Abstract
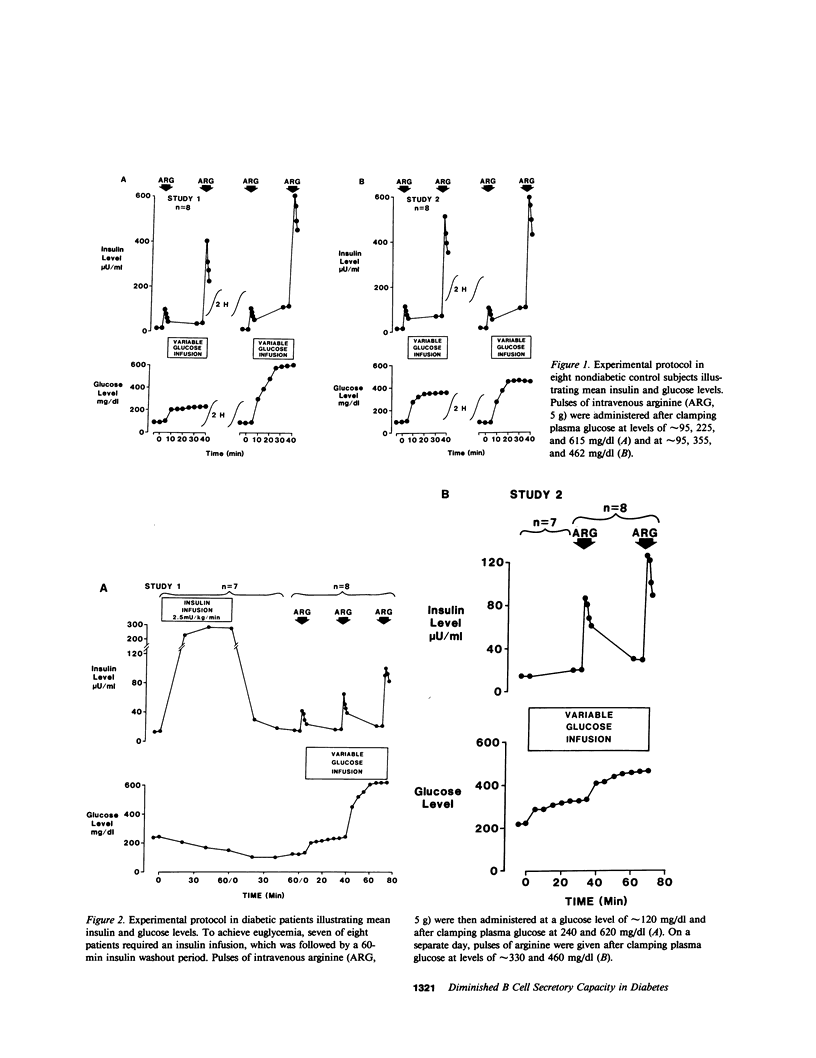
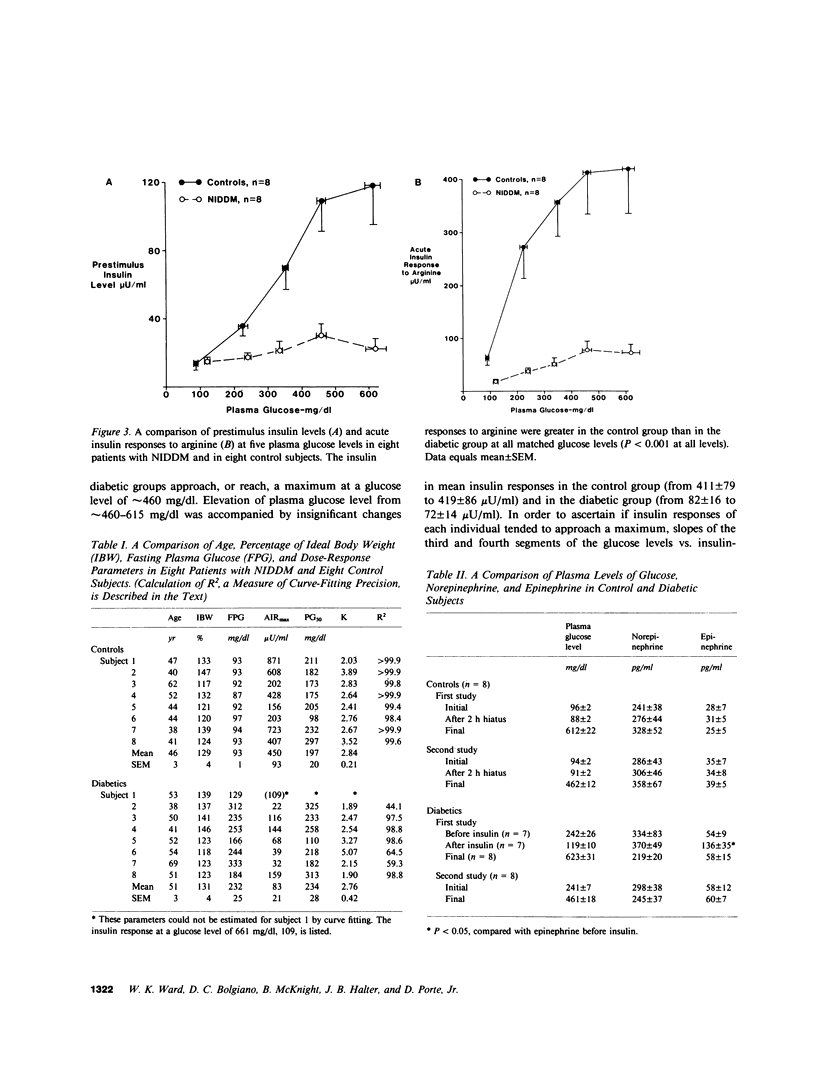
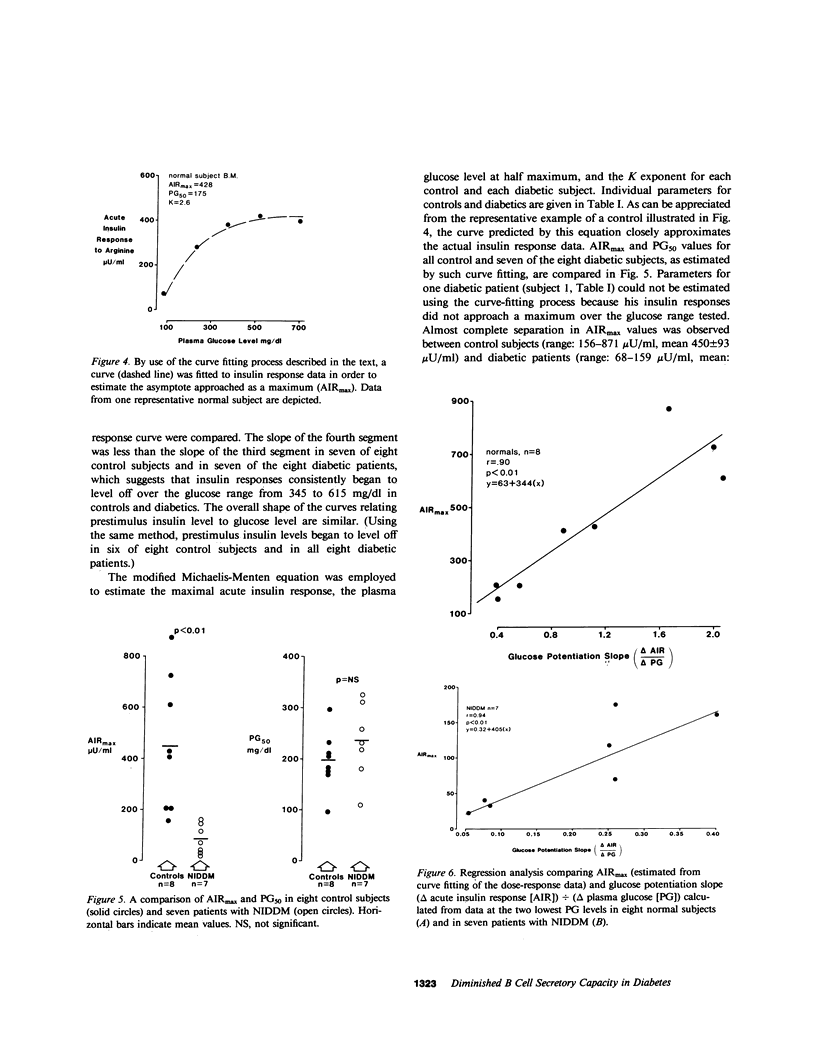
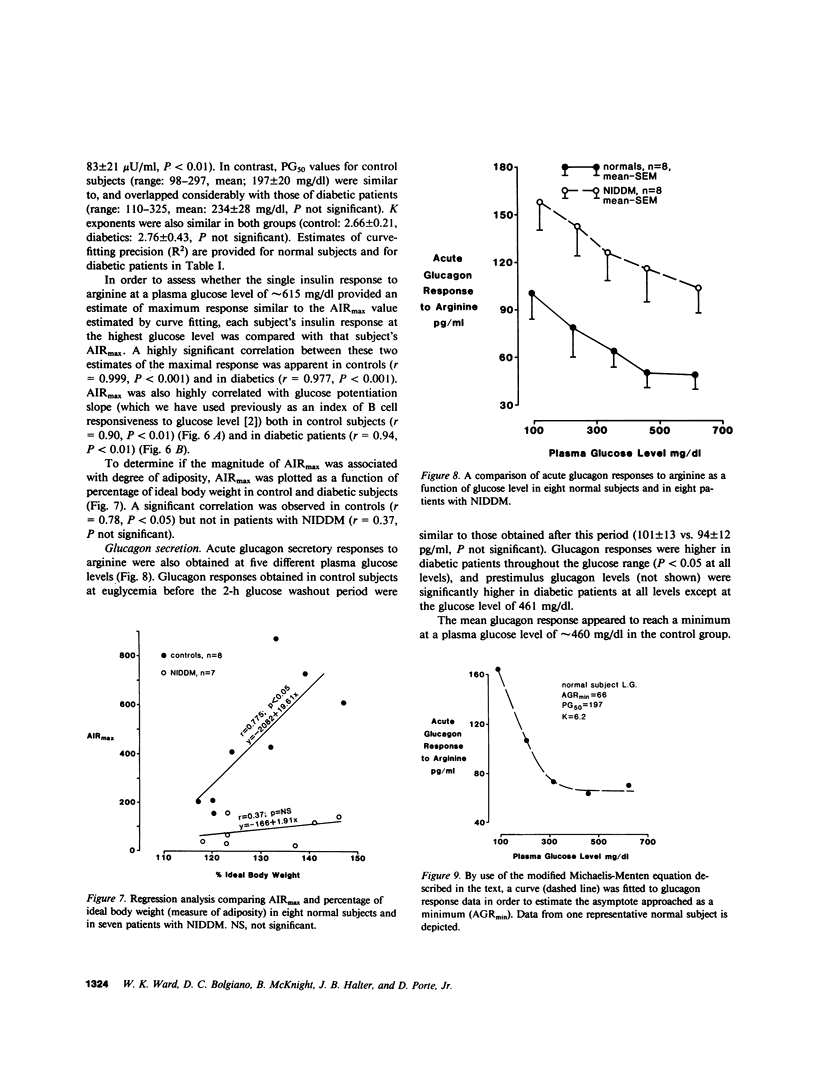
In order to assess whether patients with noninsulin-dependent diabetes mellitus (NIDDM) possess normal insulin secretory capacity, maximal B cell responsiveness to the potentiating effects of glucose was estimated in eight untreated patients with NIDDM and in eight nondiabetic controls. The acute insulin response to 5 g intravenous arginine was measured at five matched plasma glucose levels that ranged from approximately 100-615 mg/dl. The upper asymptote approached by acute insulin responses (AIRmax) and the plasma glucose concentration at half-maximal responsiveness (PG50) were estimated using nonlinear regression to fit a modification of the Michaelis-Menten equation. In addition, glucagon responses to arginine were measured at these same glucose levels to compare maximal A cell suppression by hyperglycemia in diabetics and controls. Insulin responses to arginine were lower in diabetics than in controls at all matched glucose levels (P less than 0.001 at all levels). In addition, estimated AIRmax was much lower in diabetics than in controls (83 +/- 21 vs. 450 +/- 93 microU/ml, P less than 0.01). In contrast, PG50 was similar in diabetics and controls (234 +/- 28 vs. 197 +/- 20 mg/dl, P equals NS) and insulin responses in both groups approached or attained maxima at a glucose level of approximately 460 mg/dl. Acute glucagon responses to arginine in patients with NIDDM were significantly higher than responses in controls at all glucose levels. In addition, although glucagon responses in control subjects reached a minimum at a glucose level of approximately 460 mg/dl, responses in diabetics declined continuously throughout the glucose range and did not reach a minimum. Thus, A cell sensitivity to changes in glucose level may be diminished in patients with NIDDM. In summary, patients with NIDDM possess markedly decreased maximal insulin responsiveness to the potentiating effects of glucose. Such a defect indicates the presence of a reduced B cell secretory capacity and suggests a marked generalized impairment of B cell function in patients with NIDDM.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard J. C., Weinberg C., Pfeifer M. A., Best J. D., Halter J. B., Porte D., Jr Interaction of glucose and epinephrine in the regulation of insulin secretion. Diabetes. 1982 Sep;31(9):802–807. doi: 10.2337/diab.31.9.802. [DOI] [PubMed] [Google Scholar]
- Botha J. L., Vinik A. I., Blake K. C., Jackson W. P. Kinetics of insulin secretion in chronic pancreatitis and mild maturity onset diabetes. (Evidence for "gut hormone" action beyond glucoreceptor and cyclic adenosine monophosphate mediated insulin release). Eur J Clin Invest. 1976 Sep 10;6(5):365–372. doi: 10.1111/j.1365-2362.1976.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Brooks J. R. Operative approach to pancreatic carcinoma. Semin Oncol. 1979 Sep;6(3):357–367. [PubMed] [Google Scholar]
- Cerasi E., Luft R., Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes. 1972 Apr;21(4):224–234. doi: 10.2337/diab.21.4.224. [DOI] [PubMed] [Google Scholar]
- Cerasi E. Potentiation of insulin release by glucose in man. I. Quantitative analysis of the enhancement of glucose-induced insulin secretion by pretreatment with glucose in normal subjects. Acta Endocrinol (Copenh) 1975 Jul;79(3):483–501. [PubMed] [Google Scholar]
- DeFronzo R. A., Hendler R., Christensen N. Stimulation of counterregulatory hormonal responses in diabetic man by a fall in glucose concentration. Diabetes. 1980 Feb;29(2):125–131. doi: 10.2337/diab.29.2.125. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Halter J. B., Porte D., Jr Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem. 1978 Apr;24(4):567–570. [PubMed] [Google Scholar]
- Gepts W., Lecompte P. M. The pancreatic islets in diabetes. Am J Med. 1981 Jan;70(1):105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):833–841. doi: 10.1172/JCI107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972 Aug;51(8):2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H. J., Menzel R., Gottschling H. D., Jahr D. Enhancement of glucose-stimulated insulin secretion from isolated rat pancreatic islets by human insulin antibodies. Acta Endocrinol (Copenh) 1976 Sep;83(1):123–132. doi: 10.1530/acta.0.0830123. [DOI] [PubMed] [Google Scholar]
- Halter J. B., Graf R. J., Porte D., Jr Potentiation of insulin secretory responses by plasma glucose levels in man: evidence that hyperglycemia in diabetes compensates for imparied glucose potentiation. J Clin Endocrinol Metab. 1979 Jun;48(6):946–954. doi: 10.1210/jcem-48-6-946. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Malvaux P., Lambert A. E. Glucagon immunoassay using polyethylene glycol to precipitate antibody-bound hormone. Diabetologia. 1974 Feb;10(1):61–68. doi: 10.1007/BF00421415. [DOI] [PubMed] [Google Scholar]
- Hollander P. M., Asplin C. M., Palmer J. P. Glucose modulation of insulin and glucagon secretion in nondiabetic and diabetic man. Diabetes. 1982 Jun;31(6 Pt 1):489–495. doi: 10.2337/diab.31.6.489. [DOI] [PubMed] [Google Scholar]
- Iversen J., Miles D. W. Evidence for a feedback inhibition of insulin on insulin secretion in the isolated, perfused canine pancreas. Diabetes. 1971 Jan;20(1):1–9. doi: 10.2337/diab.20.1.1. [DOI] [PubMed] [Google Scholar]
- Karam J. H., Grodsky G. M., Ching K. N., Schmid F., Burrill K., Forsham P. H. "Staircase" glucose stimulation of insulin secretion in obesity. Measure of beta-cell sensitivity and capacity. Diabetes. 1974 Sep;23(9):763–770. doi: 10.2337/diab.23.9.763. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Horwitz D. L., Jennings A. S., Chiasson J. L., Keller U., Rubenstein A. H. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes. 1978 May;27(5):563–570. doi: 10.2337/diab.27.5.563. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Nesher R., Tuch B., Hage C., Levy J., Cerasi E. Time-dependent inhibition of insulin release: suppression of the arginine effect by hyperglycaemia. Diabetologia. 1984 Feb;26(2):142–145. doi: 10.1007/BF00281122. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Walter R. M., Ensinck J. W. Arginine-stimulated acute phase of insulin and glucagon secretion. I. in normal man. Diabetes. 1975 Aug;24(8):735–740. doi: 10.2337/diab.24.8.735. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. A., Halter J. B., Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981 Mar;70(3):579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Levey G. S. Principles of membrane receptor physiology and their application to clinical medicine. Ann Intern Med. 1980 May;92(5):663–680. doi: 10.7326/0003-4819-92-5-663. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Gutzeit A., Kikuchi M., Cerasi E., Renold A. E. Defective early phase insulin release in perifused isolated pancreatic islets of spiny mice (Acomys cahirinus). Diabetologia. 1975 Oct;11(5):457–465. doi: 10.1007/BF00429916. [DOI] [PubMed] [Google Scholar]
- Saito K., Yaginuma N., Takahashi T. Differential volumetry of A, B and D cells in the pancreatic islets of diabetic and nondiabetic subjects. Tohoku J Exp Med. 1979 Nov;129(3):273–283. doi: 10.1620/tjem.129.273. [DOI] [PubMed] [Google Scholar]
- Santiago J. V., Clarke W. L., Shah S. D., Cryer P. E. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab. 1980 Oct;51(4):877–883. doi: 10.1210/jcem-51-4-877. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med. 1981 Jun 18;304(25):1518–1524. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]
- Ward W. K., Halter J. B., Beard J. C., Porte D., Jr Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol. 1984 May;246(5 Pt 1):E405–E411. doi: 10.1152/ajpendo.1984.246.5.E405. [DOI] [PubMed] [Google Scholar]
- Warren K. W., Braasch J. W., Thum C. W. Diagnosis and surgical treatment of carcinoma of the pancreas. Curr Probl Surg. 1968 Jun;:3–70. [PubMed] [Google Scholar]
- Westermark P., Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978 Nov;15(5):417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]



