Abstract
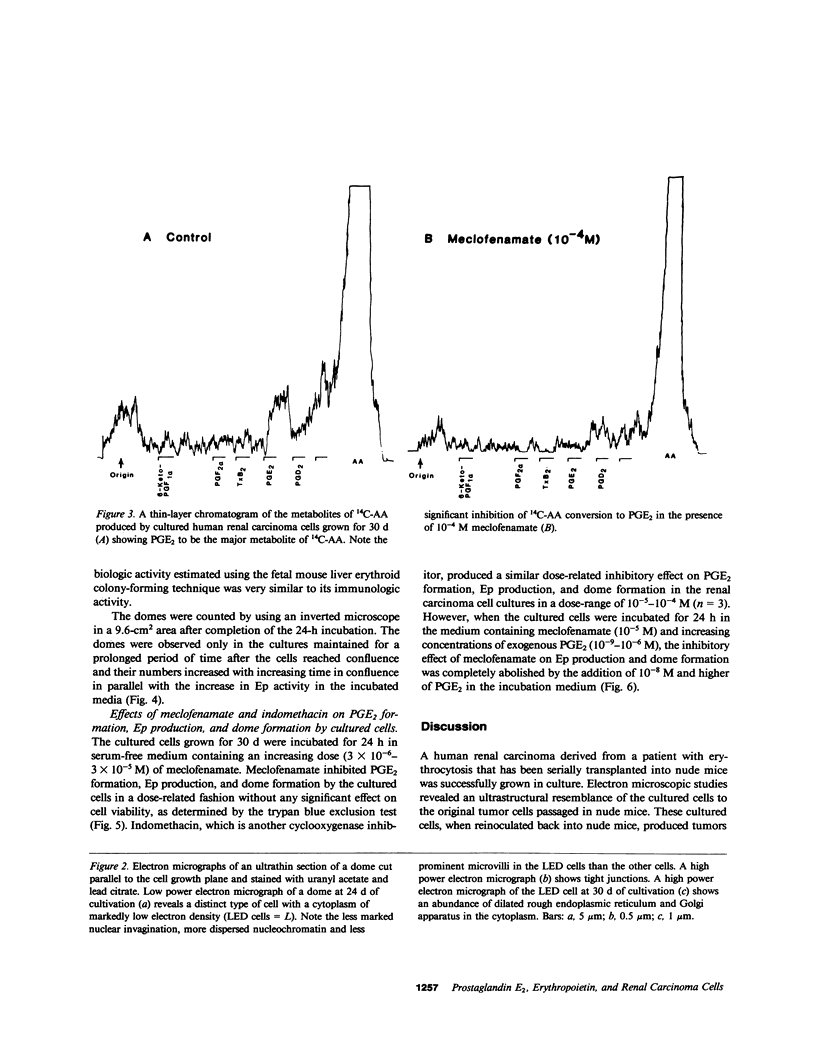
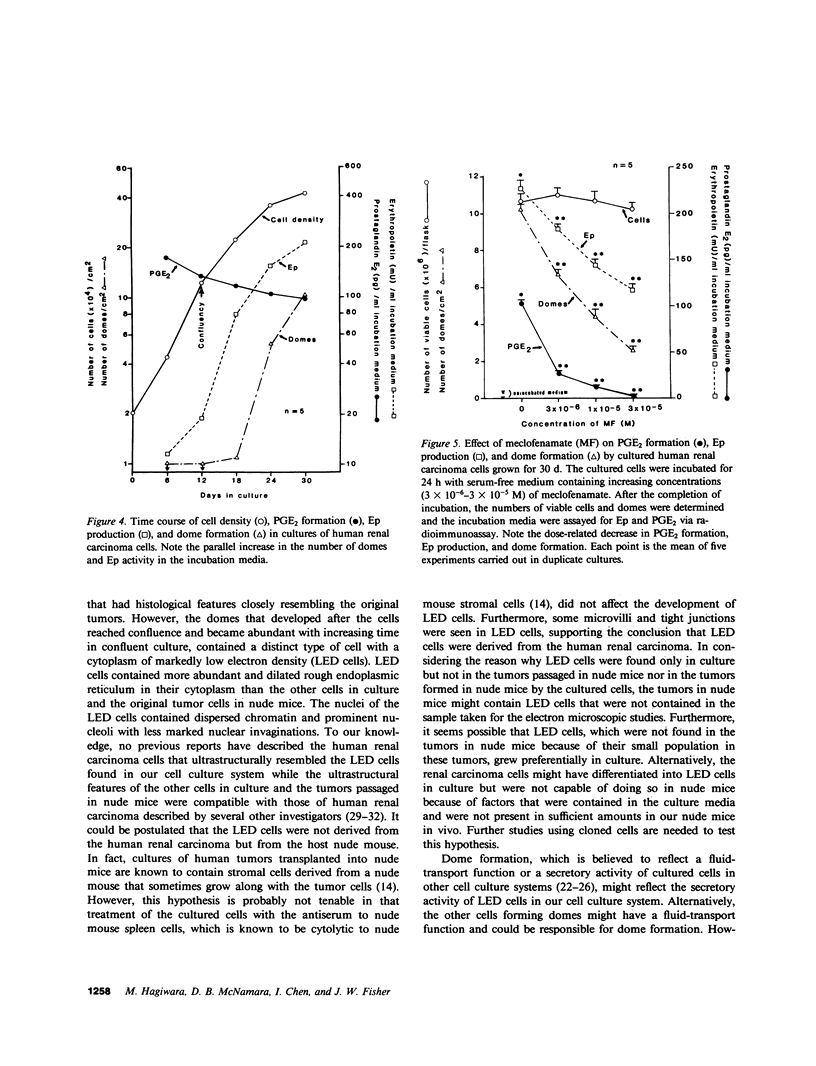
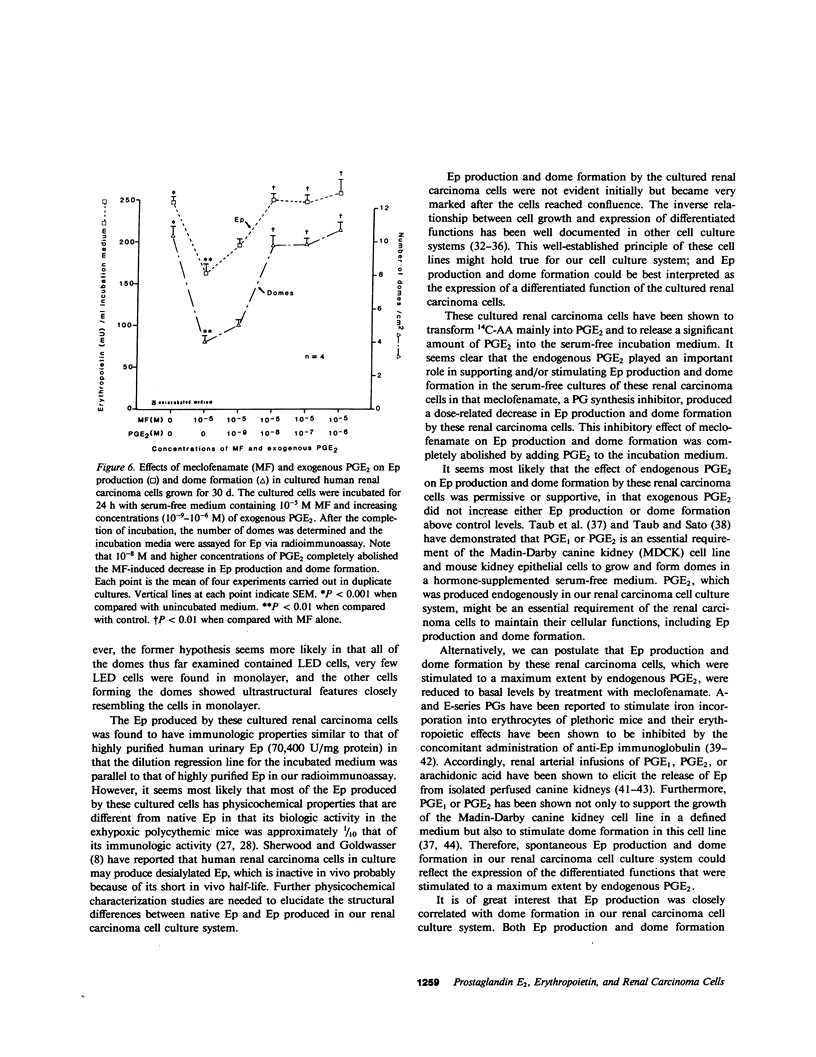
Studies were carried out on the role of endogenous prostaglandin E2 (PGE2) in erythropoietin (Ep) production and dome formation in primary monolayer cultures of a human renal carcinoma from a patient with erythrocytosis that has been serially transplanted into BALB/c athymic nude mice. The metabolism of [14C]arachidonic acid (14C-AA) by cultured renal carcinoma cells, which were plated in 25-cm2 flasks at a density of 2 X 10(4) cells/cm2 and grown for 6, 12 (confluence, 13 X 10(4) cells/cm2), 16, 24, and 30 d in Eagle's minimum essential medium (MEM) supplemented with 10% fetal bovine serum, was examined by using radiometric thin-layer chromatography (TLC). TLC revealed PGE2 to be the major metabolite of 14C-AA produced by the cultured cells throughout the 30 d of cultivation. In addition, the cultured cells at each time period were incubated for 24 h in 5 ml of serum-free Eagle's MEM and the levels of PGE2 and Ep in the incubated media were measured via radioimmunoassay. PGE2 levels in the serum-free media incubated with the cultured cells grown for 6 d were significantly (P less than 0.001) elevated (174 +/- 2.5 pg/ml, n = 5), compared with the unincubated control media (1.5 +/- 0.19 pg/ml, n = 5) and gradually decreased at each time period to 97.6 +/- 4.4 pg/ml (n = 5) at 30 d. On the other hand, the levels of Ep in the incubated media of the cells grown for 6 d were 11.5 +/- 0.52 mU/ml (n = 5) compared with 7.6 +/- 0.62 mU/ml (n = 5) in the control media. However, after the cultured cells became confluent, the levels of Ep in the incubated media showed a marked increase to 222.9 +/- 5.26 mU/ml (n = 5) at 30 d of cultivation. Multicellular hemicysts (domes) developed after the cultured cells reached confluence and their numbers increased with increasing time in confluence in parallel with the increase in Ep. Meclofenamate (MF) (3 X 10(-6)-3 X 10(-5) M), a prostaglandin synthesis inhibitor, produced a significant dose-related decrease in PGE2, Ep, and dome formation without producing a significant effect on cell viability in the 30-d cells. This inhibitory effect of MF on Ep production and dome formation was completely abolished by the addition of 10(-8) M PGE2 to the incubation medium. In conclusion, endogenous PGE2 plays an important role in supporting and/or stimulating Ep production and dome formation in cultured renal carcinoma cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton H. D., Halushka P. V., Alexander R. W., Mason D. M., Keiser H. R., DeVita V. T., Jr Indomethacin-responsive hypercalcemia in a patient with renal-cell adenocarcinoma. N Engl J Med. 1974 Jun 11;291(2):83–85. doi: 10.1056/NEJM197407112910207. [DOI] [PubMed] [Google Scholar]
- Burk J. R., Lertora J. J., Martinez I. R., Jr, Fisher J. W. Renal cell carcinoma with erythrocytosis and elevated erythropoietic stimulatory activity. South Med J. 1977 Aug;70(8):955–958. doi: 10.1097/00007611-197708000-00017. [DOI] [PubMed] [Google Scholar]
- Cummings K. B., Robertson R. P. Prostaglandin: increased production by renal cell carcinoma. J Urol. 1977 Nov;118(5):720–723. doi: 10.1016/s0022-5347(17)58172-0. [DOI] [PubMed] [Google Scholar]
- Dukes P. P., Shore N. A., Hammond D., Ortega J. A., Datta M. C. Enhancement of erythropoiesis by prostaglandins. J Lab Clin Med. 1973 Nov;82(5):704–712. [PubMed] [Google Scholar]
- Dulbecco R., Bologna M., Unger M. Differentiation of a rat mammary cell line in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1256–1260. doi: 10.1073/pnas.76.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
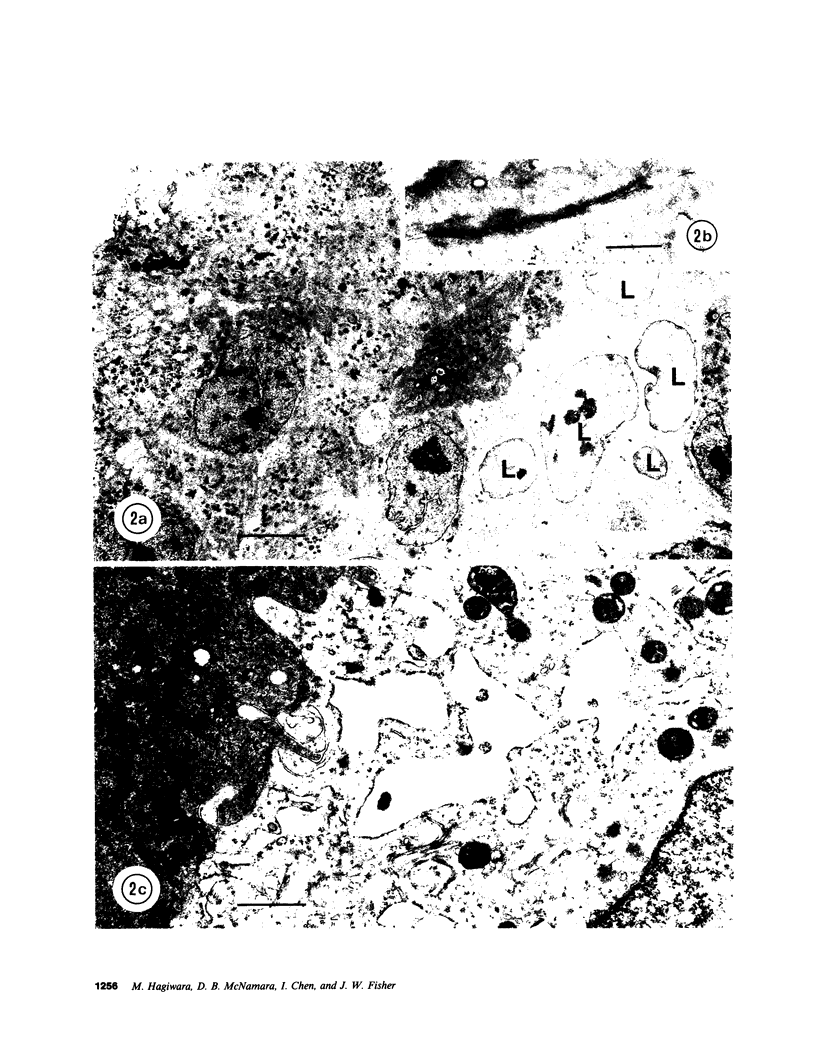
- Ericsson J. L., Seljelid R., Orrenius S. Comparative light and electron microscopic observations of the cytoplasmic matrix in renal carcinomas. Virchows Arch Pathol Anat Physiol Klin Med. 1966 Oct 10;341(3):204–223. doi: 10.1007/BF00961071. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Gross D. M., Nelson P. K., Fisher J. W. The effects of arachidonic acid on erythropoietin production in exhypoxic polycythemic mice and the isolated perfused canine kidney. J Pharmacol Exp Ther. 1978 Nov;207(2):402–409. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. M., Brookins J., Fink G. D., Fisher J. W. Effects of prostaglandins A2, E2 and F2 alpha on erythropoietin production. J Pharmacol Exp Ther. 1976 Aug;198(2):489–496. [PubMed] [Google Scholar]
- Hagiwara M., Chen I. L., McGonigle R., Beckman B., Kasten F. H., Fisher J. W. Erythropoietin production in a primary culture of human renal carcinoma cells maintained in nude mice. Blood. 1984 Apr;63(4):828–835. [PubMed] [Google Scholar]
- Hammond D., Winnick S. Paraneoplastic erythrocytosis and ectopic erythropoietins. Ann N Y Acad Sci. 1974;230:219–227. doi: 10.1111/j.1749-6632.1974.tb14452.x. [DOI] [PubMed] [Google Scholar]
- Laskin J. D., Piccinini L., Engelhardt D. L., Weinstein I. B. Control of melanin synthesis and secretion by B16/C3 melanoma cells. J Cell Physiol. 1982 Dec;113(3):481–486. doi: 10.1002/jcp.1041130318. [DOI] [PubMed] [Google Scholar]
- Leighton J., Estes L. W., Mansukhani S., Brada Z. A cell line derived from normal dog kidney (MDCK) exhibiting qualities of papillary adenocarcinoma and of renal tubular epithelium. Cancer. 1970 Nov;26(5):1022–1028. doi: 10.1002/1097-0142(197011)26:5<1022::aid-cncr2820260509>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Inducers of mammalian cell differentiation stimulate dome formation in a differentiated kidney epithelial cell line (MDCK). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1323–1327. doi: 10.1073/pnas.76.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara D. B., Boineau F. G., McMullen-Laird M., Lippton H. L., She H. S., Lewy J. E., Kadowitz P. J. Prostaglandin endoperoxide metabolism by microsomes of whole kidneys from normal, congenital unilateral hydronephrotic and unilateral ureteral obstructed rats. Prostaglandins. 1982 Nov;24(5):585–605. doi: 10.1016/0090-6980(82)90030-2. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Mullin J. M., Weibel J., Diamond L., Kleinzeller A. Sugar transport in the LLC-PK1 renal epithelial cell line: similarity to mammalian kidney and the influence of cell density. J Cell Physiol. 1980 Sep;104(3):375–389. doi: 10.1002/jcp.1041040311. [DOI] [PubMed] [Google Scholar]
- Murphy G. P., Kenny G. M., Mirand E. A. Erythropoietin levels in patients with renal tumors or cysts. Cancer. 1970 Jul;26(1):191–194. doi: 10.1002/1097-0142(197007)26:1<191::aid-cncr2820260124>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- OBERLING C., RIVIERE M., HAGUENAU F. Ultrastructure of the clear cells in renal carcinomas and its importance for the demonstration of their renal origin. Nature. 1960 Apr 30;186:402–403. doi: 10.1038/186402a0. [DOI] [PubMed] [Google Scholar]
- Okabe T., Suzuki A., Ohsawa N., Kosaka K., Terasima T. Immune elimination of host fibroblasts for the cultivation of human tumors transplanted into nude mice. Cancer Res. 1979 Oct;39(10):4189–4194. [PubMed] [Google Scholar]
- Paulo L. G., Wilkerson R. D., Roh B. L., George W. J., Fisher J. W. The effects of prostaglandin E 1 on erythropoietin production. Proc Soc Exp Biol Med. 1973 Mar;142(3):771–775. doi: 10.3181/00379727-142-37113. [DOI] [PubMed] [Google Scholar]
- Rege A. B., Brookins J., Fisher J. W. A radioimmunoassay for erythropoietin: serum levels in normal human subjects and patients with hemopoietic disorders. J Lab Clin Med. 1982 Dec;100(6):829–843. [PubMed] [Google Scholar]
- Rizzino A., Gonda M. A., Rapp U. R. Dome formation by a retrovirus-induced lung adenocarcinoma cell line. Cancer Res. 1982 May;42(5):1881–1887. [PubMed] [Google Scholar]
- Robertson R. P., Baylink D. J., Marini B. J., Adkison H. W. Elevated prostaglandins and suppressed parathyroid hormone associated with hypercalcemia and renal cell carcinoma. J Clin Endocrinol Metab. 1975 Jul;41(1):164–167. doi: 10.1210/jcem-41-1-164. [DOI] [PubMed] [Google Scholar]
- Schooley J. C., Mahlmann L. J. Stimulation of erythropoiesis in plethoric mice by prostaglandins and its inhibition by antierythropoietin. Proc Soc Exp Biol Med. 1971 Nov;138(2):523–524. doi: 10.3181/00379727-138-35931. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She H. S., McNamara D. B., Spannhake E. W., Hyman A. L., Kadowitz P. J. Metabolism of prostaglandin endoperoxide by microsomes from cat lung. Prostaglandins. 1981 Apr;21(4):531–539. doi: 10.1016/0090-6980(81)90002-2. [DOI] [PubMed] [Google Scholar]
- Sherwood J. B., Goldwasser E. Erythropoietin production by human renal carcinoma cells in culture. Endocrinology. 1976 Aug;99(2):504–510. doi: 10.1210/endo-99-2-504. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Printz M. P. PGI2 production by rat blood vessels: diminished prostacyclin formation in veins compared to arteries. Prostaglandins. 1978 Jul;16(1):1–16. doi: 10.1016/0090-6980(78)90196-x. [DOI] [PubMed] [Google Scholar]
- Sufrin G., Mirand E. A., Moore R. H., Chu T. M., Murphy G. P. Hormones in renal cancer. J Urol. 1977 Apr;117(4):433–438. doi: 10.1016/s0022-5347(17)58490-6. [DOI] [PubMed] [Google Scholar]
- Tannenbaum M. Ultrastructural pathology of human renal cell tumors. Pathol Annu. 1971;6:249–277. [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub M., Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980 Nov;105(2):369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- Thorling E. B. Paraneoplastic erythrocytosis and inappropriate erythropoietin production. A review. Scand J Haematol Suppl. 1972;17:1–166. [PubMed] [Google Scholar]
- Toyama K., Fujiyama N., Suzuki H., Chen T. P., Tamaoki N., Ueyama Y. Erythropoietin levels in the course of a patient with erythropoietin-producing renal cell carcinoma and transplantation of this tumor in nude mice. Blood. 1979 Jul;54(1):245–253. [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Visser A. S., Prop F. J. "Domes," periodically expanding and collapsing secretory structures in cell cultures of mouse mammary tumors. J Natl Cancer Inst. 1974 Jan;52(1):293–295. doi: 10.1093/jnci/52.1.293. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Rosse W. F., Swarm R. L. The erythropoiesis-stimulating factors produced by tumors. Ann N Y Acad Sci. 1968 Mar 29;149(1):509–515. doi: 10.1111/j.1749-6632.1968.tb15190.x. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Snider J. J., Cline A., Caldwell B. V., Speroff L. Antihypertensive function of renal-cell carcinoma. Evidence for a prostaglandin-A-secreting tumor. N Engl J Med. 1974 Apr 11;290(15):843–845. doi: 10.1056/NEJM197404112901509. [DOI] [PubMed] [Google Scholar]