Abstract
Rowell syndrome is a rare disease consisting of erythema multiforme-like lesions associated with lupus erythematosus. The syndrome occurs mostly in middle-aged women. The authors describe the syndrome in a 15-year-old boy who responded well to systemic steroids and hydroxychloroquine.
Keywords: Children, erythema multiforme, lupus erythematosus, Rowell syndrome
INTRODUCTION
Rowell et al.[1] described a syndrome characterized by lupus erythematosus (LE) and erythema multiforme (EM)-like lesions, positive tests for rheumatoid factor (RF), speckled antinuclear antibody (ANA), and precipitating antibody to saline extract of human tissue (anti-SjT) in 1963. Subsequently, major and minor diagnostic criteria were proposed for the diagnosis of this syndrome.[2] Major criteria include coexistence of LE and EM-like lesions, and positive ANA with a speckled pattern. Minor criteria include chilblains, positive anti-La (SS-B) or anti-Ro (SS-A) antibodies, and reactive RF. All the major criteria and at least one of the minor criteria are required to confirm the diagnosis. We describe this syndrome in a 15-year-old boy.
CASE REPORT
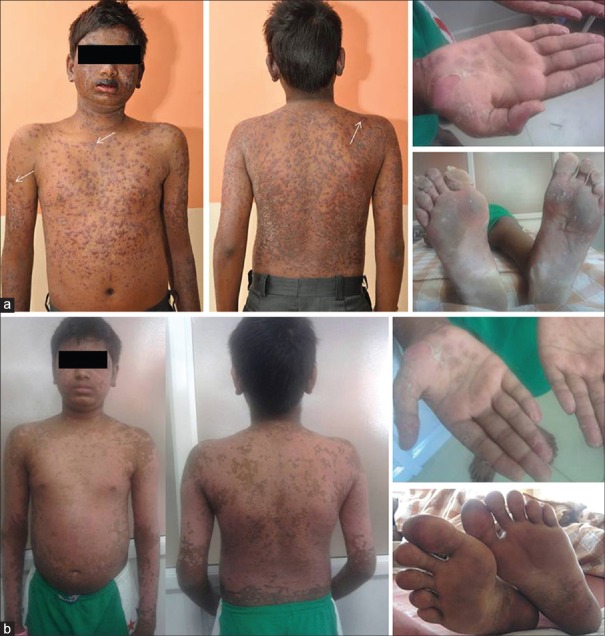
A 15-year-old boy with diagnosed systemic LE (SLE) since three years presented with painful, pruritic, ring-like erythematous plaques all over the body of 10 days duration. He also complained of pain in his knees and abdominal pain with vomiting. Three years ago, the patient had fever, facial rash, oral ulceration and arthritis along with hemolytic anemia (Hb of 8.4 g/dL), thrombocytopenia (platelet counts, 122,000/mm3), and a positive ANA and anti-double-stranded DNA (anti–dsDNA) antibodies. At that time he also developed facial puffiness, proteinuria (100 mg/dL), and red cell casts in the urine suggestive of renal involvement. He was initially treated with oral prednisolone 2 mg/kg/day and subsequently 1 mg/kg/day on alternate days. The patient stopped the treatment on his own one month ago. He gave a history suggestive of chilblain-like lesions. There was no history of upper respiratory infection, fever or drug intake in the recent past. Physical examination revealed widespread erythematous, well-defined and confluent papules and plaques with blister formation at some places [Figure 1a]. There were hemorrhagic crusts over the lips and oral mucosa. Lesions were symmetrically distributed over the face, trunk, abdomen, and extremities, including the palms and soles. Some of the lesions were “targetoid” in appearance [arrows in Figure 1a]. There was minimal epidermal detachment, especially over the sole. His vital parameters, hydration, and systemic examination were normal. Haemogram revealed hemoglobin of 11.9 g/dL, total leukocyte count of 7400 cells/mm3, platelet count of 114,000 cells/mm3, and erythrocyte sedimentation rate of 50 mm at 1 h. Peripheral smear suggested evidence of hemolysis. Serum C3 was 31 mg/dL (reference range for males: 88-252 mg/dL); C4, 8 mg/dL (reference range for males: 12-72 mg/dL); C-reactive protein, 18 mg/L (reference range: <10 mg/L), and serum creatinine was 0.6 mg/dL. Serum electrolytes, prothrombin time, and activated partial thromboplastin time were normal. Serology revealed a negative RF, positive ANA with a speckled pattern, a positive anti-La (SS-B) and a strongly positive anti-Ro (SS-A). Urine routine examination showed red cell casts with proteinuria of 100 mg/dL. 24-h urine excretion of protein was 105 mg/m2/h. Urine culture was sterile. Ophthalmological evaluation was normal. Ultrasonography of kidney, ureter, and bladder was normal. Echocardiography was normal. The patient met the diagnostic criteria for SLE by the presence of oral ulcers, skin rash, arthralgia, hemolytic anemia, renal disorder, hypocomplementemia, and a positive ANA. Skin biopsy revealed a subcorneal split containing hemosiderin pigment. The rest of the epidermis revealed a few lymphocytes and necrotic kerationocytes with evidence of diffuse keratinocyte regeneration. The dermis showed superficial oedema with a lymphohistiocytic infiltrate, along with perivascular oedema, endothelial swelling and fibrin deposits. These features suggested EM. Direct immunofluorescence (DIF) revealed deposits of IgG, IgM, IgA, and C3 in the basal membrane zone (BMZ). A renal biopsy revealed features of SLE nephritis class IV-A with an activity index of 5. A diagnosis of Rowell syndrome was established based on the clinical manifestations, confirmatory skin biopsy, and positive diagnostic criteria for SLE. The patient was treated with oral prednisolone 2 mg/kg/day in three divided doses and hydroxychloroquine 4 mg/kg/day. Significant improvement was noted in the skin, mucosal, and palmoplantar lesions after three weeks [Figure 1b], with no fresh lesions. Azathioprine was added later. Cyclophosphamide given subsequently was not well tolerated. The patient is under regular follow up with no recurrence of skin lesions.
Figure 1.
(a) Extensive erythema multiforme lesions over face, trunk, abdomen, back, and extremities with minimal epidermal detachment over soles. (b) Healing skin lesions of Rowell syndrome after 3 weeks of therapy
DISCUSSION
Rowell syndrome (RS) is a rare syndrome, in which patients with LE develop EM-like lesions. The simultaneous appearance of LE with EM was first described in 1922 by Scholtz.[3] Later in 1963, Rowell et al.[1] reported female patients with discoid LE (DLE) and EM-like lesions that were associated with a speckled pattern of ANA, positive RF, and precipitating antibodies to a saline extract of human tissues (anti-SjT); the latter is now regarded as similar to anti-Ro. EM patients do not typically show accompanying immunologic characteristics or anti-SjT antibodies. Rowell syndrome was hence considered a distinct entity with the following criteria: SLE; EM-like lesions; and immunologic serum abnormalities: A speckled ANA pattern and positive RF.[3]
Most of the cases of RS have been reported in middle-aged women.[4] The syndrome occurred in a 15 year old boy in our case. Modifications have been made to the diagnostic criteria of RS in recent years because of inconsistent features. In 1995, Lee et al.[5] suggested the inclusion of chilblains as a diagnostic feature. In 2000, Zeitouni et al.[2] proposed the following revised diagnostic criteria for RS: Major criteria that include SLE, DLE, or subacute cutaneous LE (SCLE); EM-like lesions (with or without mucosal involvement); and ANA with a speckled pattern. Minor criteria include chilblains, anti-Ro/anti-La antibody, and a positive RF. All three major criteria and at least one minor criterion are required for a diagnosis of RS.
Speckled ANA pattern is the most consistent feature of RS occurring in about 88% of the cases, whereas RF is the least preserved feature, present in only 41%.[2,4,6] The immunologic abnormalities of RS are also shared by SCLE.
Both SLE and RS respond to a similar therapeutic regimen. Azathioprine, antimalarials, prednisone, dapsone, and cyclosporine have been used with good results.[5,6,7,8,9] Our patient responded well to prednisolone, and hydroxychloroquine.
Despite the refined diagnostic criteria, recent literature has debated on whether RS is an overlap syndrome, a real association, or coincidence of DLE and EM,[9,10] Aygodan et al. proposed that the RS is a subentity of subacute LE with EM. Others have variously suggested that RS is a different variant of cutaneous LE, a subtype of chronic LE or an independent LE subtype.[7,8,9,10] Furthermore, Torchia[9] concluded that RS might be included as an autonomous type of cutaneous LE within the spectrum of LE-specific skin disease.
In conclusion, Rowell syndrome, although rare, should be suspected in all patients with LE with EM-like lesions where there is no evidence of a precipitating factor.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rowell NR, Beck JS, Anderson JR. Lupus erythematosus and erythema multiforme-like lesions. A syndrome with characteristic immunological abnormalities. Arch Dermatol. 1963;88:176–80. doi: 10.1001/archderm.1963.01590200064012. [DOI] [PubMed] [Google Scholar]
- 2.Zeitouni NC, Funaro D, Cloutier RA, Gagné E, Claveau J. Redefining Rowell's syndrome. Br J Dermatol. 2000;142:343–6. doi: 10.1046/j.1365-2133.2000.03306.x. [DOI] [PubMed] [Google Scholar]
- 3.Scholtz M. Lupus erythematosus acutus disseminates haemorrhagicus. Arch Dermatol Syphilol. 1922;6:466. [Google Scholar]
- 4.Duarte AF, Mota A, Pereira M, Baudrier T, Azevedo F. Rowell syndrome: Case report and review of literature. Dermatol Online J. 2008;14:15. [PubMed] [Google Scholar]
- 5.Lee S, Schloss E, Kowichi J. Rowell's syndrome: A case report with subacute cutaneous lupus erythematosus and erythema multiforme. Can J Dermatol. 1995;7:807–10. [Google Scholar]
- 6.Khandpur S, Das S, Singh MK. Rowell's syndrome revisited: Report of two cases from India. Int J Dermatol. 2005;44:545–9. doi: 10.1111/j.1365-4632.2004.01995.x. [DOI] [PubMed] [Google Scholar]
- 7.Aydogan K, Karadogan S, Balaban Adim S, Tunali S. Lupus erythematosus associated with erythema multiforme: Report of two cases and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:621–7. doi: 10.1111/j.1468-3083.2005.01233.x. [DOI] [PubMed] [Google Scholar]
- 8.Torchia D. Rowell syndrome categorized as cutaneous lupus erythematosus subtype. J Am Acad Dermatol. 2012;67:417–21. doi: 10.1016/j.jaad.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Müller CS, Hinterberger LR, Vogt T. Successful treatment of Rowell syndrome using oral cyclosporine A. Int J Dermatol. 2011;50:1020–2. doi: 10.1111/j.1365-4632.2010.04848.x. [DOI] [PubMed] [Google Scholar]
- 10.Antiga E, Caproni M, Bonciani D, Bonciolini V, Fabbri P. ‘Rowell's syndrome?’. Lupus. 2012;21:577–85. doi: 10.1177/0961203311430513. [DOI] [PubMed] [Google Scholar]