Abstract
Respiratory syncytial virus (RSV) is the most important pathogen for lower respiratory tract illness in infants and a high priority for vaccine development. We previously reported that RSV virus-like particles (VLPs) expressing either the fusion (F) or attachment (G) glycoprotein could confer protection against RSV challenge in BALB/c mice. Here, we tested the hypothesis that RSV VLP vaccine efficacy can be enhanced by mixing RSV VLP F and RSV VLP G, and we analyzed host responses to these RSV VLPs. Mice were immunized with VLP F, VLP G, or VLP F + VLP G. Lung viral loads in BALB/c mice following RSV strain A2-line19F challenge were lower in mice vaccinated with RSV VLP F + VLP G compared to VLP F− or VLP G-vaccinated mice. Vaccination with VLP F or VLP F + VLP G induced similar levels of neutralizing antibodies. The enhanced protection against RSV challenge induced by vaccination with RSV VLP F + VLP G correlated with CD8 T cells producing T helper type 1 cytokines. VLP G vaccination alone followed by challenge resulted in immunopathology similar to formalin-inactivated RSV vaccination and RSV challenge. Taken together, mixed VLP F + VLP G provided a high level of protection against RSV without vaccine-induced immunopathology, but VLP G vaccination enhanced disease when used alone.
Keywords: Respiratory Syncytial Virus, Virus Like Particle (VLP), Lung pathology, RSV fusion (F) protein, RSV attachment glyco (G) protein
1. Introduction
Respiratory syncytial virus (RSV) is the most important pathogen for lower respiratory tract illness (LRI) in infants and children, but there are no licensed vaccines. In the USA, RSV is associated with hospitalization of 1-2% of the winter infant cohort (Zhou et al., 2012). In the 1960s, a formalin-inactivated RSV vaccine (FI-RSV) tragically resulted in exacerbated RSV disease upon natural RSV exposure (Kapikian et al., 1969). There is an urgent need for novel vaccine approaches to control RSV infection.
Virus-like particles (VLPs) resemble native viruses in size and structure but lack viral genetic information, and non-infectious VLPs are a promising vaccine candidate platform (Schneider-Ohrum and Ross, 2012). VLP vaccines licensed for human use include the hepatitis B virus (HBV) vaccine Recombivax®, the human papillomavirus (HPV) vaccines Cervarix® and Gardisil®, and the influenza virus vaccine FluBlok®. Both Cervarix® and FluBlok® are produced using recombinant baculovirus (rBV) expression in insect cells (Monie et al., 2008; Treanor et al., 2011). The recombinant baculovirus (rBV) expression system in insect cell enables high levels of VLP expression in suspension cultures, which facilitates large-scale production (Quan et al., 2007; Vicente et al., 2011).
Previously we demonstrated that rBV-expressed VLPs containing the RSV fusion (F) protein (VLP F) or the RSV attachment (G) glycoprotein (VLP G) could provide protection against RSV A2 strain challenge in a BALB/c mouse model (Quan et al., 2011). Here, we analyzed cellular and humoral immune responses to VLP G and VLP F vaccination, and we tested whether mixing VLP G and VLP F enhances protection compared to VLP G or VLP F alone. We used the pathogenic RSV A2-line19F challenge strain, which establishes relatively high viral loads and induces airway mucus expression in mice, to determine the ability of VLP G, VLP F, and mixed VLP G + VLP F to ameliorate RSV-induced lung histopathology compared to FI-RSV (Lee et al., 2012; Moore et al., 2009).
2. Materials and Methods
2.1. Mice and virus
Pathogen-free 6 to 8-week-old female BALB/c mice were purchased from Charles River Laboratories International (Wilmington, MA). All animal procedures were conducted according to the guidelines of the Emory University Institutional Animal Care and Use Committee. RSV A2-line19F was generated as described elsewhere (Lee et al., 2012; Moore et al., 2009).
2.2. Cells and antibodies
HEp-2 cells were obtained from ATCC (CCL-23) and propagated in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and penicillin G-streptomycin sulfate-amphotericin B solution (Cellgro, 30-004-CL). Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900II medium (GIBCO-BRL,10902-096) at 27°C in flasks at a speed of 140 rpm as described previously (Quan et al., 2011). Polyclonal goat anti-RSV antibody (Millipore, AB1128) was used in virus immunoplaque assay (Lee et al., 2012). HRP conjugated anti-goat antibody (Southern Biotech, Birmingham, AL) was used as a secondary antibody.
2.3. Generation of recombinant baculoviruses
Recombinant baculoviruses (rBVs) expressing RSV F, RSV G, or influenza virus matrix (M1) were generated as described previously (Quan et al., 2011). Briefly, transfections of DNA containing the genes were accomplished using cellfectin II (Invitrogen, Grand Island, NY) with SF9 cells as recommended by the manufacturer, followed by transformation of pFastBac containing RSV-F or RSV-G or influenza M1 with white/blue screening. The rBVs were derived by using a Bac-to-Bac expression system (Invitrogen, Grand Island, NY) according to the manufacturer's protocol.
2.4. VLP production
RSV VLP F was produced by infecting Sf9 cells with rBVs expressing RSV A2 strain F and influenza virus matrix (M1) protein core. RSV VLP G was produced by infecting Sf9 cells with rBVs expressing RSV A2 strain G and influenza M1, as described (Quan et al., 2011). At day 2 post infection (p.i), cell culture supernatants were collected and cleared of cell debris by centrifugation at 6000 rpm for 20 minutes at 4°C. VLP M1 was produced by infecting insect cells with rBV expressing influenza matrix protein M1. VLPs were concentrated with QuixStand (GE) and further purification was performed by 30% and 60% sucrose gradient ultracentrifugation (30,000 rpm, for 60 min) at 4°C. The VLP bands between 30% and 60% were collected and then diluted with phosphate-buffered saline (PBS) and pelleted at 28,000 rpm for 40 minutes at 4°C. VLPs were resuspended in PBS overnight at 4°C and stored at-80 ºC (Quan et al., 2011).
2.5. Preparation of formalin-inactivated RSV (FI-RSV)
FI-RSV was generated as described previously (Peebles et al., 2000). RSV stocks (500 ml) were incubated for 72 h at 37°C, with 4% wt/vol formalin phosphate. The stocks then were centrifuged (17,700 × g) for 17 h. The pellet containing FI-RSV was resuspended in EMEM without serum (1/40 the original volume). The suspensions were diluted 4-fold, and 4 mg/mL aluminum hydroxide gel (Sigma, A8222) was added. The buffered precipitate was centrifuged at 1000 × g for 30 min, resuspended in 1/40 of the original virus stock volume of EMEM without serum, sonicated for 15 s, and stored at 4°C in 1-mL aliquots.
2.6. Vaccination, blood collection, and RSV infection
Groups of mice (n=5) were vaccinated intramuscularly (i.m) 25 μg of VLPs at day 0 and boosted with 25 μg of VLPs 3 weeks later. Unvaccinated (naïve) and influenza virus (M1) VLP-vaccinated mice were used as negative controls. For VLP F + VLP G groups, mice were given 12.5 μg of VLP F and 12.5 μg of VLP G in the same regimen described above. For FI-RSV group, mice were given 100 μl of FI-RSV i.m at day 0 and not boosted. As a control for protective vaccination, primary RSV-infected mice were used, and these mice were inoculated intranasally (i.n) with 2 × 106 PFU/100 μl of RSV A2-line19F, and there was no boost. Peripheral blood was collected from the submandibular vein before immunization and at three weeks and six weeks. For RSV challenge, were anesthetized by intramuscular injection of a ketamine-xylazine solution and infected i.n with 3 × 105 PFU RSV A2-line19F six weeks after the initial vaccination (Lee et al., 2012).
2.7. Preparation of lung lymphocytes
Lung lymphocytes were isolated described previously (Lee et al., 2012). Briefly, lung tissues were minced and ground through a sterile mesh to obtain a single-cell suspension. Cells were layered onto Fico/Lite-LM (mouse) (Atlanta Biologicals), and lung mononuclear cells were isolated by centrifugation at 2,700 rpm.
2.8. Lung IgG ELISA and RSV-neutralization antibodies
RSV-specific antibodies (IgG) were determined in lung homogenates by enzyme-linked immunosorbent assay (ELISA) as described (Quan et al., 2011). RSV-specific neutralizing antibody titers in mouse sera were measured using RSV reporter virus expressing the far-red fluorescent protein monomeric Katushka-2 (RSV A2-K-line19F) generated previously (Hotard et al., 2012). Mouse sera were heat-inactivated at 56°C for 30 min and serially diluted two-fold in MEM. Equal volumes of the diluted sera were mixed with RSV A2-K-line19F to yield 50 PFU/well. Virus only and hyperimmune serum with a known neutralizing antibody titer were included on each plate as negative and positive controls, respectively. The serum + virus (or virus only) mixtures were incubated at 37°C, 5% CO2 for 1 h. Subconfluent monolayers of HEp-2 cells prepared in separate 96-well plates were infected by spinoculation at 3000 rpm for 30 min with the RSV A2-K-line19F only or the serum + virus mixtures. The 96-well plates were incubated at 37°C, 5% CO2 for 24 h. The viral fluorescent focus units (FFU) in each well were counted. The neutralizing antibody (nAb) titers were calculated as log IC50 values of FFU reduction using a non-linear regression analysis with four parameters in GraphPad Prism software.
2.9. Intracellular cytokine staining (ICS)
ICS was performed to enumerate cytokine-producing cells as described previously (Lee et al., 2014; Lee et al., 2012). Lung lymphocytes were stimulated in vitro with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louise, MO), 500 ng/ml ionomycin (Sigma Aldrich, St. Louise, MO), and 0.5 μl Golgi Plug (BD Biosciences, Franklin Lakes, NJ). Cell surface staining was performed with PerCP-Cy5.5-anti-CD3 and APC-Cy7-anti-CD8 followed by ICS using Cytofix/Cytoperm (BD Pharmingen, San Diego, CA). FITC-anti-IFN-γ (XMG1.2) and PE-anti-IL-2 (JES6-5H4) were used. All antibodies listed were purchased from BD Biosciences (Franklin Lakes, NJ) or eBioscience (San Diego, CA). Fluorescence was measured using an LSRII cytometer (BD Immunocytometry Systems) and analyzed using FlowJo software (Tree Star, Ashlan, OR).
2.10. Lung viral load
Mice were euthanized by CO2 inhalation, and lungs were harvested at day 4 post-infection (p.i.). We used a Beadbeater (Biospec Products, Bartlesville, OK) to homogenize the lungs as described (Lee et al., 2012). Lung homogenates were immediately serially diluted and inoculated sub-confluent HEp-2 cells in 24-well plates. After 1 hr adsorption at room temperature, the cells were overlayed with complete EMEM/10% FBS/penicillin G/streptomycin sulfate/amphotericin B solution/0.75% methylcellulose. After six days, the overlay media was removed and the cells fixed with methanol. Plaques were visualized by immunodetection as described (Lee et al., 2012; Stokes et al., 2011).
2.11. Lung histopathology
Mice were euthanized by intraperitoneal (i.p.) injection of sodium pentobarbital (8.5 mg/kg body weight). Heart-lung blocks were harvested at day 4 p.i. Lungs were fixed in 10% neutral-buffered formalin for 24 hrs. Lungs were transferred to 70% ethanol and then embedded in paraffin blocks (Lee et al., 2012; Stokes et al., 2011). Lung tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) to assess histologic changes. Interstitial pneumonia (IP) was scored by a pathologist blinded to the experimental groups. Lymphocytes, neutrophils, macrophages, and eosinophils were assessed in peribronchiolar, perivascular, interstitial, and alveolar spaces as described (Cherukuri et al., 2012; Lee et al., 2012; Stokes et al., 2011). For the eosinophil score, groups were assessed for severity of eosinophilic infiltrate on a scale of 0 to 5 in the peribronchiolar, perivascular, interstitial, and alveolar spaces, where 0 = no eosinophils present, 1=1-10, 2=11-20, 3=21-30, 4=31-40, 5= 41–50 in area. Lung sections were stained with Periodic acid-Schiff (PAS) to assess airway mucin expression (Lee et al., 2012).
2.12. Statistical analyses
P values were determined by either a two-tailed t test or oneway ANOVA and Tukey multiple comparison test, using GraphPad Prism software. Data values below limits of detection were assigned a value of half the limit of detection. A P value less than 0.05 was considered to be significant.
3. Results
3.1. Enhanced protection against RSV challenge by mixing RSV VLP F and VLP G
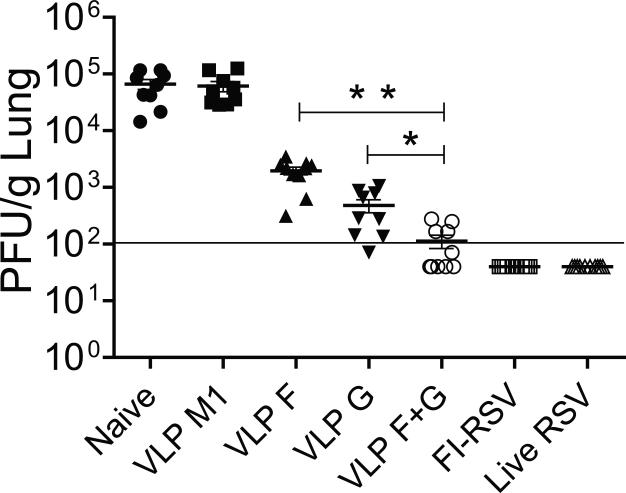
Prior to this study, we performed RSV challenge experiments in BALB/c mice using the A2 strain to define RSV VLP F and VLP G vaccine efficacy, and although VLP G and VLP F were found to be protective, the lung viral titers in control mice were relatively low in this model (Quan et al., 2011). Here, we used RSV strain A2-line19F as the challenge strain because this strain exhibits not only higher viral loads in mice but also airway mucus expression (Lee et al., 2014; Lee et al., 2012; Moore et al., 2009). Mice were unvaccinated (naïve), or vaccinated with VLP M1 (no RSV antigen), VLP F, VLP G, or mixed VLP F + VLP G then boosted 3 weeks later. Control mice were vaccinated once with FI-RSV, or infected i.n. with live RSV. The mice were challenged with RSV A2-line19F at six weeks. Lung viral loads 4 days post-challenge were significantly decreased in mice vaccinated with VLP F, VLP G, or VLP F + VLP G compared with naive or VLP M1 controls (Fig. 1). Mice vaccinated with FI-RSV or infected with live RSV were showed complete protection against A2-line19F challenge (Fig. 1). The RSV VLP F + VLP G-vaccinated group showed additive protection compared to VLP F or VLP G vaccination alone (Fig. 1).
FIG 1.
Lung viral loads after RSV A2-line19F challenge. BALB/c mice were vaccinated with VLP M1, VLP F, VLP G, VLP F + VLP G, FI-RSV, or infected with live RSV. Mice (n = 5 per group) were challenged with A2-line19F at 3 weeks after the boost. Lungs were harvested day 4 p.i., and infectious RSV was titrated by plaque assay. Each symbol represents the titer for an individual mouse, and the black bar represents the mean for the group. The horizontal line depicts the limit of detection. Data show two independent experiments combined. * Statistically significant comparing VLP G and VLP F + VLP G (P < 0.001). ** Statistically significant comparing VLP F and VLP F + VLP G immunization (P < 0.001).
3.2. Humoral responses to RSV VLP F, VLP G, and VLP F+G vaccination
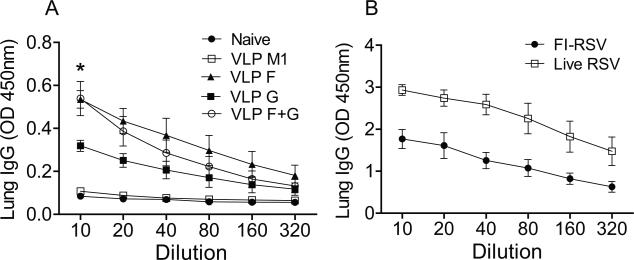
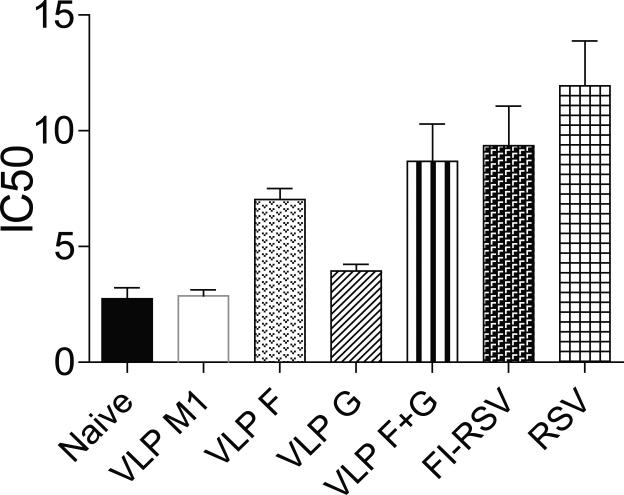
We measured RSV-specific IgG responses in lungs day 4 post RSV challenge. Mice given either live RSV i.n. or FI-RSV i.m. produced higher amount of RSV-specific IgG in lungs than mice vaccinated with RSV VLPs (Fig. 2). Higher levels of RSV-specific IgG antibodies were detected in the lungs of mice vaccinated with RSV VLP F or RSV VLP F + G than mice vaccinated with RSV VLP G (Fig. 2A) (*P < 0.01). We evaluated the serum nAb titers RSV VLP vaccinated mice. The nAb titers obtained from VLP F-vaccinated or VLP F + VLP G-vaccinated mice were higher than the groups of naïve, VLP M1-vaccinated, or VLP G-vaccinated mice (Fig. 3). Mice vaccinated with VLP G had significantly higher nAb titers than VLP M1-vaccinated mice but had lower nAb titers than VLP F-vaccinated mice (Fig. 3). Taken together, these results show that RSV VLP F vaccination induced greater RSV-specific and nAb responses than VLP G vaccination.
FIG 2.
RSV-specific IgG responses. Lung homogenates were collected at day 4 p.i. and examined for RSV-specific IgG antibodies. Total RSV-specific IgG amount was determined in serially diluted lung samples. (A) Lung IgG level in mice unvaccinated (naïve) or vaccinated with the indicated RSV VLPs. (B) Lung IgG level in mice vaccinated with FI-RSV or infected with live RSV.
FIG 3.
Serum nAb titers. Sera were obtained from naïve mice or obtained 3 weeks post-boost from VLP- or FI-RSV-vaccinated mice, or 3 weeks following primary RSV infection (n = 5 per group). Sera were heat-inactivated and assayed for RSV-neutralizing activity. The IC50 value obtained from two independent experiments combined are shown as mean with SEM. Significant differences (P < 0.05) were observed between the following groups: VLP M1 and VLP G, VLP F and VLP G, VLP G and VLP F + VLP G, RSV and FI-RSV.
3.3. CD8+ T cell responses to RSV VLP F, VLP G, and VLP F+G vaccination
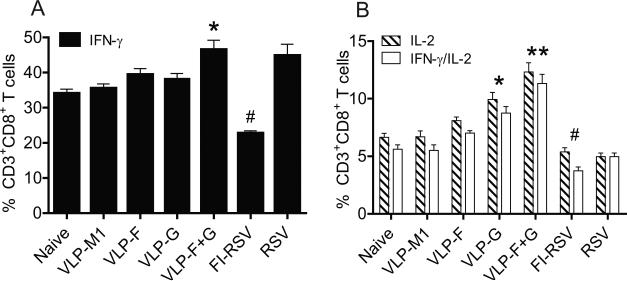
In addition to antibody, cellular immunity can play an important role in protection against RSV infection (Cherukuri et al., 2012; Lee et al., 2012). As mice vaccinated with VLP F + VLP G exhibited greater protection against RSV challenge than mice vaccinated with VLP F, but these two groups showed similar nAb titers (Fig. 1 and Fig. 3), we hypothesized that VLP G induces protective cellular immune responses. We examined cytokine production by CD8+ T cells. The percentage of CD3+ CD8+ T cells producing IFN-γ was higher in the RSV VLP F + VLPG-vaccinated group compared to other VLP groups (Fig. 4A). Also, the percentage of CD3+ CD8+ T cells producing IL-2 or IFN-γ and IL-2 were higher in VLP G-vaccinated mice and the VLP F + VLPG-vaccinated mice compared to the VLP F group (Fig. 4B). We observed significantly lower cytokine production by CD8+ T cells from mice vaccinated with FI-RSV (Fig. 4A and 4B). Thus, we found that VLP G induced CD8+ T cell cytokine expression, and the enhanced protection associated with VLP F + VLP G vaccination correlated with CD8+ T cell cytokine expression.
FIG 4.
Lung CD8+ T cell cytokine production. BALB/c mice (n=5) were unvaccinated or vaccinated with RSV VLPs and challenged with RSV A2-line19F at 3 weeks after the boost. Lung lymphocytes were isolated on day 8 p.i. and stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml). The percentage of CD3+ CD8+ T cells in lung lymphocytes from naïve, challenged mice or RSV VLP-vaccinated and challenged mice that expressed IFN-γ (A), IL-2 or IFN-γ+/IL-2+ (B) in the presence of PMA and ionomycin plus SEM. Graphs show the percentages of CD8+ T cells that produced each indicated cytokine. Data show two independent experiments combined. (*, **, #, P < 0.05, ANOVA)
3.4. RSV VLP F and VLP F+ VLP G vaccination reduced RSV lung pathology
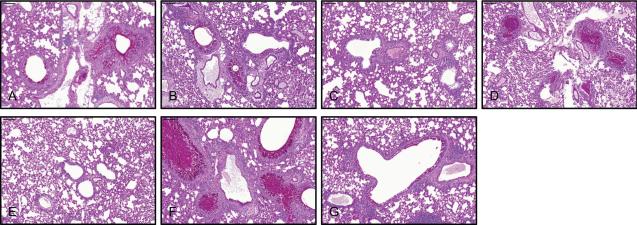
Histopathological changes in RSV-infected lungs include airway mucus production, and the immunopathology of FI-RSV enhanced disease is associated with eosinophils (Johnson et al., 2007; Lee et al., 2012; Varga et al., 2001). We examined whether mucin expression in the airways of RSV-infected mice can be controlled by RSV VLP vaccine. We detected airway mucin expression in naïve + A2-line19F challenged and RSV VLP M1 vaccinated and A2-line19F challenged mice (Fig. 5A and 5B), in agreement with our previous reports using the mucogenic A2-line19F strain (Lee et al., 2012). RSV VLP F vaccination and VLP F + VLP G vaccination ablated the RSV-induced airway mucin expression in this model (Fig. 5C and 5E). In stark contrast, RSV VLP G and FI-RSV vaccination followed by A2-line19F challenge resulted in excessive mucus production and airway mucus plugging (Fig. 5D and 5F). Thus, under these experimental conditions with mixed VLP F and VLP G, the protection induced by VLP F was dominant to the immunopathology caused by VLP G.
FIG 5.
Reduction of RSV-induced airway mucin expression by RSV VLP vaccination. Unvaccinated mice (naïve), VLP-vaccinated, FI-RSV-vaccinated, and RSV-infected mice were challenged with RSV A2-line19F at day 21 post-boost or 21 days post-primary infection in the case of RSV infection. There were 5 mice per group. Lungs were harvested day 8 p.i. and processed for PAS staining. Representative lung sections containing airways are shown. (A) naïve, (B) VLP M1, (C) VLP F, (D) VLP G, (E) VLP F + VLP G, (F) FI-RSV, and (G) RSV. Scale bars represent 100 μm. The results represent those from two independent experiments with similar data.
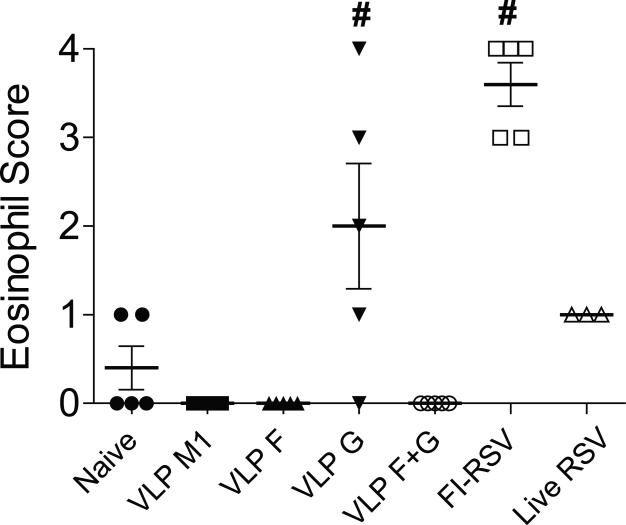
Lung eosinophilia is associated with FI-RSV vaccination, a phenotype that is independent of the G protein in BALB/c mice, and eosinophilia is also associated with G protein vaccination (Johnson and Graham, 2004; Srikiatkhachorn and Braciale, 1997a; Waris et al., 1996). In line with these observations, we found that the eosinophil score was significantly higher in mice immunized with RSV VLP G or FI-RSV (Fig. 6). Notably, eosinophils were undetectable in VLP F + VLP G vaccinated and challenged mice (Fig. 6). The results show that RSV VLP F ameliorated eosinophilia induced by RSV VLP G vaccination.
FIG 6.
Vaccine-induced eosinophils. BALB/c mice (5 per group) were naïve, VLP-vaccinated, FI-RSV-vaccinated, and RSV-infected mice and then challenged with RSV A2-line19F at day 21 post-boost or 21 days post-primary infection. Eosinophil infiltration in the lung was scored as described in the Materials and Methods. Data show mean score ± SEM of one experiment representative of two independent experiments with equivalent results. Each symbol represents one mouse. # and *, P < 0.05, ANOVA).
4. Discussion
We demonstrated that vaccination with mixed RSV VLP F and VLP G resulted in antibody and CD8+ T cell responses and protected against RSV A2-line19F strain challenge in BALB/c mice. These RSV VLPs contain either RSV F or RSV G in complex with influenza M1, and both VLP F and RSV VLP G were previously found to reduce lung viral loads after RSV strain A2 challenge (Quan et al., 2011). Here, we found that mixing VLP F and VLP G results in additive protection. However, in contrast to VLP F, vaccination with VLP G alone resulted in vaccine-enhanced immunopathology similar to FI-RSV, so application of RSV G VLPs may invoke a risk not associated with F-containing VLPs.
Currently, the best protection for children against RSV-induced LRI is RSV infection itself. Although homologous RSV strains can infect individuals repeatedly, reinfections are associated with mild symptoms, and immunity to severe disease builds rapidly in children after RSV exposure (Graham, 2011; Ohuma et al., 2012). It is well established that nAb, in the forms of maternal Ab, infection-induced nAb, and passive prophylaxis, protects against RSV. In our study, VLP F and VLP F + VLP G induced similar levels of serum nAb, and VLP G induced lower levels of nAb. These results are consistent with earlier work showing that vaccinia virus expressing RSV F (Vac-F) induces higher nAbs than Vac-G (Olmsted et al., 1986). The pre-fusion conformation of the F protein has recently been found to induce potent nAbs via a novel pre-fusion-specific epitope, and human polyclonal anti-RSV sera was found to contain activity against pre-fusion F (Magro et al., 2012; McLellan et al., 2013). At this time, we do not know whether the F incorporated in VLP F is in the pre-fusion or post-fusion form, and acquisition of a mAb specific to the pre-fusion form will enable this determination. If the VLP F particles are post-fusion, it may be possible to engineer pre-fusion F into these VLPs for further evaluation. In our previous study of RSV VLP F and VLP G, we reported that VLP G provided slightly but significantly greater protection against RSV challenge than VLP F (Quan et al., 2011). Here, we found that VLP G provided slightly greater protection than VLP F, but there was no statistically significant difference (Fig. 1). Here, we used the A2-line19F challenge strain, whereas the A2 strain was used previously (Quan et al., 2011). Also, the lung homogenization methods were not the same, which could potentially confound comparing the two studies.
Although the primary goal of RSV vaccines has been to induce protective levels of nAb, antiviral CD8+ T cell responses are considered favorable due to the role of these cells in RSV clearance (Graham, 2011). We measured IFN-γ and/or IL-2 secreting CD8+ T cell responses following RSV VLP vaccination and challenge. The percentage of CD8+ T cells expressing IFN-γ, IL-2, as well double positive IFN-γ and IL-2 expressers was higher in the VLP F + VLP G-vaccinated mice than the VLP F- or VLP G-vaccinated mice. The VLP G and VLP F + VLP G vaccination resulted in CD8+ T cells IL-2 expression following challenge, implicating VLP G in T cell stimulation that correlated with additive protection against challenge afforded by VLP F + VLP G vaccination. We assayed IFN-γ and IL-4 by ICS with CD4 and CD8 staining in mice vaccinated with VLP F or VLP G, and we found no difference in the number of CD4+ T cells in the lung or the percentage of CD4+ T cells expressing IFN-γ comparing VLP F to VLP G, and we found negligible CD4+ T cell IL-4 expression (data not shown). Overall, our data suggest that VLP F + VLP G vaccination established both nAb and CD8+ T cell response in mice.
Although the FI-RSV enhanced disease phenotype in mice did not depend on the presence of the G protein, vaccination with recombinant vaccinia virus expressing RSV G (Vac-G) primed for enhanced RSV disease, and G protein vaccination was associated with eosinophils in macaques (de Waal et al., 2004; Johnson and Graham, 2004; Srikiatkhachorn and Braciale, 1997b). Previous work with Vac-G found that CD8+ T cells can inhibit Vac-G-induced enhanced RSV disease (Olson et al., 2008; Olson and Varga, 2007; Srikiatkhachorn and Braciale, 1997a). Vaccinia virus expressing the RSV M2 protein, which harbors an immunodominant CD8 T cell epitope, was shown to inhibit Vac-G-induced enhanced disease, but Vac-F failed to do so (Olson et al., 2008). In our study, VLP F mixed with VLP G ablated the enhanced disease induced by VLP G. The difference may be that F and G displayed on VLPs may differ in antigenicity from F and G expressed intracellularly by a virus vector. Antigen dose may determine the outcome of RSV vaccine enhanced disease phenotypes in mice. Alternatively, higher levels of nAb and T helper type 1 cytokine producing CD8 T cells induced by mixed VLP F and G vaccines may inhibit VLP G-mediated enhanced disease by better clearing of incoming challenge virus, in line with the observation that Ab quality is one determinant of the vaccine-enhanced disease phenotype (Delgado et al., 2009).
Non-live RSV vaccines are being targeted to seropositive populations (e.g. pregnant mothers, the elderly, and older children) because of enhanced disease associated with inactivated RSV seronegative infants. VLPs can be a viable approach for RSV vaccines in these seropositive populations, with desirable properties of the general immunogenicity, scale-up potential, and regulatory approval of VLP vaccines. Considering application to seropositive population, we think our results with mixed VLP G and VLP F warrant further evaluation. Newcastle disease virus (NDV) VLPs have been developed that are composed of RSV G ectodomain fused to the NDV HN cytoplasmic tail and RSV F ectodomain fused to NDV F cytoplasmic tail, the NDV nucleoprotein, and the NDV matrix protein (VLP-H/G+F/F), and these VLPs were immunogenic and protective in mice (McGinnes et al., 2011; Schmidt et al., 2012). The NDV-RSV VLPs were produced in avian cells by transient DNA transfection. The rBV-produced VLP vaccines from serum-free insect cells have advantages in terms of VLP vaccine technology. RSV VLP production utilizing the rBV/insect cell system is FDA approved for human use, and the insect cell expression system results in high levels of VLP expression in the suspension cultures, which facilitates large-scale production (Vicente et al., 2011). Also, mammalian cell-produced VLPs require the validation of removal of viruses or oncogenic substances from the cells (Hesse and Wagner, 2000).
In summary, mixing respiratory syncytial virus (RSV) virus-like particle (VLP) vaccines expressing either the RSV F protein or the RSV G protein has an additive effect on protective immunity against RSV challenge in a BALB/c mouse model, compared to RSV VLP F vaccination alone or RSV VLP G vaccination alone. The enhanced protection provided by mixing RSV VLP F + VLP G correlated with CD8 T cell cytokine production. No vaccine-enhanced immunopathology was observed in a BALB/c mouse model after vaccination with VLP F or mixed RSV VLP F + VLP G followed by RSV challenge. In contrast, vaccination with VLP G alone resulted in immunopathology following challenge. These results suggest that mixed VLP F and VLP G vaccination induces better protection by effective lung viral clearance and suppressing VLP G-induced immunopathology.
Vaccination with RSV VLP G, but not VLP F, resulted in vaccine-enhanced disease following RSV challenge in mice.
Vaccination with mixed RSV VLP F and VLP G had an additive effect on protective immunity against RSV challenge in mice.
No vaccine-enhanced disease was observed in RSV challenged mice after vaccination with mixed RSV VLP F and VLP G.
Acknowledgments
We thank the Emory Children's Pediatric Research Center flow cytometry core, which is supported by Children's Healthcare of Atlanta (CHOA). We thank Carla Shoffeitt (CHOA) for histology technical assistance. This work was supported by the following grants: NIH AI087798 (M.L.M), NIH AI095227 (R. Stokes Peebles, Jr., Vanderbilt and M.L.M), and AI105170 (S.M.K).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Cherukuri A, Stokes KL, Patton K, Kuo H, Sakamoto K, Lambert S, Stillman E, Moore ML, Lee S. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immun. Ageing. 2012;9:21. doi: 10.1186/1742-4933-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal L, Power UF, Yuksel S, van Amerongen G, Nguyen TN, Niesters HG, de Swart RL, Osterhaus AD. Evaluation of BBG2Na in infant macaques: specific immune responses after vaccination and RSV challenge. Vaccine. 2004;22:915–922. doi: 10.1016/j.vaccine.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse F, Wagner R. Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Trends Biotechnol. 2000;18:173–180. doi: 10.1016/s0167-7799(99)01420-1. [DOI] [PubMed] [Google Scholar]
- Hotard AL, Shaikh FY, Lee S, Yan D, Teng MN, Plemper RK, Crowe JE, Jr., Moore ML. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology. 2012;434:129–136. doi: 10.1016/j.virol.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod.Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:S46–57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Lee S, Mittler RS, Moore ML. Targeting CD137 enhances vaccine-elicited anti-respiratory syncytial virus CD8+ T cell responses in aged mice. J Immunol. 2014;192:293–299. doi: 10.4049/jimmunol.1300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Stokes KL, Currier MG, Sakamoto K, Lukacs NW, Celis E, Moore ML. Vaccine-Elicited CD8+ T Cells Protect against Respiratory Syncytial Virus Strain A2-Line19F-Induced Pathogenesis in BALB/c Mice. J. Virol. 2012;86:13016–13024. doi: 10.1128/JVI.01770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A. 2012;109:3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol. 2011;85:366–377. doi: 10.1128/JVI.01861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monie A, Hung CF, Roden R, Wu TC. Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics. 2008;2:97–105. [PMC free article] [PubMed] [Google Scholar]
- Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE, Jr., Goleniewska K, Williams JV, Collins PL, Peebles RS., Jr. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J. Virol. 2009;83:4185–4194. doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuma EO, Okiro EA, Ochola R, Sande CJ, Cane PA, Medley GF, Bottomley C, Nokes DJ. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol. 2012;176:794–802. doi: 10.1093/aje/kws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted RA, Elango N, Prince GA, Murphy BR, Johnson PR, Moss B, Chanock RM, Collins PL. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Hartwig SM, Varga SM. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol. 2008;181:7958–7968. doi: 10.4049/jimmunol.181.11.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol. 2007;179:5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- Peebles RS, Jr., Sheller JR, Collins RD, Jarzecka K, Mitchell DB, Graham BS. Respiratory syncytial virus (RSV)-induced airway hyperresponsiveness in allergically sensitized mice is inhibited by live RSV and exacerbated by formalin-inactivated RSV. J.Infect.Dis. 2000;182:671–677. doi: 10.1086/315783. [DOI] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis. 2011;204:987–995. doi: 10.1093/infdis/jir474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. Long-term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J Virol. 2012;86:11654–11662. doi: 10.1128/JVI.01510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Ohrum K, Ross TM. Virus-like particles for antigen delivery at mucosal surfaces. Curr Top Microbiol Immunol. 2012;354:53–73. doi: 10.1007/82_2011_135. [DOI] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997a;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Braciale TJ. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997b;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS, Jr., Moore ML. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, El Sahly H, King J, Graham I, Izikson R, Kohberger R, Patriarca P, Cox M. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok(R)) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011;29:7733–7739. doi: 10.1016/j.vaccine.2011.07.128. [DOI] [PubMed] [Google Scholar]
- Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- Vicente T, Roldao A, Peixoto C, Carrondo MJ, Alves PM. Large-scale production and purification of VLP-based vaccines. J Invertebr Pathol. 2011;107(Suppl):S42–48. doi: 10.1016/j.jip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]