Abstract
Objectives:
To investigate the effect of acidic solution on surface roughness and microleakage of tooth-colored restorative materials.
Materials and Methods:
A 160 box-shaped cavities were prepared on the buccal surfaces of 160 human molars, and assigned to four groups: Group A restored with Ketac™ Molar Easymix, Group B with Fuji II™ LC, Group C with Ketac™ N100, and Group D with Filtek™ Z250, and subdivided into study and control groups (n = 20). Study groups were immersed in lemon juice (pH = 2.79) for 24 h, whilst controlgroups in deionized distilled water. All samples were immersed in 2% methylene blue dye, sectioned into two equal halves for surface roughness, and microleakage tests. Data were analyzed using Mann–Whitney and Kruskal–Wallis tests at P < 0.05.
Results:
There was a significant difference in surface roughness of Ketac™ Molar, Fuji II™ LC, and Ketac™ N100. No significant difference was found in microleakage of Ketac™ Molar and Fuji II™ LC; however, there were significant differences in the gingival margin of Ketac™ N100, and the occlusal margin of Filtek™ Z250.
Conclusions:
All glass ionomer cements were eroded after exposure to the acidic drink. Filtek™ Z250 and Ketac™ Molar Easymix showed more microleakage. All materials showed more microleakage at the gingival margins.
Keywords: Composite resin, erosion, glass ionomer, microleakage, surface roughness
INTRODUCTION
Tooth-colored restorative materials such as glass ionomer cements (GICs) and composite resins (CRs) have gained popularity due to increasing demand of esthetics. Under acidic conditions, dentalrestorative materialsmay suffer erosion which could be predicted by changes in surface roughness.[1] Atomic force microscope (AFM) is considered an important and applicable method for Ra assessment. Rough surface of restorations with loss of contour will lead to high friction and results in high surface wear rate. Rough surface will influence bacterial adhesion by increasing plaque retention and can cause staining.[2] Microleakage can also develop which increases the probability of recurrent caries and postoperative hypersensitivity.[3]
However, no study was found that investigated the capacity of acidic soft drinks to produce microleakage or evaluating the surface roughness of the eroded tooth-colored restorations. Hence, the aims of this study were to evaluate the effect of an acidic soft drink (lemon juice) on the surface roughness and microleakage of cavities restored with four types of widely used tooth-colored dental restorative materials.
It is hypothesized that there are no changes in the surface roughness and marginal integrity of the eroded tooth-colored dental restorative materials.
MATERIALS AND METHODS
One hundred and sixty freshly extracted human molars, free from cracks, caries, and fillings were cleaned withultrasonic and hand scalers, and then kept in thymol solution (1%) at room temperature.
Standardized box-shaped cavity preparations were made on the buccal surfaces of tooth samples with 5.0 mm in mesiodistal width, 3.0 mm in occlusogingival height, and 2.0 mm in depth, measured with periodontal probe. Samples were randomly assigned to four groups: Group A was restored with conventional glass ionomer restorative material (Ketac™ Molar Easymix, 3M-ESPE, Germany); Group B with resin-modified glassionomer restorative material (Fuji™ II LC, GC Corporation, Japan); Group C with nanoionomer restorative material (Ketac™ N100, 3M-ESPE, USA); and Group D with microhybridCR (Filtek™ Z250, 3M-ESPE, USA). All restorative materials were placed according to manufacturers’ instructions, and the details of those materials are shown in Table 1.
Table 1.
Test materials
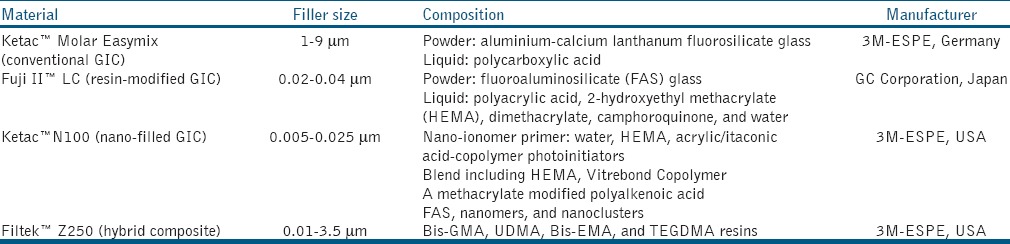
In Group A; Ketac™ Molar Easymix liquid was applied to the cavity for 10 s, rinsed and dried with cotton pellet, prior to application of Ketac™ Molar Easymix. In Group B; GC Dentin Conditioner (GC Corporation, Japan;10% polyacrylic acid) was applied to the cavity for 10 s, rinsed, and dried. Fuji II™ LC capsule was triturated, and then injected into the cavity preparation, light-cured for 20 s. Fuji Coat LC (GC Corporation, Japan) was applied to the restoration and light-cured for 10 s. In Group C; GC Dentin Conditioner was applied, followed by Ketac™ N100 primer (3M ESPE, USA) which was light-cured for 10 s. Ketac™ N100 pastes were mixed, and then applied to the preparation and light-cured for 20 s. For Group D; the cavity was acid-etched with Scotchbond™ etchant (3M ESPE, USA) and bonded with Adper Single Bond Adhesive (3M ESPE, USA) before placement of Filtek™ Z250, which was then light-cured for 40 s.
All light-curing procedures were performed using light-cure unit (Elipar™ Free Light 2 LED, 3M-ESPE, USA) with a light intensity of 1123 mW/cm2. The output from the curing device was validated using a visible curing light meter (CURE RITE/Caulk). All restorations were finished and polished after 24 h using Sof-Lex (3M-ESPE, USA) discs. Each group was further subdivided into study and control groups (n = 20).
Lemon juice purchased from local grocery stores with pH 2.79 measured using an analog pH meter (Hanna Microprocessor pH Meter, USA) was used in the study.
All areas of the teeth were covered with two coats of acid resistant nail polish except the restoration and 1mm rim of the tooth structure around. Apices were sealed with sticky wax (Kemdent, UK). The control samples were kept in deionized distilled water (DDW) and were tested for surface roughness at baseline using AFM. All study group samples were immersed in the lemon juice at room temperature for 24 h,[4] then washed with DDW and dried with cotton pellets, then immersed in 2% methylene blue dye solution at room temperature for 24 h, removed, and rinsed under running water.
Assessment of surface roughness and marginal integrity
Each sample was sectioned longitudinally at the middle in a buccolingual direction using a diamond disc (Exakt hard material cutter, Germany) under water spray to give two equal halves (A and B). Segment A was once more sectioned to provide a 1.0mm thickness segment for the surface roughness test (Ra), while segment B was used for the microleakage measurement. All samples for Ra test were embedded in cylindrical wax block (CollegeWax Metrowax, England), and scanned under AFM (AMBIOS Technology Inc, USA) in its contact mode except forKetac™ Molars which were measured using Profilometer (S3000 Mitutoyo, Japan). Each sample was measured at three different locations and the mean was taken.[5]
The degree of marginal leakage was evaluated by the penetration of the dye stain from the occlusal and gingival cavosurface margins to the base of the cavity preparation. Each specimen was viewed under a stereomicroscope (Leica Imaging System, LEICA, UK) at ×20 magnification by one examiner and photographs taken. It is scored according to the depth of dye penetration.[6] The scoring criteria were:
0- No evidence of dye penetration at tooth/restoration interface.
1- Dye penetration along the interface to ≤halfdepths of cavity.
2- Dye penetration to full depth of cavity.
3- Dye penetration to base of cavity and beyond.
Data were analyzed with Statistical Package for Social Sciences (SPSS) version 18.0 (SPSS Inc, 2010) using Mann-Whitney and Kruskal-Wallis statistical tests of the surface roughness (Ra) and microleakage, with a significance level set at P < 0.05.
RESULTS
Surface roughness
There was a significant difference in the surface roughness of Ketac™ Molar, Fuji II™ LC, and Ketac™ N100 (P < 0.001), while there was no difference in Filtek™ Z250 (P = 0.053), when the study groups were compared to the control groups [Table 2 and Figure 1]. Comparison among study groups showed significant difference between the groups (P < 0.001). Further, pair-wise comparison using Mann–Whitney test revealed that Ketac™ Molar Easymix exhibited the highest Ra among the groups, followed byFuji II™ LC, Ketac™ N100, withFiltek™ Z250 being the least (P < 0.001).
Table 2.
Comparison of median (IqR) of surface roughness (nm) between study and control groups of eroded tooth-colored dental restorative materials
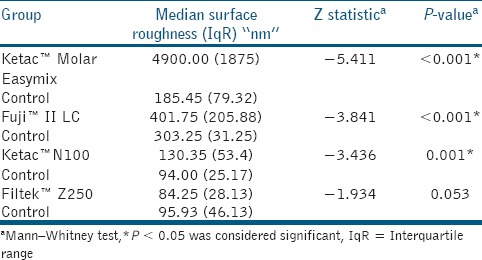
Figure 1.
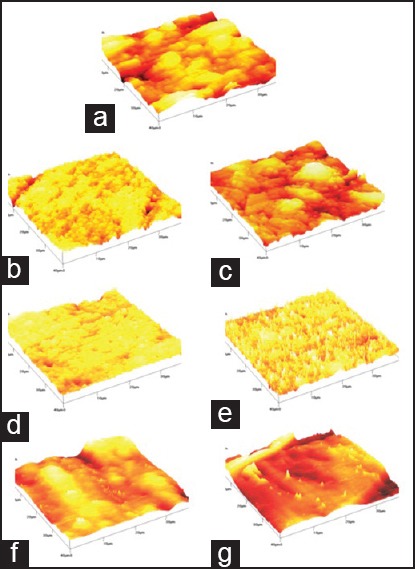
AFM photographs. (a) Ketac™ Molar before immersion (b) Fuji II™ LC before immersion. (c) Fuji II™ LC after immersion. (d) Ketac™ N100 before immersion. (e) Ketac™ N100 after immersion. (f) Filtek™ Z250 before immersion.(g) Filtek™ Z250 after immersion in the acidic drink. AFM = Atomic force microscope
Microleakage
The degree of intraexaminer reproducibility of measurements, that is, intra class correlation coefficient (ICC) was close to 1.00, indicating high reliability. There were no significant differences in microleakage of Ketac™ Molar Easymix and Fuji II™ LC after exposure to the acidic drink (on both occlusal and gingival margins P > 0.05). In Ketac™ N100, no significant difference was demonstrated at the occlusal margin (P > 0.05), but there was a significant difference at the gingival margin (P = 0.029). In Filtek™ Z250 samples; there was a significant difference in microleakage occlusally (P = 0.035), but no difference gingivally (P > 0.05) [Table 3]. Figure 2 shows the microleakage of Fuji II™ LC in control and study groups.
Table 3.
Comparison of median (IqR) of microleakage scores between study and control groups of eroded tooth-colored dental restorative materials
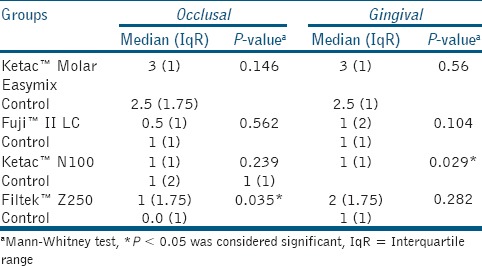
Figure 2.
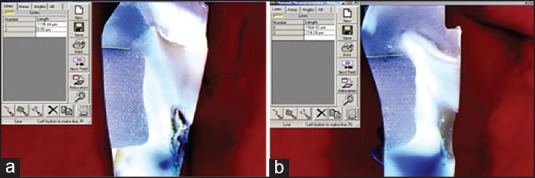
Stereomicroscope photographs showing microleakage measurement. (a) Fuji™ II LC before immersion in the acidic drink. (b) Fuji™ II LC after immersion in theacidic drink
Comparison among study groups showed that Ketac™ Molar Easymix samples exhibited the highest microleakage scores, followed by Filtek™ Z250 and Ketac™ N100, while the least was demonstrated in Fuji II™ LC (P < 0.001). No statistical significant difference was observed between occlusal and gingival margins in Ketac™ Molar Easymix (P = 0.524) and Ketac™ N100 (P = 0.906), while the differences were observed in both Fuji II™ LC (P = 0.047) and Filtek™ Z250 (P = 0.008).
DISCUSSION
After exposure to the acidic drinks, there was a significant change in the Ra of Ketac™ Molar Easymix which exceeded the AFM limits, therefore, these samples were measured with profilometer. These changes are due to the dissolution of cement matrix and leaching of the principal forming components (F1−, Sr2+, Al3+, Ca2+, and Na1+) leaving the fillers protruded from the surface. The larger filler particles of this material (2.8 μm in average) could be considered as an additional factor for higher Ra and it comes in agreement with a previous study.[7]
The Ra values of Fuji II™ LC were significantly lower than Ketac™ Molar Easymix, which may be due to its smaller filler size (0.02-0.04 μm). The incorporation of resin has added resin polymerization setting reaction, making the material mature faster.
The Ra values of Ketac™ N100 were the lowest among all GICs tested in this study, due to its nanosized filler of 0.005-0.025 μm. Furthermore, the filler loading is approximately 69% (by weight) and all of the nanofillers are further surface-modified with methacrylate silane coupling agents that might be the influencing factor for better interaction between nanofillers and matrix.[8]
The Ra values of Filtek™ Z250 did not differ significantly after exposure to the acidic drink. The resin matrix of Filtek™ Z250 is a combination of difunctional monomers Bisphenol-glycidyl methacrylate (Bis-GMA), Ethoxylated bisphenol A glycol dimethacrylate (Bis-EMA), Urethane dimethacrylate (UDMA), Triethylene glycol dimethacrylate (TEGDMA). TEGDMA decreases the surface softening by acids[9] and increases the degree of polymerization of resin-based materials improving their physical properties, thereby minimizing their abrasion rate.[10] Precoating the silica particles with silane coupling agents had resulted in a very strong coherence between these particles and organic matrix which helps against hydrolytic degradation.[11] This result is in agreement with previous studies.[5,12,13] However, this result is in contrast with the findings of the surface roughness of composites simulated with toothbrush abrasion.[14]
For microleakage, there were no significant changes in Ketac™ Molar EasyMix and Fuji™ II LC at either margin, as well as at the occlusal margin of Ketac™ N100 after exposure to acidic drinks. However, Ketac™ Molar EasyMix showed high microleakage scores both in the control and study groups. The possible explanation for Fuji™ II LC and Ketac™ N100 could be the two-fold mechanism of bonding for GICs; chemically via ionic interaction of the carboxyl groups of the polyalkenoic acid with calcium ions of hydroxyapatite that remained attached to the collagen fibrils[15] and second is the micromechanical bonding via application of polyalkenoic acid that removes the smear layer and exposes collagen fibrils up to about 0.5-1μm depth.[16] However, there was a significant difference demonstrated at the gingival margins of Ketac™ N100 samples, which could be attributed to the presence of resin which causeshigher polymerization shrinkage and also due to their superficial mechanical interlocking.[17]
Microleakage of Filtek™ Z250 was higher at occlusal margin after exposure to the acidic drink. There was a possibility of erosion of enamel and subsequent loss of the adhesive material resulting in higher microleakage levels. These findings meet those reported in a previous study.[18] Polymerization shrinkage can also create a gap at tooth/restoration interface resulting in bond failure and subsequent microleakage. The difference of microleakage at the gingival margins of Filtek™ Z250 was not significant. Here, the major tooth substrate is dentin, which has the ability to buffer the acidic effects and prevent demineralization,[19] thereby preserving the marginal integrity.
Ketac™ Molar EasyMix exhibited the highest microleakage scores when comparison was made among study groups. The slow setting mechanism of conventional GIC (acid-base reaction) may result in the delay of material adhesion to tooth structure.[20] The lowest microleakage was observed in Fuji™ II LC samples, which could be due to resin incorporation and the polymerization reaction which is considered as dominant reaction.[21] Similar results were found in a study,[22] but another study found contrasting results.[23]
In Fuji II™ LC, microleakage was higher gingivally, where dentin represented the major tooth substrate to which the material was bonded. Since dentin contains a substantial proportion of water and organic material, the adhesion of a hydrophilic material like Fuji™ II LC (due to hydroxyethyl methacrylate (HEMA) incorporation) could have resulted in water sorption, swelling, weakening of the restorations, and resulting in gaps formation and changed markedly on exposure to moisture.[24] Additionally, dentin is more porous, which could have enhanced the permeability to dye penetration compared to enamel.[25]
There was no difference in microleakage between either margins of Ketac™ N100. Pretreatment of the prepared cavities with Ketac™ N100 primer, where copolymer of acrylic/itaconic acid dissolved in HEMA and water resulted in partial demineralization of both enamel and dentin. The tooth tissue is easier to be wetted by the restorative material which might improve the adhesion, as supported by a previous study.[26]
Higher microleakage demonstrated gingivally in Filtek™ Z250 could be due to the fact that the gingival margins were located further in dentin. Intrinsically hydrated dentin makes bonding of a hydrophobic resin substrate difficult. Furthermore, the collagen content of dentin is higher than enamel (18%) which is normally inaccessible, owing to the surrounding hydroxyapatite crystals. This study agrees with a study[25] where microleakage was found to be more cervically than occlusally in CR restorations.
Suggestion for future research
Use of pH cycling or toothbrush abrasion cycling should be used in future studies to directly extrapolate the findings of the study from a clinical standpoint.[27]
CONCLUSIONS
By the limitation of this study, all GIC restorative materials were eroded after exposure to the acidic drink, where Ketac™ Molar showed the highest surface roughness, followed by Fuji™ II LC, and minimum in Ketac™ N100. However, there was no significant difference in surface roughness of Filtek™ Z250, which shows that it is stable in an acidic environment.
Fuji™ II LC showed the leastmicroleakage followed by Ketac™ N100 and Filtek™ Z250, while the worst material was the conventional GIC (Ketac™ Molar Easymix). In general, all materials showed more microleakage at the gingival margins than occlusal margins.
ACKNOWLEDGEMENT
The authors would like to thank Universiti Sains Malaysia for the financial support given through the SHORT TERM GRANT-304/PPSG/61311041.
Footnotes
Source of Support: Financial support given by Universiti Sains Malaysia through the SHORT TERM GRANT-304/PPSG/61311041
Conflict of Interest: None declared.
REFERENCES
- 1.Rahim TN, Mohamad D, Md Akil H, Ab Rahman I. Water sorption characteristics of restorative dental composites immersed in acidic drinks. Dent Mater. 2012;28:e63–70. doi: 10.1016/j.dental.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Sidhu SK, Sherriff M, Watson TF. In vivo changes in roughness of resin-modified glass ionomer materials. Dent Mater. 1997;13:208–13. doi: 10.1016/S0109-5641(97)80028-0. [DOI] [PubMed] [Google Scholar]
- 3.Mali P, Deshpande S, Singh A. Microleakage of restorative materials: An in vitro study. J Indian Soc Pedod Prev Dent. 2006;24:15–8. doi: 10.4103/0970-4388.22828. [DOI] [PubMed] [Google Scholar]
- 4.Buchalla W, Attin T, Hellwig E. Brushing abrasion of luting cements under neutral and acidic conditions. Oper Dent. 2000;25:482–7. [PubMed] [Google Scholar]
- 5.Zuryati AG, Qian OQ, Dasmawati M. Effects of home bleaching on surface hardness and surface roughness of an experimental nanocomposite. J Conserv Dent. 2013;16:356–61. doi: 10.4103/0972-0707.114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidhu SK. Sealing effectiveness of light-cured glass ionomer cements liners. J Prosthet Dent. 1992;68:891–4. doi: 10.1016/0022-3913(92)90545-l. [DOI] [PubMed] [Google Scholar]
- 7.Kula K, Nelson S, Kula T, Thompson V. In vitro effect of phosphate fluoride gel on the surface of composites with different filler particles. J Prosthet Dent. 1986;56:161–9. doi: 10.1016/0022-3913(86)90465-8. [DOI] [PubMed] [Google Scholar]
- 8.Yap SH, Yap AU, Teo CK, Ng JJ. Polish retention of new aesthetic restorative materials over time. Singapore Dent J. 2004;26:39–43. [PubMed] [Google Scholar]
- 9.Asmussen E. Softening of BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res. 1984;92:257–61. doi: 10.1111/j.1600-0722.1984.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawai K, Iwami Y, Ebisu S. Effect of resin monomer composition on toothbrush wear resistance. J Oral Rehabil. 1998;25:264–8. doi: 10.1111/j.1365-2842.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Lambrechts P, Debels E, Van Landuyt K, Peumans M, Van Meerbeek B. How to simulate wear? Overview of existing methods. Dent Mater. 2006;22:693–701. doi: 10.1016/j.dental.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Turssi CP, Hara AT, de Magalhaes CS, Serra MC, Rodrigues AL., Jr Influence of storage regime prior to abrasion on surface topography of restorative materials. J Biomed Mater Res B Appl Biomater. 2003;65:227–32. doi: 10.1002/jbm.b.10005. [DOI] [PubMed] [Google Scholar]
- 13.Rai R, Gupta R. In vitro evaluation of the effect of two finishing and polishing systems on four esthetic restorative materials. J Conserv Dent. 2013;16:564–7. doi: 10.4103/0972-0707.120946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uppal M, Ganesh A, Balagopal S, Kaur G. Profilometric analysis of two composite resins’ surface repolished after tooth brush abrasion with three polishing systems. J Conserv Dent. 2013;16:309–13. doi: 10.4103/0972-0707.114356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida Y, Van Meerbeek B, Nakayama Y, Snauwaert J, Hellemans L, Lambrechts P, et al. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res. 2000;79:709–14. doi: 10.1177/00220345000790020301. [DOI] [PubMed] [Google Scholar]
- 16.Inoue S, Van Meerbeek B, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, et al. Effect of remaining dentin thickness and the use conditioner on micro-tensile bond strength of a glass-ionomer adhesive. Dent Mater. 2001;17:445–55. doi: 10.1016/s0109-5641(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho E, Cardoso MV, De Munck J, Neves AA, Van Landuyt KL, Poitevin A, et al. Bonding effectiveness and interfacial characterization of a nano-filled resin-modified glass ionomer. Dent Mater. 2009;25:1347–57. doi: 10.1016/j.dental.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Navarro R, Vicente A, Ortiz AJ, Bravo LA. The effects of two soft drinks on bond strength, bracket microleakage, and adhesive remnant on intact and sealed enamel. Eur J Orthod. 2011;33:60–5. doi: 10.1093/ejo/cjq018. [DOI] [PubMed] [Google Scholar]
- 19.Ganss C, Klimek J, Starck C. Quantitative analysis of the impact the organic matrix on the fluoride effect on erosion progression in human dentine using longitudinal microradiography. Arch Oral Biol. 2004;49:931–5. doi: 10.1016/j.archoralbio.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Wilson AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972;132:133–5. doi: 10.1038/sj.bdj.4802810. [DOI] [PubMed] [Google Scholar]
- 21.Cho E, Kopel H, White SN. Moisture susceptibility of resin modified glass-ionomer materials. Quintessence Int. 1995;26:351–8. [PubMed] [Google Scholar]
- 22.Kusgoz A, Tuzuner T, Ulker M, Kemer B, Saray O. Conversion degree, microhardness, microleakage and fluoride release of different fissure sealants. J Mech Behav Biomed Mater. 2010;3:594–9. doi: 10.1016/j.jmbbm.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Toledano M, Osorio E, Osorio R, Garcia-Godoy F. Microleakage of class V resin-modified glass ionomer and compomer restorations. J Dent. 1999;81:610–5. doi: 10.1016/s0022-3913(99)70217-9. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson JW, Anstice HM, McLean JW. A preliminary report on the effect of storage in water on the properties of commercial light-cured glass-ionomer cements. Br Dent J. 1992;173:98–101. doi: 10.1038/sj.bdj.4807956. [DOI] [PubMed] [Google Scholar]
- 25.da Silva AF, Piva E, Demarco FF, Correr Sobrinho L, Osinga PW. Microleakage in conventional and bonded amalgam restorations: Influence of cavity volume. Oper Dent. 2006;31:377–83. doi: 10.2341/05-49. [DOI] [PubMed] [Google Scholar]
- 26.Korkmaz Y, Ozel E, Attar N, Ozge Bicer C. Influence of conditioning methods on the shear bond strength of novel light-curing nano ionomer restorative to enamel and dentin. Lasers Med Sci. 2009;25:861–6. doi: 10.1007/s10103-009-0718-8. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho FG, Sampaio CS, Fucio SB, Carlo HL, Correr-Sobrinho L, Puppin-Rontani RM. Effect of chemical and mechanical degradation on surface roughness of three glass ionomers and a nanofilled resin composite. Oper Dent. 2012;37:509–17. doi: 10.2341/10-406-L. [DOI] [PubMed] [Google Scholar]


