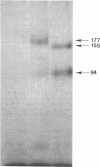
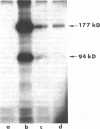
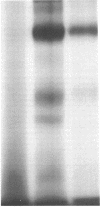
Abstract
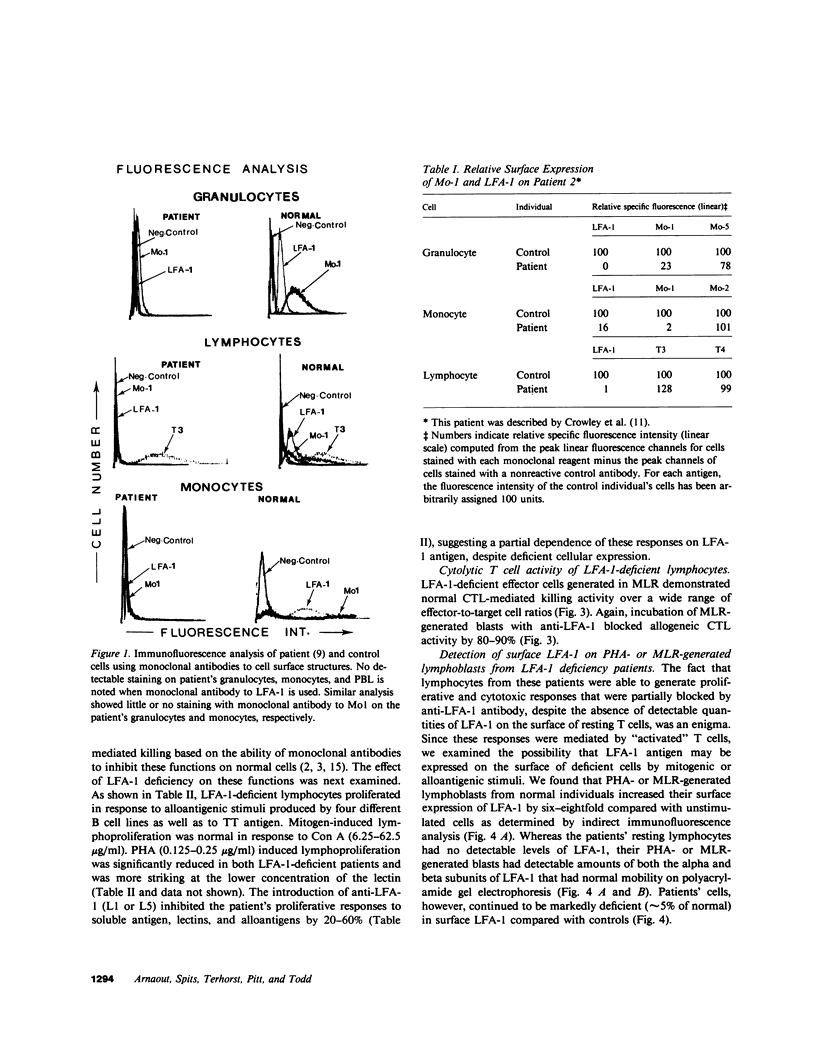
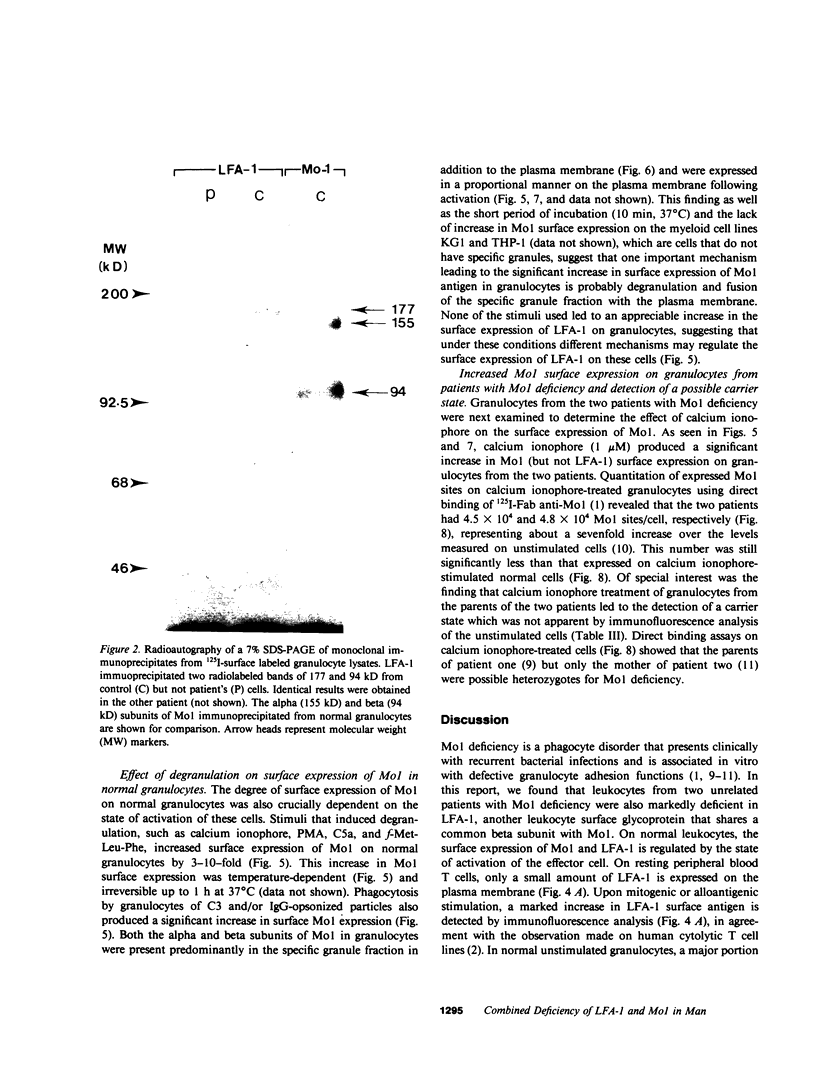
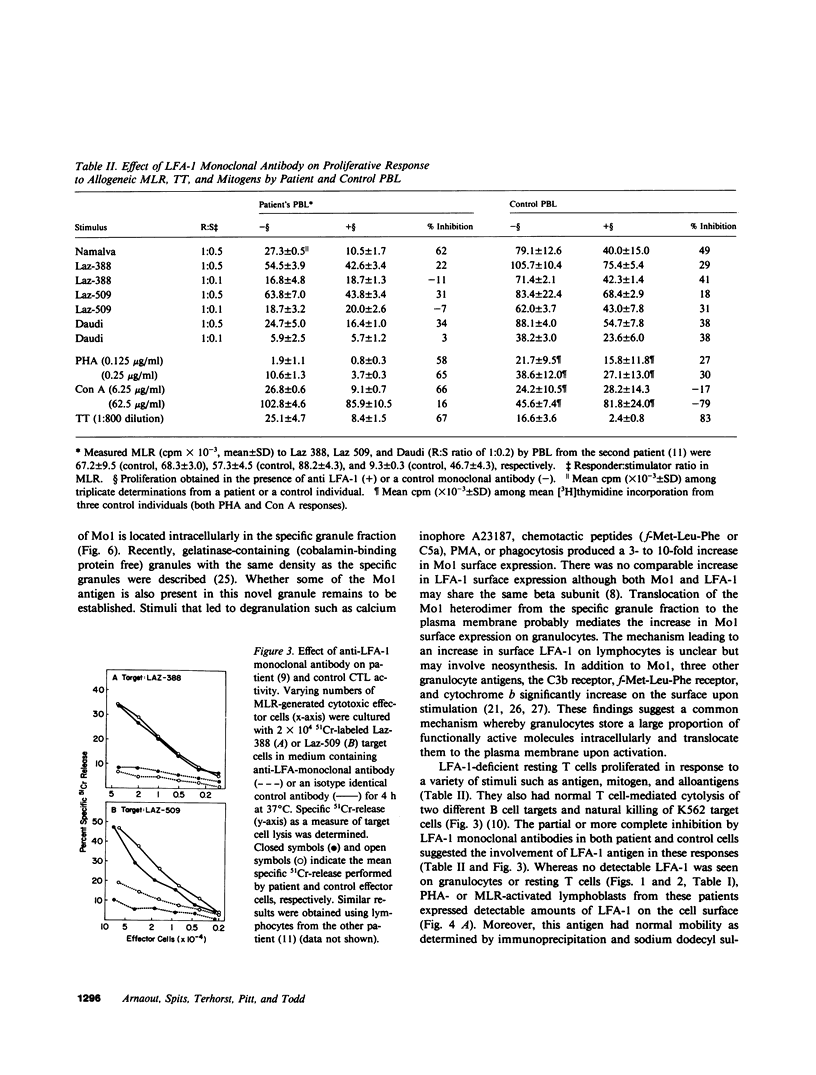
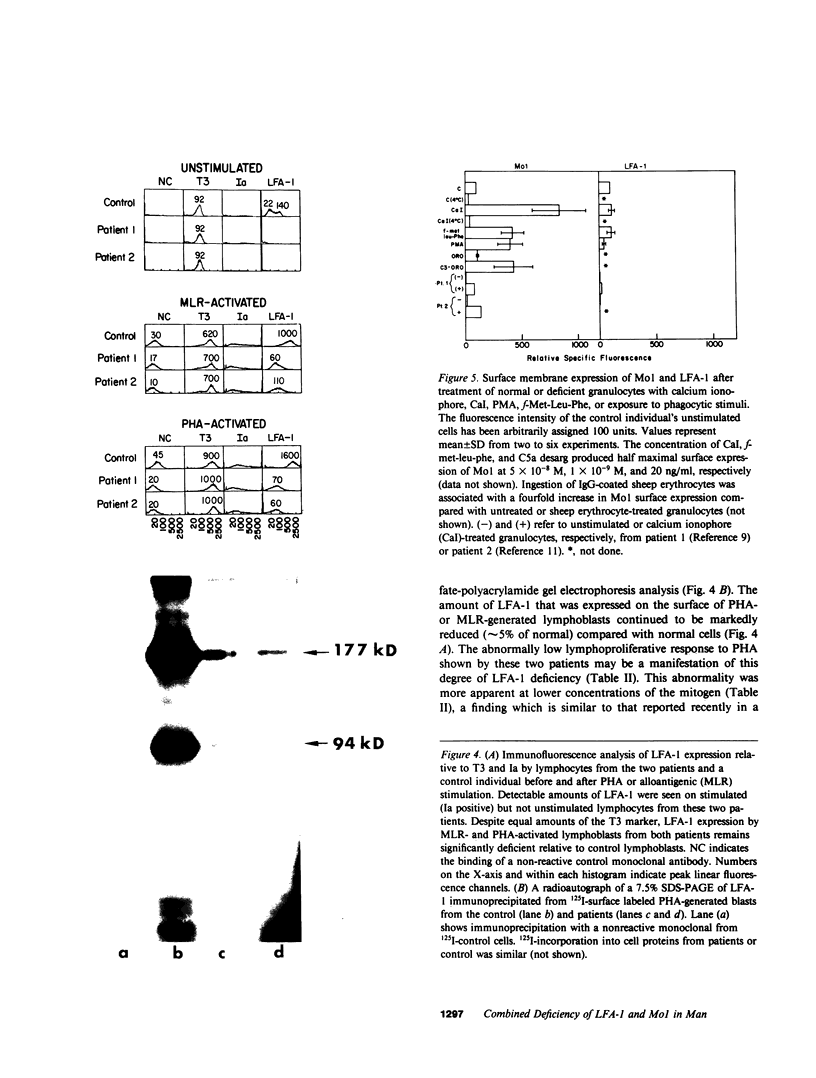
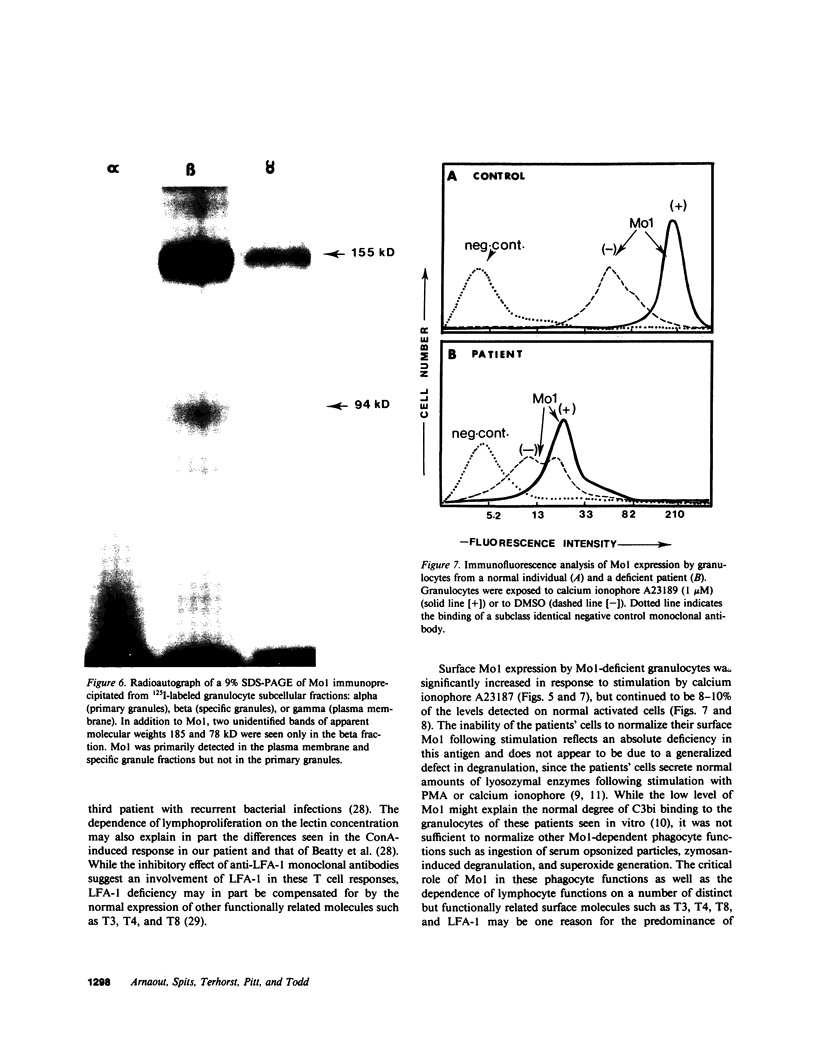
Mo1, a phagocyte surface glycoprotein heterodimer, is involved in a number of phagocyte adhesion functions such as binding and ingestion of serum-opsonized particles, zymosan-induced degranulation, and superoxide generation. Deficiency of this antigen in humans has been associated with increased susceptibility to recurrent bacterial infections. The beta subunit of Mo1 is shared by another surface glycoprotein named LFA-1, which is involved in lymphocyte proliferation, cytolytic T cell, and natural killing activities. Two unrelated patients with Mo1 deficiency were found to be deficient in LFA-1 as well as in the common beta subunit. Investigation of lymphocyte functions in these two patients revealed normal mixed leukocyte culture-generated cytolytic T cell and natural killing activities and significantly reduced proliferative response to phytohemagglutinin. LFA-1-deficient cells also proliferated in response to soluble antigen and different alloantigens. These responses were partially blocked by anti-LFA-1 antibody. Whereas LFA-1 was undetectable by immunofluorescence and immunoprecipitation on the patients' resting T cells, significantly reduced (approximately 5% of normal) but detectable amounts of the heterodimeric LFA-1 antigen were found on mitogen and alloantigen-activated T cells. On granulocytes, Mo1 surface expression was also dependent on the state of cellular activation. The amount of surface Mo1 present on resting normal granulocytes increased by 3-10-fold following exposure to stimuli that induced degranulation, suggesting the presence of a major intracellular pool for this antigen. Analysis of subcellular fractions from granulocytes showed that intracellular Mo1 is located primarily in the specific granule fraction. Activated granulocytes had little or no increase in their surface expression of LFA-1 antigen. Deficient granulocytes had significantly increased numbers of Mo1 antigen expressed on the surface following stimulation with calcium ionophore (1 microM). However, the amount expressed continued to be significantly reduced compared with normal cells. Quantitation of surface Mo1 on granulocytes exposed to calcium ionophore (1 microM) showed that both parents in one family but only the mother in the other family had significantly reduced levels of Mo1, suggesting heterogeneity in the inheritance of this disorder. Whereas LFA-1 deficiency on lymphocytes was associated with normal alloantigen-induced cytolytic T cell and natural killing activities in these two patients, functions which were in part dependent on small amounts of detectable LFA-1 antigen, the Mo1 deficiency state led to significant defects in phagocyte adhesion functions. Hence, the clinical symptoms associated with this combined deficiency state reflect a more profound phagocyte than lymphocyte disorder.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty P. G., Ochs H. D., Harlan J. M., Price T. H., Rosen H., Taylor R. F., Hansen J. A., Klebanoff S. J. Absence of monoclonal-antibody-defined protein complex in boy with abnormal leucocyte function. Lancet. 1984 Mar 10;1(8376):535–537. doi: 10.1016/s0140-6736(84)90933-4. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crowley C. A., Curnutte J. T., Rosin R. E., André-Schwartz J., Gallin J. I., Klempner M., Snyderman R., Southwick F. S., Stossel T. P., Babior B. M. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980 May 22;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Monoclonal antibody to a novel lymphocyte function-associated antigen (LFA-1): mechanism of blockade of T lymphocyte-mediated killing and effects on other T and B lymphocyte functions. J Immunol. 1981 Aug;127(2):590–595. [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Fletcher M. P., Seligmann B. E., Gallin J. I. Correlation of human neutrophil secretion, chemoattractant receptor mobilization, and enhanced functional capacity. J Immunol. 1982 Feb;128(2):941–948. [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Kürzinger K., Ho M. K., Springer T. A. Structural homology of a macrophage differentiation antigen and an antigen involved in T-cell-mediated killing. Nature. 1982 Apr 15;296(5858):668–670. doi: 10.1038/296668a0. [DOI] [PubMed] [Google Scholar]
- LeBien T. W., Bradley J. G., Koller B. Preliminary structural characterization of the leukocyte cell surface molecule recognized by monoclonal antibody TA-1. J Immunol. 1983 Apr;130(4):1833–1836. [PubMed] [Google Scholar]
- Muirhead K. A., Schmitt T. C., Muirhead A. R. Determination of linear fluorescence intensities from flow cytometric data accumulated with logarithmic amplifiers. Cytometry. 1983 Jan;3(4):251–256. doi: 10.1002/cyto.990030404. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Johnsson A., Wärmegård B., Seljelid R. Separation of human monocytes on density gradients of Percoll. J Immunol Methods. 1980;33(3):221–229. doi: 10.1016/0022-1759(80)90209-4. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Analysis of antigenic determinants on human monocytes and macrophages. Blood. 1982 Apr;59(4):775–786. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Van Agthoven A., Schlossman S. F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1(3):329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Ware C. F., Sanchez-Madrid F., Krensky A. M., Burakoff S. J., Strominger J. L., Springer T. A. Human lymphocyte function associated antigen-1 (LFA-1): identification of multiple antigenic epitopes and their relationship to CTL-mediated cytotoxicity. J Immunol. 1983 Sep;131(3):1182–1188. [PubMed] [Google Scholar]