Abstract
Embryonic stem (ES) cells are characterized by pluripotency, defined as the developmental potential to generate cell lineages derived from all three primary germ layers. In the past decade, great progress has been made on the cell culture conditions, transcription factor programs and intracellular signaling pathways that control both murine and human ES cell fates. ES cells of mouse vs. human origin have distinct culture conditions, responding to some tyrosine kinase signaling pathways in opposite ways. Previous work has implicated the Src family of non-receptor protein-tyrosine kinases in mouse ES cell self-renewal and differentiation. Seven members of the Src kinase family are expressed in mouse ES cells, and individual family members appear to play distinct roles in regulating their developmental fate. Both Hck and c-Yes are important in self-renewal, while c-Src activity alone is sufficient to induce differentiation. While these findings implicate Src-family kinase signaling in mouse ES cell renewal and differentiation, the role of this kinase family in human ES cells is largely unknown. Here, we explored Src-family kinase expression patterns and signaling in human ES cells during self-renewal and differentiation. Of the eleven Src-related kinases in the human genome, Fyn, c-Yes, c-Src, Lyn, Lck and Hck were expressed in H1, H7 and H9 hES cells, while Fgr, Blk, Srm, Brk, and Frk transcripts were not detected. Of these, c-Yes, Lyn, and Hck transcript levels remained constant in self-renewing human ES cells vs. differentiated EBs, while c-Src and Fyn showed a modest increase in expression as a function of differentiation. In contrast, Lck expression levels dropped dramatically as a function of EB differentiation. To assess the role of overall Src-family kinase activity in human ES cell differentiation, cultures were treated with inhibitors specific for the Src kinase family. Remarkably, human ES cells maintained in the presence of the potent Src-family kinase inhibitor A-419259 retained the morphology of domed, pluripotent colonies and continued to express the self-renewal marker TRA-1-60 despite culture under differentiation conditions. Taken together, these observations support a role for Src-family kinase signaling in the regulation of human ES cell fate, and suggest that the activities of individual Src-family members are required for initiation of the differentiation program.
Keywords: Src-family kinases, human ES cells, Lck, embryoid bodies, pluripotency
Introduction
Human ES (hES) cells are pluripotent stem cells derived from the inner cell mass of blastocyst stage human embryos produced by in vitro fertilization (Thomson et al., 1998). Like mouse ES (mES) cells, hES cells are pluripotent and can form embryoid bodies in vitro and teratomas in vivo upon injection into immunodeficient mice. Although hES cells are of the same blastocyst origin as mES cells, they depend on distinct receptor tyrosine kinase signaling pathways for maintenance in culture. For example, hES cells require bFGF and TGFβ/Activin signals to maintain the undifferentiated state. In contrast, factors essential for mES cell self-renewal, such as LIF and BMPs, either have no effect on hES cells or induce their differentiation, respectively (Yu and Thomson, 2008). In hES cells, bFGF signals through the FGF receptor tyrosine kinase to activate Erk signaling which inhibits differentiation and the PI3K-Akt pathway to promote survival (Dvorak et al., 2005; Li et al., 2007). In addition, the TGFβ/Nodal/Activin signaling axis inhibits neuronal differentiation, and works synergistically with bFGF to maintain hES cell pluripotency (Vallier et al., 2005). Despite these differences in growth factor requirements between mES and hES cells, the core transcription factors governing pluripotency are similar, with both mES and hES cells expressing the master pluripotency factors, Oct4, Nanog and Sox2 (Boyer et al., 2005). While the growth factor conditions, receptor kinase signaling, and transcription factor networks governing hES cell fate have been examined in detail, the intracellular signaling pathways downstream of receptor tyrosine kinases have not been fully explored.
The Src family of non-receptor tyrosine kinases is coupled to many growth factor receptors (including the FGFR) to regulate cell adhesion, proliferation, growth and survival (Parsons and Parsons, 2004; Boggon and Eck, 2004). There are eleven Src-related kinases in the human genome (Manning et al., 2002), eight of which have been studied extensively in mammalian cells (Blk, Fgr, Fyn, Lck, Lyn, Hck, c-Src and c-Yes) plus three phylogenetically related kinases (Srm, Frk and Brk). In adult mice, c-Src, Fyn and c-Yes are ubiquitously expressed, while Lck, Lyn, Hck, Blk and Fgr are more restricted in their expression patterns, primarily to hematopoietic cells (Lowell and Soriano, 1996). Surprisingly, at least seven members of the Src kinase family are expressed in mES cells, and individual family members appear to play distinct roles in regulating their developmental fate (Meyn, III et al., 2005). For example, expression of Hck is rapidly silenced as mES cells differentiate to embryoid bodies (EBs), suggesting a role in self-renewal or the suppression of differentiation. In contrast, c-Src is expressed and active in both pluripotent mES cells and EBs derived from them, and its activity alone is sufficient to induce differentiation of mES cells to primitive ectoderm and endoderm (Meyn, III et al., 2005; Meyn, III and Smithgall, 2009). Other studies have shown that both Hck and c-Yes are important for mES cell self-renewal downstream of LIF (Ernst et al., 1994; Anneren et al., 2004; Tamm et al., 2011), and that c-Yes may have a similar role in human ES cells (Anneren et al., 2004).
In this study, we performed a comprehensive analysis of Src-family kinase (SFK) expression and signaling during hES cell self-renewal and differentiation. The hES cell lines H1, H7 and H9 all express comparable mRNA levels of the Src-family members c-Src, c-Yes, Fyn, Lck and Lyn under culture conditions for self-renewal. Remarkably, Lck expression decreased dramatically as hES cells differentiated; Lck transcript and protein levels were lost more rapidly than those of the well-known pluripotency marker Oct4 during EB formation. The observed changes in Lck expression and activity are unique to hES cells as compared to mES cells, and suggest a possible role in maintenance of the undifferentiated state. In contrast, c-Src, c-Yes and Fyn message levels did not change during differentiation, similar to previous observations in mES cells. We also found differences in SFK activity as a function of hES cell differentiation. c-Src, Fyn and Lyn were active in renewing hES cells and differentiated EBs, while Lck and c-Yes were active only in self-renewing hES cells. To investigate the requirement for SFK activity in differentiation, hES cell cultures were treated with small molecule SFK inhibitors previously tested in mES cells (Meyn, III and Smithgall, 2009; Meyn, III et al., 2005). Remarkably, hES cells treated with the potent pan-SFK inhibitor A-419259 retained colony morphology and marker expression characteristic of pluripotency even under culture conditions for differentiation. Immunoblots confirmed that this compound blocked all endogenous SFK activity, including that of Lck and c-Src. These observations demonstrate that the activities of several Src-family members are required for hES cells to initiate the differentiation program, and that SFK signals for differentiation may be dominant to those for self-renewal.
Materials and Methods
Cell culture
Human ES cell lines H1, H7, and H9 (WiCell WA01, WA07 and WA09) were maintained in feeder-free culture conditions with mTeSR medium (Stemcell Technologies) on 6-well plates coated with hESC-qualified Matrigel (BD Biosciences) as described (Schatten et al., 2005). The culture medium was changed daily, and cells were passaged every 5–7 days thereafter using Dispase (Stemcell Technologies) as per the manufacturer’s instructions.
EB formation
ES cells were dissociated into single cells with Accutase (Stemcell Technologies), pelleted and re-suspended in mTeSR medium containing the Rho kinase inhibitor, Y27632 (10 μM). Viable cells were counted using Trypan Blue (Invitrogen) and 2,000 cells were loaded into each microwell of AggreWell plates (Stemcell Technologies). EBs were harvested 24 hours later, re-suspended in AggreWell medium (Stemcell Technologies) or differentiation medium (described below) and maintained on ultra-low attachment plates (Corning). EBs were harvested 3, 9 and 12 days after initiation and were washed using a cell strainer (BD Biosciences) to remove single cells before lysis.
Inhibitor treatment
ES cells were passaged onto Matrigel-coated 6-well plates and maintained in mTeSR medium for three days. Culture medium was then replaced with fresh mTeSR or differentiation medium with or without SFK inhibitors for an additional three days. Differentiation medium was composed of 5% knockout serum replacement, 15% fetal bovine serum, 1% non-essential amino acid, 1% L-glutamine, and 1% Pen/Strep in DMEM/F-12 medium (all components from Life Technologies). The SFK inhibitors SKI-1 and PP2 were purchased from EMD-Millipore-Calbiochem while A-419259 was purchased from Santa Cruz Biotechnology.
RT-PCR
RNA isolation, RT-PCR, quantitative real-time RT-PCR, and data analysis were performed as described in detail elsewhere (Zhang et al., 2014).
Immunoprecipitation and immunoblots
Cells were washed with phosphate-buffered saline (PBS) and lysed with RIPA buffer supplemented with phosphatase and protease inhibitors as described (Meyn, III et al., 2005). SFK proteins were immunoprecipitated from equivalent amounts of clarified ES cell lysates with isoform-specific antibodies and protein G-Sepharose as described elsewhere (Zhang et al., 2014; Meyn, III and Smithgall, 2009; Meyn, III et al., 2005). Immunoprecipitated proteins as well as cell lysates were separated by SDS-PAGE, transferred to PVDF membranes and probed with antibodies to the SFK activation loop, each SFK protein, Oct4, and actin as a loading control. Antibodies used for these studies are summarized in Table S1.
Immunofluorescence microscopy
Cells were plated onto Matrigel-coated coverslips in 6-well plates and maintained in mTeSR medium for three days. The cell culture medium was then switched to differentiation medium or fresh mTeSR with or without inhibitors for three additional days. Cells were then washed with PBS, fixed in 4% paraformaldehyde (Sigma) for 15 min, and blocked with PBS containing 5% bovine serum albumin (Fisher) and 5% normal goat serum (Invitrogen) for 1 h at room temperature. Cells were then incubated with primary antibodies overnight at 4 °C in blocking buffer, followed by 3 washes with PBS. Cells were then incubated with secondary antibodies coupled to various fluorescent probes at 37 °C for 1 h followed by 3 additional washes. Cells were then sealed with coverslips using mounting medium containing DAPI to stain the nuclei (Vector Laboratories). Antibodies used for immunocytochemistry fluorescence are summarized in Table S1.
Results
Human ES cells express multiple Src-family kinases
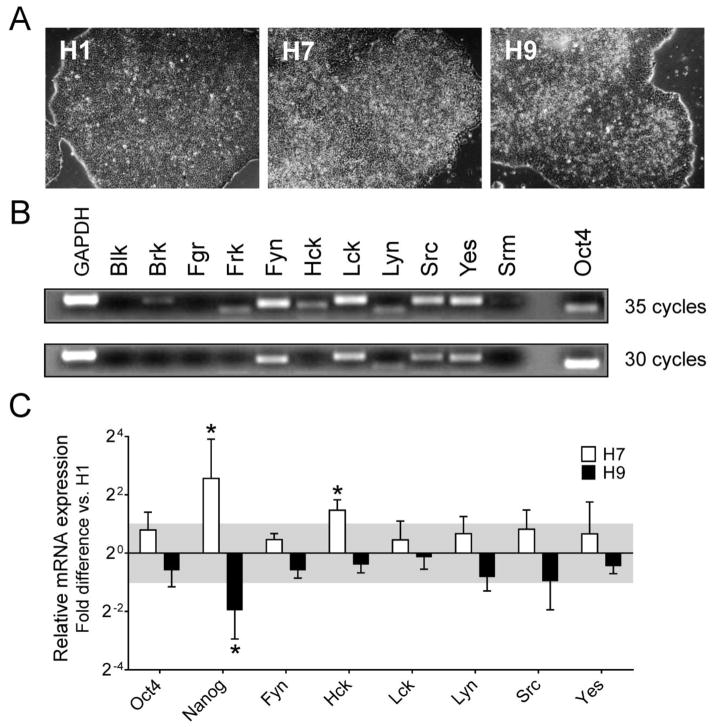
Despite the expression of eleven Src-related kinases in the human genome, only c-Yes has been studied in the context of hES cell biology (Anneren, 2008). While the c-Yes protein is expressed in both pluripotent hES cells and differentiated EBs derived from them, c-Yes kinase activity is downregulated as hES cells differentiate to EBs (Anneren et al., 2004; Zhang et al., 2014). This observation provided the first evidence that c-Yes, and possibly other members of the Src kinase family, may regulate hES cell fate. To study SFK signaling in hES cells more broadly, we first determined which family members are expressed in hES cells using RT-PCR. For this study, we cultured the hES cell lines H1, H7 and H9 in chemically defined mTeSR self-renewal medium under-feeder free conditions on Matrigel-coated plates (Ludwig et al., 2006). All three cell lines grew as domed colonies, with some evidence of differentiation at the edge of each colony (Figure 1A). RT-PCR analysis revealed that H1 hES cells express six SFKs, including c-Src, c-Yes, Fyn, Lck, Hck and Lyn. Of these, c-Src, c-Yes, Fyn and Lck are the most strongly expressed (Figure 1B). The pluripotency marker Oct4 was included in this experiment as a positive control.
Figure 1. Profile of SFK gene expression in hES cells.
A) The hES cell lines H1, H7 and H9 were maintained under feeder-free conditions on Matrigel-coated plates using mTeSR1 medium. All three lines grew as large domed colonies with well-circumscribed boundaries. B) H1 hES cells were harvested 5 days after passage for RNA isolation. SFK (Blk, Fgr, Fyn, Hck, Lck, Lyn, c-Src, c-Yes), Src-related kinase (Brk, Frk, Srm) and pluripotency marker (Oct4) expression were determined by RT-PCR with GAPDH as a positive control. Images of agarose gels of the PCR products are shown after 30 and 35 PCR cycles. C) Relative SFK (Fyn, Hck, Lck, Lyn, c-Src, c-Yes) and pluripotency marker (Oct4, Nanog) expression levels across three hES cell lines. Transcript levels for the indicated genes were determined by quantitative real-time RT-PCR for the H1, H7 and H9 hES cell lines. The results are expressed as the average fold-change in mRNA level in H7 and H9 cells relative to H1 cells ± SEM (n = 3, *p < 0.05; pairwise xed reallocation randomization test). SFK expression varied by less than 2-fold across these hES cell lines (gray area), with the exception of Hck expression in H7 cells.
We next determined the relative expression levels of these six SFKs in comparison to the pluripotency markers Oct4 and Nanog across all three hES cell lines using quantitative real-time RT-PCR (qPCR, Figure 1C). The H7 and H9 cell lines were observed to express the pluripotency marker Oct4 as well as the SFKs Fyn, c-Src, c-Yes, Lck and Lyn at levels comparable to H1 cells by this approach, with less than two-fold variation across the three hES cell lines. In contrast, expression of the pluripotency marker Nanog and the SFK Hck was more variable, indicative of some heterogeneity between these hES cell lines. Nevertheless, these results show that hES cells express multiple Src-family members, with the expression levels of c-Src, c-Yes, Fyn and Lck most consistent among the three hES cell lines tested.
Src family kinase expression during hES cell differentiation
Embryoid body (EB) formation is a convenient model for ES cell differentiation in vitro. When cultured in suspension without feeder layers, hES cells spontaneously form aggregates known as EBs similar to those described for mES cells (Itskovitz-Eldor et al., 2000). Developing EBs express differentiation markers characteristic of all three germ layers, and mimic the early developmental stages of gastrulation and preimplantation embryogenesis (Murry and Keller, 2008).
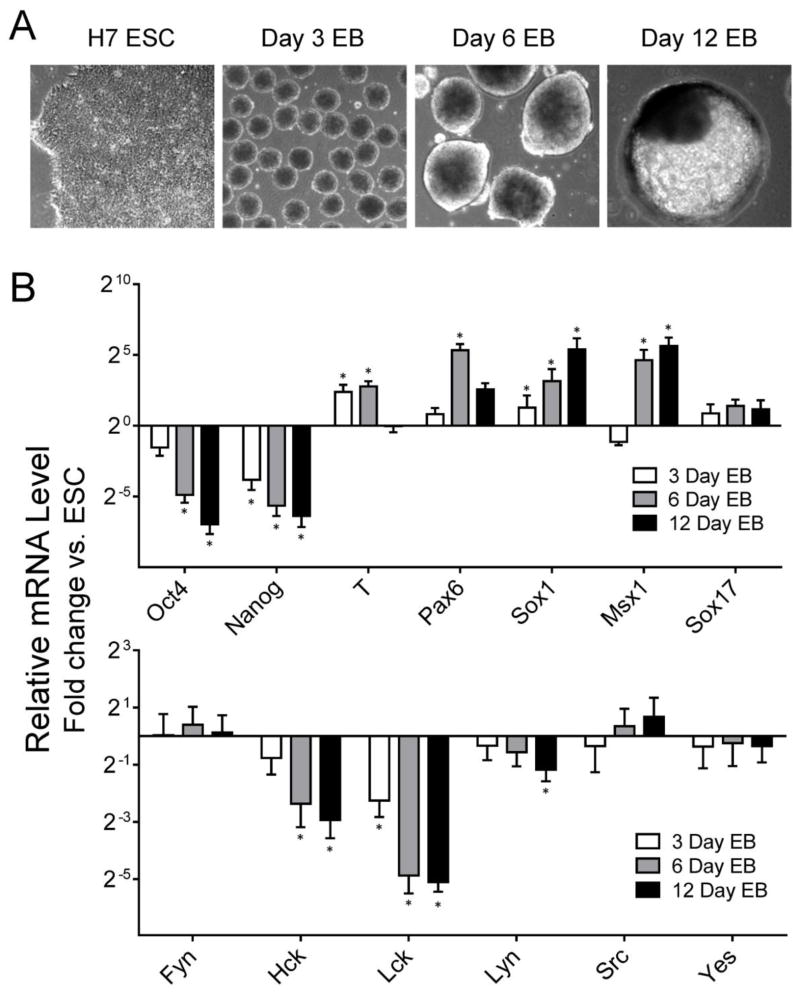
Previous work has shown that individual Src family members exhibit dynamic expression changes as mES cells differentiate to EBs (Meyn, III et al., 2005). To investigate whether SFKs are also differentially regulated during hES cell differentiation, EB formation was initiated from H7 hES cells using AggreWell microplates starting with an equal number of cells per well (Mohr et al., 2010). EBs were harvested 3, 6 and 12 days later (see Figure 2A for morphology), and total RNA was extracted for qPCR analysis of gene expression. As controls for the differentiation process, we first assessed the expression of pluripotency and germ layer markers (Figure 2B). The self-renewal markers Oct4 and Nanog were down-regulated in EBs, while the differentiation markers T (mesoderm), Pax6, Sox1 and Msx1 (ectoderm) increased significantly, consistent with EB differentiation. Lck and Hck expression was rapidly downregulated in response to EB formation, suggesting that that these SFKs may be important for hES cell renewal (Figure 2B). Lyn expression was also downregulated in day 12 EBs compared with control ES cells, but to a lesser extent than Hck and Lck. In contrast, c-Src c-Yes and Fyn expression was maintained throughout EB formation, indicating these three kinases may have roles to play in both self-renewing hES cells and differentiated EBs. Similar results were observed during EB formation from H1 hES cells (data not shown).
Figure 2. SFK expression during EB formation from H7 hES cells.
A) EB formation was initiated from H7 hES cells using Aggrewell plates as described under Materials and Methods. Representative images of H7 hES cells and EBs after 3, 6 and 12 days of culture later are shown; all images recorded at same magnification (100 ×). B) Total RNA was extracted from renewing hES cells and 3, 6, and 12-day EBs. Expression of self-renewal (Oct4, Nanog) and differentiation markers (T, mesoderm; Pax6, Sox1, Msx1, ectoderm; and Sox17, endoderm) as well as SFKs (Fyn, Hck, Lck, Lyn, c-Src, c-Yes) was determined by qPCR relative to control H7 hES cells maintained in mTeSR medium. Results are expressed as the average fold change relative to the undifferentiated hES cell population ± S.E.M. (n = 3; *p < 0.05; Pairwise Fixed Reallocation Randomization Test).
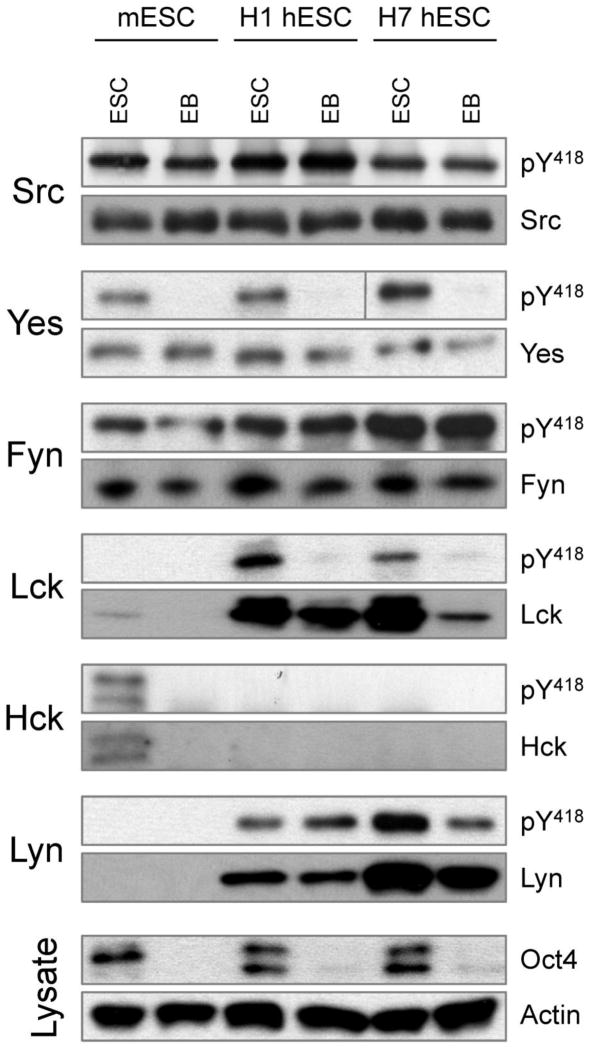
We next explored SFK protein expression patterns and activity levels in immuno-precipitates from the hES cell lines H1 and H7 as well as 6-day EBs derived from them. We also included the mES cell line D3 in this study for comparison. Three Src-family members showed similar expression and activity profiles in human and mouse ES cells and EBs (Figure 3). c-Src and Fyn were strongly expressed and active in both self-renewing and differentiated hES cells. The same is true for mES cells, consistent with our previous results (Meyn, III and Smithgall, 2009; Meyn, III et al., 2005). While the c-Yes protein is present in self-renewing mouse and human ES cells as well as EBs, it is only active in self-renewing stem cells, which is also consistent with an earlier report (Anneren et al., 2004). Interestingly, three other SFKs showed differences in protein expression and activity between mouse and human ES cells. Hck was present and active in self-renewing mES cells, but was not detected in differentiated day 6 mouse EBs as observed previously (Meyn, III et al., 2005). Unlike mES cells, however, we could not detect Hck protein in self-renewing human ES cells or EBs, despite the presence of Hck transcripts by RT-PCR. In contrast to Hck, Lck was highly expressed and active in both lines of self-renewing hES cells but not in mES cells. Similar to c-Yes, Lck activity was completely repressed in differentiated EBs derived from both hES cell lines. Lck protein levels were also decreased as a function of differentiation, consistent with the decreased transcript levels observed by qPCR. However, Lck protein was still detectable in 6-day EBs, even though its activity appears to be downregulated based on loss of activation loop phosphorylation on Tyr418. These observations suggest that regulation of Lck signaling in hES cells may be complex, involving both gene transcription and regulation of kinase function.
Figure 3. SFK protein expression and activity in ES cells and 6 day EBs.
Lysates were prepared from mouse ES cells (D3 line), human ES cells (H1 and H7 lines) and 6-day EBs derived from them. Endogenous SFKs (c-Src, c-Yes, Fyn, Lck, Hck, Lyn) were immunoprecipitated and blotted with a phosphospecific antibody for the active form of each kinase (pY418 antibody, which recognizes the conserved phosphotyrosine residue in the activation loop of the kinase domain) as well as the respective SFK protein. Cell lysates were also blotted for Oct4 as marker of ES cell self-renewal plus actin as a loading control. This experiment was repeated twice with comparable results; a representative example is shown.
Inhibition of Src-family kinase activity blocks hES cell differentiation
The dynamic changes in SFK expression patterns (Lck) and activity (Lck and c-Yes) that occur during EB formation from hES cells suggest that the activity of this kinase family might influence hES cell growth and differentiation. To test this idea, we employed the pyrrolopyrimidine compound A-419259, which is a potent, cell-active inhibitor selective for the Src-kinase family (Meyn, III and Smithgall, 2009). Previous studies showed that inhibition of endogenous SFK activity with this compound maintained mES cell pluripotency in the absence of LIF while reversibly inhibiting EB formation (Meyn, III et al., 2005). Subsequent work attributed the effects of A-419259 treatment to inhibition of c-Src, which drives differentiation of mES cells towards primitive ectoderm-like cells (Meyn, III and Smithgall, 2009). Using A-419259 concentrations determined in the previous study with mES cells, we tested whether this SFK inhibitor produced similar effects on hES cells.
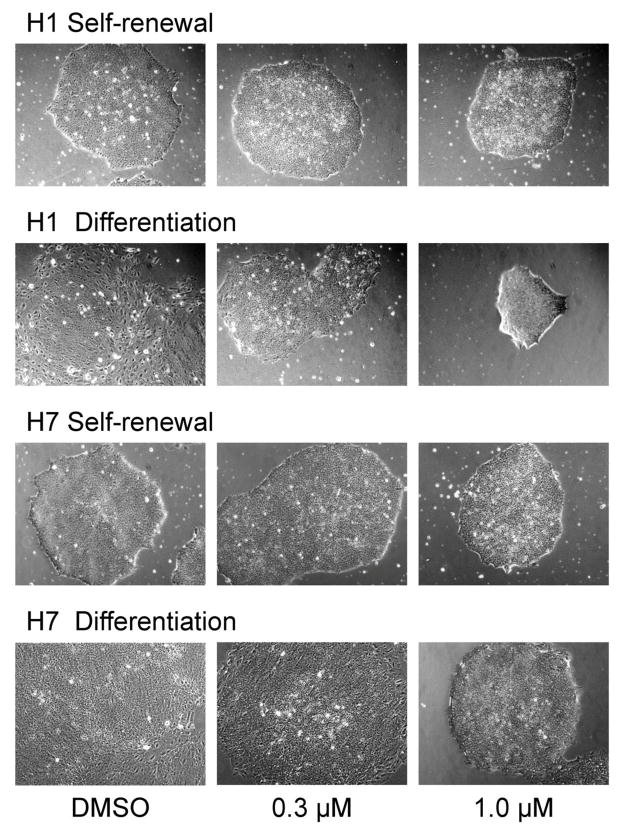
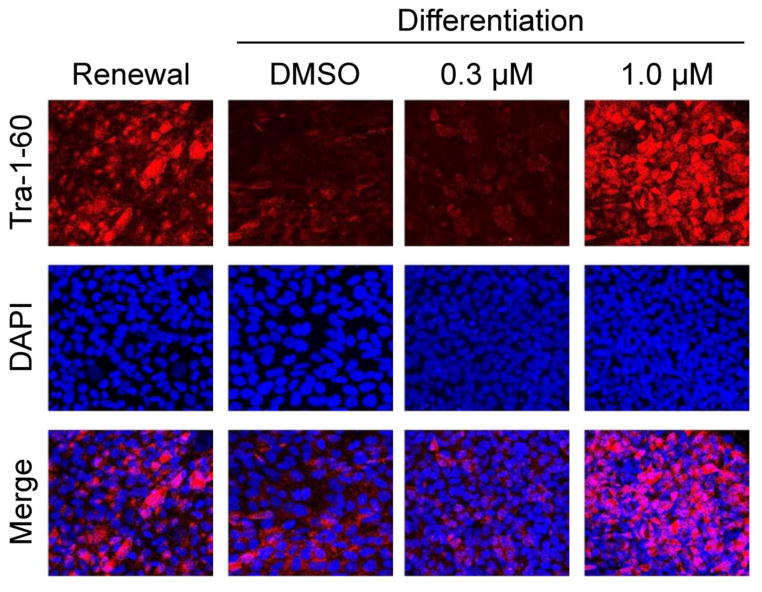
For inhibitor experiments, the H1 and H7 lines of hES cells were passaged onto Matrigel-coated plates and grown in self-renewal medium (mTeSR) for three days. The culture medium was then replaced with either differentiation medium or fresh renewal medium in the presence or absence of A-419259 for an additional three days, followed by assessment of colony morphology and pluripotency marker expression. As shown in Figure 4, A-419259 treatment had no effect on the undifferentiated colony morphology of hES cells grown in mTeSR medium. However, when hES cells were switched to differentiation medium in the presence of 1 μM A-419259, they retained the domed morphology characteristic of pluripotent colonies. This effect was not observed when the inhibitor concentration was lowered to 0.3 μM, suggesting a threshold effect similar to that observed previously in mES cells (Meyn, III et al., 2005).
Figure 4. hES cells treated with the small molecule SFK inhibitor A-419259 retain undifferentiated colony morphology.
The H1 and H7 hES cell lines were grown in mTeSR self-renewal medium or switched to differentiation medium in the absence or presence of the pan-SFK inhibitor, A-419259, at the concentrations shown. Note that both hES cells lines maintained undifferentiated colony morphology when cultured in the presence of A-419259 under differentiation conditions.
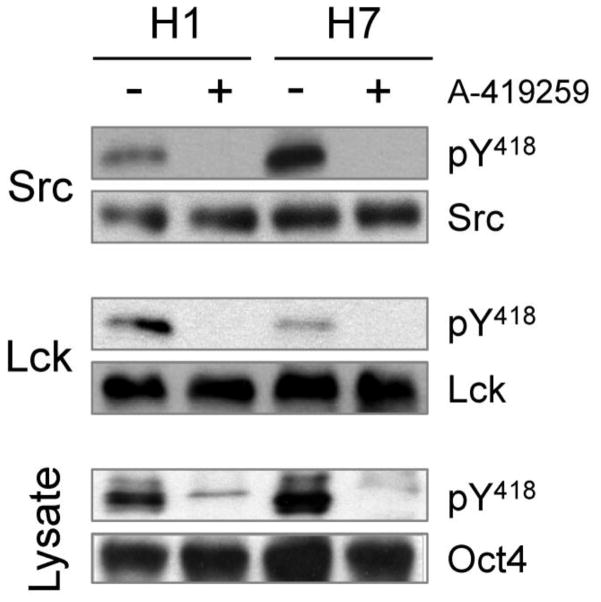
We next investigated whether the observed effect of A-419259 treatment on hES cell colony morphology correlated with inhibition of endogenous SFK activity. For this experiment, self-renewing H1 hES cells were treated overnight with A-419259 followed by immunoblotting of cell lysates with a phosphospecific antibody to detect active SFKs. As shown in Figure 5, constitutive SFK activity was readily detected in untreated hES cells that was almost completely blocked in the presence of A-419259. We also examined the effect of A-419259 treatment on Lck and c-Src kinase activities individually. For this experiment, Lck and c-Src were immunoprecipitated from control and inhibitor-treated cells, followed by immunoblotting with the phosphospecific antibody. Lck and c-Src activity were completely inhibited in immunoprecipitates from A-419259-treated cells. These findings are consistent with our previous observations with this inhibitor in mES cells (Meyn, III and Smithgall, 2009; Meyn, III et al., 2005), and support a role for c-Src in the initiation of hES differentiation.
Figure 5. A-419259 inhibits endogenous SFK activity in hES cells.
Self-renewing hES cells (H1 and H7 lines) were cultured in the absence or presence of A-419259 (1.0 μM) for 16 h, followed by lysis and immunoprecipitation of c-Src and Lck. Each immunoprecipitate was then blotted with a phosphospecific antibody for the active form of each kinase (pY418 antibody, which recognizes the conserved phosphotyrosine residue in the activation loop of the kinase domain) as well as the respective SFK protein. Cell lysates were also blotted for pY418 as well as Oct4 as a marker of ES cell self-renewal. This experiment was repeated twice with comparable results; a representative example is shown.
Human ES cells maintain cell-surface renewal marker expression following SFK inhibition under differentiation culture conditions
To determine whether SFK inhibition affects pluripotency marker expression in hES cells, we first determined the effect of A-419259 treatment on cell-surface expression of Tra-1-60, one of the most stringent markers for hES cell pluripotency (Chan et al., 2009). H1 hES cells were cultured on mTeSR renewal medium or under differentiation conditions in the presence or absence of A-419259 for three days as described above. Cells were then fixed and stained for Tra-1-60 as well as DAPI (Figure 6). Untreated hESC cultures stained strongly with Tra-1-60 under self-renewal conditions while the switch to differentiation medium resulted in a dramatic loss of Tra-1-60 expression. However, hES cells grown in the presence of A-419259 continued to express TRA-1-60 despite the switch to differentiation culture conditions (Figure 6), consistent with the maintenance of rounded undifferentiated colonies observed in the presence of this inhibitor (Figure 4). Treatment of the H7 line of hES cells with A-419259 also resulted in sustained expression of cell-surface Tra-1-60 despite the switch to differentiation medium (data not shown).
Figure 6. hES cells retain Tra-1-60 expression following SFK inhibition.
The H1 line of hES cells was grown in mTeSR self-renewal medium or switched to differentiation medium in the absence (DMSO carrier) or presence of the pan-SFK inhibitor, A-419259, at the concentrations shown. Cells were fixed and immunostained for the cell-surface pluripotency marker Tra-1-60 three days later (magnification 400×). This experiment was repeated three times with comparable results; representative fields of cells from each condition are shown.
Loss of Lck expression is an early indicator of hES cell differentiation
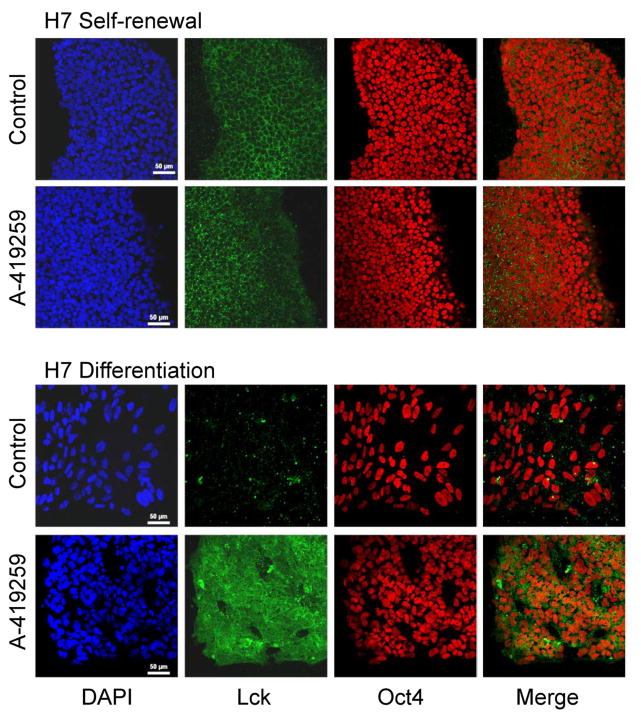
In a final series of experiments, we investigated changes in Lck protein expression at the single cell level as a function of differentiation. For these studies, H7 hES cells were cultured under renewal and differentiation conditions for three days in the presence or absence of A-419259. The cells were then fixed, permeabilized and immunostained for Lck protein expression, as well as Oct4 as a marker of pluripotency. As shown in Figure 7, Lck expression was readily detected in self-renewing ES cells, with clear subcellular localization to the cell periphery. Nuclear Oct4 staining was also observed, and neither Oct4 nor Lck expression was affected by A-419259 treatment under self-renewal culture conditions. On the other hand, hES cells grown for three days under differentiation conditions showed a dramatic reduction in Lck immunofluorescence, even though Oct4 expression was still evident. Remarkably, hES cells cultured in differentiation medium containing A-419259 retained very strong expression of Lck, with expression now observed throughout the cells and not just limited to the cell periphery. These results are consistent with the reduction in Lck expression and activity observed during EB formation from hES cells described earlier (Figures 2 and 3) and identify the Src-family member Lck as a very sensitive indicator of hES cell pluripotency. As a control, we also looked at the expression and subcellular localization of c-Src by immunofluorescence under the same four culture conditions. Unlike Lck, neither the staining intensity nor the subcellular localization of c-Src to the cell periphery changed as a function of differentiation (data not shown).
Figure 7. Loss of Lck expression accompanies hES cell differentiation.
The H7 line of hES cells was grown in mTeSR self-renewal medium or switched to differentiation medium in the absence (Control) or presence of A-419259 (1 μM). Cells were fixed, permeabilized and immunostained for the SFK Lck and the nuclear pluripotency factor Oct4 four days later. DAPI-stained nuclei are also shown. The merged image combines cells in the Lck and Oct4 panels only. This experiment was repeated three times with comparable results; a representative field of cells is shown for each condition.
A final point relates to morphological changes shown in Figure 7 in relation to overall colony morphology presented in Figure 4 under similar culture conditions. Changes to H7 hESC colony morphology or “compactness” as a function of differentiation or inhibitor treatment can be inferred from the size of and distance between the nuclei in Figure 7. Individual nuclei are clearly visible in the images stained with both DAPI and Oct4. Under conditions of self-renewal, the nuclei appear smallest and most closely packed together, consistent with tight colony morphology associated with self-renewal as shown in Figure 4. Under self-renewal conditions, A-419259 treatment had no discernable effect on colony morphology. In contrast, nuclei from cells grown under differentiation conditions (without A-419259) appear larger and further apart (Figure 7), consistent with the loss of compact colony morphology and cell spreading that accompanies differentiation. On the other hand, nuclei in Figure 7 from cultures treated with A-419259 under differentiation conditions appear smaller, closer together and more closely resemble the size and packing of those in undifferentiated colonies. Careful inspection of colony morphology in Figure 4 also shows that self-renewing colonies are more clearly circumscribed and tightly packed than those from cells grown under differentiation conditions with A-419259.
Discussion
In this study, we found that hES cells express multiple Src-family members, with c-Src, c-Yes, Fyn, Lck and Lyn readily detected at both the transcript and protein levels. Using a phosphospecific antibody for the active form of each kinase, we also observed that all five kinases appear to be constitutively active in self-renewing ES cells. Differentiation of hES cells to EBs was associated with loss of Lck and c-Yes kinase activity, as well as potent silencing of Lck gene expression. In contrast, c-Src and Fyn gene expression, protein levels and kinase activity were maintained throughout differentiation. These results suggest that Lck and c-Yes activity may influence self-renewal, while c-Src and Fyn signaling may be related to differentiation. Previous results have shown c-Yes is expressed and active in both mouse and human ES cells under self-renewal conditions (Anneren et al., 2004), and that enforced expression of c-Yes interferes with EB formation from mES cells (Zhang et al., 2014). On the other hand, both c-Src and Fyn are present and active in self-renewing mES cells as well as EBs formed from them. Other studies have shown that c-Src activity alone is sufficient to drive mES cells towards primitive ectoderm and endoderm, and also induces the expression of several genes that regulate the epithelial to mesenchymal transition associated with early embryogenesis (Zhang et al., 2014; Meyn, III and Smithgall, 2009). Our findings that c-Src is also constitutively expressed and active in EBs derived from hES cells supports a similar role for this family member in the control of human EB formation. Other work has shown that c-Src is highly expressed and active following induction of hES cell differentiation with retinoic acid, consistent with our findings (Son et al., 2008). Interestingly, Zhang et al. recently demonstrated that the differentiation-promoting activity of c-Src is antagonized by c-Yes in mES cells, despite their very close phylogenetic relationship (Zhang et al., 2014). A similar balance between c-Yes and c-Src signaling may control the differentiation of hES cell as well.
Our observation that self-renewing hES cells express high levels of active Lck is surprising given that Lck expression is largely restricted to T-lymphocytes in adults. Immunofluorescence microscopy demonstrates that Lck localizes to the cell periphery, suggesting that it may interact with growth factor or cytokine receptors in the ES cell context. Previous microarray studies have identified Lck as a highly expressed gene in pluripotent hES cells vs. their differentiated counterparts (Cao et al., 2008). In addition, a global phosphoproteomic study of hES cells identified Lck as a signaling protein with more abundant phosphorylation site identifications in pluripotent hES cells compared to differentiated hES cell derivatives (Brill et al., 2009). These results are consistent with our findings that Lck activity and gene expression are rapidly downregulated as ES cells differentiate, and further implicate Lck in signaling events related to hES cell self-renewal or suppression of differentiation.
Other studies have implicated another hematopoietic SFK, Hck, in mES cell self-renewal. Hck activity is stimulated by the self-renewal cytokine LIF in mES cells (Ernst et al., 1994), and Hck gene expression is rapidly silenced as mES cells differentiate to EBs (Meyn, III et al., 2005). In contrast to mES cells, however, we detected only low levels of Hck transcripts in self-renewing hES cells and were unable to detect Hck protein or activity in hESCs or EBs. This remarkable difference in Hck expression may reflect an important difference in the pluripotent state exemplified by human vs. mouse ES cells - the naïve and primed states (Nichols and Smith, 2009). Whereas mES cells represent the naïve pluripotent state, hES cells typify primed pluripotency and are more like mouse epiblast stem (EpiS) cells. Interestingly, while Hck is highly expressed in mES cells, we found that it is down-regulated in mouse EpiS cells (data not shown). Thus, Hck may represent a specific marker of naïve pluripotency, which explains why it is strongly expressed in mES cells yet only expressed in hES cells at very low levels.
Using the potent Src-family kinase inhibitor, A-419259, we also explored the role of SFK activity in hES cell fate. Treatment of hES cells with this inhibitor blocked all endogenous Src-family kinase activity, including that of c-Src and Lck. As a consequence, hES cells treated with the inhibitor grew as domed, morphologically undifferentiated colonies even when cultured in differentiation medium. Similar results were obtained with two additional Src-family kinase inhibitors from other chemical classes: the pyrazolopyrimidine, PP2, and the anilino-quinazoline, SKI-1 (data not shown) (Meyn, III et al., 2005). Furthermore, hES cells continued to express the well-established cell-surface marker for self-renewal, Tra-1-60, when cultured with A-419259 under differentiation conditions. Finally, addition of A-419259 to Aggrewell cultures of hES cells completely blocked the formation of EBs (data not shown), a result similar to that observed previously with mES cells (Meyn, III et al., 2005). Taken together, these observations strongly suggest that SFK activity is required for hES cell differentiation to occur.
A-419259 inhibits all members of the Src-kinase family to a similar extent, including kinases linked to opposing functions in ES cell self-renewal (e.g., Lck or c-Yes) vs. differentiation (c-Src). However, the net effect of global SFK inhibition is suppression of hES cell differentiation, a phenotype very similar to that reported previously in mES cells (Zhang et al., 2014; Meyn, III and Smithgall, 2009; Meyn, III et al., 2005). This observation supports the idea that a hierarchy of SFK signaling exists in ES cells, in which kinases that influence self-renewal are dominant over those that initiate differentiation. When both classes of kinases are blocked, then the ES cells become trapped in the undifferentiated state.
A complete understanding of the complex and opposing roles of individual Src-family members in hES cell differentiation will require future studies of individual kinase functions. One approach is use RNA interference to selectively block the expression of individual kinases. For example, RNAi knockdown of c-Yes caused ES cells to lose self-renewal marker expression and undergo differentiation (Anneren et al., 2004). However, knockdown approaches may suffer from the same limitations as knockout mice, in that functional compensation through upregulation of redundant kinases may occur during the selection process required to establish the knockdown cell population. In addition, knockdown and knockout approaches, in contrast to pharmacological inhibition, eliminate the kinase protein entirely thus removing possible adaptor or other functions that are independent of protein kinase activity. Another approach is the use of selective kinase inhibitors. However, because of the highly conserved nature of Src-family kinase domains, truly selective ATP-competitive inhibitors for individual SFKs are not currently available. An alternative is to use a chemical genetics approach, in which small molecule kinase inhibitors are combined with genetic mutations to achieve selective inhibition or resistance for a specific kinase. Previous work showed that expression of an inhibitor-resistant mutant of c-Src rescued mES cells from the A-419259-induced differentiation block, providing important evidence that c-Src activity is essential for mES cell differentiation (Meyn, III and Smithgall, 2009). Future studies will explore the development of isoform-selective inhibitors and activators of individual Src-family members. Such compounds may represent valuable chemical probes for the role of individual Src-family kinases in both mouse and human ES cell differentiation and may ultimately have translational value in the control of hES cell fate.
Supplementary Material
Highlights.
Expression and activity of Src-family kinases were explored in human ES cell lines
Fyn, Hck, Lck, Lyn, Src, and Yes are present and active in self-renewing hES cells
Lck & Yes activity decline as hES cells differentiate suggesting a role in renewal
Src, Fyn and Lyn are active in both hES cells and EBs and may drive differentiation
Inhibition of Src, Fyn and Lyn blocks hES cell differentiation supporting this view
Acknowledgments
This work was supported in part by National Institutes of Health grant GM077629 to T.E.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anneren C. Tyrosine kinase signalling in embryonic stem cells. Clin Sci (Lond) 2008;115:43–55. doi: 10.1042/CS20070388. [DOI] [PubMed] [Google Scholar]
- Anneren C, Cowan CA, Melton DA. The Src family of tyrosine kinases is important for embryonic stem cell self-renewal. J Biol Chem. 2004;279:31590–31598. doi: 10.1074/jbc.M403547200. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell. 2009;5:204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, Miller JD, Hartung O, Rho J, Ince TA, Daley GQ, Schlaeger TM. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- Dvorak P, Dvorakova D, Koskova S, Vodinska M, Najvirtova M, Krekac D, Hampl A. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells. 2005;23:1200–1211. doi: 10.1634/stemcells.2004-0303. [DOI] [PubMed] [Google Scholar]
- Ernst M, Gearing DP, Dunn AR. Functional and biochemical association of Hck with the LIF/IL-6 receptor signal transducing subunit gp130 in embryonic stem cells. EMBO J. 1994;13:1574–1584. doi: 10.1002/j.1460-2075.1994.tb06420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Lowell CA, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Meyn MA, III, Schreiner SJ, Dumitrescu TP, Nau GJ, Smithgall TE. SRC family kinase activity is required for murine embryonic stem cell growth and differentiation. Mol Pharmacol. 2005;68:1320–1330. doi: 10.1124/mol.104.010231. [DOI] [PubMed] [Google Scholar]
- Meyn MA, III, Smithgall TE. Chemical genetics identifies c-Src as an activator of primitive ectoderm formation in murine embryonic stem cells. Sci Signal. 2009;2:ra64. doi: 10.1126/scisignal.2000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- Schatten G, Smith J, Navara C, Park JH, Pedersen R. Culture of human embryonic stem cells. Nat Methods. 2005;2:455–463. doi: 10.1038/nmeth0605-455. [DOI] [PubMed] [Google Scholar]
- Son MY, Kim J, Han HW, Woo SM, Cho YS, Kang YK, Han YM. Expression profiles of protein tyrosine kinase genes in human embryonic stem cells. Reproduction. 2008;136:423–432. doi: 10.1530/REP-08-0080. [DOI] [PubMed] [Google Scholar]
- Tamm C, Bower N, Anneren C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J Cell Sci. 2011;124:1136–1144. doi: 10.1242/jcs.075796. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Meyn MA, III, Smithgall TE. c-Yes tyrosine kinase is a potent suppressor of ES cell differentiation and antagonizes the actions of its closest phylogenetic relative, c-Src. ACS Chem Biol. 2014;9:139–146. doi: 10.1021/cb400249b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.