Abstract
We assessed whether red cell distribution width (RDW) is associated with serum uric acid (UA) level in a group of 512 patients with newly diagnosed hypertension, recruited in Beijing. Patients were divided into high uric acid group and low uric acid group according to the median (334.9 μmol/L) of serum uric acid. Compared with the low uric acid group, the patients with high uric acid had higher red blood cell count (P < 0.001) and RDW (P = 0.032). The multiple linear regression analysis showed that RDW (P = 0.001) was positively correlated with uric acid level after the adjustment of related factors. Stepwise multiple logistic regression model confirmed that RDW (odds ratio: OR = 1.75) was independent determinants of high serum uric acid as well as sex (OR = 6.03), triglycerides (OR = 1.84), and Blood Urea Nitrogen (BUN, OR = 1.30). RDW may be independently associated with serum UA level in patients with newly diagnosed hypertension. To firmly establish the causal role of RDW in the incidence of high uric acid level among hypertensive patients, large cohort studies are needed.
Uric acid is the final product of endogenous and dietary purine metabolism. Uric acid is a weak acid, and the majority of uric acid (about 98%) circulates in the blood as ionised urate. Because of the high concentration of sodium in the extracellular compartment, urate is largely present as monosodium urate with a low solubility1,2. The consumption of foods rich in purines and protein3, obesity4, alcohol5, and soft drinks sweetened with fructose could make serum uric acid level a dramatic increase6. In addition, elevated uric acid may also be closely associated with low-level lead intoxication, which causes a decline in renal function7. High uric acid levels have been traditionally considered a risk factor for gout and hyperturicemia8. However, it has emerged recently that high serum uric acid level is a risk factor of cardiovascular disease9. It is suggested that the relation between uric acid and cardiovascular disease (CVD) is evident not only in the presence of overt hyperuricemia but also with serum uric acid levels considered in the normal to high range (>5.2 to 5.5 mg/dL)10.
Red cell distribution width (RDW), an index of the routine blood cell count, is not only used to evaluate different types of anemia but also is a potential predictor of morbidity and mortality in a variety of settings, especially in many cardiovascular diseases. Several studies have suggested increased RDW was associated with a higher possibility of adverse clinical outcomes such as heart failure, hypertension and coronary artery disease11. The specific mechanism of RDW with these negative outcomes had not been fully clear. Several lines of evidences found that Inflammatory status is significantly related to ineffective erythropoiesis, and it has been suggested that inflammatory cytokines, such as interleukin (IL)-1 β, IL-6, tumor necrosis factor (TNF)-α, desensitize bone marrow elytroid progenitors to erythropoiesis, inhibit red blood cell maturation and thereby promote anysocytosis12. Elevated RDW may be due to an underlying inflammatory state. High serum uric acid is one of independent risk factors of cardiovascular disease. Hyperuricemia is closely associated with inflammatory process. Some inflammatory cytokines could activate xanthine oxidase enzyme in epithelial cells with serum uric acid elevated13. Considering the above situation, we hypothesis that high blood pressure does damage to endothelia cells promoting the secretin of inflammatory cytokines, and some inflammatory cytokines such as interleukin-6 play an important role in inducing hyperuricemia14. Therefore, as a sensitive index of inflammatory status in this process, RDW could be a potential predictor of high uric acid in newly diagnosed patients. The aim of the present study was to evaluate the relationship between uric acid levels with RDW in newly diagnosed hypertension patients.
Results
General characteristics of the subjects
As shown in Table 1, 256 patients with low uric acid and 256 ones with high uric acid were rolled in the study. Among the 512 hypertension patients, 39 had diabetes mellitus. In the comparisons of demographic and clinical characteristic between hypertension patients with low uric acid level and with high uric acid level, the average age for two groups was 46.8 ± 7.8 years and 46.9 ± 7.8 years (P = 0.843), respectively. The male ratio in the low uric acid group is 45.7% and 78.5% (P < 0.001) in the high uric acid group. Compared with the low uric acid group, the patients with high uric acid tend to be smokers (P = 0.001) and had higher body mass index (BMI) (25.6 ± 3.7 vs 26.2 ± 3.1, P = 0.049), serum creatinine (73.0 ± 12.4 vs 66.9 ± 12.9, P < 0.001), triglycerides (TG) (1.9 ± 0.6 vs 1.8 ± 0.5, P = 0.002), aspartate aminotransferase (AST) (26.5 ± 11.4 vs 24.2 ± 10.4, P = 0.004), alanine aminotransferase (ALT) (34.1 ± 26.0 vs 27.7 ± 22.9, P = 0.020), blood urea nitrogen (BUN) (4.9 ± 1.1 vs 4.7 ± 1.2, P = 0.011), red blood cell count (RBC) (5.0 ± 0.5 vs 4.9 ± 0.4, P < 0.001), RDW (12.9 ± 0.9 vs 12.6 ± 0.8, P = 0.032), hemoglobin (Hb) (153.7 ± 15.5 vs 146.5 ± 15.5, P < 0.001) and lower high density lipoprotein cholesterol (HDL- cholesterol) (1.0 ± 0.2 vs 1.1 ± 0.2, P = 0.002). There were no differences between the high uric acid group and the low group with regard to age, blood pressure, estimated glomerular filtration rate (eGFR), albumin to creatinine ration (ACR), total cholesterol (TC), low density lipoprotein cholesterol (LDL-cholesterol), white blood cell count (WBC), platelet count (Plt) and C-reactive protein (CRP).
Table 1. Baseline characteristics of men with newly diagnosed hypertension according to uric acid median.
| Parameters | Low UA (n = 256) | High UA (n = 256) | P value |
|---|---|---|---|
| Age (years) | 46.8 ± 7.8 | 46.9 ± 7.8 | 0.843 |
| Male (%) | 117(45.7%) | 201(78.5%) | 0.000 |
| Smoking, yes | 25(9.8%) | 51(19.9%) | 0.001 |
| Body weight index (kg/m2) | 25.6 ± 3.7 | 26.2 ± 3.1 | 0.049 |
| Diabetes mellitus (%) | 23(9.0%) | 16(6.3%) | 0.244 |
| Systolic blood pressure (mmHg) | 152.6 ± 12.1 | 152.4 ± 11.7 | 0.795 |
| Diastolic blood pressure (mmHg) | 99.7 ± 8.9 | 100.2 ± 8.7 | 0.532 |
| Uric acid (μmol/L) | 303.6 ± 28.2 | 382.5 ± 27.5 | 0.000 |
| Creatinine (μmol/L) | 66.9 ± 12.9 | 73.0 ± 12.4 | 0.000 |
| eGFR (mL/min/1.73 m2) | 105.6 ± 24.7 | 104.5 ± 24.6 | 0.490 |
| ACR (mg/g) | 34.7 ± 41.7 | 40.9 ± 48.4 | 0.120 |
| TC (mmol/L) | 5.1 ± 0.9 | 5.2 ± 0.8 | 0.502 |
| TG (mmol/L) | 1.8 ± 0.5 | 1.9 ± 0.6 | 0.002 |
| HDL-cholesterol (mmol) | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.002 |
| LDL-cholesterol (mmol) | 3.1 ± 0.8 | 3.2 ± 0.8 | 0.524 |
| AST (U/L) | 24.2 ± 10.4 | 26.5 ± 11.4 | 0.004 |
| ALT (U/L) | 27.7 ± 22.9 | 34.1 ± 26.0 | 0.020 |
| BUN (mmol/L) | 4.7 ± 1.2 | 4.9 ± 1.1 | 0.011 |
| Fasting glucose (mmol/L) | 7.7 ± 2.1 | 7.3 ± 2.3 | 0.180 |
| WBC count (103/mm3) | 6.4 ± 1.8 | 6.5 ± 1.7 | 0.901 |
| RBC count (103/mm3) | 4.9 ± 0.4 | 5.0 ± 0.5 | 0.000 |
| RDW (%) | 12.6 ± 0.8 | 12.9 ± 0.9 | 0.032 |
| Plt count (103/mm3) | 238.9 ± 53.1 | 235.2 ± 53.5 | 0.548 |
| CRP (mg/dL) | 2.5 ± 5.0 | 2.3 ± 4.7 | 0.758 |
| Hb (g/L) | 146.5 ± 15.5 | 153.7 ± 15.5 | 0.000 |
Correlations between serum uric acid and other variables in newly diagnosed hypertensive patients
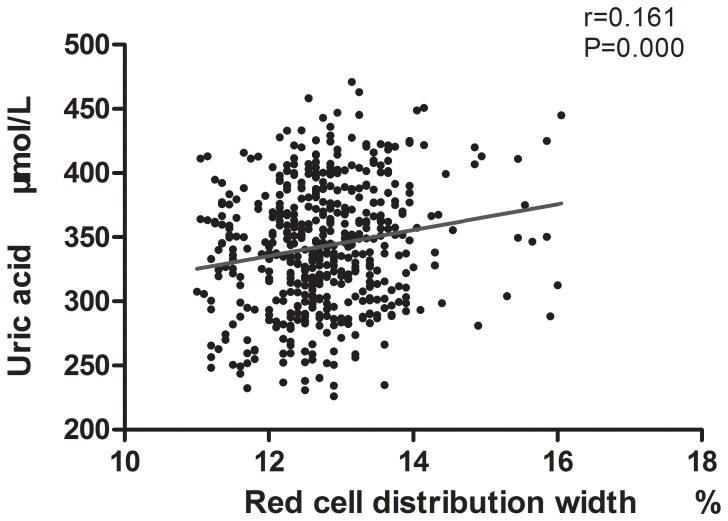
Person correlation analysis demonstrated that serum uric acid was positively correlated with RDW (r = 0.161, P < 0.001, Figure 1), body weight index, creatinine, ACR, triglycerides, AST, ALT, BUN, red blood cell count, and hemoglobin. The negative relationship was found between serum uric acid and HDL-cholesterol, platelet count. The specific analysis results were shown in Table 2.
Figure 1. Correlation between red cell distribution width (RDW) level and serum uric acid level.
Table 2. Partial correlation analysis between serum uric acid concentration and other variables in patients with untreated essential hypertension.
| Variables | Correlation coefficient | P value |
|---|---|---|
| Age (years) | 0.043 | 0.330 |
| Body weight index (kg/m2) | 0.098 | 0.026 |
| Systolic blood pressure (mmHg) | 0.023 | 0.610 |
| Diastolic blood pressure (mmHg) | 0.062 | 0.159 |
| Creatinine (μmol/L)* | 0.339 | 0.000 |
| eGFR (mL/min/1.73 m2)* | −0.071 | 0.107 |
| ACR (mg/g)* | 0.128 | 0.004 |
| TC (mmol/L) | 0.011 | 0.803 |
| TG (mmol/L)* | 0.134 | 0.002 |
| HDL-cholesterol (mmol) | −0.189 | 0.000 |
| LDL-cholesterol (mmol) | 0.023 | 0.608 |
| AST (U/L) | 0.137 | 0.002 |
| ALT (U/L) | 0.140 | 0.002 |
| BUN (mmol/L)* | 0.153 | 0.000 |
| Fasting glucose (mmol/L)* | 0.019 | 0.666 |
| WBC count (103/mm3) | 0.024 | 0.674 |
| RBC count (103/mm3) | 0.258 | 0.000 |
| RDW (%)* | 0.161 | 0.000 |
| Plt count (103/mm3) | −0.132 | 0.018 |
| CRP (mg/dL)* | 0.082 | 0.074 |
| Hb (g/L) | 0.286 | 0.000 |
*Spearman rank correlation coefficients.
Multiple linear regression analysis for serum uric acid level
The collinearity diagnostics suggested that there are no obvious multi-collinearity among variables. The maximum condition index is 1.435, and the minimum eigenvalue is 0.636. In a multiple linear regression analysis, Log RDW (β = 319.040, P = 0.019) was statistically significant parameters after the adjustment of related factors (age, sex, BMI, smoking status, creatinine, fasting glucose, eGFR, CRP, TG, HDL-cholesterol, TC, LDL-cholesterol, ACR, ALT, AST, BUN, WBC count, Plt count, RBC count, Hb, Plt count.) Other significant variables factors were shown in Table 3.
Table 3. Stepwise multiple linear regression analysis for the effect of independent variables on serum uric acid.
| Variable | B | S.E. | t | P |
|---|---|---|---|---|
| Constant | 69.439 | 58.522 | 1.187 | 0.236 |
| Age | 0.755 | 0.308 | 2.450 | 0.015 |
| Sex | 44.770 | 5.116 | 8.751 | 0.000 |
| RDW* | 319.040 | 134.726 | 2.368 | 0.019 |
| BUN* | 53.572 | 23.370 | 2.292 | 0.023 |
*transformed by log.
Multiple logistic regression analysis for high serum uric acid
Defining serum uric acid as the dependent variable and all other factors with uric acid were treated as covariates in a stepwise multiple regression model, the results suggested that RDW (odds ratio (OR) = 1.75, 95% confidence internal (CI): 1.10–2.79) was a risk factor of high serum uric acid, independent of sex (OR = 6.03, 95% CI: 3.41–10.63), TG (OR = 1.84, 95%CI: 1.10–3.06), and BUN (OR = 1.30, 95%CI: 1.01–1.67) (Table 4).
Table 4. Stepwise multiple logistic regression for uric acid in patients with untreated essential hypertension.
| Variables | B | S.E. | Wald | P value | Odds ratio | 95%CI |
|---|---|---|---|---|---|---|
| Sex | 1.796 | 0.290 | 38.491 | 0.000 | 6.03 | 3.41–10.63 |
| Triglycerides | 0.609 | 0.260 | 5.466 | 0.019 | 1.84 | 1.10–3.06 |
| RDW | 0.559 | 0.237 | 5.538 | 0.019 | 1.75 | 1.10–2.79 |
| BUN | 0.259 | 0.129 | 4.066 | 0.044 | 1.30 | 1.01–1.67 |
| Constant | −9.047 | 3.218 | 7.905 | 0.005 |
Discussion
This is the first study to demonstrate a significantly positive association between RDW and serum uric acid level in patients without antihypertensive treatment. RDW was significantly increased in hypertensive patients with high uric acid levels compared with those of low high uric acid level. RDW (OR = 1.75, 95%CI: 1.10–2.79) was an independent determinant of high serum uric acid in newly diagnosed hypertensive.
Although the notion that uric acid is a risk factor of poor health outcome in the general population15,16 is not universally acknowledged, a number of epidemiological dada show that high uric acid is an independent risk factor of hypertension17, diabetes18, CVD19, and morality15,16. Even after adjustment for some risk factors, high uric acid is still an independent risk factor of cardiovascular events in patients affected by hypertension, diabetes, pre-existing cerebrovascular, and CVD20,21,22. RDW shows variability in the size of circulating erythrocytes and is routinely measured by automated hematology analyzers as part of a complete blood count23. Just like uric acid, RDW is also a potent predictor of morbidity and mortality in a variety of settings, especially in many cardiovascular diseases. Several lines of evidences have suggested increased RDW was associated with a higher possibility of variety of diseases24. Moreover, elevated RDW may reflect a status of high inflammation and oxidative stress, both known to reduce red blood cell survival25, and elevated serum uric acid levels have also been shown to be associated with inflammation even in populations with no hypertension17. Chronic inflammation could cause RDW level elevation and increased RDW reflect an underlying chronic inflammation, which could result in a higher risk of cardiovascular diseases26. Lappe et al. found that RDW was associated with mortality (correlating with high-sensitivity C-reactive protein levels) in patients with coronary artery disease27. Semba et al. investigated whether serum antioxidants and inflammation predicted RDW values in older women, and the patients in higher quartile of RDW were more likely to have a higher interleukin-6 level28. Uric acid and RDW share with the same inflammatory factors. In community-dwelling older persons, randomly selected from the general population, a positive and significant association between uric acid and inflammatory markers (WBC, neutrophil count, CRP, IL-6, IL-1ra, IL-18, and TNF-α) was found25. CRP inflammatory marker is included in this study. However, CRP was not correlated with uric acid (Table 2). On one hand our results were obtained in a newly diagnosed hypertension population without severe renal function impairment, free of cardiovascular diseases, and the association between CRP and uric acid would be more significant with the progress of hypertension. On the other hand, this also may highlight that RDW could be more sensitive indicator in predicting elevated uric acid level than CRP.
Another important determinant of adverse outcomes in hypertensive patients might be oxidative stress that cause oxidative damage. Semba et al. measured RDW at baseline, 12 months and 24 months in 786 moderately to severely disabled women aged more than 65 years old and found serum selenium was an underlying predictor of RDW, which suggests that oxidative stress may be a potential biological mechanism for increased RDW28. With difference from RDW, urate has a more complex relationship to oxidative stress, possessing both scavenging properties and oxidizing and radical forming properties. An in vitro study by Patterson et al.29 suggested that uric acid can play either of these roles depending on the oxidative status of the lipoprotein. They found that uric acid functioned as an antioxidant in the presence of native LDL taken from human plasma, but in response to mildly oxidized LDL, when the oxidation had occurred via copper, uric acid became a pro-oxidant. The present study showed that uric acid have a positive relationship with RDW. When paired with these factors, uric acid may play a pro-oxidant role in the process of adverse outcomes affected by hypertension.
There are several limitations in the present study. Potential limitations of the present study may not permit the global application of our findings. First, an important limitation of this study is its cross-sectional nature, with the known limitations of establishing causal or temporal relationships between elevated RDW and serum uric acid level. Second, only a single 24-h urine sample was used for the assessment of uric microalbuminuria, instead of consecutive collections that would have been preferable. Third, it is important to note that our results were obtained in a selected population of Asian middle-aged patients, without severe renal function impairment, free of cardiovascular diseases. As we known, hypertension is also a kind of hereditary disease, and several genes are suggested to be associated with hypertension30,31,32,33,34.Therefore, the conclusions of our study cannot be extrapolated to non-Asian populations and caution should be needed when applying the results of our findings to other clinical settings. Finally, it is more meaningful if a prospective study will be conducted to confirm our findings. The main strength is that patients with anti-hypertensive treatment were not included in the study, which eliminated the influence of antihypertensive treatment on the serum uric acid level. Otherwise, this observational study is well planned, executed with a proper statistical analysis and control a number of potential cofounders.
In conclusion, our study show that the association between RDW and serum uric acid level exists in patients with untreated essential hypertension. This association is independent of a number of potential confounding factors including eGFR, ACR, diabetes mellitus status, markers of inflammation, smoking, serum lipids and blood pressure. RDW may be considered as an important predictive factor in the evaluation of the total cardiovascular risk in essential hypertensive patients. To firmly establish the causal role of RDW in the incidence of high uric acid level among hypertensive patients, large cohort studies are needed.
Methods
Study population
In a retrospective cross-sectional design, 711 consecutive patients with newly diagnosed untreated hypertension were recruited in the hospital of Anzhen Hospital of Beijing from November 2012 and October 2013. Among 711 patients who underwent physical examination and laboratory tests, 512 patients' data were collected according to an exclusion criteria. The exclusion criteria is: patients with diabetic micro-and and macro-vascular complications (Micro-vascular complications: nephropathy, retinopathy. Macro-vascular complications: coronary heart disease, cerebrovascular disease), malignant neoplasms, renal dysfunction or liver diseases, and urinary tract infection. Patients who were taking medications such as uric acid-lowering agents were also excluded. We sub-divided the patients into high uric acid group and low uric acid group according to the median(334.9 μmol/L) of serum uric acid. 256 essential hypertension patients with low uric acid and 256 ones with high uric acid were included in the present study.
Newly diagnosed hypertension was defined as systolic blood pressure ≥140 mmHg and (or) diastolic blood pressure ≥90 mmHg. The patients' clinical and demographic characteristics encompassing age, sex, and smoking habits were obtained by a standardized questionnaire. Patients who had smoked at least on cigarette daily for 1 year were considered smokers.
Ethics statement
This study protocol was approved by the institutional review board of Xiangya Medical School, and written informed consent was obtained from all participants. The study was performed in accordance with approved guidelines.
Laboratory examination
Hematologic testing was performed on the ADVIA 120 (Bayer Diagnostics, Newbury, Berkshire, UK) automated hematology analyzer, which measures hemoglobin photometrically, including white blood cell counts, platelet counts, hemoglobin, mean corpuscular volume and RDW, optical laser light scattering for cell enumeration, flow cytometer and laser diffraction for red blood cell (RBC) counts. Serum creatinine was measured on a Roche/Hitachi Modular System P (Roche Diagnostics GmbH, Mannheim, Germany) by creatinine Jaffé, rate blanked and compensated assay. Microalbuminuria was defined as an ACR more between 30 μg/mg creatinine and 300 μg/mg creatinine35. Urine concentrations of albumin were measured by immunoturbidimetric method. In addition, fasting blood glucose level, creatinine level and fasting serum lipid status including TC, LDL-cholesterol, HDL-cholesterol, TG, and CRP levels were also recorded. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Estimated glomerular filtration rate (eGFR) was calculated from SCr, sex, and patients' age. The calculation formula defined: 186 × SCr−1.154 × age in years−0.203 × 1.210(if black) × 0.742(if female)36, the definition of renal dysfunction (low GFR) was an eGFR < 60 ml/min/1.73 m2.
Statistical analysis
The normality test of all continuous variables was conducted by using kurtosis and skewness coefficients. Variables were log-transformed before statistical analysis when not conforming the normal distribution (RDW, BUN, TG, CRP, serum creatinine, eGFR, and fasting glucose). Data were reported as mean ± standard deviation for qualitative variables and as percentages for quantitative variables. The subjects were divided into low uric acid group and high uric acid group according to uric acid median. Differences between qualitative variables were tested by Student t test. Pearson's correlation or Spearman rank correlation coefficients were calculated between uric acid and other index. The collinearity diagnostics was conducted before regression model was built. Eigenvalue and condition index are used to decide whether multi-collinearity exits or not. If eigenvalue is close to zero or condition index is greater than 30, it will mean that multi-collinearity exist. Stepwise multiple linear regression analyses were used to identify significant determinants for uric acid. Differences between quantitative variables were tested using χ2 test. Stepwise multiple logistic regression analysis was used to calculate the odds ratio of independent variables. Both of the above two models include the following variables: age, sex, BMI, smoking status, creatinine, fasting glucose, eGFR, CRP, TG, HDL-cholesterol, TC, LDL-cholesterol, ACR, ALT, AST, BUN, WBC count, Plt count, RBC count, Hb, Plt count. Statistical analysis was performed using SPSS version 17.0 (SPSS Inc, USA), and the level of statistical significance was defined as P < 0.05.
Author Contributions
Z.Z.L. and L.Z.C. conceived the study, Y.Y.L. participated in the design; S.P.Y., Y.Y.H. and P.C. collected the data. Z.Z.L. and L.Z.C. drafted the manuscript. M.L. revised the manuscript and language. All authors reviewed the manuscript.
Acknowledgments
We thank all our colleagues working in the Department of Epidemiology and Health Statistics, School of public health of Central South University. This work was supported by National Natural Science Foundation of China (No.81373855 and No.81273713), Hunan Province Science and Technology Project (2014SK2013), fundamental research funds for the central universities of central south university (2014zzts069), and Graduate Innovation Project of Hunan Province (CX2014B097).
References
- Richette P. & Bardin T. Gout. Lancet. 375, 318–328 (2010). [DOI] [PubMed] [Google Scholar]
- Grassi D. et al. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. Curr Pharm Des. 19, 2432–2438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K. et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 350, 1093–1103 (2004). [DOI] [PubMed] [Google Scholar]
- Choi H. K., Atkinson K., Karlson E. W. & Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals' follow-up study. Arch Intern Med. 165, 742–748 (2005). [DOI] [PubMed] [Google Scholar]
- Choi H. K., Atkinson K., Karlson E. W., Willett W. & Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 363, 1277–1281 (2004). [DOI] [PubMed] [Google Scholar]
- Choi H. K. & Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 336, 309–312 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan E., Lingala B. & Bhalla V. Low-level lead exposure and the prevalence of gout: an observational study. Ann Intern Med. 157, 233–241(2012). [DOI] [PubMed] [Google Scholar]
- de Oliveira E. P. & Burini R. C. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 4, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yıldız A. et al. Association of serum uric acid level and coronary blood flow. Coron Artery Dis. 18, 607–13 (2007). [DOI] [PubMed] [Google Scholar]
- Niskanen L. K. et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 164, 1546–1551 (2004). [DOI] [PubMed] [Google Scholar]
- Felker G. M. et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 50, 40–47 (2007). [DOI] [PubMed] [Google Scholar]
- Macdougall I. C. & Cooper A. The inflammatory response and erythropoiesis sensitivity. Nephrol Dial Transplant. 17, 48–52 (2002). [DOI] [PubMed] [Google Scholar]
- Wu L. L. & Wu J. T. Serum uric acid is a marker of inflammation and a marker predicting the risk of developing CVD, stroke, renal failure and cancer. J Biomed Lab Sci. 20, 1–6 (2008). [Google Scholar]
- Kanellis J. & Kang D. H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 25, 39–42 (2005). [DOI] [PubMed] [Google Scholar]
- Fang J. & Alderman M. H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 283, 2404–2410 (2000). [DOI] [PubMed] [Google Scholar]
- Freedman D. S., Williamson D. F., Gunter E. W. & Byers T. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 141, 637–644 (1995). [DOI] [PubMed] [Google Scholar]
- Masuo K., Kawaguchi H., Mikami H., Ogihara T. & Tuck M. L. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42, 474–480 (2003). [DOI] [PubMed] [Google Scholar]
- Kodama S. et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 32, 1737–1742 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachimescu A. G., Brennan D. M., Hoar B. M., Hazen S. L. & Hoogwerf B. J. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 58, 623–630 (2008). [DOI] [PubMed] [Google Scholar]
- Verdecchia P. et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 36, 1072–1078 (2000). [DOI] [PubMed] [Google Scholar]
- Struthers A. D. et al. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart. 87, 229–234 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K. & Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 116, 894–900 (2007). [DOI] [PubMed] [Google Scholar]
- Turhan H. et al. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int J Cardiol. 108, 224–230 (2006). [DOI] [PubMed] [Google Scholar]
- Patel K. V. et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 65, 258–265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C. et al. Uric acid and inflammatory markers. Eur Heart J. 27, 1174–1181 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker G. M. et al. Red cell distribution width as a novel prognostic marker in heart failure: Data from the charm program and the duke databank. J Am Coll Cardiol. 50, 40–47 (2007). [DOI] [PubMed] [Google Scholar]
- Lappe J. M. et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 412, 2094–2099 (2011). [DOI] [PubMed] [Google Scholar]
- Semba R. D. et al. Serum antioxidants and inflammation predict red cell distribution width in older women: The Women's Health and Aging Study I. Clin Nutr. 29, 600–604 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. A., Horsley E. T. & Leake D. S. Prooxidant and antioxidant properties of human serum ultra filtrates toward LDL: important role of uric acid. J Lipid Res. 44, 512–21 (2003). [DOI] [PubMed] [Google Scholar]
- Santulli G. et al. CaMK4 Gene Deletion Induces Hypertension. J Am Heart Assoc. 1, e1081 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M. & Mayer G. Significance of genetic polymorphisms of the renin-angiotensin-aldosterone system in cardiovascular and renal disease. Pharmacogenomics. 10, 463–476 (2009). [DOI] [PubMed] [Google Scholar]
- Patel S. K. et al. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 5, 227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- antulli G., Trimarco B. & Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev. 20, 5–12 (2013). [DOI] [PubMed] [Google Scholar]
- Lobmeyer M. T. et al. Polymorphisms in genes coding for GRK2 and GRK5 and response differences in antihypertensive-treated patients. Pharmacogenet Genomics. 21, 42–49 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi F. Quantitation of microalbuminuria using random urine samples. Pediatr Nephrol. 17, 107–10 (2002). [DOI] [PubMed] [Google Scholar]
- NKF-K/DOQI clinical practice guidelines for chronic kidney disease. Am J Kidney Dis. 39, S76 (2002). [PubMed] [Google Scholar]