Abstract
Introduction:
Irrigation plays an indispensable role in removal of tissue remnants and debris from the complicated root canal system. This study compared the human pulp tissue dissolution by different concentrations of chlorine dioxide, calcium hypochlorite and sodium hypochlorite.
Materials and Methods:
Pulp tissue was standardized to a weight of 9 mg for each sample. In all,60 samples obtained were divided into 6 groups according to the irrigating solution used- 2.5% sodium hypochlorite (NaOCl), 5.25% NaOCl, 5% calcium hypochlorite (Ca(OCl)2), 10% Ca(OCl)2, 5%chlorine dioxide (ClO2) and 13% ClO2. Pulp tissue was placed in each test tube carrying irrigants of measured volume (5ml) according to their specified subgroup time interval: 30 minutes (Subgroup A) and 60 minutes (Subgroup B). The solution from each sample test tube was filtered and was left for drying overnight. The residual weight was calculated by filtration method.
Results:
Mean tissue dissolution increases with increase in time period. Results showed 5.25% NaOCl to be most effective at both time intervals followed by 2.5% NaOCl at 60 minutes, 10%Ca(OCl)2 and 13% ClO2 at 60 minutes. Least amount of tissue dissolving ability was demonstrated by 5% Ca(OCl)2 and 5% ClO2 at 30 minutes. Distilled water showed no pulp tissue dissolution.
Conclusion:
Withinthe limitations of the study, NaOCl most efficiently dissolved the pulp tissue at both concentrations and at both time intervals. Mean tissue dissolution by Ca(OCl)2 and ClO2 gradually increased with time and with their increase in concentration.
Keywords: Calcium hypochlorite, chlorine dioxide, sodium hypochlorite, tissue dissolution
INTRODUCTION
Disinfecting and cleaning the root canal system of microbial flora and pulpal tissue are prerequisites for successful root canal treatment.[1] Although root canal shaping can be efficiently attained with advanced instrumentation technology, effective cleaning of the entire root canal system still remains a challenge.[2]
Irrigation plays an indispensable role in removal of tissue remnants and debris from the complicated structure of root canal anatomy. The most favorable features of irrigants are their flushing action, tissue-dissolving ability, antimicrobial effect and low toxicity.[3,4]
Sodium hypochlorite is the most commonly used endodontic irrigant because of its well-known antimicrobial and tissue-dissolving activity.[5] But, the disadvantage of using 5.25% NaOCl as an irrigant is its extreme cytotoxicity.[6]
Calcium hypochlorite is used for industrial sterilization, bleaching and water purification treatment.[7] It is available as granules, and has high concentration of available chlorine.
Chlorine dioxide is chemically similar to chlorine or hypochlorite, the familiar household bleach. It is reported to be tuberculoidal, bactericidal, virucidal and fungicidal.[6] It is mostly used for sewage water disinfection, industrial process water treatment, industrial air treatment, mussel control, foodstuffs production and treatment, industrial waste oxidation and gas sterilization of medical equipment.
In most of the previous studies, tissue solubility of various irrigants has been measured using bovine pulp tissue and bovine mucosa. Fewer studies have been done on the direct solubilizing effects of various irrigants on human pulp tissue. Till date, there has been no study comparing the human pulp tissue dissolving ability of chlorine dioxide, calcium hypochlorite and sodium hypochlorite.
Therefore the aim of this in vitro study is to compare the human pulp tissue dissolution by different concentrations of chlorine dioxide, calcium hypochlorite and sodium hypochlorite.
MATERIALS AND METHODS
Freshly extracted, intact vital premolars, extracted for orthodontic reasons were collected from the Department of Oral and Maxillofacial Surgery. Any carious/fractured teeth were discarded. The methodology employed was similar to that undertaken by Tanejaetal.[8] Two longitudinal grooves on the proximal surfaces of the teeth were made with round bur. The teeth were split into two halves with chisel and mallet. Pulp tissue was removed in toto. It was cut with the 15 no. BP blade and placed on a pre-weighed filter paper. The weight of freshly extracted pulp was standardized to 9.0 mg. In all, seventy samples of standardized weight were taken. The samples were divided into different groups and subgroups as shown in Figure 1.
Figure 1.
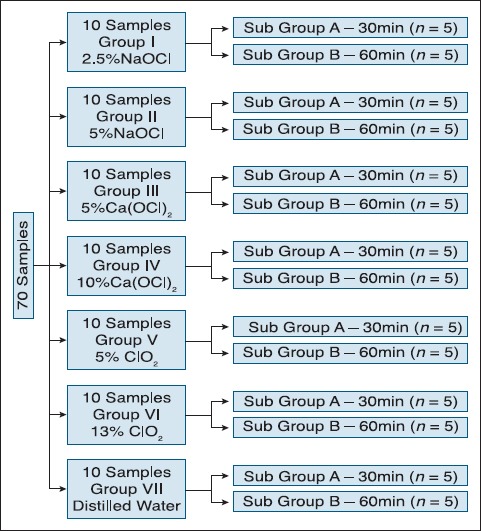
Distribution of the samples
In all, seventy test tubes were taken, ten for each group:
In Group I 10 test tubes were filled with measured volume (5 ml each) of 2.5% NaOCl
In Group II 10 test tubes were filled with measured volume (5 ml each) of 5% NaOCl
In Group III 10 test tubes were filled with measured volume (5 ml each) of 5% Ca(OCl)2
In Group IV 10 test tubes were filled with measured volume (5 ml each) of 10% Ca(OCl)2
In Group V 10 test tubes were filled with measured volume (5 ml each) of 5% ClO2
In Group VI 10 test tubes were filled with measured volume (5 ml each) of 13% ClO2
In Group VII 10 test tubes were filled with measured volume (5 ml each) of distilled water
All the solutions were freshly prepared. Solutions of 2% NaOCland 5% NaOClwereprepared by diluting 10% NaOCl (Avarice Laboratories Private Limited, Ghaziabad, India). Solutions of 5% Ca(OCl)2 and 10% Ca(OCl)2 were prepared from calcium hypochlorite powder (Central Warehouse Private Limited, Baroda, India). Solutions of 5% and 13% ClO2 were prepared by mixing solutions A and B (Rapid Oxide, Kresko Projects Private Limited, Ahmedabad, India) according to the manufacturer's instructions.
Each group had 2 subgroups having 5 test tubes each according to different time periods of 30 min and 60 min, respectively, for which the sample was immersed in the irrigant. Pulp tissue was placed into each test tube. These were then kept in an incubator at 37°C. After the passage of specified time, the solution from each sample test tube was filtered through a Wattman filter paper. This was followed by overnight drying of the filter paper which was then measured in an analytical balance. The difference in the weight of the dried filter paper (with residue) and initial filter paper (before filtration) gave the weight of residue left subsequent to filtration, which is the sum of the weight of pulpal residue and irrigating solution residue. A similar procedure was carried out for all the samples.
Irrigating solution (5 ml) was filtered through a pre-weighed filter paper. This was followed by overnight drying of the filter paper which was then measured. The difference in the weight of the dried filter paper and initial filter paper (before filtration) gave the dry weight of residue of that irrigating solution. The methodology employed was similar to that undertaken by Taneja et al. This procedure was repeated for irrigants of all groups. Weight of the residual pulp left on the filter paper of each sample was calculated by subtracting the weight of residue of respective irrigating solution from the weight of the total residue on filtration paper, measured earlier. Difference in the weight of pulp tissue before immersion and the weight of residual pulp left on the filter paper gave the amount of pulp dissolved by the respective irrigant of that group. Similarly readings for all samples of each subgroup were taken. Thus, by filtration method, the amount of pulp dissolved by various irrigating solution at different time intervals was measured quantitatively [Figure 2].
Figure 2.
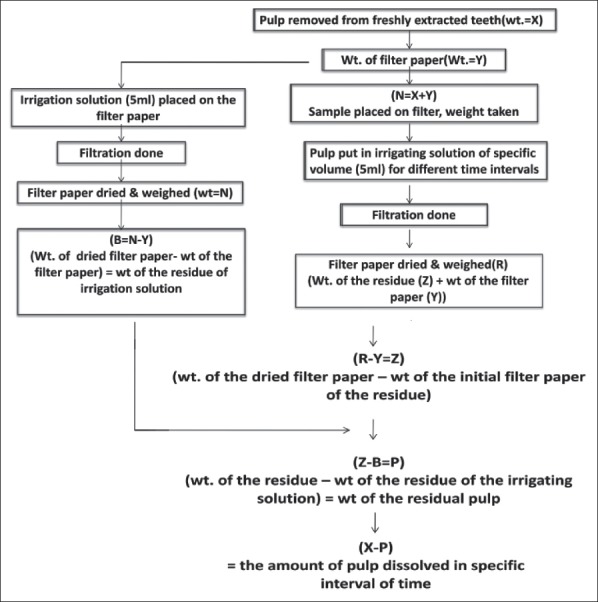
Flowchart of the methodology
Statistics
The mean dissolution time for pulp tissue by different groups at different time intervals was statistically analyzed by ANOVA and Post Hoc analysis (Tukey HSD) was done for intergroup comparison by using SPSS Version 15.0 statistical Analysis Software.
RESULTS
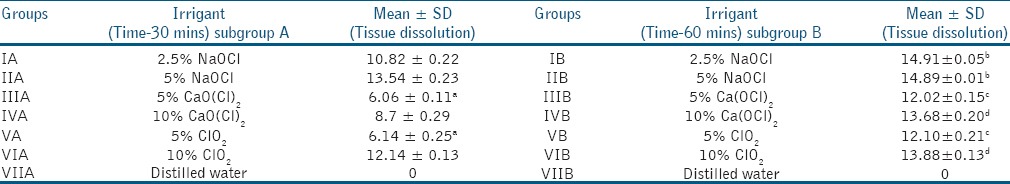
The mean dissolution time for pulp tissue was found to increase with passage of time for all the groups. Statistically significant difference was found between all the groups except for subgroups IIIA and VA, IB and IIB, IIIB and VB, and, IVB and VIB [Table 1]. Distilled water showed no dissolution of the pulp.
Table 1.
Mean dissolution time for pulp tissue using different irrigants at two time periods
DISCUSSION
Chemomechanical preparation forms the key requisite for the success of root canal instrumentation. Therefore, some sort of irrigation/disinfection is necessary to remove tissue from inaccessible areas of the root canal and to kill microorganisms.
Callahan[9] and Grossman[10] demonstrated the importance of the solvent ability of endodontic irrigant. In vital root canal therapy, the presence of pulpal remnants may lead to post-operative pain and also has the potential to form a periapical lesion as suggested by Strindberg.[11]
The contact time of an irrigant is limited and the vascularity of vital tissue resists the action of certain irrigants. Therefore, the speed/time of dissolution of vital tissue by different irrigants is an important factor.[12]
A unique feature of the present study is the use of filtration method for determining the dissolution of pulp where the weight of the residue from the irrigant was calculated and subtracted from the total weight of the pulp along with the solution's residue, so that the exact dissolution of pulp tissue can be calculated. This unique filtration technique helped us to overcome the limitations of other studies where because of the residue of the irrigant, the exact dissolution of pulp tissue could not be calculated.
In this study, chlorine dioxide and calcium hypochlorite were chosen as an irrigant and compared with sodium hypochlorite.
The ClO2 is far less toxic and irritating when applied to the human body.[6] It does not form chlorinated hydrocarbons, which are carcinogenic, when in contact with organic matter. Ca(OCl)2 has greater available chlorine than NaOCl (upto 65% available chlorine).[13] No study comparing the two materials’ tissue dissolving capacity has been undertaken so far.
All the solutions were freshly prepared.
Results from the present study showed that 5% sodium hypochlorite was the most effective pulp tissue solvent among all irrigants at both time intervals. This is because of the ionization of NaOCl to liberate hypochlorous acid (HOCl) and hydroxyl ions in an aqueous environment.[14] When hydroxyl ion levels decrease as a result of the saponification and amino acid neutralization reactions, the pH also decreases, thereby favoring the formation of HOCl molecules. The chloramination reaction is then initiated which results in degradation and hydrolysis of amino acids.[12]
This result is in agreement with Rosenfeld[5] who reported 5% NaOCl as an effective solvent of human pulp tissue. According to the results of our study 2.5% NaOCl was less effective than 5% NaOCl at 30 minutes. Hand et al.[15] have also suggested that 5.25% NaOCl was a more effective solvent than 2.5% NaOCl. Previous studies have shown that the tissue-dissolving ability of sodium hypochlorite solution decreases if it is diluted.[15,16]
The results of our study demonstrated that 2.5% and 5% NaOCl showed no significant difference in pulp dissolution at 60 minutes. This is in accordance with Sirtes et al.[17] who found that 1%, 2.62%, and 5.25% solutions had an unchanged quantity of available chlorine for one hour.
Mean tissue dissolution with calcium hypochlorite was significantly less as compared with sodium hypochlorite at both concentrations and at both time intervals. This might be because of the release of large amount of hydroxyl ions as a result of dissolution of calcium hypochlorite granules in aqueous solution, which would take longer time to be exhausted.[13]
An in vitro study undertaken by Dutta and Saunders[13] demonstrated that NaOCl dissolved tissue faster than the Ca(OCl)2 solution over the first 35 minutes, but there was no significant differences thereafter.
Solution of 5% Ca(OCl)2 when used for 30 minutes showed less tissue dissolution than 10% at 30 minutes. The hyperosmotic effect of 10% solution might have caused tissue dehydration resulting in more weight loss than 5% solution.[13]
Solutions of Ca(OCl)2 at both 5% and 10% showed more amount of tissue dissolution at 60 minutes because Ca(OCl)2 has an initial slower rate of tissue dissolution.
Mean tissue dissolution by ClO2 was significantly less than NaOCl. The reason for this might also be the low pH of ClO2 as compared to high pH (pH = 12) of NaOCl. In a study by Nishikiori et al,[18] the pH of ClO2 was raised upto 12 by using NaOH and it was found equivalent to NaOCl for dissolving bovine pulp tissue. But according to Deininger et al.[19] ClO2 exhibits biocidal efficacy only over a pH range of 3-9. Between pH 4-7, chlorine exists predominantly as HClO, the active moiety responsible for bacterial inactivation, whereas above pH 9, OCl− predominates.[20] Also the addition of NaOH will result in making the aqueous solution of ClO2 ineffective by breaking it down into sodium chlorate, sodium chlorite and water.
Among the ClO2 group, large amount of mean tissue dissolution was demonstrated by 13% ClO2 at 60 minutes.5% ClO2 showed lower pulp dissolving capacity at 30 minutes, followed by 5% ClO2 at 60 minutes and 13% ClO2 at 30 minutes, which were comparable. Studies have suggested that lower the pH, more time was needed for solution contact for tissue dissolution.[6] This might be the reason for less effectiveness of ClO2 in dissolution of pulp tissue at 30 minutes than at 60 minutes. No significant difference was seen with 13% ClO2 at both 30 minutes and 60 minutes.
Statistically insignificant results were obtained between calcium hypochlorite and chlorine dioxide group at both concentrations. The reason for this is not clear but it may be because of the amount of available chlorine which is nearly same for both the materials. But less tissue dissolution was seen at lower concentrations for both groups, thus indicating that high concentrations for both should be used if the contact time is less.
Results of our study demonstrated no dissolution of the pulp by distilled water at all time intervals. This is in agreement with the study by Gordon et al.[21] who confirmed that distilled water is an ineffective solvent of vital tooth pulp.
CONCLUSION
NaOClmost effectively dissolved the pulp tissue at both concentrations and at both time intervals.
Ca(OCl)2 at both concentrations dissolved the pulp tissue more effectively at 60 minutes.
ClO2 was most effective at higher concentration and at 60 minutes
Tissue dissolving ability of ClO2 and Ca(OCl)2 are comparable at high concentration and at 60 minutes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ricucci D, Siqueira JF, Jr, Bate AL, Pitt Ford TR. Histologic investigation of root canal-treated teeth with apical periodontitis: A retrospective study from twenty-four patients. J Endod. 2009;35:493–502. doi: 10.1016/j.joen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Hargreaves KM. Pathways of the pulp. In: Kenneth M, Hargreaves, Stephen Cohen, editors; Louis H, Berman, editors. 10th ed. St. Louis: Mosby Elsevier; 2011. It is a single volume text book of 10th ed. [Google Scholar]
- 3.Turkun M, Cengiz T. The effects of sodium hypochlorite and calcium hydroxide on tissue dissolution and root canal cleanliness. Int Endod J. 1997;30:335–42. doi: 10.1046/j.1365-2591.1997.00085.x. [DOI] [PubMed] [Google Scholar]
- 4.Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JA. Dissolution ofpulptissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int Endod J. 2004;37:38–41. doi: 10.1111/j.1365-2591.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld EF, James GA, Burch BS. Vital pulp tissue response to sodium hypochlorite. J Endod. 1978;4:140–6. doi: 10.1016/S0099-2399(78)80129-0. [DOI] [PubMed] [Google Scholar]
- 6.Cobankara FK, Ozkan HB, Terlemez A. Comparison of organic tissue dissolution capacities of sodium hypochlorite and chlorine dioxide. J Endod. 2010;36:272–4. doi: 10.1016/j.joen.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker HA, Mohler BM. The sterilization of milk bottles with calcium hypochlorite. Am J Public Health (N Y) 1912;2:282–7. doi: 10.2105/ajph.2.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taneja S, Chadha R, Dixit S, Gupta R, Nayar R. An in vitro comparison of quantitative dissolution of human pulp in different irrigating solutions. J Oral Health Community Dent. 2010;4:28–33. [Google Scholar]
- 9.Callahan JR. Sulphuric acid for opening root canals. Dent Cosmos. 1894;36:957–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman LI, Meiman BW. Solution of pulp tissue by chemical agents. J Am Dent Assoc. 1941;28:223–5. [Google Scholar]
- 11.Strindberg Z. The dependence of the results of pulp therapy on certain factors. Acta Odontol Scand. 1956;14:1–175. [Google Scholar]
- 12.Grey GC. Thesis: Boston University; 1970. The capabilities of sodium hypochlorite to digest organic debris from root canals with emphasis on accessory canals. [Google Scholar]
- 13.Dutta A, Saunders WP. Comparative evaluation of calcium hypochlorite and sodium hypochlorite on soft-tissue dissolution. J Endod. 2012;38:1395–8. doi: 10.1016/j.joen.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Estrela C, Estrela CR, Barbin EL, Spano JC, Marchesan MA, Pecora JD. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13:113–7. doi: 10.1590/s0103-64402002000200007. [DOI] [PubMed] [Google Scholar]
- 15.Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4:60–4. doi: 10.1016/S0099-2399(78)80255-6. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Rass M, Oglesby SW. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod. 1981;7:376–7. doi: 10.1016/S0099-2399(81)80059-3. [DOI] [PubMed] [Google Scholar]
- 17.Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005;31:669–71. doi: 10.1097/01.don.0000153846.62144.d2. [DOI] [PubMed] [Google Scholar]
- 18.Nishikiori R, Nomura Y, Sawajiri M, Masuki K, Hirata I, Okazaki M. Influence of chlorine dioxide on cell death and cell cycle of human gingival fibroblasts. J Dent. 2008;36:993–8. doi: 10.1016/j.jdent.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Deininger R, Ancheta A, Ziegler A, editors. Conference Paper. Chlorine dioxide. Paper presented at the PAN American Health Organization (PAHO) Symposium: Water Quality: Effective Disinfection. 1998. Available from: http://www.bvsd.ops.oms.org .
- 20.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 2009;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon TM, Damato D, Christner P. Solvent effect of various dilutions of sodium hypochlorite on vital and necrotic tissue. J Endod. 1981;7:466–9. doi: 10.1016/S0099-2399(81)80308-1. [DOI] [PubMed] [Google Scholar]


