) although it is evident that carotid body chemoafferents play an essential role in the expression of sleep apnea (Smith et al., 2003). Despite considerable normal physiology, the C57BL6 mouse is of major interest to sleep-apnea researchers because it displays disordered breathing (irregular breaths and irregular breathing patterns including apneas, sighs, sniffs) during sleep and wakefulness and markedly disordered breathing upon return to room-air after exposure to HX gas challenges (Han et al., 2000, 2001, 2002; Tagaito et al., 2001; Yamauchi et al., 2008a,b,c, 2010). Post-HX breathing in sleep-apnea patients is associated with episodes of glottal closures (Dempsey et al., 2010; Strohl et al., 2012). The finding that the working heart-brainstem preparation from C57BL6 mice displays spontaneous central apneas associated with active laryngeal closure (Stettner et al., 2008) further supports the concepts that disordered breathing in conscious unrestrained C57BL6 mice include obstruction of the upper airway, and that this strain is a true model of sleep apnea (Stettner et al., 2008).
The genetic bases for sleep disordered breathing in mouse strains have received extensive investigation (Tankersley et al., 1994, 1998, 2000, Tankersley, 2000, 2001, 2003; Han and Strohl, 2000; Han et al., 2001, 2002; Tagaito et al., 2001; Schneider et al., 2003; Strohl, 2003; Tankersley and Broman, 2004; Balbir et al., 2006; Yamauchi et al., 2008b; Gillombardo et al., 2012) as have the neurochemical processes (Tankersley et al., 2002b; Price et al., 2003; Groeben et al., 2005; Yamauchi et al., 2007, 2008a, 2012; Moore et al., 2012, 2014), and structural features of respiratory structures such as the carotid bodies (Yamaguchi et al., 2003, 2006; Chai et al., 2011).
Despite intensive investigation of post-HX breathing, the possibility that disordered breathing in C57BL6 mice increases upon return to room-air following exposures to HC challenges and especially more physiological/pathophysiological relevant H-H challenges (see Dempsey et al., 2010) has received minimal attention (see Yamauchi et al., 2007, 2008c). Analyses of disordered breathing patterns following return to room-air after HX, HC and H-H challenges will allow for examination of the dynamic interplay of HX and HC on the occurrence of apneas, sighs and irregular breathing per se. Moreover, the issue as to whether the actual level of the frequency of breathing (fR) is associated with or is essential to the expression of disordered breathing upon return to room-air after HX, HC or H-H challenges is not settled (Han et al., 2001, 2002; Tagaito et al., 2001; Yamauchi et al., 2008a,b, 2010). The analysis of breathing patterns is a complicated process that certain groups have mastered (e.g., Tankersley et al., 1994, 1998, 2000, Tankersley, 2000, 2001, 2003; Han and Strohl, 2000; Han et al., 2001, 2002; Tagaito et al., 2001; Schneider et al., 2003; Strohl, 2003; Tankersley and Broman, 2004; Balbir et al., 2006; Yamauchi et al., 2008b; Gillombardo et al., 2012). One less intense (and less informative) method to examine disordered breathing is the Rejection Index (Rinx) feature in whole body plethysmography systems including the Buxco system recently acquired by Data Sciences International (St. Paul, MN). As will be described, the Buxco system allows the investigator to set key ventilatory parameters to include or reject irregular breathing patterns, apneas, sighs and sniffs. The number of rejected breaths in each recording epoch (e.g., 15 sec) is counted and presented as the Rinx. To our knowledge, the applicability of this method of detecting disordered breathing has not been examined previously. In strict terms, Rinx does not give information about individual breathing events (e.g., apneas, sniffing) but rather is an index of the time that is spent in non-eupneic breathing rather than an actual measurement of the events that define disordered breathing. As such, will use the term disordered breathing only when events such as apneas and sighs were actually determined (including the post-HX phase in C57BL6 mice of the present study). We will use the term “non-eupneic breathing” when referring to our findings pertaining to Rinx that were not accompanied by actual calculations of individual events.
The aims of this study were to determine whether (1) an increase in the level of non-eupneic breathing occurs upon return to room-air after HC or H-H challenges as well as HX challenge, (b) Rinx is applicable for the detection of non-eupneic breathing at rest, during HX-challenge and upon return to room-air in conscious C57BL6 mice, and (c) the level of fR is a determinant of Rinx before and during the HX and post-HX phases in C57BL6 mice, Swiss-Webster mice, and in B6AF1 mice which are derived from a C57BL6 dam and an A/J sire. The data from the three strains is presented because they demonstrate that Rinx and thereby non-eupneic breathing is not simply a function of the level of fR. It should be noted that unlike C57BL6 mice, the B6AF1 and A/J mouse show little spontaneous or post-HX-associated disordered breathing (Han et al., 2002, Yamauchi et al., 2008a,b) and we serendipitously determined that Swiss Webster mice displayed substantial post-HX elevations in fR that were accompanied by minimal increases in Rinx.
2. Methods
2.1. Mice
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the Animal Care and Use Committees of the University of Virginia, and Case Western Reserve University. Adult male C57BL6 mice and B6AF1 mice were from Jackson Laboratories (Bar Harbor, ME). Male Swiss-Webster mice were from Harlan (Indianapolis, IN). The mice were exposed to the protocols when they were approximately 90 days of age to eliminate development influences on the results. It should be noted that corrections for body weights are not necessary for the parameters under investigation in this study, namely fR and Rinx (although volume parameters were computed to determine Rinx, each mouse served as its own control).
2.2. Whole-body plethysmography
Ventilatory parameters were recorded in conscious mice by whole body plethysmography (PLY3223; Data Sciences International, St. Paul, MN) as described previously (Palmer et al., 2013a,b, 2014; Gaston et al., 2014). The parameters were fR; tidal volume (VT) , minute ventilation (VE), inspiratory time (TI), expiratory time (TE) and peak inspiratory (PIF) and peak expiratory (PEF) flows. The provided software (Fine Pointe, BUXCO) constantly corrected digitized values for changes in chamber temperature and humidity. Pressure changes associated with the respiratory waveform were converted to volumes (i.e., VT, PIF and PEF) using the algorithm of Epstein and colleagues (Epstein and Epstein, 1978; Epstein et al., 1980). More specifically, factoring in chamber temperature and humidity, the cycle analyzers filtered the acquired signals, and algorithms (Fine Pointe, BUXCO) generated an array of box flow data that identified a waveform segment as an acceptable breath. From that data vector, the minimum and maximum values were determined. The flows at this point were box-flow signals. From this array, the minimum and maximum box flow values were determined and then multiplied by the compensation factor provided by the selected algorithm (Epstein and Epstein, 1978; Epstein et al., 1980), thus producing VT, PIF and PEF that are used to determine accepted and rejected waveforms. In all protocols described below, the conscious unrestrained mice were placed in the plethysmography chambers and allowed at least 60 min to acclimatize before exposure to the gas challenges.
2.3 Protocols for gas challenges
2.3.1. Study 1
Conscious unrestrained C57BL6 mice (n=16) were exposed to a hypoxic (10% O2, 90% N2) challenge for 5 min after which time they were re-exposed to room air for 15 min.
2.3.2. Study 2
Conscious unrestrained C57BL6 mice were exposed to either a hypoxic (10% O2, 90% N2; n= 16 mice), hypercapnic (5% CO2, 21% O2, 74% N2, n=16) or hypoxic-hypercapnic challenge (5% CO2, 10% O2, 85% N2, n=16) for 15 min after which time they were re-exposed to room air for 15 min.
2.3.3. Study 3
Conscious unrestrained C57BL6 mice, Swiss Webster mice and B6AF1 mice (n=16 mice per group) were exposed to a hypoxic (10% O2, 90% N2; n= 16 mice) challenge for 15 min after which time they were re-exposed to room air for 15 min.
2.4. Rejection Index
The Buxco system includes a Rinx algorithm that was set to reject breaths that did not reflect normal tidal volume breathing and as such typically rejected (a) abnormal breaths (abnormal balance of inspiratory and expiratory volumes), apneas, sighs, post-sighs, sniffs and waveforms that most likely arose from activities such as grooming the face and hands, and rearing. For each accepted breath, the soft-ware determined the number of rejected breaths since the last accepted breath and calculated the percentage of breaths accepted. This percentage of accepted breaths is then aver-aged over each epoch (e.g. 15 s) to arrive at the value reported asRinx. In the present studies, minimum Vt was set at 0.05 ml, mini-mum Ti was set at 0.04 s, maximum Te was set at 0.5 s, the ratio of exspiratory volume/inspiratory volume (volume balance) was set to the range of 60% to 140%. Values were rejected when they fell outside the above criteria and when (a) Ti was greater than two times Te, (b) PIF could not be distinguished from PEF, (c) expiratory flow at 50% Vt, time to maximum expiratory flow, relaxation time or conditioning coefficient could not be computed. Using in-house soft-ware programs, analyses of the waveforms over 5 min periods before and 5–10 min after return to room-air following HX challenge was performed on 16 C57BL6 mice (88 ± 2 days of age) to compare the values with Rinx values generated by the Buxco soft-ware for these mice. One mouse was tested at a time in order to allow monitoring of behaviors (see below).
2.6. Activity Scores
The Activity Scores during the 5 min periods before and 5-10 min after return to room-air following HX challenge were performed on the same 16 C57BL6 mice (88 ± 2 days of age) used to determine non-eupneic breathing events (see above). Initial analyses in which we simultaneously monitored behaviors of mice in the plethysmography chambers and the accompanying respiratory wave-forms, we determined that non-eupneic breathing was most marked during body and/or paw grooming, and progressively less intense during moving and rearing, moving or head movements. Accordingly, the observers (2 per mouse) were asked to note the presence of these activities during each 15 sec epoch. Each mouse was also simultaneously filmed with a VIXIA HF R500 HD Flash Memory Camcorder (Canon Model: 9176B001s) so that (1) the behaviors displayed by each mouse could be scored by another two observers blinded as to (1) which of the 16 mice was being viewed, and (2) whether the recording was from the pre- or post-HX phases. The recorded behaviors were then given a score based on our initial findings regarding the increasing magnitude and duration of effects of each behavior on the plethysmography wave-form. The scores ascribed to each behavior were, head movements (score 1), moving (score 2), moving and rearing (score 3), and episodes of body and/or paw grooming (score 4). The four sets of results from each mouse per epoch were averaged.
2.6. Statistics
All data are presented as mean ± SEM. To determine the total responses (cumulative %changes from pre-hypoxia values) during gas challenge and return to room air for each mouse, we summed the values recorded before and during the challenge and those upon return to room air. We then determined the cumulative response by the formula, total response (%change) = {[(sum of values during hypoxic challenge or return to room air) − (sum of values before hypoxic challenge)]/sum of values before hypoxic challenge} × 100. We then determined the mean and SEM of the group data. All data were analyzed by one-way or two-way ANOVA followed by Student's modified t-test with Bonferroni corrections for multiple comparisons between means (Palmer et al., 2013a,b).
3. Results
3.1. Relationship between Rinx and disordered breathing
The total duration of spontaneous pauses (apneas), post-sighs, irregular breaths and sniffing determined by our in-house analyses was increased during the post-HX period (i.e., return to room-air after HX challenge) whereas the time due to movement artifacts was not different between the two periods (Table 1). The total times of disordered breathing before and during the post-HX period were consistent with the Rinx values determined by the plethysmography software (see last two rows of Table 1).
Table 1.
Breathing events before and after return to room-air following exposure to a hypoxic challenge
| Phase | Number | Pre | Post-HX |
|---|---|---|---|
| Spontaneous pause | Number/min | 1.82 ± 0.18 | 5.22 ± 0.65* |
| Duration | 0.99 ± 0.12 | 1.62 ± 0.18* | |
| Total, sec | 1.76 ± 0.27 | 8.36 ± 1.9* | |
| Post-sigh without apnea | Number/min | 0.36 ± 0.04 | 1.53 ± 0.22* |
| Duration | 0.68 ± 0.73 | 0.89 ± 0.12* | |
| Total, sec | 0.25 ± 0.36 | 1.37 ± 0.24* | |
| Post-sigh - type 1 | Number/min | 0.25 ± 0.03 | 1.04 ± 0.18* |
| Duration | 0.63 ± 0.08 | 0.69 ± 0.08* | |
| Total, sec | 0.16 ± 0.03 | 0.72 ± 0.11* | |
| Post-sigh - type 2 | Number/min | 0.17 ± 0.02 | 0.83 ± 0.11* |
| Duration | 0.65 ± 0.09 | 0.71 ± 0.12* | |
| Total, sec | 0.11 ± 0.02 | 0.59 ± 0.08* | |
| Total per min | 2.28 ± 0.39 | 10.97 ± 2.3* | |
| %Time per min | 3.8 ± 0.5 | 18.3 ± 3.3* | |
| Irregular breathing | Total, sec | 2.69 ± 0.32 | 7.46 ± 0.93* |
| Sniffing | Total, sec | 2.44 ± 0.26 | 6.23 ± 0.83* |
| Movement artifacts | Total, sec | 1.11 ± 0.21 | 1.27 ± 0.15 |
| Total per min | 6.24 ± 0.83 | 14.96 ± 2.33* | |
| %Time per min | 10.4 ± 2.2 | 24.9 ± 3.8* | |
| Overall Total, min | 8.52 ± 1.12 | 25.93 ± 4.43 | |
| %Time per min | 14.1 ± 2.3 | 43.2 ± 6.6* | |
| Rinx, % | 13.5 ± 1.8 | 41.9 ± 5.2* | |
The data are presented as mean ± SEM. HX, hypoxic gas challenge. There were 16 male C57BL6 mice in the group.
P < 0.05, post-HX values versus Pre values.
3.2. Hypoxic challenge in C57BL6 mice
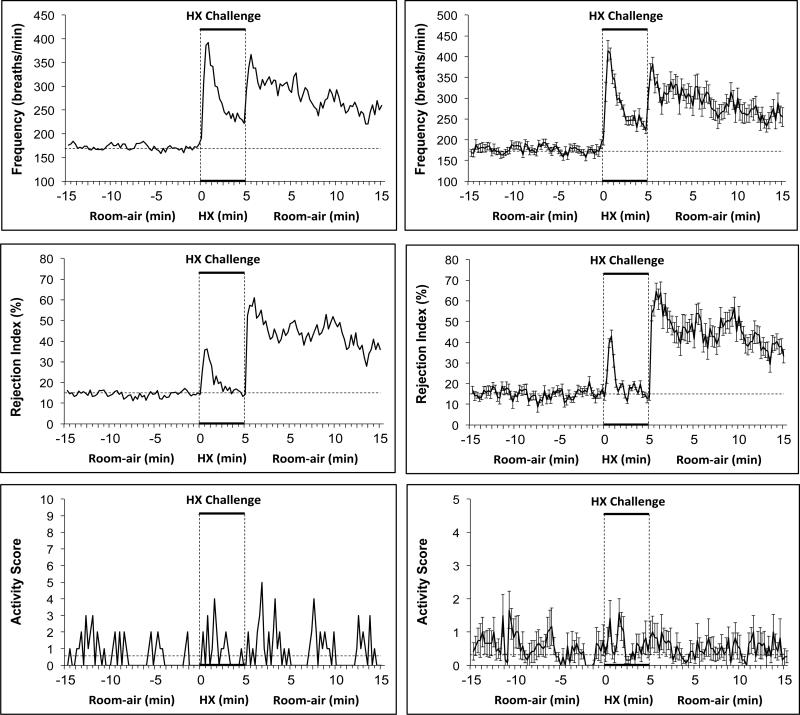
Typical examples of resting fR (values were those not rejected by the Buxco soft-ware), Rinx and Activity Score of a conscious C57BL6 mouse before, during and after a 5 min exposure to the HX gas (10% O2, 90% N2) challenge are shown in the left hand columns of Figure 1. Prior to HX, resting fR was stable at about 170 breaths/min and Rinx was stable at about 15%. Within some 15 sec recording periods, the mouse displayed episodes of head movements (score 1), moving (score 2), moving and rearing (score 3), or body and/or paw grooming (score 4). This activity was not overtly influential on Rinx suggesting that the majority of rejected breaths were not due to movement or grooming which can elicit an artifactual effect on breathing waveforms. Consistent with established findings, exposure of the C57BL6 mice to HX challenge elicited an initial increase in fR that waned substantially during the challenge, a phenomenon known as “roll-off” (Palmer et al., 2013a,b; Gaston et al., 2014). Rinx initially increased during the HX challenge but subsided substantially well before roll-off in fR was prominent. Again, the sporadic increases in Activity Score were not obviously related to Rinx. Upon return to room-air, there was an abrupt increase in fR and Rinx that was accompanied by sporadic increases in Activity Score. Rinx remained elevated during many time periods in which Activity Score was zero.
Figure 1.
Typical examples (left-hand panels) and summary graphs (right-hand panels) of frequency of breathing, Rejection Index (0% = no disordered breathing, 100% = completely disordered breathing) and Activity Score (see Methods for details) of conscious C57BL6 mice (n=16) before, during a 5 min challenge with a hypoxic (HX) gas mixture (10% O2, 90% N2), and following return to room-air. The data in the right-hand panels are presented as mean ± SEM.
A summary of findings with 16 male C57BL6 mice is shown in the right-hand panels of Figure 1. The major conclusions regarding Rinx were: (1) Rinx occurs about 15% of time in conscious unrestrained C57BL6 mice, (2) Rinx increases during the initial exposure to hypoxic gas but declines rapidly to baseline values while fR is still elevated, (3) Rinx is markedly elevated following return to room-air, and (4) the steady state Rinx and the changes during and following HX challenge were not obviously related to Activity Score.
3.3. Comparison of hypoxic, hypercapnic or hypoxic-hypercapnic challenges in C57BL6 mice
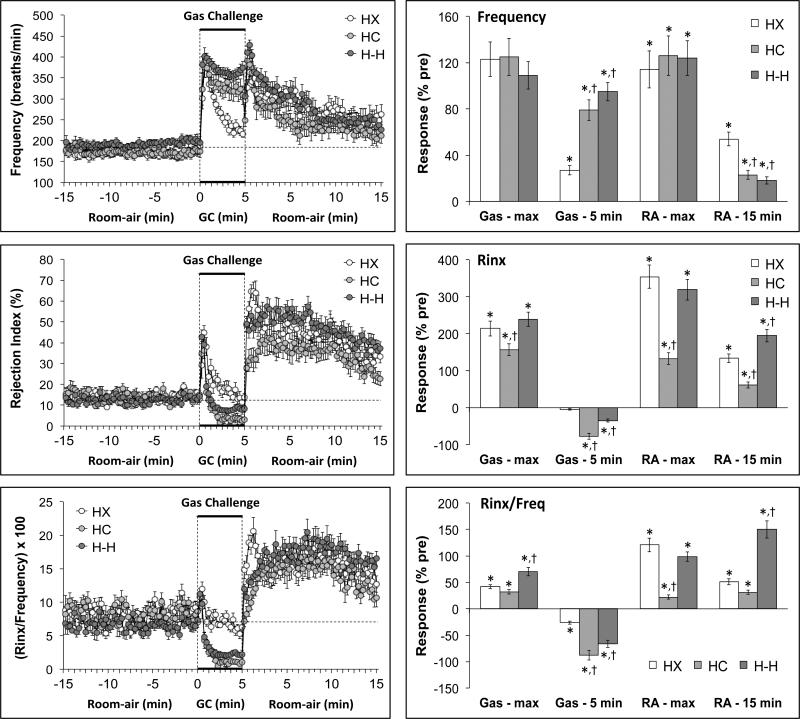
The summaries of fR, Rinx and Rinx/fR values of conscious C57BL6 mice recorded before, during and following a 5 min exposure to a (1) HX challenge (10% O2, 90% N2), (2) HC challenge (5% CO2, 21% O2, 74% N2) or (3) H-H challenge (10% O2, 5%CO2, 85% N2) are shown in the left-hand panels of Figure 2. Resting values prior to gas challenges were similar to one another (Table 2). HX challenge elicited initial increases in fR and Rinx that were subject to roll-off (roll off was more rapid and complete for Rinx). HC and H-H challenges elicited sustained increases in fR accompanied by an initial increase and then sustained decreases in Rinx (Figure 2, Table 2). The return to room-air after the HX, HC and H-H challenges were associated with sudden increases in fR that gradually declined toward pre-challenge values and abrupt and sustained increases in Rinx. The maximal changes at key points are summarized in the right-hand panels of Figure 2. The HX, HC and H-H challenges elicited robust and similar maximal increases in fR (column Gas – max). There was substantial roll-off during the HX challenge (column GAS - 5 min) but minimal roll-off during the HC and H-H challenges. The maximal increases in fR that occurred upon return to room-air were similar following exposure to the HX, HC or H-H challenges (column RA - max). The return to pre-challenge levels over 15 min (column RA - 15 min) was substantial for each group but less pronounced in the HX than the HC and H-H groups.
Figure 2.
Left-hand panels. Changes in frequency of breathing, Rejection Index and Rejection Index/frequency (Rinx/frequency) in conscious C57BL6 mice before, during a 5 min challenge with a hypoxic (HX) gas mixture (10% O2, 90% N2), a hypercapnic (HC) mixture (5% CO2, 21% O2, 74% N2), or a hypoxic-hypercapnic (H-H) mixture (5% CO2, 10% O2, 85% N2), and following return to room-air. Right-hand panels. Maximal responses recorded during the hypoxic challenge and upon return to room-air (HX – max and RA - max, respectively). The values at the end of the hypoxic challenge and the return to room-air (HX – 5 min and RA - 15 min, respectively) are also shown. The data are presented as mean ± SEM. There were 16 mice in each group. *P < 0.05, significant response. †P < 0.05, HC or HH versus HX.
Table 2.
Values at baseline (Pre) and during exposure to gas challenge and return to room-air
| Parameter | Variables | HX | HC | H-H |
|---|---|---|---|---|
| Frequency (breaths/min) | Pre | 170 ± 9 | 173 ± 11 | 192 ± 11 |
| GC - max | 380 ±14* | 388 ± 19* | 401 ± 21* | |
| GC - 5 min | 216 ± 8* | 308 ± 23 | 373 ± 16 | |
| RA - max | 364 ± 17* | 391 ± 20* | 428 ± 22* | |
| RA - 15 min | 262 ± 24* | 212 ± 18* | 226 ± 19 | |
| Rejection Index (%) | Pre | 14 ± 2 | 14 ± 2 | 13 ± 2 |
| GC - max | 45 ± 3* | 36 ± 4* | 43 ± 3 | |
| GC - 5 min | 14 ± 2 | 3 ± 1* | 8 ± 2* | |
| RA - max | 65 ± 6* | 33 ± 5* | 53 ± 4* | |
| RA - 15 min | 33 ± 3* | 23 ± 3* | 37 ± 4* | |
| (Rejection Index/Frequency) × 100 | Pre | 8 ± 1 | 8 ± 1 | 7 ± 1 |
| GC - max | 12 ± 1* | 11 ± 1* | 11 ± 1* | |
| GC - 5 min | 6 ± 1* | 1 ± 0* | 2 ± 1 | |
| RA - max | 18 ± 2* | 10 ± 2 | 13 ± 2* | |
| RA - 15 min | 13 ± 2* | 11 ± 2 | 17 ± 1* |
The data are presented as mean ± SEM. GC, gas challenge. RA, return to room-air. HX, hypoxic-gas challenge. HC, hypercapnic gas challenge. H-H, hypoxic-hypercapnic gas challenge. There were 16 male C57BL6 mice in each gas challenge (mice only received one of the challenges).
P < 0.05, significantly different from Pre values.
The HX, HC and H-H challenges elicited robust maximal increases in Rinx that were slightly less in the HC group (column Gas - max). Rinx gradually returned to pre-gas levels during the HX challenge whereas Rinx values fell below zero in the HC group (column Gas - 5 min). The fall in the H-H group was less than in the HC group. The maximal increases in Rinx upon return to room-air were similar following exposure to the HX or H-H challenges but smaller in the HC group (column RA – max). The return to pre-challenge levels (column RA - 15 min) was substantial for HX group, more so for the HC group but less in the H-H group. As a result of these changes, the maximal increases in Rinx/fR were greater in the H-H group than the HX or HC groups (column Gas - max). Rinx/fR subsequently fell during the HX, HC and H-H challenges with the falls being greater in the HC and H-H groups (Gas - 5 min). The maximal increases in Rinx/fR that occurred upon return to room-air were similar following exposure to the HX or H-H challenges but substantially smaller in the HC group (column RA – max). The return to pre-challenge levels (column RA - 15 min) was substantial for the HX group, more so for the HC group but substantially less in the H-H group.
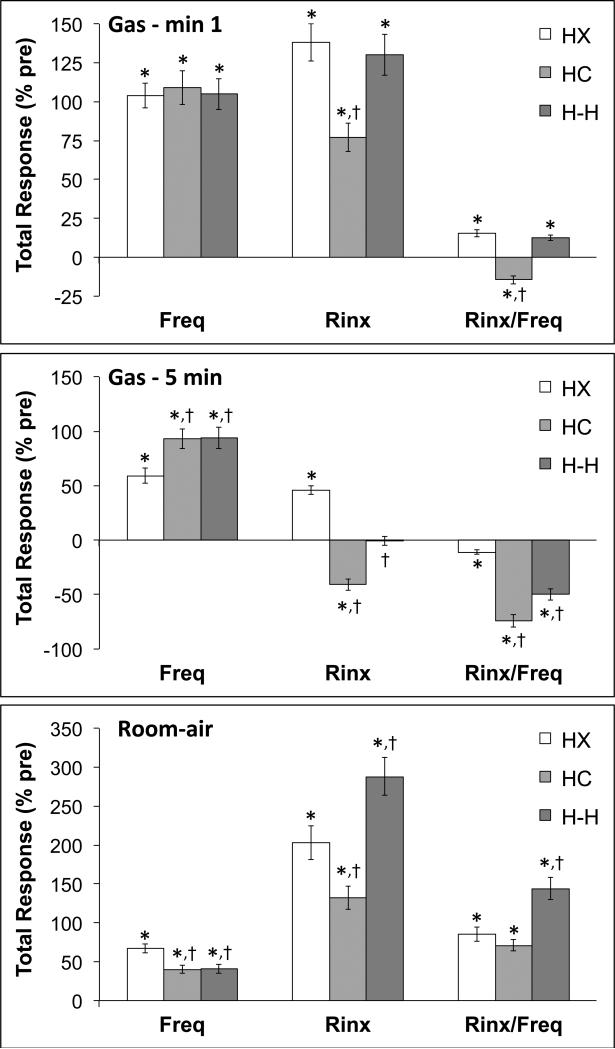
The total changes in fR, Rinx and Rinx/fR during the gas challenges and upon return to room-air are summarized in Figure 3. The total changes in fR were similar in the HX, HC and H-H groups over the first min (top panel: Gas - min 1). Because of the pronounced roll-off, the total response over the 5 min HX challenge was smaller than in the HC and H-H groups (Middle panel: Gas – 5 min). The initial total increase in Rinx was smaller in the HC than the HX or H-H groups (top panel: Gas - min 1) whereas there was an increase in total Rinx during the entire HX challenge (middle panel: Gas – 5 min), a fall in total Rinx during HC challenge but no overall change in total Rinx during H-H challenge. Taken together, there were small initial increases in total Rinx/fR in the HX and H-H groups but a decrease in the HC group (top panel: Gas - min 1). There was a small decrease in total Rinx/fR during the entire HX challenge but substantial decreases in total Rinx/fR during the entire HC and H-H challenges. The decrease in the H-H group was less than that in the HC group. The total increase in fR following return to room-air was greater in the HX group than in the HC or H-H groups (Bottom panel: Room-air). The total increase in Rinx was greatest in the H-H group and lowest in the HC group with the value in the HX group being between the HC and H-H groups. The increase in total Rinx/fR was higher in the H-H group than either the HX or HC groups.
Figure 3.
Total changes in frequency of breathing (Freq), Rejection Index (Rinx) and Rejection Index/frequency (Rinx/freq) in C57BL6 mice before, during the first minute (Gas – 1 min) and for the entire 5 min (Gas – 5 min) challenge with a hypoxic (HX) gas mixture (10% O2, 90% N2), a hypercapnic (HC) mixture (5% CO2, 21% O2, 74% N2), or a hypoxic-hypercapnic (H-H) mixture (5% CO2, 10% O2, 85% N2), and upon return to room-air (15 min total). The data are mean ± SEM. There were 16 mice in each group. *P < 0.05, significant response. †P < 0.05, HC or HH versus HX.
3.4. Hypoxic gas challenges and return to room-air in C57BL6, Swiss Webster and B6AF1 mice
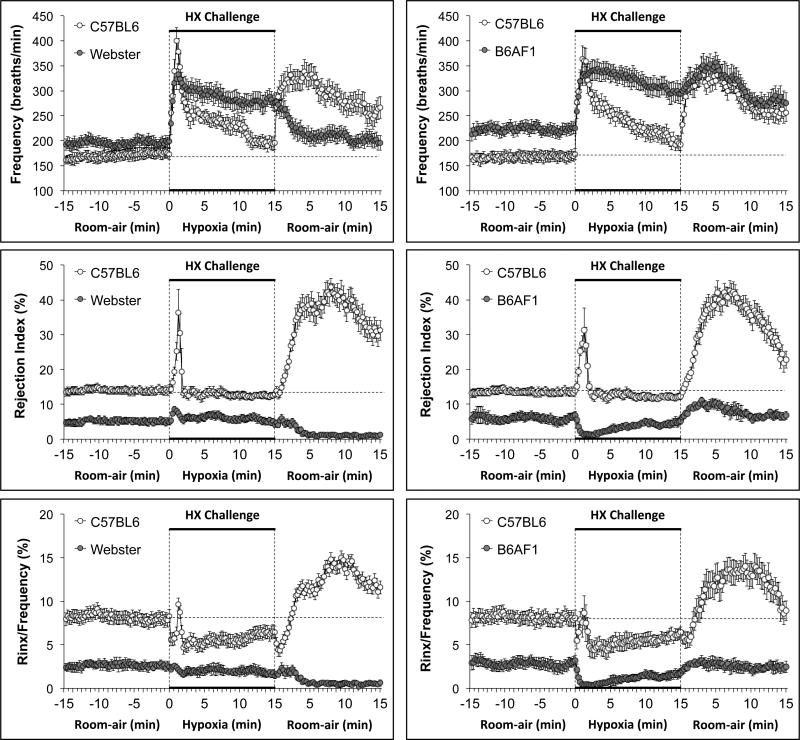
Summary statistics for the C57BL6, Swiss-Webster and B6AF1 mice are provided in Table 3. The mice were of equivalent age. The body weights of the Swiss-Webster mice were greater than the C57BL6 or B6AF1 mice. Resting fR was higher in B6AF1 mice than C57BL6 or Swiss Webster mice. Rinx was higher in C57BL6 mice than in Swiss-Webster or B6AF1 mice, which were similar to one another. Rinx/fR was higher in C57BL6 mice than in Swiss-Webster mice, which in turn was higher than in B6AF1 mice. The changes in fR, Rinx and Rinx/fR during hypoxic challenge (10% O2, 90% N2) in C57BL6 and Swiss Webster mice (left-hand panels) and C57CL6 and B6AF1 mice (right hand panels) are summarized in Figure 4. Both groups of C57BL6 mice showed initial increases in fR upon HX challenge that displayed substantial roll-off. Both groups of C57BL6 mice showed a transient increase in Rinx during the challenge but minimal changes thereafter. Both groups of C57BL6 mice showed sustained decreases in Rinx/fR during HX challenge. The Swiss-Webster mice displayed an increase in fR that did not roll-off substantially. There were small increases in Rinx and Rinx/fR during initiation of the HX challenge in the Swiss Webster mice that were followed by gradual declines thereafter. The B6AF1 mice also displayed an increase in fR with minimal roll-off during the HX challenge. Both Rinx and Rinx/fR fell substantially during the HX challenge.
Table 3.
Parameters relevant to the Swiss-Webster and B6AF1 studies
| Swiss-Webster Study |
B6AF1 study |
|||
|---|---|---|---|---|
| Parameter | C57BL6 | Webster | C57BL6 | B6AF1 |
| N | 16 | 16 | 16 | 16 |
| Age (days) | 88 ± 1 | 85 ± 2 | 88 ± 1 | 86 ± 1 |
| Weight (g) | 28.2 ± 0.6 | 36.4 ± 0.8* | 28.1 ± 0.5 | 29.6 ± 0.6† |
| Frequency (breaths/min) | 172 ± 11 | 196 ± 13 | 168 ± 12 | 213 ± 12* |
| Rejection Index (%) | 14.2 ± 1.3 | 5.1 ± 0.9* | 13.6 ± 1.2 | 6.0 ± 1.3* |
| (Rejection Index/Frequency) × 100 | 8.1 ± 0.8 | 5.0 ± 0.6* | 8.4 ± 0.9 | 2.8 ± 0.6*,† |
The data are presented as mean ± SEM. There were 16 male C57BL6 mice in each group.
P < 0.05, significantly different from appropriate C57BL6 group.
P < 0.05, B6AF1 versus Swiss Webster.
Figure 4.
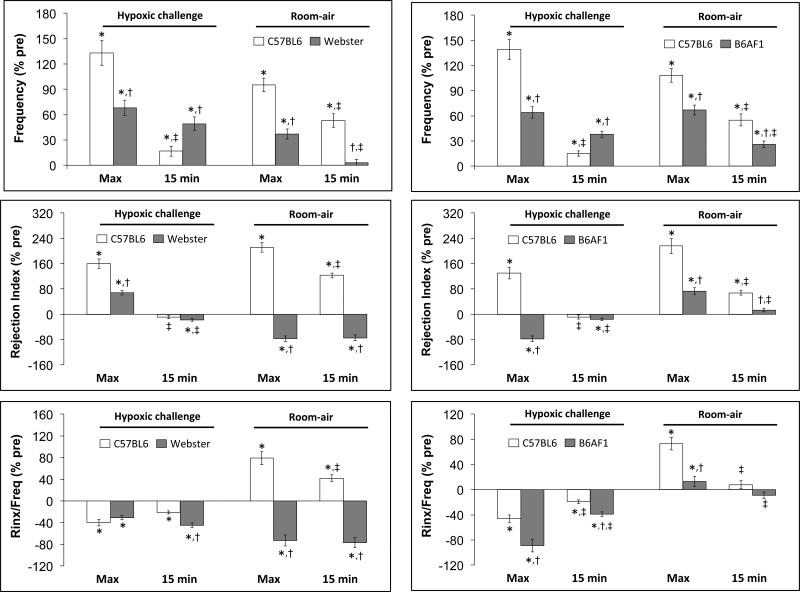
Left-hand panels. Changes in the frequency of breathing, Rejection Index (0% = no disordered breathing, 100% = completely disordered breathing) and Rejection Index/frequency (Rinx/frequency) in conscious C57BL6 mice and Swiss-Webster (Webster) mice before, during a 15 min challenge with a hypoxic (HX) gas mixture (10% O2, 90% N2), and following return to room-air. Right-hand panels. Changes in frequency, Rejection Index and Rejection Index/Frequency in conscious C57BL6 mice and B6AF1 mice before, during a 15 min challenge with a hypoxic (HX) gas mixture (10% O2, 90% N2), and following return to room-air. There were 16 mice in each of the above groups. The data in all panels are presented as mean ± SEM.
The changes in fR, Rinx and Rinx/fR upon return to room-air in following the hypoxic challenges in C57BL6 and Swiss Webster mice and C57BL6 and B6AF1 mice are also summarized in Figure 4. Both groups of C57BL6 mice showed initial increases in fR upon return to room-air that were sustained for at least 15 min. Both groups of C57BL6 mice also displayed somewhat gradually occurring (compared to the rate of increase in fR) increases in Rinx and Rinx/fR that remained elevated for over 10 min. Upon return to room-air, the Swiss-Webster mice displayed simple time-dependent decreases in fR toward baseline levels whereas Rinx and Rinx/fR fell virtually to zero. Upon return to room-air, the B6AF1 mice maintained the high level of fR that was reached during the HX challenge whereas Rinx and Rinx/fR merely returned toward baseline values.
The percent changes in fR, Rinx and Rinx/fR (maximal responses and those at 15 min) that occurred upon exposure to the HX challenge and upon return to room-air in the C57BL6 and Swiss-Webster study are shown in the left-hand panels of Figure 5. The maximal increases in fR (column “Max”) elicited by the HX challenge was greater in C57BL6 than in Swiss-Webster mice. However, roll-off was greater in C57BL6 than Swiss-Webster mice (column 15 min, under Hypoxic Challenge). The maximal increases in Rinx were greater in C57BL6 mice than in Swiss-Webster mice whereas there was a minor decrease in Rinx in Swiss-Webster but not C57BL6 mice at 15 min. The maximal changes in Rinx/fR upon exposure to the HX challenge were similar in both groups whereas the fall in Rinx at 15 min was greater in the Swiss-Webster than the C57BL6 mice. Upon return to room-air, fR, Rinx and Rinx/fR values all increased in C57BL6 mice and remained elevated at 15 min (left-hand panels). In the Swiss-Webster mice, fR rose initially and then subsided by 15 min whereas there was an immediate and sustained fall in Rinx and Rinx/fR.
Figure 5.
Left-hand panels. Maximal changes in frequency of breathing, Rejection Index (0% = no disordered breathing, 100% = completely disordered breathing) and Rejection Index/frequency (Rinx/freq) in conscious C57BL6 and Swiss-Webster (Webster) mice recorded during a hypoxic challenge (10% O2, 90% N2) and upon return to room-air (HX – max and RA - max, respectively). The values at the end of the hypoxic challenge and the return to room-air (HX – 15 min and RA - 15 min, respectively) are also shown. Right-hand panels. Maximal changes in frequency of breathing, Rejection Index and Rejection Index/Frequency (Rinx/freq) in conscious C57BL6 and B6AF1 mice recorded during a hypoxic challenge (10% O2, 90% N2) and upon return to room-air (HX – max and RA - max, respectively). The values at the end of the hypoxic challenge and the return to room-air (HX – 15 min and RA - 15 min, respectively) are also shown. The data in all panels are presented as mean ± SEM. There were 16 mice in each group. *P < 0.05, significant response. †P < 0.05, Swiss-Webster or B6AF1 versus C57BL6. ‡P < 0.05, 15 min values versus Max values.
The percent changes in fR, Rinx and Rinx/fR (maximal responses and those at 15 min) that occurred upon exposure to the HX challenge and upon return to room-air in the C57BL6 and B6AF1 study are shown in the right-hand panels of Figure 5. The maximal increases in fR elicited by the HX challenge was greater in C57BL6 mice than in B6AF1 mice whereas roll-off was greater in C57BL6 mice. Rinx initially rose upon exposure to the HX challenge in C57BL6 mice but had declined at 15 min. In contrast, Rinx fell initially in the B6AF1 mice but normalized by 15 min. The maximal decreases in Rinx/fR and those recorded at 15 min of the HX challenge were greater in B6AF1 mice than in C57BL6 mice. Upon return to room-air, fR, Rinx and Rinx/fR values all increased in C57BL6 mice and except for Rinx/fR remained elevated at 15 min (right-hand panels). The increases in fR, Rinx and Rinx/fR in the B6AF1 mice were smaller than those in C57BL6 mice.
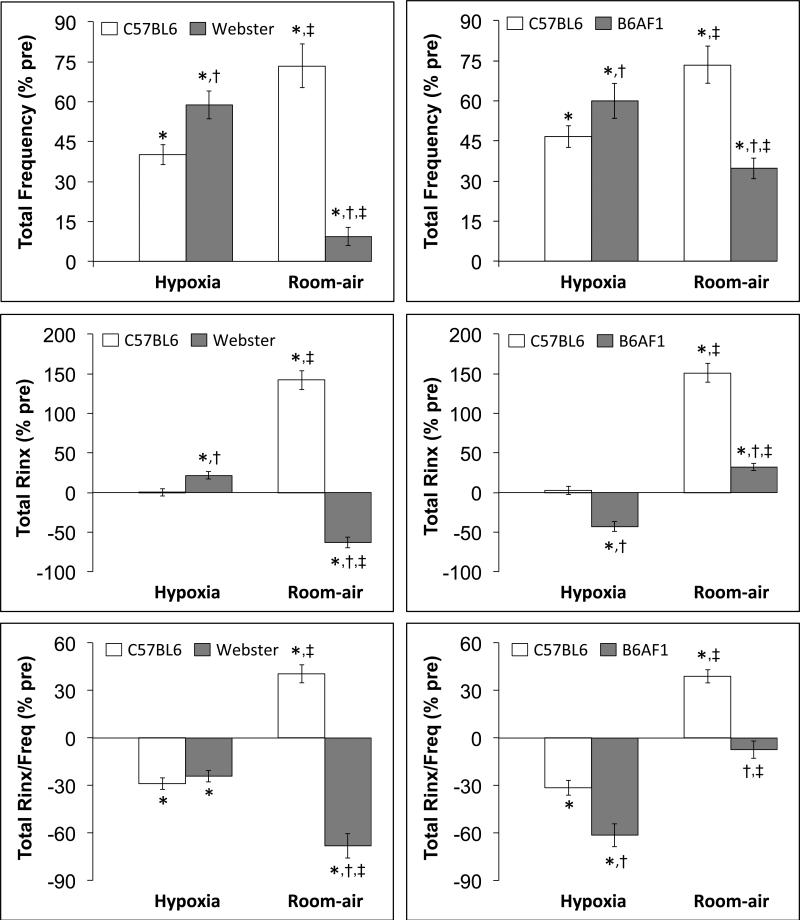
The total percent changes in fR, Rinx and Rinx/fR that occurred during the HX challenge and upon return to room-air in the C57BL6 and Swiss-Webster study are shown in the left-hand panels of Figure 6. During the HX challenge, the total increases in fR were greater in Swiss Webster than in C57BL6 mice, the total increase in Rinx was slightly greater in Swiss-Webster than in C57BL6 mice, whereas the total decline in Rinx/fR were similar in both groups. Upon return to room-air, C57BL6 mice displayed increases in fR, Rinx and Rinx/fR. In contrast, Swiss-Webster mice displayed minor total increases in fR that were accompanied by substantial falls in total Rinx and Rinx/fR.
Figure 6.
Left-hand panels. Total changes in frequency of breathing, Rejection Index (Total Rinx) and Rejection Index/Frequency (Total Rinx/freq) in conscious C57BL6 and Swiss-Webster (Webster) mice recorded during a hypoxic challenge (10% O2, 90% N2) and upon return to room-air (HX – max and RA - max, respectively). Right-hand panels. Total changes in frequency of breathing, Rejection Index (Total Rinx) and Rejection Index/Frequency (Total Rinx/freq) in conscious C57BL6 and B6AF1 mice recorded during a hypoxic challenge (10% O2, 90% N2) and upon return to room-air (HX – max and RA - max, respectively). The data in all panels are presented as mean ± SEM. There were 16 mice in each group. *P < 0.05, significant response. †P < 0.05, Swiss-Webster or
The total percent changes in fR, Rinx and Rinx/fR that occurred during the HX challenge and upon return to room-air in the C57BL6 and B6AF1 study are shown in the right-hand panels of Figure 6. During the HX challenge, the total increases in fR were greater in B6AF1 mice than in C57BL6 mice. The total change in Rinx was not significant in C57BL6 mice whereas there was a fall in B6AF1 mice. As a result, there was a greater fall in Rinx/fR in B6AF1 mice than in C57BL6 mice during the HX challenge. Upon return to room-air, the C57BL6 mice displayed substantial increases in fR, Rinx and Rinx/fR. The increases in fR were smaller in the B6AF1 mice than in the C57BL6 mice and the B6AF1 mice displayed minor total changes in Rinx and Rinx/fR.
4. Discussion
4.1. Rinx and its relationship to disordered breathing
The present study provides compelling evidence that Rinx may be a valuable parameter for investigating the genetic and mechanistic processes underlying disordered breathing in mice. In addition, these studies pave the way for using Rinx as a marker for the efficacy of drugs on disordered breathing. In this study, the Buxco parameter settings used to define disordered breathing (Rinx) were set to pick up any abnormal breaths as well as apneas/pauses, sighs and sniffs, and irregular breaths due to abrupt movement. Indeed, these settings gave Rinx values for the percent time spent in disordered breathing at rest and post-HX that were very similar to those determined by manual analyses. With respect to these analyses, it is evident that the post-HX period in C57BL6 mice was associated with substantial increases in the total time associated with spontaneous pauses (apneas), post sighs and sniffing, and a marked increase in irregular breathing (unbalanced inspiratory/expiratory volumes). Since movement artifacts were minimal at rest and upon return to room-air, it is evident that the occurrence of this markedly disordered breathing is a unique feature of the physiological/pathophysiological make-up of the C57BL6 mouse.
It should be noted that the manual analyses of the C57BL6 mice were performed between 5 and 10 min post-HX, a period of enhanced and relatively stable Rinx recorded in these mice. The question of the relationships between fR and Rinx in the first few min of post-HX as a function of the duration of the HX challenge will be discussed below. It should also be noted that the input variables for assessing Rinx (e.g., VT, TI, TE, balance between inspiratory and expiratory volumes, and minimum box pressure change) can be modified to selectively reject a particular event such as a sniff thereby allowing more detailed analyses of the disordered breathing events.
4.2. Ventilatory responses during and following gas challenges in C57BL6 mice
The changes in fR during the 5 and 15 min HX challenges in conscious C57BL6 mice (increases followed by roll-off) and upon return to room-air (substantial increase from roll-off levels for at least 10 min) are consistent with our previous findings as to the effects of 15 min HX challenges and post-HX phase in conscious C57BL6 mice (Palmer et al., 2013a,b; Gaston et al., 2014). Although the pattern of changes of fR and Rinx (initial increases followed by roll-off) were similar during the HX challenge, it was evident that the degree of Rinx per level of fR (Rinx/fr) fell during HX challenge, but, not as markedly as during HC or H-H challenge (see Figure 2). It is therefore apparent that HX does not have the impact on disordered breathing elicited by HC or H-H. Since the degree of post-HX changes in fR were similar after the 5 and 15 min HX challenges, it is apparent that the mechanisms responsible for the post-HX responses are recruited rapidly in these mice. However, the duration of HX exposure dramatically influenced the time to appearance of Rinx in the post-HX phase. More specifically, fR and Rinx rose immediately following return to room-air after the 5 min HX challenge (Figure 2). In contrast, whereas fR rose immediately after return to room-air following the 15 min HX challenge, Rinx actually fell initially for 1-2 min before gradually rising with significant increases in Rinx not occurring for 3-4 min (Figure 4). Extrapolating to the generation of disordered breathing in animals and humans, it is possible that more brief episodes of apnea destabilize breathing more effectively than longer apneas, which suggests that neurochemical processes recruited to defend against ventilatory instability upon cessation of the apnea take a definite amount of time to become effective. It should be noted that return to room air after even more brief (1 min) episodes of HX is associated with pronounced ventilatory instability in C57BL6 mice (Yamauchi et al., 2007, 2008c).
In contrast to HX challenge, the HC challenge elicited an increase in fR that was not subject to roll-off and which was accompanied by robust decreases in Rinx and Rinx/fR. As such, it is evident that hypercapnia elicits dramatic effects on disordered breathing in C57BL6 mice, most probably via actions within the carotid bodies and brain. Nonetheless, the return to room-air following the HC challenge also elicited a pronounced increase in fR that was associated with an equally pronounced increase in Rinx. These findings raise the possibility that hypercapnia and resultant generation of H+ ions via the carbonic anhydrase system plays a vital role in the etiology of disordered breathing. Moreover, evidence that inhibition of carbonic anhydrase markedly diminishes disordered breathing in C57BL6 mice upon return to room-air after 1 min HX or HC challenges (Yamauchi et al., 2007, 2008c) strongly suggests that H+ ions and acid-sensing ion channels (ASICs), which exist within the carotid bodies (Lu et al., 2013; Tan et al., 2014) and central neurons involved in ventilatory control (Huda et al., 2012; Song et al., 2012), play a ubiquitous role in disordered breathing. Indeed, HX stimulates the activity of the H+ ion extruding Na+-H+ exchanger (NHE) in neocortical (Jørgensen et al., 1999), and CA1 hippocampal (Yao et al., 2001) neurons from the mouse, which in turn could activate ASICs during HX challenge, and depending on the rapidity of change in extracellular H+-ion concentrations, the return to room-air phase. Indeed, in rat CA1 hippocampal neurons, stimulation of NHE only occurred after the neurons were returned to normoxia (Diarra et al., 1999; Sheldon and Church, 2002). Moreover, although acute HX challenge (5-10 min induration) had little effect on steady-state pHi of rat hippocampal astrocytes, HX stimulated HCO3–-independent acid extrusion (NHE activity) and inhibited HCO3–-dependent acid extrusion (Bevensee and Boron, 2008). Moreover, intermittent HX also increases the expression of ASICS in neurons of trapezoid body and lateral paragigantocellular nuclei in the rat brain (Cao et al., 2009). Of direct relevance to the present study is evidence that HX can decrease intracellular pH of carotid body glomus cells and most likely extracellular extrusion of H+-ions (Pang and Eyzaguirre, 1993), which would allow for activation of glomus cell ASICs (Lu et al., 2013; Tan et al., 2014). It is also relevant to our H-H findings in particular, that HX augments H+-ion induced activation of transient receptor potential vanilloid receptors (TRPV1) in rat dorsal root ganglia (Henrich and Buckler, 2009). In light of existing evidence at the time, Yamauchi et al (2007) postulated that unstable breathing is caused by carbonic anhydrase (H+ ion-dependent) pathways to or within the central respiratory controller, which encourages reentry into apnea and unstable breathing. The more recently acquired data discussed above strongly support this postulation.
A key observation of the present study was that exposure to H-H challenge elicited an increase in fR that was reminiscent of that elicited by HC challenge rather than HX challenge (i.e., no roll-off during H-H or HC challenge) and a decrease in Rinx that was observed for the HC but not the HX challenge. These findings support a wealth of evidence that HC activates neurochemical processes that dominate those elicited by the HX challenge (see Campen et al., 2004, 2005; Dempsey et al., 2010). These interactions may be in the brain rather than the carotid bodies since hypoxia augments the effects of hypercapnia on glomus cell activity and vice versa (Dasso et al., 2000; Roy et al., 2000). However, similar to HX and HC challenges, the return to room-air after H-H challenge elicited robust increases in Rinx and Rinx/fR, which at 15 min was substantially greater than for the post-HX or post-HC phases. It appears that HX and HC play a synergistic role in the expression of disordered breathing, which may involve the above mentioned synergisms between HX and HC on carotid body glomus cell/chemoafferent activity. However the possibility of direct brain involvement is suggested by evidence that the gain of brain CO2/H+ chemoreceptors in dogs is critically dependent on carotid body afferent activity and that brain-carotid body interaction results in hyper-additive ventilatory responses to central HC (Blain et al., 2010).
With respect to the return to room-air, we found that post-HX tachypnea (1) is markedly diminished in C57BL6 mice in which the carotid sinus nerves were transected several days before-hand (Gaston et al., 2014), (2) is markedly diminished in C57BL6 mice in which the cysteine at the 93 position in the β-chain of hemoglobin in red blood cells was converted into an alanine, thereby preventing HX-induced generation of S-nitrosothiols (Gaston et al., 2014), and (3) markedly augmented in C57BL6 mice null in S-nitrosoglutathione reductase, an enzyme playing a major role in the catalysis of S-nitrosoglutathione and overall S-nitrosylation status of functional proteins (see Palmer et al., 2014). Taken together, these findings raise the possibility that HX generates blood S-nitrosothiols, which in turn activate carotid body glomus cells and/or chemoafferent terminals to elicit the post-HX ventilatory response. This may involve the ability of S-nitrosothiols to activate ASICs on glomus cells since S-nitroso-N-acetylpenicillamine has been shown to potentiate H+-gated currents in dorsal root ganglion neurons and H+-gated currents in CHO cells expressing ASIC sub-units, most probably via S-nitrosylation events (Cadiou et al., 2007). This possibility is supported by earlier evidence that HX challenge may involve direct activation of carotid body chemoafferents via HX-sensitive proteins or external chemical influences (Sun and Reis, 1994; Roy et al., 2000). We are currently determining whether the generation of circulating S-nitrosothiols is responsible for the post-HX increases in disordered breathing (Rinx) using the mouse models described above.
4.3. Comparisons between C57BL6, Swiss-Webster and B6AF1 mice
C57BL6 mice have been used extensively to study the effects of HX, HC and H-H gas challenges on ventilatory function (see Palmer et al., 2013a,b, 2014; Gaston et al., 2014) and disordered breathing (Han et al., 2001, 2002; Tagaito et al., 2001; Schneider et al., 2003; Yamauchi et al., 2007, 2008a, 2012; Moore et al., 2012, 2014). Although Swiss-Webster mice have been used in studies concerned with the effects of 2 min episodes of HX and HC challenges on ventilatory parameters (Schenkler et al., 2002) and the effects of 5 min episodes of hypoxia-induced gasping (Jacobi and Thach, 1989), no studies have addressed the relationships between ventilatory performance and level of disordered breathing at rest, during HX or HC challenges, and upon return to room-air. Our findings comparing the three mouse strains was that resting Rinx was not necessarily a function of the magnitude of resting fR since Swiss-Webster (and in particular B6AF1 mice) had higher fR at rest than C57BL6 mice but substantially lower Rinx values than the C57BL6 mice. This would suggest that resting Rinx is a function of intrinsic mechanisms regulating breathing patterns rather than the level of fR per se. A key finding of the present study was that Swiss-Webster mice did not display of roll-off during HX challenge and displayed a gradual subsidence of fR upon return to room-air, which were in stark contrast to the substantial roll-off and pronounced post-HX tachypnea observed in C57BL6 mice. Other than initial short-lived increases, Rinx did not change markedly during the HX challenge in Swiss-Webster mice (as in C57BL6 mice). It could be expected that Rinx would fall during the HX challenge due to enhanced tidal volume breathing, which would diminish the relative occurrence of disordered breathing including apneas. However, as with C57BL6 mice, this did not happen in Swiss-Webster mice, which suggests that neural pathways recruited by HX are not sufficient to overcome the mechanisms responsible for disordered breathing in these mice. Whereas C57BL6 mice displayed pronounced increases in fR, Rinx and Rinx/fR upon return to room-air after the HX challenge, fR gradually declined in the Swiss-Webster mice and Rinx and Rinx/fR gradually fell to virtually zero values during the post-HX phase. These dramatic differences in post-HX changes in fR and levels of disordered breathing between C57BL6 and Swiss-Webster mice certainly reinforce extensive knowledge that genetic factors play a vital role in determining ventilatory patterns (Han and Strohl, 2000; Han et al., 2001, 2002; Groeben et al., 2005; Gillombardo et al., 2012).
The B6AF1 mouse (off-spring of a C57BL6 dam, A/J sire) more closely behaves like the A/J mouse than the C57BL6 mouse in that it shows minimal disordered breathing at rest and a minimal expression of disordered breathing during the post-HX phase (Han et al., 2002; Yamauchi et al., 2008a,b). As such, the issue that was addressed in the B6AF1 mice used in this study was whether Rinx and Rinx/fR values at rest and during the post-HX phase would be consistent with direct measurements of disordered breathing (Han et al., 2002; Yamauchi et al., 2008a,b). First, it should be noted that the B6AF1 mice had higher resting fR but lower resting Rinx and Rinx/fR values than C57BL6 mice. Second, unlike C57BL6 mice, fR did not show roll-off during the HX challenge and moreover, Rinx and Rinx/fR values fell dramatically at the onset of the HX challenge, which probably reflects a dominance of normal tidal volume breathing (e.g., matching of inspiratory and expiratory volumes), before recovering toward pre-HX values during the later period of the challenge (the Rinx/fR values of the C57BL6 mice also showed this trend). Taken together, it is evident that B6AF1 mice behave more like A/J mice than C57BL6 mice during HX challenge. The data from the B6AF1 mice clearly demonstrate that HX can elicit a substantial decrease in disordered breathing in a mouse strain, with the reason(s) for the slow recovery of Rinx and Rinx/fR during the challenge indicative of adaptive mechanisms taking place.
A vital set of findings pertained to the differences between C57BL6 mice and B6AF1 mice during the post-HX phase. More specifically, the B6AF1 mice displayed substantial post-HX elevations in fR values that were similar qualitatively and quantitatively to the C57BL6 mice. However, in contrast to the C57BL6 mice, the B6AF1 mice displayed only minor increases in Rinx and Rinx/fR values merely retuned to pre-HX values. These findings demonstrate that post-HX elevations in breathing are not necessarily associated with disordered breathing (elevated Rinx) and that extend previous evidence that the B6AF1 mouse behaves like the A/J mouse rather than the C57BL6 mouse with respect to post-HX breathing patterns (Han et al., 2002; Yamauchi et al., 2008a,b). The mechanisms responsible for the differences in expression of disordered breathing in C57BL6, A/J and B6AF1 in the post-HX phase may be multifactorial, however there is evidence that the genetic mechanisms that produce strain differences in ventilatory function do not associate with carotid body structure or tyrosine hydroxylase morphology and that the A/J chromosome 1 contributes little to the morphology of the C57BL6 carotid body (Chai et al., 2011).
4.4. Perspectives
The present study provides evidence that Rinx and Rinx/fR are reliable analytic tools, which detect breathing patterning and disordered breathing in mice placed in whole-body plethysmography chambers. The parameters are reproducible within a strain and distinguish breathing differences among strains; and may be valuable for detection of non-eupneic breathing during the various stages of the awake-sleep cycle. The use of Rinx and Rinx/fR may be considered as a first-level phenotyping for breathing at rest, during a gas challenge and upon return to room-air, and would complement the detailed analyses of waveforms as elegantly performed by several groups (Dick and Coles, 2000; Tankersley et al., 2000, Strohl, 2003; Balbir et al., 2006). Moreover, use of Rinx and Rinx/fR may be valuable in pharmacological studies aimed at uncovering the mechanisms of disordered breathing, in assessment of gene-by-drug interactions, and in studies designed to establish the efficacy of drugs on respiratory disorders such as sleep apnea.
4.5. Study Limitations
The major limitations of Rinx as a stand-alone parameter are that (1) the individual events that make up a disordered breathing pattern such as apneas are not identified, and (2) without concomitant recording of behaviors, Rinx cannot be a true index of disordered breathing since tachypnea and sniffing for example have a close temporal association with outward behaviors such as grooming and motor activity in rats (see Kabir et al., 2010) and most probably mice. Nonetheless, our studies in C57BL6 mice clearly demonstrate that Rinx and individual breathing events such as apneas during the post-hypoxic phase are not overtly related to behavioral activity and indeed there are many epochs during which Rinx and the expression of disordered breathing events are high even though the mice are not displaying any outward behaviors. Since Rinx can only be equated to disordered breathing if concomitant measures of individual breathing events and behaviors are recorded we used the term non-eupneic breathing rather than disordered breathing on the basis that behavioral contributions to Rinx did not change during the post-hypoxia period (see Table 1). Nonetheless, once an initial finding of an elevated Rinx is established then more detailed analyses of individual breathing events and behaviors must be performed to establish that the increase in Rinx was indeed indicative of greater disordered breathing.
In any experiment, it is pertinent to question whether a change in Rinx reflects the effects of a particular challenge on peripheral or central respiratory homeostatic mechanisms, altered behavioral/locomotor patterns, or effects on the level of arousal/anxiety, knowing for example that hypoxic (Roth et al., 2002; Kumar and Goyal, 2008a,b; Ninot, 2011) and hypercapnic (Battaglia and Ogliari, 2005; Johnson et al., 2012; Battaglia et al., 2014) challenges are anxiogenic stimuli in humans and mice. As mentioned above, our studies show that the marked increase in disordered breathing events (e.g., apneas, sighs) during the post-hypoxic phase are not obviously related to behavioral activity at specific times during this period but it is certainly possible that anxiety elicited during the hypoxic and/or hypercapnic challenges are intimately involved in the expression of breathing pattern upon return to room-air in C57BL6 mice, whereas this may not be the case for Swiss-Webster or B6AF1 mice which show little disordered breathing during the post-hypoxia phase. Moreover, the finding that the number of sighs and %sniffing is a reflection of anxiety states in rats (Carnevali et al., 2013) is no doubt relevant to our studies which show a substantial increase in the number of sighs and %sniffing during the post-hypoxia phase in C57BL6 mice.
During the HX challenge, it would be expected that the vital ventilatory response consisting of more rapid and deeper breathing would dominate non-eupneic breathing events (i.e., diminish behaviors such as sniffing and sighing) via the increase in chemical drive designed to maintain PO2. Moreover, anxiety-related breathing events initiated during the HX challenge would be un-masked during the post-HX phase and strongly influence breathing. This anxiety-related (emotional) component of post-HX breathing may be unique to C57BL6 mice since there was little evidence of non-eupneic breathing during the post-HX phase in Swiss-Webster or more strikingly in B6AF1 mice, which displayed robust post-HX increases in fR but minimal changes in Rinx (see Figure 4). However, even though the HX challenge diminished Rinx in B6AF1 mice it did not do so in C57BL6 or Swiss-Webster mice. As such, the ability of HX to mask non-eupneic breathing may not only be absent in the latter two strains but also have little effect on post-HX enhancement of non-eupneic breathing because C57BL6 mice did whereas the Swiss-Webster did not display this phenomenon.
Highlights.
C57BL6 mice display marked non-eupneic breathing upon cessation of a hypoxic challenge.
They also showed non-eupneic breathing after hypercapnic or hypoxic-hypercapnic challenges.
B6AF1 and Swiss-Webster mice displayed reduced non-eupneic breathing post-hypoxia.
Post-hypoxic non-eupneic breathing is independent of resting breathing frequency.
Acknowledgement
This study was supported by a National Institutes of Health (NIH) Program Project Grant (1P-01-HL-101871; L. A. Palmer, S. J. Lewis), and a grant from Galleon Pharmaceuticals (S. J. Lewis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None
References
- Balbir A, Okumura M, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O'Donnell CP, Shirahata M. Genetic regulation of chemoreceptor development in DBA/2J and A/J strains of mice. Advances in Experimental Medicine and Biology. 2006;580:99–104. doi: 10.1007/0-387-31311-7_15. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A. Anxiety and panic: from human studies to animal research and back. Neuroscience and Biobehavioral Reviews. 2005;29:169–179. doi: 10.1016/j.neubiorev.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, D'Amato F, Kinkead R. Early-life risk factors for panic and separation anxiety disorder: Insights and outstanding questions arising from human and animal studies of CO2 sensitivity. Neuroscience and Biobehavioral Reviews. 2014 doi: 10.1016/j.neubiorev.2014.04.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Boron WF. Effects of acute hypoxia on intracellular-pH regulation in astrocytes cultured from rat hippocampus. Brain Research. 2008;1193:143–152. doi: 10.1016/j.brainres.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Pallot DJ. The carotid body chemoreceptor: an investigation in the mouse. Quarterly Journal of Experimental Physiology. 1982;67:557–576. doi: 10.1113/expphysiol.1982.sp002676. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. Journal of Physiology. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Forster HV, Greene AS, Lowry TF. Breathing periodicity in intact and carotid body-denervated ponies during normoxia and chronic hypoxia. Journal of Applied Physiology. 1993;74:1073–1082. doi: 10.1152/jappl.1993.74.3.1073. [DOI] [PubMed] [Google Scholar]
- Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA. Modulation of acid-sensing ion channel activity by nitric oxide. Journal of Neuroscience. 2007;27:13251–13260. doi: 10.1523/JNEUROSCI.2135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Tagaito Y, Li J, Balbir A, Tankersley CG, Smith P, Schwartz A, O'Donnell CP. Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiological Genomics. 2004;20:15–20. doi: 10.1152/physiolgenomics.00197.2003. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Tagaito Y, Jenkins TP, Balbir A, O'Donnell CP. Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. Journal of Applied Physiology. 2005;99:807–813. doi: 10.1152/japplphysiol.00039.2005. [DOI] [PubMed] [Google Scholar]
- Cao XL, Chen Q, Zhou H, Tang YH, Xu JG, Zheng Y. Expression of acid-sensing ion channels in neurons of trapezoid body and lateral paragigantocellular nuclei in rat brain, and effects of intermittent hypoxia on their expression. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:662–666. [PubMed] [Google Scholar]
- Carnevali L, Sgoifo A, Trombini M, Landgraf R, Neumann ID, Nalivaiko E. Different patterns of respiration in rat lines selectively bred for high or low anxiety. PLoS One. 2013;8:e64519. doi: 10.1371/journal.pone.0064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S, Gillombardo CB, Donovan L, Strohl KP. Morphological differences of the carotid body among C57/BL6 (B6), A/J, and CSS B6A1 mouse strains. Respiratory Physiology and Neurobiology. 2011;177:265–272. doi: 10.1016/j.resp.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. Journal of Physiology. 1996;497:79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso LL, Buckler KJ, Vaughan-Jones RD. Interactions between hypoxia and hypercapnic acidosis on calcium signaling in carotid body type I cells. American Journal of Physiology. 2000;279:L36–L42. doi: 10.1152/ajplung.2000.279.1.L36. [DOI] [PubMed] [Google Scholar]
- Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience. 1999;93:1003–1016. doi: 10.1016/s0306-4522(99)00230-4. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respiratory Physiology. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell C. Pathophysiology of sleep apnea. Physiology Reviews. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Rigual R. Single-unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. Journal of Applied Physiology. 2000;88:1489–1495. doi: 10.1152/jappl.2000.88.4.1489. [DOI] [PubMed] [Google Scholar]
- Duling LC, Cherng TW, Griego JR, Perrine MF, Kanagy NL. Loss of α2B-adrenoceptors increases magnitude of hypertension following nitric oxide synthase inhibition. American Journal of Physiology. 2006;291:H2403–H2408. doi: 10.1152/ajpheart.01066.2005. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Epstein RA. A theoretical analysis of the barometric method for measurement of tidal volume. Respiratory Physiology. 1978;32:105–210. doi: 10.1016/0034-5687(78)90103-2. 1978. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Epstein MA, Haddad GG, Mellins RB. Practical implementation of the barometric method for measurement of tidal volume. Journal of Applied Physiology. 1980;49:1107–1115. doi: 10.1152/jappl.1980.49.6.1107. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, 3rd, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3 - networks within networks: the neuronal control of breathing. Progress in Brain Research. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ. Essential role of hemoglobin beta-93-cysteine in post-hypoxia facilitation of breathing in conscious mice. Journal of Applied Physiology. 2014;116:1290–1299. doi: 10.1152/japplphysiol.01050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillombardo CB, Yamauchi M, Adams MD, Dostal J, Chai S, Moore MW, Donovan LM, Han F, Strohl KP. Identification of novel mouse genes conferring posthypoxic pauses. Journal of Applied Physiology. 2012;113:167–174. doi: 10.1152/japplphysiol.01394.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. Heritable and pharmacological influences on pauses and apneas in inbred mice during anesthesia and emergence. Experimental Lung Research. 2005;31:839–853. doi: 10.1080/01902140600586458. [DOI] [PubMed] [Google Scholar]
- Han F, Strohl KP. Inheritance of ventilatory behavior in rodent models. Respiratory Physiology. 2000;121:247–256. doi: 10.1016/s0034-5687(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. Journal of Applied Physiology. 2001;91:1962–1970. doi: 10.1152/jappl.2001.91.5.1962. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. Journal of Applied Physiology. 2002;92:1133–1140. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- Henrich M, Buckler KJ. Acid-evoked Ca2+ signalling in rat sensory neurones: effects of anoxia and aglycaemia. Pflugers Archives. 2009;459:159–181. doi: 10.1007/s00424-009-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda R, Pollema-Mays SL, Chang Z, Alheid GF, McCrimmon DR, Martina M. Acid-sensing ion channels contribute to chemosensitivity of breathing-related neurons of the nucleus of the solitary tract. Journal of Physiology. 2012;590:4761–4775. doi: 10.1113/jphysiol.2012.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi MS, Thach BT. Effect of maturation on spontaneous recovery from hypoxic apnea by gasping. Journal of Applied Physiology. 1989;66:2384–2390. doi: 10.1152/jappl.1989.66.5.2384. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012;37:1911–1922. doi: 10.1038/npp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen NK, Petersen SF, Damgaard I, Schousboe A, Hoffmann EK. Increases in [Ca2+]i and changes in intracellular pH during chemical anoxia in mouse neocortical neurons in primary culture. J Neuroscience Research. 1999;56:358–370. doi: 10.1002/(SICI)1097-4547(19990515)56:4<358::AID-JNR4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kabir MM, Beig MI, Baumert M, Trombini M, Mastorci F, Sgoifo A, Walker FR, Day TA, Nalivaiko E. Respiratory pattern in awake rats: effects of motor activity and of alerting stimuli. Physiology and Behavior. 2010;101:22–31. doi: 10.1016/j.physbeh.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. Journal of Physiology. 2002;539:309–315. doi: 10.1113/jphysiol.2001.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Goyal R. Possible GABAergic modulation in the protective effect of zolpidem in acute hypoxic stress-induced behavior alterations and oxidative damage. Neurochemical Research. 2008a;33:370–377. doi: 10.1007/s11064-007-9431-9. [DOI] [PubMed] [Google Scholar]
- Kumar A, Goyal R. Gabapentin attenuates acute hypoxic stress-induced behavioral alterations and oxidative damage in mice: possible involvement of GABAergic mechanism. Indian Journal of Experimental Biology. 2008b;46:159–163. [PubMed] [Google Scholar]
- Liu L, Yan Y, Zeng M, Ahang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Whiteis CA, Sluka KA, Chapleau MW, Abboud FM. Responses of glomus cells to hypoxia and acidosis are uncoupled, reciprocal and linked to ASIC3 expression: selectivity of chemosensory transduction. Journal of Physiology. 2013;591:919–932. doi: 10.1113/jphysiol.2012.247189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Berger AJ. Neural regulation of respiration. American Review of Respiratory Disease. 1975;111:206–224. doi: 10.1164/arrd.1975.111.2.206. [DOI] [PubMed] [Google Scholar]
- Moore MW, Chai S, Gillombardo CB, Carlo A, Donovan LM, Netzer N, Strohl KP. Two weeks of buspirone protects against posthypoxic ventilatory pauses in the C57BL/6J mouse strain. Respiratory Physiology and Neurobiology. 2012;183:35–40. doi: 10.1016/j.resp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Moore MW, Akladious A, Hu Y, Azzam S, Feng P, Strohl KP. Effects of orexin 2 receptor activation on apnea in the C57BL/6J mouse. Respiratory Physiology and Neurobiology. 2014;200:118–125. doi: 10.1016/j.resp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninot G. Anxiety and depression in COPD: a review. Review de Maladies Respiratoires 2011. 2011;28:739–748. doi: 10.1016/j.rmr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Palmer LA, May WJ, deRonde K, Brown-Steinke K, Lewis SJ. Hypoxia-induced ventilatory responses in conscious mice: Gender differences in ventilatory roll-off and facilitation. Respiratory Physiology and Neurobiology. 2013a;185:497–505. doi: 10.1016/j.resp.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LA, May WJ, deRonde K, Brown-Steinke K, Gaston B, Bates JN, Lewis SJ. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respiratory Physiology and Neurobiology. 2013b;185:571–581. doi: 10.1016/j.resp.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LA, deRonde K, Brown-Steinke K, Gunter S, Jyothikumar V, Forbes M, Lewis SJ. Hypoxia-induced changes in protein S-nitrosylation in female mouse brainstem. American Journal of Respiratory Cell and Molecular Biology. 2014 doi: 10.1165/rcmb.2013-0359OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Eyzaguirre C. Hypoxia affects differently the intracellular pH of clustered and isolated glomus cells of the rat carotid body. Brain Research. 1993;623:349–355. doi: 10.1016/0006-8993(93)91453-y. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respiratory Physiology. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Price ER, Han F, Dick TE, Strohl KP. 7-nitroindazole and posthypoxic ventilatory behavior in the A/J and C57BL/6J mouse strains. Journal of Applied Physiology. 2003;95:1097–1104. doi: 10.1152/japplphysiol.00166.2003. [DOI] [PubMed] [Google Scholar]
- Roth WT, Gomolla A, Meuret AE, Alpers GW, Handke EM, Wilhelm FH. High altitudes, anxiety, and panic attacks: is there a relationship? Depression and Anxiety. 2002;16:51–58. doi: 10.1002/da.10059. [DOI] [PubMed] [Google Scholar]
- Roy A, Rozanov C, Mokashi A, Lahiri S. PO2-PCO2 stimulus interaction in [Ca2+]i and CSN activity in the adult rat carotid body. Respiratory Physiology. 2000;122:15–26. doi: 10.1016/s0034-5687(00)00116-x. [DOI] [PubMed] [Google Scholar]
- Schneider H, Patil SP, Canisius S, Gladmon EA, Schwartz AR, O'Donnell CP, Smith PL, Tankersley CG. Hypercapnic duty cycle is an intermediate physiological phenotype linked to mouse chromosome 5. Journal of Applied Physiology. 2003;95:11–19. doi: 10.1152/japplphysiol.01144.2002. [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Hansen SN, Pfaff DW. Gender comparisons of control of breathing and metabolism in conscious mice exposed to cold. Neuroendocrinology. 2002;76:381–389. doi: 10.1159/000067580. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Church J. Intracellular pH response to anoxia in acutely dissociated adult rat hippocampal CA1 neurons. Journal of Neurophysiology. 2002;87:2209–2224. doi: 10.1152/jn.2002.87.5.2209. [DOI] [PubMed] [Google Scholar]
- Smith CA, Nakayama H, Dempsey JA. The essential role of carotid body chemoreceptors in sleep apnea. Canadian Journal of Physiology and Pharmacology. 2003;81:774–779. doi: 10.1139/y03-056. [DOI] [PubMed] [Google Scholar]
- Soliz J, Soulage C, Borter E, van Patot MT, Gassmann M. Ventilatory responses to acute and chronic hypoxia are altered in female but not male Paskin-deficient mice. American Journal of Physiology. 2008;295:R649–R658. doi: 10.1152/ajpregu.00876.2007. [DOI] [PubMed] [Google Scholar]
- Soliz J, Thomsen JJ, Soulage C, Lundby C, Gassmann M. Sex-dependent regulation of hypoxic ventilation in mice and humans is mediated by erythropoietin. American Journal of Physiology. 2009;296:R1837–R1846. doi: 10.1152/ajpregu.90967.2008. [DOI] [PubMed] [Google Scholar]
- Song N, Zhang G, Geng W, Liu Z, Jin W, Li L, Cao Y, Zhu D, Yu J, Shen L. Acid sensing ion channel 1 in lateral hypothalamus contributes to breathing control. PLoS One. 2012;7:e39982. doi: 10.1371/journal.pone.0039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respiratory Physiology and Neurobiology. 2004;143:321–332. doi: 10.1016/j.resp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Huppke P, Gärtner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respiratory Physiology Neurobiology. 2008;160:21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Strohl KP. Periodic breathing and genetics. Respiratory Physiology and Neurobiology. 2003;135:179–185. doi: 10.1016/s1569-9048(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Butler JP, Malhotra A. Mechanical properties of the upper airway. Comparative Physiology. 2012;2:1853–1872. doi: 10.1002/cphy.c110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. Dopamine or transmitter release from rat carotid body may not be essential to hypoxic chemoreception. American Journal of Physiology. 1994;267:R1632–R1639. doi: 10.1152/ajpregu.1994.267.6.R1632. [DOI] [PubMed] [Google Scholar]
- Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. Journal of Applied Physiology. 2001;91:2758–2766. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circulation Research. 2007;101:1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- Tankersley CG. A genomic model for differential hypoxic ventilatory responses. Advances in Experimental Medicine and Biology. 2000;475:75–85. doi: 10.1007/0-306-46825-5_8. [DOI] [PubMed] [Google Scholar]
- Tankersley CG. Selected contribution: variation in acute hypoxic ventilatory response is linked to mouse chromosome 9. Journal of Applied Physiology. 2001;90:1615–1622. doi: 10.1152/jappl.2001.90.4.1615. [DOI] [PubMed] [Google Scholar]
- Tankersley CG. Genetic aspects of breathing: on interactions between hypercapnia and hypoxia. Respiratory Physiology and Neurobiology. 2003;135:167–178. doi: 10.1016/s1569-9048(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. American Journal of Physiology. 1994;267:R1371–R1377. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, DiSilvestre DA, Jedlicka AE, Wilkins HM, Zhang L. Differential inspiratory timing is genetically linked to mouse chromosome 3. Journal of Applied Physiology. 1998;85:360–365. doi: 10.1152/jappl.1998.85.1.360. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Elston RC, Schnell AH. Genetic determinants of acute hypoxic ventilation: patterns of inheritance in mice. Journal of Applied Physiology. 2000;88:2310–2318. doi: 10.1152/jappl.2000.88.6.2310. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Irizarry R, Flanders S, Rabold R. Circadian rhythm variation in activity, body temperature, and heart rate between C3H/HeJ and C57BL/6J inbred strains. Journal of Applied Physiology. 2002a;92:870–877. doi: 10.1152/japplphysiol.00904.2001. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Haxhiu MA, Gauda EB. Differential CO2-induced c-fos gene expression in the nucleus tractus solitarii of inbred mouse strains. Journal of Applied Physiology. 2002b;92:1277–1284. doi: 10.1152/japplphysiol.00609.2001. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Broman KW. Interactions in hypoxic and hypercapnic breathing are genetically linked to mouse chromosomes 1 and 5. Journal of Applied Physiology. 2004;97:77–84. doi: 10.1152/japplphysiol.01102.2003. [DOI] [PubMed] [Google Scholar]
- Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Progress in Brain Research. 2014;209:207–233. doi: 10.1016/B978-0-444-63274-6.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari SG, Bugenhagen SM, Wang Z, Schreier DA, Carlson BE, Chesler NC, Beard DA. Analysis of cardiovascular dynamics in pulmonary hypertensive C57BL6/J mice. Frontiers in Physiology. 2013;4:355. doi: 10.3389/fphys.2013.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. Journal of Applied Physiology. 1987;63:1658–1664. doi: 10.1152/jappl.1987.63.4.1658. [DOI] [PubMed] [Google Scholar]
- Wilkinson MH, Berger PJ, Blanch N, Brodecky V, Jones CA. Paradoxical effect of oxygen administration on breathing stability following post-hyperventilation apnoea in lambs. Journal of Physiology. 1997;504:199–209. doi: 10.1111/j.1469-7793.1997.199bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Balbir A, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O'Donnell CP, Shirahata M. Structural and functional differences of the carotid body between DBA/2J and A/J strains of mice. Journal of Applied Physiology. 2003;9:1536–1542. doi: 10.1152/japplphysiol.00739.2002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Balbir A, Okumura M, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O'Donnell CP, Shirahata M. Genetic influence on carotid body structure in DBA/2J and A/J strains of mice. Advances in Experimental Medicine and Biology. 2006;580:105–109. doi: 10.1007/0-387-31311-7_16. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Strohl KP. Acetazolamide protects against posthypoxic unstable breathing in the C57BL/6J mouse. Journal of Applied Physiology. 2007;103:1263–1268. doi: 10.1152/japplphysiol.01287.2006. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. Journal of Applied Physiology. 2008a;105:518–526. doi: 10.1152/japplphysiol.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respiratory Physiology and Neurobiology. 2008b;162:117–125. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Strohl KP. Post-hypoxic unstable breathing in the C57BL/6J mouse: effects of acetazolamide. Advances in Experimental Medicine and Biology. 2008c;605:75–79. doi: 10.1007/978-0-387-73693-8_13. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Kimura H, Strohl KP. Mouse models of apnea: strain differences in apnea expression and its pharmacologic and genetic modification. Advances in Experimental Medicine and Biology. 2010;669:303–307. doi: 10.1007/978-1-4419-5692-7_62. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu XQ, Douglas RM, Haddad GG. Role of Na+/H+ exchanger during O2 deprivation in mouse CA1 neurons. American Journal of Physiology. 2001;281:C1205–C1210. doi: 10.1152/ajpcell.2001.281.4.C1205. [DOI] [PubMed] [Google Scholar]
- Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. Journal of Applied Physiology. 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]