To the Editor:
Natural killer (NK) cells are lymphocytes that are important for host defense against viral and bacterial infections as well as malignant transformation. They have the capacity to kill target cells and to produce cytokines upon activation without previous sensitization. Although NK cells have been traditionally categorized as members of the innate immune system, recent studies have shown that NK cells have the ability to modify their effector function based on previous cytokine and/or activating receptor-mediated stimulation (1–3). The first evidence that NK cells can remember came from studies showing that they mediated the recall responses to haptens in mice lacking T and B cells using a contact hypersensitivity model (4). NK cell memory has also been demonstrated in viral infections using murine models (3). In these models, NK cells acquired long-lived memory to influenza A, vesicular stomatitis virus and human immunodeficiency virus antigens (5). Additionally, development of long-lived NK cell memory to mouse cytomegalovirus (MCMV) infection was shown to be dependent on IL-12 mediated signals (3, 6). Even without exposure to specific antigens, ex vivo short exposure of mouse and human NK cells to activating cytokines, such as IL-12, IL-15 and IL-18, elicits “memory-like” properties that are defined as enhanced effector functions after restimulation (1, 7, 8).
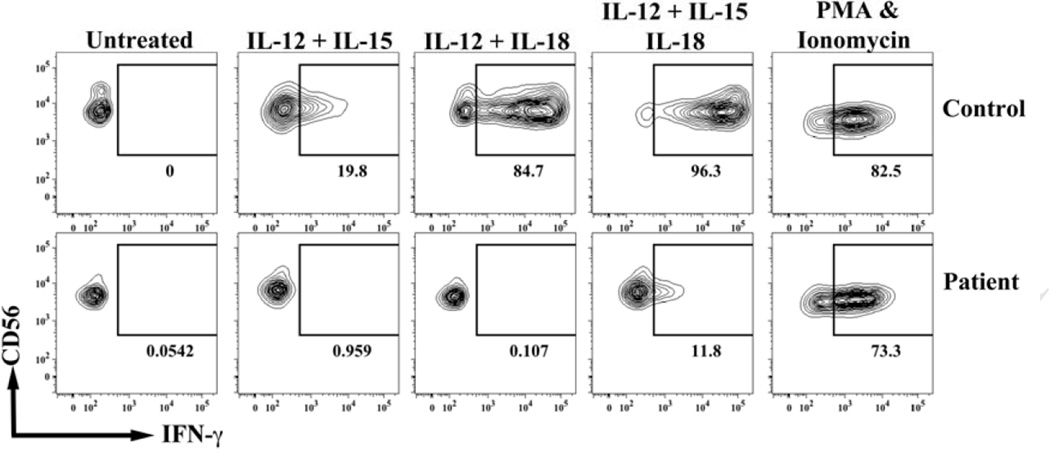
In this study we tested the role of IL-12 mediated signals in the in vitro generation of human NK cells with enhanced effector function after restimulation. In our opinion, the best method to address this issue would involve the use of PBMCs from patients with a deficiency in IL-12 or IL-12 receptor (IL-12R), since we could then exclude that the NK cells used in the experiments had been activated at any time in vivo by IL-12. We have previously described a 19-month-old patient with an IL-12Rβ1 deficiency due to a complex mutation at exon 14 in the IL12RB1 gene (c.1623_1624delinsTT; p.Q541X) (9). We showed that IFN-γ production was markedly decreased after stimulation of PBMCs with phytohemagglutinin (PHA) or PHA plus IL-12 and that there was diminished STAT4 phosphorylation after IL-12 stimulation (9). Here, we specifically tested if the NK cells from this patient responded to IL-12 stimulation. Flow cytometric analyses showed this patient’s NK cells did not produce IFN-γ when they were stimulated with IL- 12 + IL-15 or IL-12 + IL-18 (Fig 1). On the other hand, approximately 20% of the NK cells from the age-matched healthy control produced IFN-γ when they were stimulated with IL-12 + IL-15, and 85% of the NK cells produced IFN-γ when they were stimulated with IL-12 + IL-18 (Fig 1). When NK cells were stimulated with a combination of the three cytokines, almost all NK cells from the healthy control produced IFN-γ, while only 12% of the NK cells from the patient expressed IFN-γ. Furthermore, on a per cell basis the NK cells from the patient produced less IFN-γ than the NK cells from the control, as shown by median fluorescence intensity (MFI) of 921 for the IFN-γ producing NK cells from the patient versus a MFI of 35205 for the IFN-γ producing NK cells from the healthy control. We excluded that the patient had a defect in the IFNG gene or its regulation because her NK cells and those from the control produced similar amounts of IFN-γ in response to phorbol 12-myristate 13-acetate (PMA) plus ionomycin (Fig 1). As expected, these results corroborated that NK cells from an IL-12Rβ1 deficient patient do not respond to IL-12.
FIG. 1.
Decreased IFN-γ production by IL-12Rβ1 deficient NK cells in response to IL-12 in combination with IL-15 and/or IL-18. PBMCs were stimulated with different combinations of IL-12 (10 ng/mL), IL-15 (10 ng/mL) and IL-18 (50 ng/mL) for 16 hours or with PMA (50 ng/mL) and ionomycin (1 µM) for 6 hours. For the last 6–8 hours monensin was added to the culture. Then, cell surface receptor expression and intracellular IFN-γ production were determined by flow cytometry. Lymphocytes were electronically gated based on the forward and side scatter parameters and NK cells were identified by CD3-CD56+ phenotype.
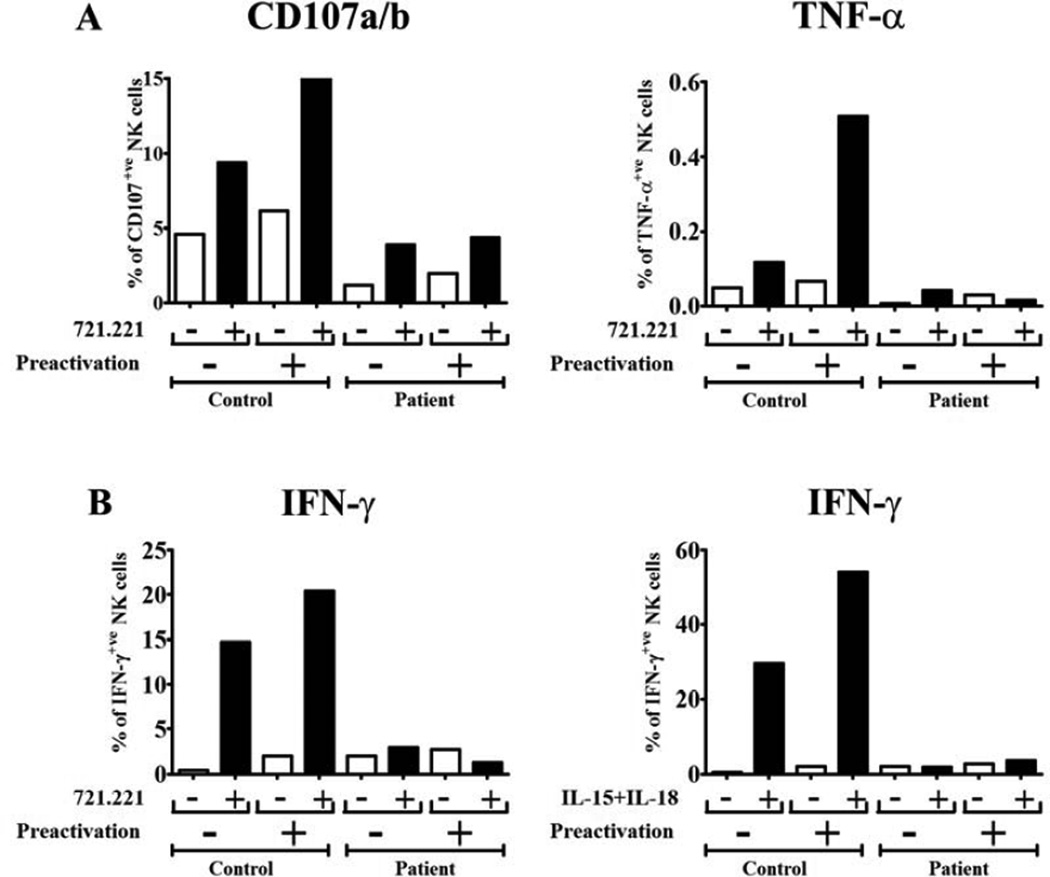
Next, we evaluated the role of IL-12-mediated signals in the generation of NK cells with enhanced effector functions after restimulation, or “memory-like” NK cells as reported (1, 8), following preactivation with cytokines (Fig 2). We stimulated PBMCs with IL-12 + IL-15 + IL-18 for 16-hours, followed by washes and a 7-day rest period in vitro with survival supported by low concentrations of IL-15. After the resting period, cells were harvested and co-incubated with 721.221 target cells (EBV-transformed B cells). The expression of CD107 (CD107a and CD107b), as surrogates for degranulation, triggered by 721.221 tumor cells was measured by flow cytometry. Preactivated NK cells from the healthy control degranulated more than non-preactivated NK cells after co-incubation with 721.221 cells (15.4% versus 9.4%) (Fig 2A, left panel). However, preactivated and non-preactivated NK cells from the patient showed similar degranulation after co-incubation with 721.221 cells (4.3% versus 4%) (Fig 2A, left panel). These results suggest there is no significant increase in the degranulation capabilities after pre-activation with cytokines in NK cells lacking IL-12 mediated signaling.
FIG. 2.
Absence of enhanced effector functions after re-stimulation by IL-12Rβ1 deficient NK cells preactivated with IL-12 + IL-15 + IL-18. A) PBMCs were cultured for 16 hours with either low concentrations of IL-15 (1 ng/mL) (non-preactivated) or with IL-12 (10 ng/mL), IL-15 (10 ng/mL) and IL-18 (50 ng/mL) (preactivated). Next, cells were washed with PBS and cultured in Iscove’s modified medium supplemented with 10% of human AB serum plus 1 ng/mL of IL-15. After 7 days, cells were harvested and incubated with 721.221 cells for 6 hours in the presence of anti-CD107a and anti- CD107b mAbs. The secretion inhibitor monensin was added for the last 5 hours. Then, the CD107a/b expression (left panel) and TNF-α production (right panel) by NK cells were determined by flow cytometry. Lymphocytes were electronically gated based on the forward and side scatter parameters and NK cells were identified by CD3-CD56+ phenotype. B) PBMCs from the healthy control and the second patient were cultured as in A. After 7 days, cells were harvested and incubated with 721.221 cells or with IL-15 + IL-18 for 16 hours. Monensin was added for the last 5 hours. Then, the IFN-γ production by NK cells in response to re-stimulation with 721.221 cells (left panel) and IL-15 + IL- 18 (right panel) was measured by flow cytometry.
We also measured TNF-α production by NK cells after co-incubation with 721.221 cells (Fig 2A, right panel). Similar to the degranulation results, preactivated NK cells from the healthy control produced more TNF-α than non-preactivated NK cells (0.51% versus 0.1%) (Fig 2A, right panel), while there were no differences in the TNF-α production when preactivated and non-preactivated NK cells from the patient were compared (0.02% versus 0.04%) (Fig 2A, right panel). In order to confirm that the observed results were related with the impaired IL-12 signaling rather than with a particular mutation, a patient with a different homozygous null IL12RB1 mutation (c.1495C>T; p. Q499X) and impaired STAT4 phosphorylation upon IL-12 stimulation (see Fig E1 in this article´s Online Repository at www.jacionline.org), was also tested. Similar to the results obtained with cells from the first patient, preactivated NK cells from the healthy control produced more IFN-γ than non-preactivated NK cells after restimulation with 721.221 cells (20.4% versus 14.7%) and IL-15 + IL-18 (54.1% versus 29.7%) (Fig 2B). However, preactivated and non-preactivated NK cells from the second patient showed similar IFN-γ production after restimulation with 721.221 cells (1.29% versus 3.06%) and IL-15 + IL-18 (3.60% versus 1.94%) (Fig. 2B). These results indicate that IL-12Rβ1-deficient NK cells do not have the ability to acquire enhanced effector functions after restimulation. In addition to the defect in restimulation, the IL-12Rβ1- deficient NK cells also have a defect in primary activation. The deficiency in IFN-γ production after target and IL-15 + IL-18 stimulation, and in the absence of preactivation, suggests that human NK cells require in vivo priming with IL-12 to acquire full functional reactivity, and is consistent with a study by Guia et al (10).
Our observations are partly in contradiction to those published by Romee et al. (8), who showed that after preactivation of human NK cells with IL-12 + IL-15, re- stimulation with K562 target cells led to an increase in IFN-γ production without an increase in the cell surface expression of CD107a on NK cells. Although we do not fully understand the reasons for this discrepancy, the use of different target cells and the staining with both anti-CD107a and anti-CD107b mAbs in our experiments may explain the differences. In fact, our results are in accordance with those obtained with the MCMV infection model which demonstrated that degranulation by mouse memory NK cells was enhanced after stimulation with anti-NK1.1 mAb, (3) and the generation of memory NK cells was dependent on IL-12-mediated signals (6).
Here, using human IL-12Rβ1-deficient NK cells, we have demonstrated the essential role of IL-12-mediated signals in the generation of human NK cells with enhanced effector function after restimulation. These findings provide the rationale for including brief stimulations or preactivation with IL-12, in combination with other cytokines such as IL-15 and IL-18, to generate NK cells with enhanced effector functions, i.e. “memory-like”, that may be of great relevance in the field of NK-cell based immunotherapy and may also unveil a new aspect of pathophysiology to be considered in IL-12Rβ1 deficient patients.
Supplementary Material
Acknowledgments
Funding: This work was funded by the intramural programs of the Food and Drug Administration and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Health Department, Basque Government (Grant 2013111034).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011 Jun;12(6):500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 3.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009 Jan 29;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell- independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006 May;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 5.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010 Dec;11(12):1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012 May 7;209(5):947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012 Dec 17;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012 Dec 6;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruenberg DA, Anover-Sombke S, Gern JE, Holland SM, Rosenzweig SD, Torgerson TR, et al. Atypical presentation of IL-12 receptor beta1 deficiency with pneumococcal sepsis and disseminated nontuberculous mycobacterial infection in a 19- month-old girl born to nonconsanguineous US residents. J Allergy Clin Immunol. 2010 Jan;125(1):264–265. doi: 10.1016/j.jaci.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guia S, Cognet, de Beaucoudrey L, Tessmer MS, Jouanguy E, Berger C, et al. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008 May 15;111(10):5008–5016. doi: 10.1182/blood-2007-11-122259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.