Abstract
Objective
Temporomandibular joint diseases predominantly afflict women, suggesting a role for female hormones in the disease process. However, little is known about the role of estrogen receptor (ER) signaling in regulating mandibular condylar cartilage growth. Therefore, the goal of this study was to examine the effects of altered estrogen levels on the mandibular condylar cartilage in WT and ER beta KO mice.
Materials and Methods
21-day-old female WT (n=37) and ER beta KO mice (n=36) were either sham operated or ovariectomized, and treated with either placebo or estradiol. The mandibular condylar cartilage was evaluated by histomorphometry, proliferation was analyzed by double EdU/BrdU labeling, and assays on gene and protein expression of chondrocyte maturation markers were performed.
Results
In WT mice, ovariectomy caused a significant increase in mandibular condylar cartilage cell numbers, a significant increase in Sox9 expression and a significant increase in proliferation compared with sham operated WT mice. In contrast, ovariectomy did not cause any of these effects in the ER beta KO mice. Estrogen replacement treatment in ovariectomized WT mice caused a significant decrease in ER alpha expression and a significant increase in Sost expression compared with ovariectomized mice treated with placebo. Estrogen replacement treatment in ovariectomized ER beta KO mice caused a significant increase in Col2 expression, no change in ER alpha expression, and a significant increase in Sost expression.
Conclusion
Estrogen via ER beta inhibits proliferation and ER alpha expression while estrogen independent of ER beta induces Col2 and Sost expression.
Introduction
The mandibular condylar cartilage plays a central role in craniofacial disorders such as mandibular growth abnormalities (micrognathia or prognathia) and temporomandibular joint (TMJ) degeneration, afflicting over 50 million Americans 1, 2. The mandibular condylar cartilage differs from other articular cartilages in that it undergoes endochondral ossification, and robustly remodels during growth to mechanical loading cues 3. It is believed that there are a finite number of mandibular condylar cartilage progenitor cells; once the cells are depleted, repair remodeling and growth diminish in capacity. Like other joints, the TMJ degenerates with age 4, 5. However, women between the ages of 44–55 years are most likely to seek treatment 6, 7, suggesting a role for estrogen in the disease process.
In long bones, estrogen is responsible for endochondral growth plate fusion, in both males and females and the role of estrogen receptors in mediating long bone growth plate fusion has been characterized 8, 9. For example, estrogen via the estrogen receptor alpha is responsible for growth plate fusion in both male and female mice 8. In contrast, estrogen receptor beta is involved in growth inhibition in only young female mice 10. In fact, little is known about the role of estrogen and estrogen receptor signaling in the mandibular condylar cartilage. In our previous study, we focused on the role of ER beta in regulating mandibular condylar growth. We found that female ER beta KO mice had larger mandibular condylar cartilages, due to decreased cartilage turnover, compared with sex- and age-matched WT mice 11. However, the role of estrogen in mediating these effects is not known. Therefore, the purpose of this study was to characterize the mandibular condylar cartilage phenotype of female ER beta KO mice exposed to altered estrogen levels. A greater understanding of the role of estrogen receptors in regulating mandibular growth is desperately needed in order to take best advantage of the promising pharmacological advances provided by selective estrogen receptor modulators 12 in the treatment of mandibular growth abnormalities, and to understand the etiology behind the sex predilection of TMJ degeneration.
Material and Methods
Mice
Breeding pairs of C57Bl/6 wild type (Cat # 000664) and ERβ KO mice (homozygous male, heterozygous female, Cat # 004745) were purchased from Jackson Labs. Homozygous ERβ KO females were used for the experiments. 21-day-old female C57Bl/6 WT (total n=37) and ERβ KO mice (total n=36) were sham-operated or ovariectomized, and treated with placebo or 17β-estradiol for 28 days at a dose of 10-nanogram/gram body weight/day (60 day release, Innovative Research of America, FL). The mice were euthanized at 49 days of age (Table 1 for experiment design). This age was chosen because the vast majority of mandibular condylar cartilage growth in mice is complete by 60 days of age13. Sixteen hours prior to euthanasia, the mice were injected intraperitoneally with 10-ug 5-ethynyl-2′-deoxyuridine (EdU) per gram body weight. Three hours prior to euthanasia, mice were injected intraperitoneally with 0.1 mg bromodeoxyuridine (BrdU) per gram body weight. All experiments were performed in accordance with animal welfare based on an approved IACUC protocol #AAAD0950 (from the Columbia University animal care committee).
Table 1.
Experimental Design for mice of wild type and ER-beta knockout.
| Age of mice | 21-day old | 49-day old
|
||
|---|---|---|---|---|
| 16 hour prior to euthanasia | 3 hour prior to euthanasia | 49-day old | ||
| Treatment | Sham (WT, n=12; KO, n=12) Or OVX-Placeboa (WT, n=12; KO, n=12) Or OVX-Estradiola (WT, n=13; KO, n=12) |
EdU injection | BrdU injection | Euthanasia |
17β-Estradiol (SE-121) and placebo (SC-111) pellets were products of Innovative Research of America. The pellets were implanted under skin with a trochar and are designed to release continuously for 60 days.
Abbreviations: OVX: ovariectomy; EdU: 5-ethynyl-2′-deoxyuridine; BrdU: bromodeoxyuridine.
RNA Extraction and PCR Amplification
For each of the mice, the mandibular condylar heads (left and right) containing both the mandibular condylar cartilage and subchondral bone were carefully harvested and all the soft tissues were removed under a dissecting microscope. Total RNA was obtained from pooled left and right condylar heads and extracted with TRIzol Reagent (Invitrogen life technologies, Carlsbad, CA) following the manufacturer’s protocol. All mRNA samples were treated with DNA-free DNase Treatment & Removal Kit (Ambion by Life Technologies) to remove any possible DNA contamination. Total RNA was reverse transcribed into cDNA using the ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster city, CA) following the manufacturer’s protocol. Real-Time PCR gene expression for different genes was performed in separate wells (singleplex assay) of a 96-well-plate, in a reaction volume of 20 μl. Gapdh was used as endogenous control (Housekeeping gene, Applied Biosystems, Mm99999915_g1). Three replicates of each sample were amplified using Assays-on-Demand Gene Expression for the particular gene of interest and predesigned unlabeled gene-specific PCR primers and TaqMan MGB FAM dye-labeled probes. The PCR reaction mixture (including 2X TaqMan Universal PCR Master Mix, 20X Assays-on-Demand Gene Expression Assay Mix, 50 ng of cDNA) was run in an Applied Biosystems ABI Prism 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters. For the genes for which the efficiencies of target and endogenous control amplification were approximately equal, relative expression of a test sample compared to a reference calibrator sample (ΔΔCt Method) was used for data analysis. For the genes that were not amplified with the same efficiency as the endogenous control, the Relative Standard Curve method, in which target quantity was determined from the standard curve and divided by the target quantity of the calibrator, was used. Primers for Real-Time PCR were purchased from Applied Biosystems. Gene expression was performed for SRY-box containing gene 9 (Sox9—Mm00448840_m1), collagen type II (Col2a1—Mm00491889_m1), collagen type X (Col10a1—Mm00487041_m1), estrogen receptor alpha (Esr1—Mm00433149_m1), sclerostin (Sost—Mm00470479_m1), runt-related transcription factor 2(Runx2—Mm00501578_m1), and dentin matrix acidic phosphoprotein-1 (Dmp-1—Mm00803833_g1).
Histology and immunohistochemistry
Whole mouse heads were sectioned into two halves, fixed in 10% formalin for 4 days at room temperature and decalcified in 14% EDTA (pH 7.1) (Sigma, St Louis, MO) for 10 days (49 day samples). Subsequently, the samples were processed through progressive concentrations of ethanol, cleared in xylene, and embedded in paraffin. 5μm sagittal serial sections of the TMJ were performed by Microm HM 355s microtome (Thermo Fisher Scientific, Waltham, MA) and every 5th section stained with H&E.
Histomorphometry measurements were made in a blinded, nonbiased manner using the BioQuant computerized image analysis system (BioQuant, Nashville, TN). Mandibular condylar cartilage analysis was performed on H&E sagittal sections corresponding to the mid-coronal portion of the mandibular condylar head. The entire mandibular condylar cartilage area was divided into three layers, superficial layer (layer S), flattened layer (layer F), and hypertrophic layer (layer H) based on the cartilage cell size, shape, and staining. The entire anterior-posterior condylar cartilage area was measured for each section and the average of at least three sections was taken for that mouse. Six to seven condyles from each genotype and treatment groups were analyzed.
For immunohistochemistry, tissue sections were deparaffinized with xylene and rehydrated with decreasing concentrations of ethanol. Following rehydration, the sections were treated with 3 % peroxide to block endogenous peroxidase activity and digested for 60 minutes with pepsin for unmasking (Lab Vision, Cat # AP-9007-006, Fremont, CA). All sections were blocked with Protein Block Serum-Free (DakoCytomation, code # X0909, Carpinteria, CA). Immunohistochemical staining was performed using the LSAB + System-HRP Kit (DakoCytomation, code # K0690) following the procedure recommended by the manufacturer. The primary antibody used in this study was collagen type II antigen (Millipore, MAB8887, 1:200 dilution). BrdU immunohistochemical analysis was completed using a BrdU staining kit following the manufacturer’s instructions (Zymed Laboratories-Invitrogen Corporation, Carlsbad, CA, USA). EdU immunohistochemical analysis was completed using a Click-iT EdU Alexa Fluor@488 Imaging Kit following the manufacturer’s instructions (Invitrogen, CA). Negative controls consisted of omitting the primary antibody step and incubating with blocking solution. To quantify BrdU and EdU staining, the labeling index (number of BrdU or EdU positive cells/total number of cells) was calculated. Three to five sections, corresponding to the same anatomical area (mid sagittal), were counted for each condyle and the average of these sections was taken as the labeling index.
Statistical Analyses
Values are presented as the mean and 95% confidence intervals (CI) (lower and upper limits), with n=number of mice. For RNA analysis, RNA was pooled from both left and right condyles from the same mouse; however, one condyle from separate mice was sectioned for histologic analysis. Normality of distribution was confirmed and statistical significance of differences among means was determined by one-way analysis of variance (ANOVA), with, post hoc comparison of more than two means by the Bonferroni method using GraphPad Prism (San Diego, Ca). The statistical significance was defined as P < 0.05.
Results
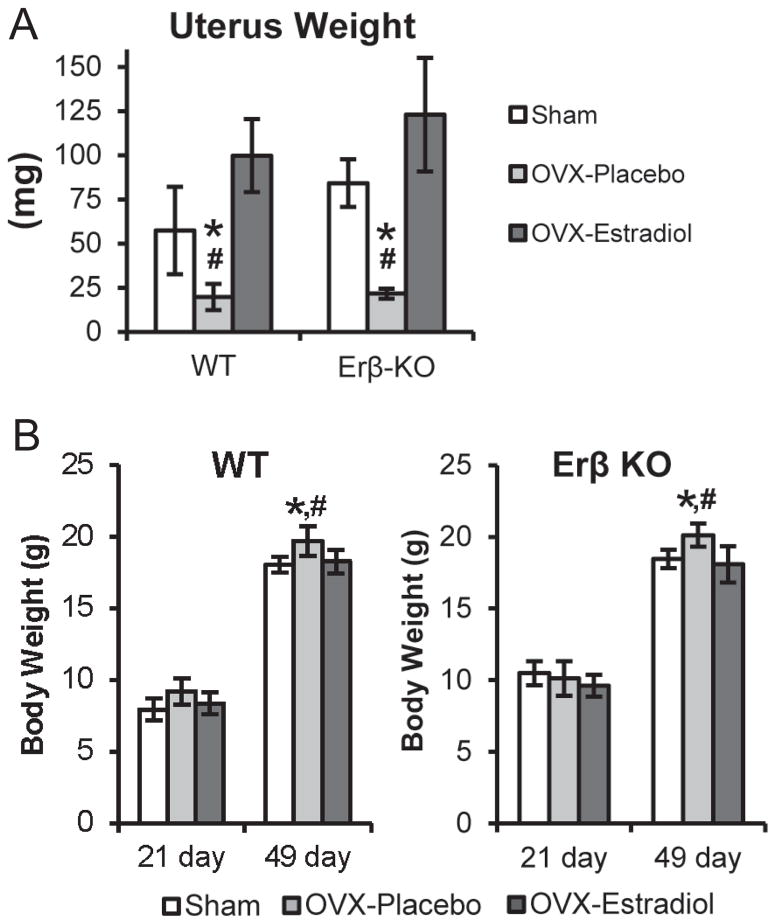
In both the WT and ER beta KO mice, uterus weights were significantly decreased by ovariectomy and returned back to basal levels by estrogen replacement therapy (Figure 1A). Ovariectomy also caused a significant increase in body weight in both 49-day-old WT and ER beta KO mice (Figure 1B).
Figure 1.
Uterus weights (A) of 49-day-old, and Body weights (B) of 21- and 49-day-old female WT and ER beta KO that were either sham-operated or ovariectomized and treated with placebo or estradiol (10 nanogram/gram body weight/day) for 4 weeks. n= 12–13 mice for each age, genotype and treatment. Values are the means with lower and upper limits of 95% CI. * = significant difference between Sham and OVX-placebo groups. #= significant difference between OVX-placebo and OVX-estradiol groups. In (A) WT, * p=0.004, # p=0.007; ER-beta KO, *and # p<0.001. In (B) WT, * p=0.005, # p=0.024; ER-beta KO, * p<0.001, # p=0.004.
Ovariectomy caused an increase in mandibular condylar cartilage growth in WT but not in ER beta KO mice
In WT mice, ovariectomy caused a significant increase in total cell numbers and in the number of cells in superficial zone compared with sham-operated WT mice. In contrast, ovariectomy in ER beta KO mice caused a significant decrease in the number of cells in the flattened zone compared with sham-operated ER beta KO mice.
Estradiol replacement treatment in ovariectomized WT mice caused a significant decrease in total cell numbers and a significant decrease in the number of cells in the superficial and hypertrophic zones compared with ovariectomized WT mice treated with placebo. Estradiol replacement treatment in ovariectomized ER beta KO mice caused a significant decrease in total cell numbers and a decrease in the number of cells in the flattened zone compared with sham-operated ER beta KO mice (Figure 2).
Figure 2.
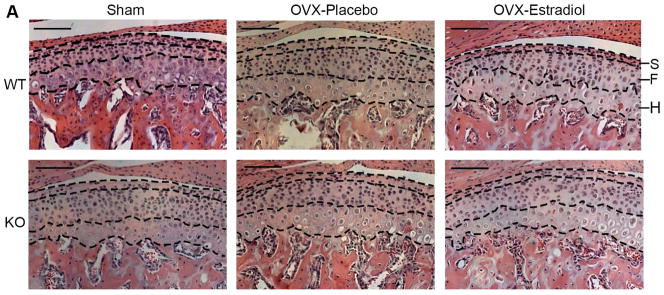
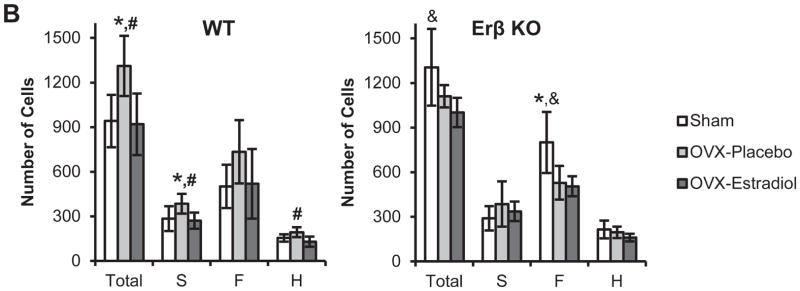
H & E staining (A) and cell counts (B) of the mandibular condylar cartilage from 49-day-old female WT and ER beta KO mice that were sham-operated or ovariectomized, and treated with either placebo or estradiol (10 nanogram/gram body weight/day). Cell counts were performed for the Superficial Layer (S, Articular and Polymorphic zones), Flattened Zone (F) and Upper Hypertrophic zone (H). The zones were delineated with black dashed lines. n=6–8 for each age and genotype. All images were taken under 10X magnification. Bars = 100μm. In (B) values are the means with lower and upper limits of 95% CI. * = significant difference between Sham and OVX-placebo groups. #= significant difference between OVX-placebo and OVX-estradiol groups. &= significant difference between Sham and OVX-estradiol groups. In WT total cell count, * and # p=0.006; S layer cell count, * p=0.029, # p=0.021; H layer cell count, # p=0.005. In ER-beta KO total cell count, & p=0.011; F layer cell count, * p=0.005, & p=0.004.
Proliferation
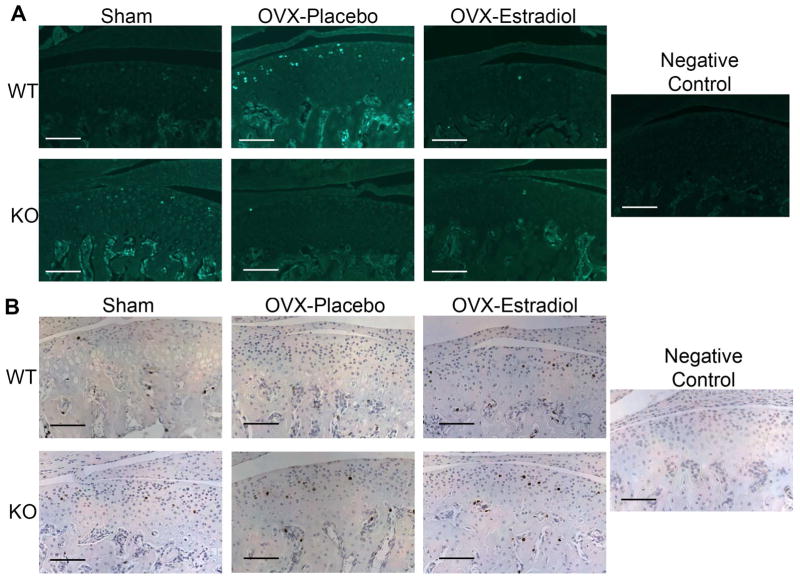
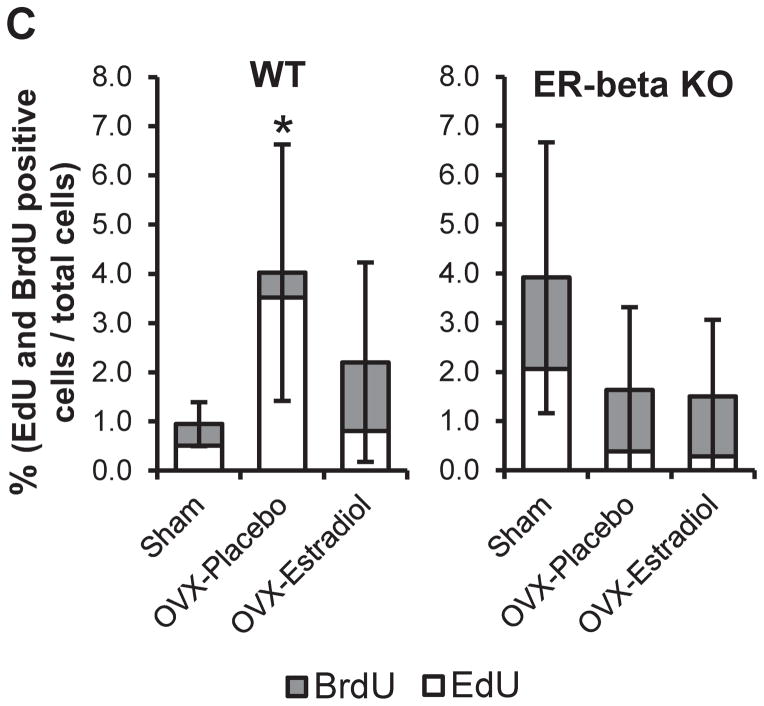
There was a significant increase in the percentage of EdU/BrdU labeled cells in the ovariectomized WT mice, compared to sham operated WT mice. However, no significant difference in EdU/BrdU labeling was evident in the ovariectomized ER beta KO mice treated with placebo or estradiol (Figure 3).
Figure 3.
EdU staining (A), BrdU staining (B) and Quantification of EdU/BrdU labeling (C). Mice were injected with EdU at 16 hours and BrdU at 3 hours prior to sacrifice. Immunohistochemistry for BrdU and EdU was performed on the mandibular condyles of female 49-day-old WT and ERβ KO mice that were sham-operated or ovariectomized, and treated with either placebo or estradiol (10 nanogram/gram body weight/day). (A) EdU positive cells were labeled with green fluorescence. (B) BrdU positive cells were labeled brown. All images were taken under 10X magnification. Bars = 100μm. (C) Labeling index = number of positive EdU or BrdU/total number of cells was calculated for each of the groups. n=6–8 for each age and genotype. Values are means with upper and lower limits of 95% CI. * significant difference (p=0.017) between WT Sham and OVX-placebo groups.
Gene and Protein expression
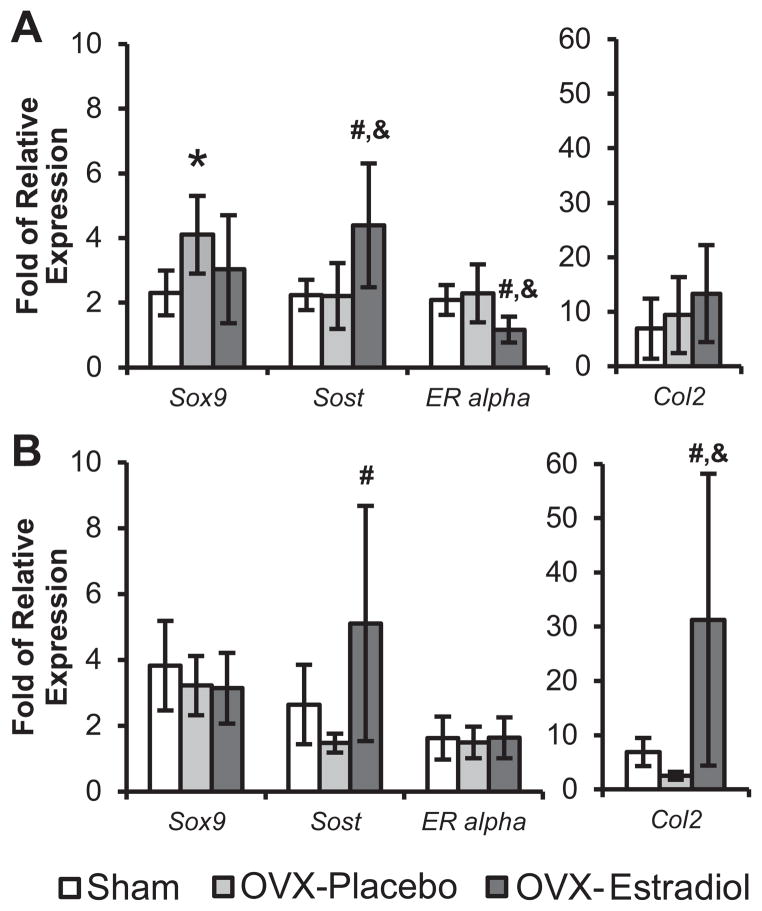
Ovariectomy caused a significant increase in Sox9 expression, compared with sham-operated WT mice. In ovariectomized WT mice, estradiol replacement treatment caused a significant decrease in ER alpha expression, a significant increase in Sost expression, and no effect on Col2 expression, compared with ovariectomized WT mice treated with placebo. In ovariectomized ER beta KO mice, estradiol replacement treatment caused a significant increase in Col 2 expression, no effect on ER alpha expression, and a significant increase in Sost expression, compared with ovariectomized ER beta KO mice treated with placebo (Figure 4). Altering the estrogen levels caused no significant changes in the expression of Col 10, Runx2, and Dmp-1 in both ER beta KO and WT mice (data not shown).
Figure 4.
Quantification of Sox9, Sost, Estrogen Receptor Alpha (ER Alpha), and Collagen type II (Col2) by Q-PCR was performed on the mandibular condyles of 49-day-old female sham-operated or ovariectomized WT and ERβ KO mice treated with placebo or estradiol. (A) WT. (B) ERβ KO. n=6–8 for each age and genotype. Values are means with upper and lower limits of 95% CI. * = significant difference between Sham and OVX-placebo groups. #= significant difference between OVX-placebo and OVX-estradiol groups. &= significant difference between Sham and OVX-estradiol groups. In (A), for Sox9, * p=0.049; for Sost, # p= 0.016, & p=0.015; for ER alpha, # p= 0.018, & p=0.047. In (B), for Sost, # p= 0.026; for Col2, # p= 0.013, & p=0.025.
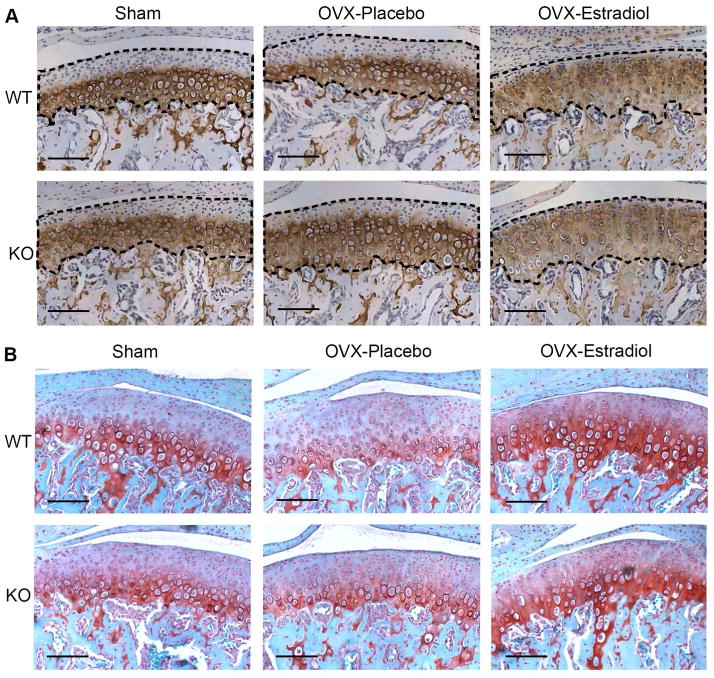
Immunohistochemistry for Collagen type 2 and Safranin O staining revealed that estradiol replacement treatment resulted in Col2 and Safranin O localization in the superior zones of the mandibular condylar cartilages of ovariectomized ER Beta KO and ovariectomized WT mice, compared with ovariectomized mice treated with placebo (Figure 5).
Figure 5.
Col2 Immunohistochemistry (A) and Safranin O staining (B) were performed on the mandibular condyles of 49-day-old female sham-operated or ovariectomized WT and ERβ KO mice treated with placebo or estradiol. In (A), the cartilage area was demarcated with black dashed line. All images were taken under 10X magnification. Bars= 100μm.
Discussion
Mandibular cartilage cell maturation proceeds through proliferation and migration of Sox9-expressing progenitor cells, from the polymorphic zone to the flattened zone where they undergo chondrogenesis. From there, they exit the proliferative pool and enter the hypertrophic zone where they undergo terminal maturation 14. We found that, similar to other studies, estrogen deficiency caused increased mandibular condylar cartilage growth 15, 16. In addition, we found that ovariectomy in WT mice resulted in an increase in Sox9 expression and a significant increase in proliferation, which were reversed by estradiol treatment. In contrast, neither of these effects occurred in the ER beta KO mice. Taken together, the results suggest that estrogen via the estrogen receptor inhibits the proliferation of Sox9 expressing cells.
In our previous study, we found that ER beta deficiency caused an increase in mandibular condylar cartilage growth in female mice 11. In that same study, we found that female WT mice expressed a relative decrease in the number of BrdU positive cells when injected with BrdU at 16 hours prior to sacrifice compared with 3 hours prior to sacrifice in which the injections were performed on two separate mice. This difference was not observed in the female ER beta KO mice. In our current study, we intended to similarly analyze proliferative pool exit by injecting EdU 16 hours and BrdU 3 hours prior to sacrifice (BrdU) in the same mice. However, due to possible cell cycle arrest caused by EdU injection 17 and because the average mandibular condylar cartilage cell cycle length is greater than 16 hours18, we combined the EdU and BrdU data as a measure of proliferation, as has been described in other studies 10.
Gene expression analysis revealed that estradiol replacement treatment caused a significant increase in Col2 expression in ovariectomized ER beta KO mice, and immunocytochemistry results indicated superior localization of Col2 in the mandibular condylar cartilage in ovariectomized WT mice. Taken together, the results suggest that the estradiol-induced increase in Col2 expression in the ER beta KO mice may be due to increased expression of Col2 in fibrocartilage cells. However, it remains to be determined why there is a discrepancy between the mRNA data and the immunocytochemistry results. During active growth, endochondral ossification occurs in the mandibular condylar cartilage and the superficial zones are devoid of Col2 expression. However, after the cessation of mandibular condylar cartilage growth and the depletion of polymorphic progenitor cells, the mandibular condylar cartilage becomes phenotypically similar to other articular hyaline cartilages and Col2 expression is detected throughout the mandibular condylar cartilage 19, 20. Therefore, in the TMJ, estradiol-induced superior localization of the Col2 may indicate accelerated cartilage differentiation.
There are two main estrogen receptors, alpha and beta. Recent research, on both humans and mice, has shown that growth plate fusion is mediated by ER alpha 8. Signaling interactions between ER beta and ER alpha may explain why estradiol replacement treatment did not cause a significant increase in Col2 expression in the ovariectomized WT mice. We found that estradiol treatment caused a significant decrease in ER alpha expression in WT mice. No such effect on ER alpha expression was observed in the estradiol treated ER beta KO mice, suggesting that ER beta signaling down regulates ER alpha expression. In addition, ER beta deficient osteoblastic cells exhibit an increase in ER alpha-mediated gene transcription, by an average of 85 % 21, suggesting that ER beta inhibits ER alpha activity. Future experiments are planned with ER alpha, ER beta and ER alpha/beta DKO mice in order to delineate the roles of ER alpha in regulating mandibular condylar cartilage growth and chondrogenesis.
A possible mechanism for the increase in chondrogenesis following estradiol treatment of the mandibular condylar cartilage is the inhibition of canonical wnt signaling by Sost 22. Studies have shown that Sost is more highly expressed in the articular and polymorphic zones than in the flattened and hypertrophic zones of mandibular condylar cartilage 23. However, the increase in Sost may also be due to increased expression in the subchondral bone. Future experiments are planned in order to determine the localization of estradiol-induced Sost expression in the TMJ. Since estradiol treatment was found to increase Sost expression in both ER beta KO and WT mice, we posit that Sost may be downstream of estrogen receptor alpha signaling. Studies have also shown that inhibition of canonical wnt signaling inhibits proliferation 24 and delays chondrocyte hypertrophy 25. This may help explain why estradiol treatment in the ovariectomized ER beta KO mice caused a decrease in the number flattened zone cells. The reason why ovariectomized ER beta KO also had a decrease in the number of flattened zone cells compared to sham operated mice is unknown and is being further investigated.
Similar to other joints, the TMJ degenerates with age, with over 70% of people between the ages of 73–75 showing signs of TMJ degeneration 4. Recent research has clearly dismissed the dogma that women of childbearing age are most likely to suffer from TMJ diseases; instead, it is most likely to occur in women between 35–54 years of age 6, 26, 27. This new data may lead to a shift in the role of estrogen in TMJ disease: from estrogen potentiating the inflammation that occurs during TMJ disease 28 to estrogen accelerating the disease process. This is in contrast to what typically occurs in articular hyaline cartilages, where estrogen is believed to have a protective effect on joint homeostasis 29. This distinction may be due to the fact that the mandibular condylar cartilage undergoes endochondral ossification during growth. We posit that the earlier cessation of mandibular condylar cartilage growth in women, due to estrogen, predisposes them to an increased likelihood of developing accelerated age-related TMJ degeneration. It is important to note that our study was performed on young growing mice when the bone and cartilage development was not complete, which does not rule out the possibility that estrogen may have a different role in the TMJ of older non-growing mice. Future work is planned to examine the effects of estrogen signaling in older mice.
In conclusion, we found that estrogen inhibits proliferation, via an ER beta dependent mechanism, and regulates chondrogenesis, independent of ER beta. A greater understanding of estrogen signaling in the mandibular condyle is needed in order to develop novel treatment modalities for patients with mandibular condylar growth abnormalities and TMJ degeneration, and benefit from advances in selective estrogen receptor modulator pharmacological therapies.
Acknowledgments
This work was supported by 1R01DE020097 (SW, HD) grant from the NIH.
Footnotes
Author contributions
J. Chen was involved in study design, data collection, data analysis, drafting and approval of the manuscript.
Y. Kamiya was involved in data collection, drafting and approval of the manuscript.
I. Polur was involved in data collection, drafting and approval of the manuscript.
M. Xu was involved in data collection, drafting and approval of the manuscript.
T. Choi was involved in data collection, drafting and approval of the manuscript.
Z. Kalajzic was involved in data collection, drafting and approval of the manuscript.
H. Drissi was involved in study design, drafting and approval of the manuscript.
S. Wadhwa was involved in study design, data analysis, drafting and approval of the manuscript.
J. Chen and S. Wadhwa take responsibility for the integrity of the work as a whole.
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 12(11 Suppl):T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proffit WR, Fields HW, Jr, Moray LJ. Prevalence of malocclusion and orthodontic treatment need in the United States: estimates from the NHANES III survey. Int J Adult Orthodon Orthognath Surg. 1998;13(2):97–106. [PubMed] [Google Scholar]
- 3.Owtad P, Park JH, Shen G, Potres Z, Darendeliler MA. The biology of TMJ growth modification: a review. J Dent Res. 92(4):315–21. doi: 10.1177/0022034513476302. [DOI] [PubMed] [Google Scholar]
- 4.Schmitter M, Essig M, Seneadza V, Balke Z, Schroder J, Rammelsberg P. Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofac Radiol. 39(4):231–4. doi: 10.1259/dmfr/16270943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luder HU. Factors affecting degeneration in human temporomandibular joints as assessed histologically. Eur J Oral Sci. 2002;110(2):106–13. doi: 10.1034/j.1600-0722.2002.11212.x. [DOI] [PubMed] [Google Scholar]
- 6.Manfredini D, Piccotti F, Ferronato G, Guarda-Nardini L. Age peaks of different RDC/TMD diagnoses in a patient population. J Dent. 38(5):392–9. doi: 10.1016/j.jdent.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Lamot U, Strojan P, Surlan Popovic K. Magnetic resonance imaging of temporomandibular joint dysfunction-correlation with clinical symptoms, age, and gender. Oral Surg Oral Med Oral Pathol Oral Radiol. 116(2):258–63. doi: 10.1016/j.oooo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Borjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor alpha in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 70(21):4023–37. doi: 10.1007/s00018-013-1317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chagin AS, Savendahl L. Oestrogen receptors and linear bone growth. Acta Paediatr. 2007;96(9):1275–9. doi: 10.1111/j.1651-2227.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 10.Chagin AS, Lindberg MK, Andersson N, et al. Estrogen receptor-beta inhibits skeletal growth and has the capacity to mediate growth plate fusion in female mice. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2004;19(1):72–7. doi: 10.1359/JBMR.0301203. [DOI] [PubMed] [Google Scholar]
- 11.Kamiya Y, Chen J, Xu M, et al. Increased mandibular condylar growth in mice with estrogen receptor beta deficiency. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 28(5):1127–34. doi: 10.1002/jbmr.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson ER, Wardell SE, McDonnell DP. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis. Bone. 53(1):42–50. doi: 10.1016/j.bone.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Festing M. A multivariate analysis of subline divergence in the shape of the mandible in C57BL-Gr mice. Genet Res. 1973;21(2):121–32. doi: 10.1017/s0016672300013306. [DOI] [PubMed] [Google Scholar]
- 14.Shibukawa Y, Young B, Wu C, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Developmental dynamics: an official publication of the American Association of Anatomists. 2007;236(2):426–34. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 15.Fujita T, Kawata T, Tokimasa C, Kaku M, Kawasoko S, Tanne K. Influences of ovariectomy and orchiectomy on the remodeling of mandibular condyle in mice. J Craniofac Genet Dev Biol. 1998;18(3):164–70. [PubMed] [Google Scholar]
- 16.Okuda T, Yasuoka T, Nakashima M, Oka N. The effect of ovariectomy on the temporomandibular joints of growing rats. J Oral Maxillofac Surg. 1996;54(10):1201–10. doi: 10.1016/s0278-2391(96)90352-3. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 17.Kohlmeier F, Maya-Mendoza A, Jackson DA. EdU induces DNA damage response and cell death in mESC in culture. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2013;21(1):87–100. doi: 10.1007/s10577-013-9340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182(3):197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- 19.Wadhwa S, Embree MC, Kilts T, Young MF, Ameye LG. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2005;13(9):817–27. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Wadhwa S, Embree M, Ameye L, Young MF. Mice deficient in biglycan and fibromodulin as a model for temporomandibular joint osteoarthritis. Cells Tissues Organs. 2005;181(3–4):136–43. doi: 10.1159/000091375. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg MK, Moverare S, Skrtic S, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17(2):203–8. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 22.Costa AG, Bilezikian JP. Sclerostin: therapeutic horizons based upon its actions. Curr Osteoporos Rep. 10(1):64–72. doi: 10.1007/s11914-011-0089-5. [DOI] [PubMed] [Google Scholar]
- 23.Hinton RJ, Serrano M, So S. Differential gene expression in the perichondrium and cartilage of the neonatal mouse temporomandibular joint. Orthod Craniofac Res. 2009;12(3):168–77. doi: 10.1111/j.1601-6343.2009.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Zhu M, Awad H, et al. Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. Journal of cell science. 2008;121(Pt 9):1455–65. doi: 10.1242/jcs.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu C, Wan Y, Cao J, et al. Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification. Bone. 2013;53(2):566–74. doi: 10.1016/j.bone.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Manfredini D, Arveda N, Guarda-Nardini L, Segu M, Collesano V. Distribution of diagnoses in a population of patients with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol. 114(5):e35–41. doi: 10.1016/j.oooo.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Guarda-Nardini L, Piccotti F, Mogno G, Favero L, Manfredini D. Age-related differences in temporomandibular disorder diagnoses. Cranio. 30(2):103–9. doi: 10.1179/crn.2012.015. [DOI] [PubMed] [Google Scholar]
- 28.Kou XX, Wu YW, Ding Y, et al. 17beta-estradiol aggravates temporomandibular joint inflammation through the NF-kappaB pathway in ovariectomized rats. Arthritis and rheumatism. 2011;63(7):1888–97. doi: 10.1002/art.30334. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen RH, Christiansen C, Stolina M, Karsdal MA. Oestrogen exhibits type II collagen protective effects and attenuates collagen-induced arthritis in rats. Clinical and experimental immunology. 2008;152(1):21–7. doi: 10.1111/j.1365-2249.2008.03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]