Abstract
Genetically engineered viruses and viral genes inserted into retroviral vectors are increasingly being considered for experimental therapy of brain tumors. A primary target of these viruses and vectors is human gliomas, the most frequently occurring primary human brain tumor. To investigate the potential of genetically engineered herpes simplex viruses (HSVs) in the therapy of these tumors, we compared the attributes of two viruses, a recombinant from which the gamma 1(34.5) gene had been deleted (R3616) and a recombinant in which the gamma 1(34.5) gene had been interrupted by a stop codon (R4009). Previous studies have shown that these recombinants were completely devoid of the ability to multiply in the central nervous system of rodents. To pursue these studies, we developed a scid mouse glioma model. Tumor cell response (survival) for 10(3), 10(4), and 10(5) implanted MT539MG glioma cells was 38, 23, and 15 days, respectively. The results were as follows: (i) both R3616 and R4009 replicate and cause cytolysis in diverse glioma cell lines of murine and human origin in vitro, and (ii) Winn-type assays 10(5) MT539MG cells coinoculated with R3616 or R4009 as compared to saline significantly prolonged survival in a dose-dependent fashion. Mice that received only tumor cells or the wild-type parent strain of the recombinants, HSV-1(F), died within 15 days. Survival was greatest with R4009. These experiments define both a model for screening oncolytic viruses and a genetically engineered virus of significant potential use as an oncolytic agent.
Full text
PDF
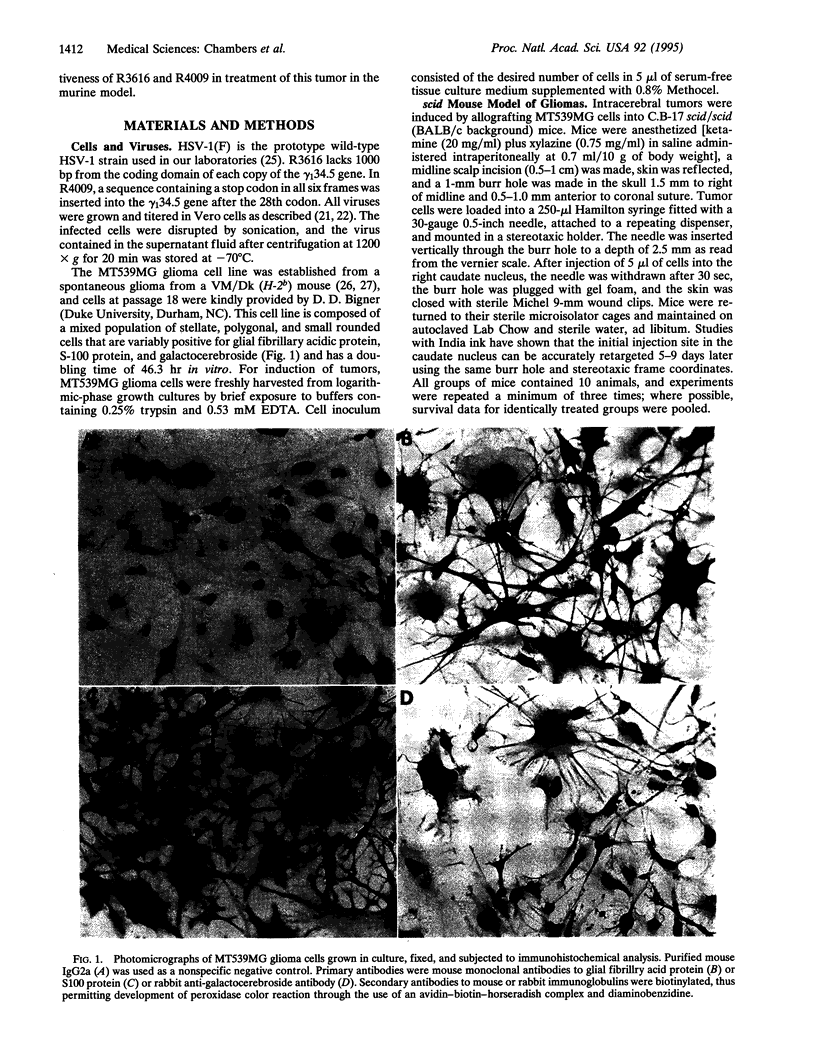
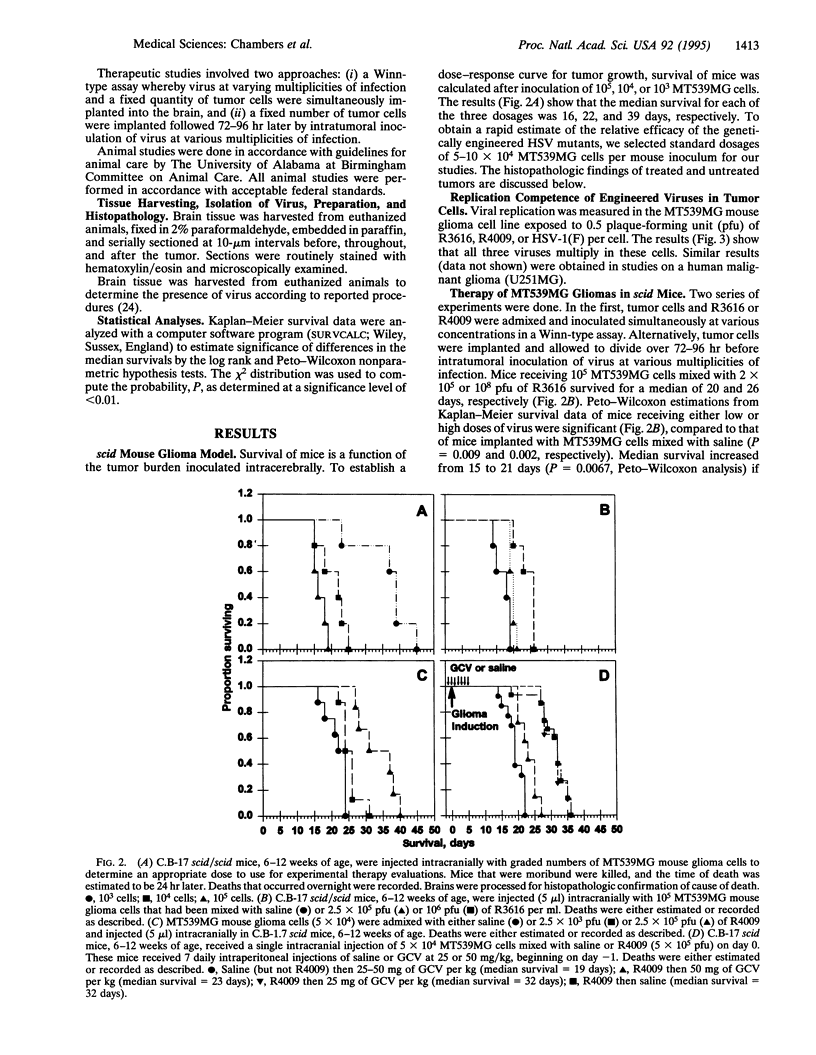
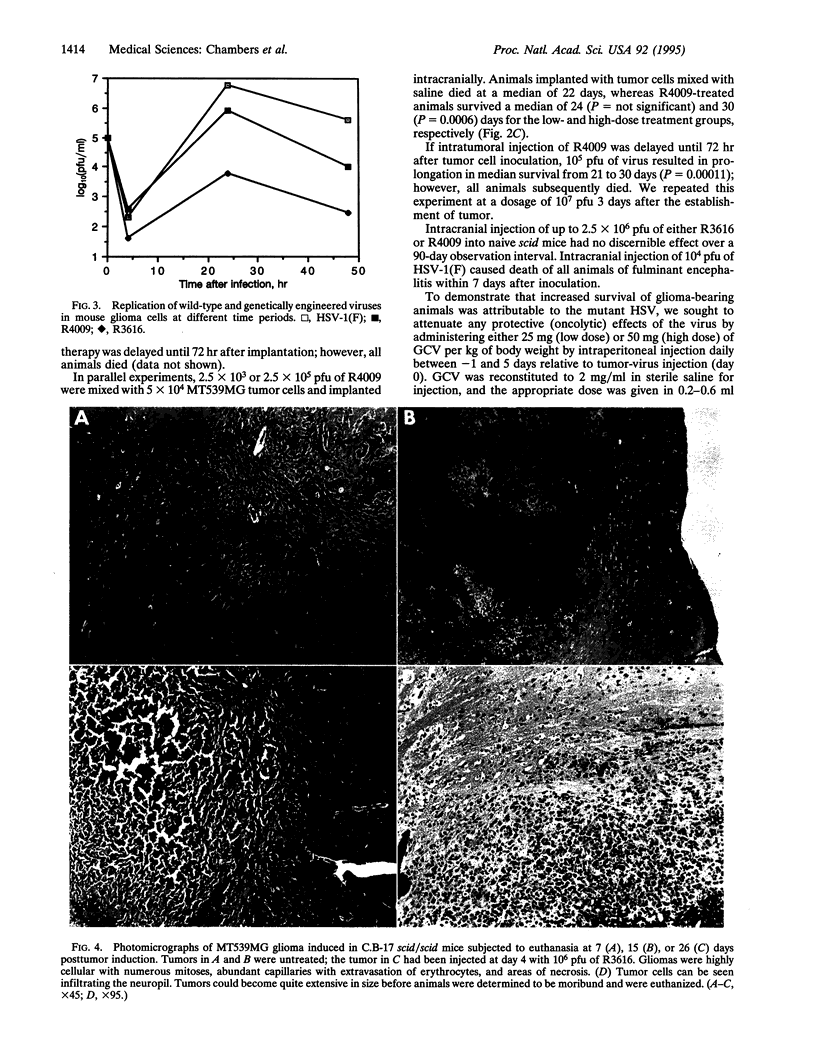

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Chou J., Sarmiento M., Lerner R. A., Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986 Jun;58(3):843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Kern E. R., Whitley R. J., Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990 Nov 30;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Chou J., Poon A. P., Johnson J., Roizman B. Differential response of human cells to deletions and stop codons in the gamma(1)34.5 gene of herpes simplex virus. J Virol. 1994 Dec;68(12):8304–8311. doi: 10.1128/jvi.68.12.8304-8311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The herpes simplex virus 1 gene for ICP34.5, which maps in inverted repeats, is conserved in several limited-passage isolates but not in strain 17syn+. J Virol. 1990 Mar;64(3):1014–1020. doi: 10.1128/jvi.64.3.1014-1020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The terminal a sequence of the herpes simplex virus genome contains the promoter of a gene located in the repeat sequences of the L component. J Virol. 1986 Feb;57(2):629–637. doi: 10.1128/jvi.57.2.629-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Daumas-Duport C., Scheithauer B., O'Fallon J., Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988 Nov 15;62(10):2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- Kim T. S., Halliday A. L., Hedley-Whyte E. T., Convery K. Correlates of survival and the Daumas-Duport grading system for astrocytomas. J Neurosurg. 1991 Jan;74(1):27–37. doi: 10.3171/jns.1991.74.1.0027. [DOI] [PubMed] [Google Scholar]
- Mahaley M. S., Jr, Mettlin C., Natarajan N., Laws E. R., Jr, Peace B. B. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989 Dec;71(6):826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- Markert J. M., Malick A., Coen D. M., Martuza R. L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993 Apr;32(4):597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Martuza R. L., Malick A., Markert J. M., Ruffner K. L., Coen D. M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991 May 10;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Mineta T., Rabkin S. D., Martuza R. L. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994 Aug 1;54(15):3963–3966. [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Frank J. A., Blaese R. M., Oldfield E. H. Toxicity studies of retroviral-mediated gene transfer for the treatment of brain tumors. J Neurosurg. 1993 Sep;79(3):400–407. doi: 10.3171/jns.1993.79.3.0400. [DOI] [PubMed] [Google Scholar]
- Ram Z., Walbridge S., Heiss J. D., Culver K. W., Blaese R. M., Oldfield E. H. In vivo transfer of the human interleukin-2 gene: negative tumoricidal results in experimental brain tumors. J Neurosurg. 1994 Mar;80(3):535–540. doi: 10.3171/jns.1994.80.3.0535. [DOI] [PubMed] [Google Scholar]
- Ram Z., Walbridge S., Shawker T., Culver K. W., Blaese R. M., Oldfield E. H. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg. 1994 Aug;81(2):256–260. doi: 10.3171/jns.1994.81.2.0256. [DOI] [PubMed] [Google Scholar]
- Salazar O. M., Rubin P., Feldstein M. L., Pizzutiello R. High dose radiation therapy in the treatment of malignant gliomas: final report. Int J Radiat Oncol Biol Phys. 1979 Oct;5(10):1733–1740. doi: 10.1016/0360-3016(79)90554-6. [DOI] [PubMed] [Google Scholar]
- Serano R. D., Pegram C. N., Bigner D. D. Tumorigenic cell culture lines from a spontaneous VM/Dk murine astrocytoma (SMA). Acta Neuropathol. 1980;51(1):53–64. doi: 10.1007/BF00688850. [DOI] [PubMed] [Google Scholar]
- Short M. P., Choi B. C., Lee J. K., Malick A., Breakefield X. O., Martuza R. L. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990 Nov;27(3):427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]
- Takamiya Y., Short M. P., Ezzeddine Z. D., Moolten F. L., Breakefield X. O., Martuza R. L. Gene therapy of malignant brain tumors: a rat glioma line bearing the herpes simplex virus type 1-thymidine kinase gene and wild type retrovirus kills other tumor cells. J Neurosci Res. 1992 Nov;33(3):493–503. doi: 10.1002/jnr.490330316. [DOI] [PubMed] [Google Scholar]
- Takamiya Y., Short M. P., Moolten F. L., Fleet C., Mineta T., Breakefield X. O., Martuza R. L. An experimental model of retrovirus gene therapy for malignant brain tumors. J Neurosurg. 1993 Jul;79(1):104–110. doi: 10.3171/jns.1993.79.1.0104. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Green S. B., Byar D. P., Alexander E., Jr, Batzdorf U., Brooks W. H., Hunt W. E., MacCarty C. S., Mahaley M. S., Jr, Mealey J., Jr Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980 Dec 4;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Kern E. R., Chatterjee S., Chou J., Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993 Jun;91(6):2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]